Abstract
This study aimed to investigate the underlying molecular mechanism of ouabain-induced apoptosis and inhibited viability of tubulointerstitial cells in lupus nephritis (LN). Serum ouabain expression in 21 LN patients and 20 healthy controls (HCs) was detected by ELISA. Na-K-ATPase α1 (NKA) expression in kidney tissue from 21 LN patients and 6 controls who underwent nephrectomy was determined by immunohistochemistry assay. HK-2 cells were treated by ouabain with concentration 0 nM (control), 0.1 nM, 1 nM, 10 nM, 100 nM and 1000 nM for 72 h; MTT assay was performed at 24 h, 48 h and 72 h to detect cells viability, AV/PI assay was conducted at 24 h to detect the cell apoptosis. NKA expression was detected by immunofluorescence assay and western blot (WB) while pSrc, pERK, pAkt, pS6K and caspase 3 expression was detected by WB after 100 nM ouabain treatment. Serum ouabain was elevated in LN patients compared to HCs, while kidney NKA was reduced in LN patients compared with controls. HK-2 cell viability by MTT assay was decreased while cell apoptosis by AV/PI was increased at each time point after ouabain 0.1 nM, 1 nM, 10 nM, 100 nM and 1000 nM treatment compared to control group. Both immunofluorescence and WB disclosed that NKA expression was decreased at 12 h and 24 h compared to 0 h after 100 nM ouabain treatment. Moreover, pSrc, pERK, pAkt, pS6K and caspase 3 expressions were elevated after 100 nM ouabain treatment. In conclusion, ouabain may contribute to LN etiology by inhibiting human proximal tubular cell viability and promoting cells apoptosis through regulating NKA, pSrc, pERK, pAkt, pS6K and caspase 3.
Keywords: Lupus nephritis (LN), ouabain, Na-K-ATPase α1 (NKA), cell viability, apoptosis
Introduction
Lupus nephritis (LN), as a prototypical human autoimmune disease, is a common and severe manifestation of systemic lupus erythematosus (SLE), which is the leading cause of morbidity and mortality in SLE patients [1-3]. The development and progression of nephritis in patients with SLE results from multiple pathogenic pathways including aberrant apoptosis, autoantibody production, immune complex deposition, and complement activation. Among these causes, aberrant apoptosis which leads to glomerular and/or tubulointerstitial injury plays a critical role in LN etiology [1,4].
Ouabain, as a cardiotonic steroid endogenously produced in mammals and circulating in plasma, has been observed to regulate a myriad of cell functions by binding/regulating Na-K-ATPase α1 (NKA), including cell proliferation, hypertrophy, apoptosis, mobility, and metabolism [5,6]. A recent report discloses that ouabain promotes cell apoptosis and autophagy in Burkitt’s lymphoma Raji cells [7]. Another study reveals that ouabain elicits human glioblastoma cell apoptosis by generating reactive oxygen species in an ERK-p66SHC-dependent manner [8]. In kidney diseases, ouabain was discovered to contribute to renal damage in a rat model of renal ischemia-reperfusion injury and it couldserve as convincing biomarker for acute kidney injury (AKI) in post cardiac surgery patients [9,10]. However, the role of ouabain in LN pathogenesis is seldom reported, and considering the role of ouabain in regulating apoptosis and causing renal injury, we hypothesized that ouabain may be involved in the etiology of LN by regulating tubulointerstitial apoptosis through modulating NKA or its related pathways. Thus this study aimed to investigate the underlying molecular mechanism of ouabain induced apoptosis and inhibited viability of tubulointerstitial cells in LN.
Patients and methods
Participants
A total of 21 LN patients underwent biopsy in The Central Hospital of Wuhan from Dec 2015 to Jun 2016 were consecutively enrolled in this case-control study. SLE was diagnosed according to the 1982 American College of Rheumatology classification criteria, and nephritis was confirmed by pathologic examination of biopsies. In addition, 6 controls without LN who had nephrectomy were consecutively recruited during the same period to compare the Na+-K+-ATPase α1 (NKA) expression in kidney tissue between LN patients and controls. Another 20 healthy controls (HCs) were consecutively included from the same time interval to compare the ouabain concentration in serum between LN patients and HCs.
This study was approved by the Medical Ethics Committee of The Central Hospital of Wuhan and conducted according to the Declaration of Helsinki. All participants or their statutory guardians provided informed consent.
Samples
Kidney tissue samples from 21 LN patients and 6 controls were obtained by biopsy or during the nephrectomy operation, fixed, and embedded in paraffin. Serum samples were acquired from 21 LN patients and 20 HCs when included in this present study. All samples were collected before any therapies such as high-dose corticosteroid, immunoglobulin and others.
Enzyme linked immunosorbent assay (ELISA)
Concentrations of ouabain in serum from LN patients and HCs were detected by ELISA using commercial Human Ouabain ELISA Kit (Beijing Dongge Bio-tech Company, China) according to manufacturer’s instructions.
Immunohistochemistry (IHC) assay
The paraffin-embedded kidney tissue sections (3 mm thick) from LN patients and controls were deparaffinized, treated with 3% H2O2 for 15 min at room temperature, and then microwaved in citrate buffer (0.01 mol/L, pH 6.0) for 10 min for antigen retrieval. After blocking with 5% bovine serum albumin, the sections were incubated with NKA primary antibody (dilution 1:400, Milipore, Germany) overnight at 4°C. Subsequently, they were incubated with HRP-Anti-Mouse/Rabbit IgG secondary antibody (Abcam, China) for 30 min. After DAB (ZSGB, China) staining, sections were stained with hematoxylin. The data were then analyzed using Image-Pro Plus 5.10 software (Media Cybernetics, USA).
Cell culture
The human proximal tubular cells (HK-2 cells) were kindly provided by Renmin Hospital of Wuhan University. The cells were cultured at 37°C in low glucose DMEM medium (HyClone, USA) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, USA), 100 U/mL penicillin G, 100 mg/mL streptomycin in the presence of 5% CO2.
Ouabain treatment and subsequent assays
HK-2 cells were serum-starved for 12 h before treatment and then switched to low glucose DMEM medium with 10% FBS. Subsequently different concentrations of ouabain (0 nM (control), 0.1 nM, 1 nM, 10 nM, 100 nM and 1000 nM) were added to treat HK-2 cells for 72 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed at 24 h, 48 h and 72 h to detect the cell viability; Annexin V-FITC (AV)/propidium iodide (PI) assay was conducted at 24 h to detect cell apoptosis.
The effect of ouabain on NKA, pSrc, pERK, pAkt, pS6K and caspase 3 expressions
To further investigate the effect of ouabain on cells apoptosis, 100 nM ouabain was added to treat HK-2 cells. NKA protein expression was detected by immunofluorescence (IF) assay and western blot (WB) at 0 h, 0.5 h, 1 h, 6 h, 12 h and 24 h after treatment. pSrc, pERK, pAkt, pS6K and caspase 3 protein expressions were determined by WB assay at 0 h, 0.5 h, 1 h, 6 h, 12 h and 24 h after treatment.
PP2 and rapamycin on regulating the effect of ouabain on pSrc, pERK, pAkt, pS6K and caspase 3 expressions
Src kinase inhibitor (PP2) (Abcam, USA) and mTOR inhibitor (Rapamycin) (Sigma, USA) were then used to treat HK-2 cells with or without ouabain. Cells were divided into 6 groups according to the treatment: control, ouabain treatment group, PP2 treatment group, rapamycin treatment group, PP2+ ouabain treatment group, rapamycin + ouabain treatment group. pSrc, pERK, pAkt, pS6K, and caspase 3 protein expression were determined by WB at 24 h after treatment. For cells treated by ouabain and PP2 or rapamycin, the inhibitor was added 1 h before the addition of ouabain.
MTT assay
Cells viability was assessed by MTT reagent (Sigma, USA). First, 100 mg of MTT was dissolved in 20 mL phosphate-buffered saline (PBS) to make a 5 mg/mL solution. Next, 10 μl of MTT solution was added to each well, which contained 100 μl culture medium, and incubated at 37°C for 4 h. Then the culture medium in the wells was removed, and 100 μl of DMSO (Dimethyl sulfoxide) was added to each well, the solution was dissolved for 10 min. Finally, the plates were analyzed using a microplate reader (Molecular Devices, USA) at 490 nm.
AV/PI assay
Cells were digested by pancreatin and subsequently washed by PBS. After the cells were suspended in 100 μl Blinding Buffer, 5 μl AV (KeyGen Biotech, China) was added and placed in the dark for 15 mins at room temperature. 5 μl PI (KeyGen Biotech, China) was added just before flow cytometry assay. Flow cytometry was used to analyze the results.
IF assay
The cell films were fixed in 4% paraformaldehyde with 0.3% Triton X-100 (Goodbio, China) for 30 min at 4°C and stained with NKA antibody (dilution 1:100, DSHB, USA) overnight at 4°C. The films were then subjected to Alexa 488-conjugated IgG (Goodbio, China) at 37°C for 50 min. All microscopic images were recorded using Nikon eclipse CI (Nikon, Japan).
WB assay
The cells were harvested and rinsed with ice-cold PBS and lysed in RIPA buffer (150 mM sodium chloride; 1.0% Triton X-100; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 50 mM Tris, pH 8.0) mixed with protease inhibitors (Merk-Millipore, USA) and phosphatase inhibitors (Roche, USA) for 30 min on ice. Samples were collected and centrifuged at 12,000 rpm for 15 min at 4°C. Supernatant protein was boiled in loading buffer at 100°C for 10 min. The protein samples were subjected to SDS-PAGE and transferred to PVDF membranes (Roche, USA). After blocking with 5% nonfat dried milk in TBS-T (10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, pH 8.0) for 1 h at room temperature, membranes were incubated with primary antibodies against NKAα1 (DSHB, USA), Phospho-Src/Src (Abcam, USA), Phospho-ERK/ERK (CST, USA), Phospho-Akt (Ser473)/Akt (Thr308) (CST, USA), Phospho-S6k (CST, USA)/S6K (Abcam, England), caspase 3 (CST, USA), GAPDH (Millipore, Germany) overnight at 4°C. HRP AffiniPure Goat Anti-Mouse/Rabbit IgG (Dilution 1:5000, Dako, USA) was used as secondary antibodies. The protein signals were detected using an ECL kit (GE, USA) and quantified using a Bio-Rad imaging densitometer (Bio-Rad, USA). The bands were analyzed with quantity one software Version 4.6.6 (Bio-Rad, USA).
Statistics
Each experiment was performed as triplicate. The statistical analysis was carried out by SPSS 22.0 (IBM, USA) and GraphPad Prism 6.0 (Graphpad, USA). Data were mainly presented as mean ± standard deviation or mean ± standard error. Comparison between two groups was determined by t test. P<0.05 was considered significant.
Results
Serum ouabain expression in LN patients and HCs
As shown in Figure 1, the level of ouabain in serum from LN patients was elevated compared with HCs (12.80 ± 1.49 vs 10.27 ± 2.33, P<0.001).
Figure 1.
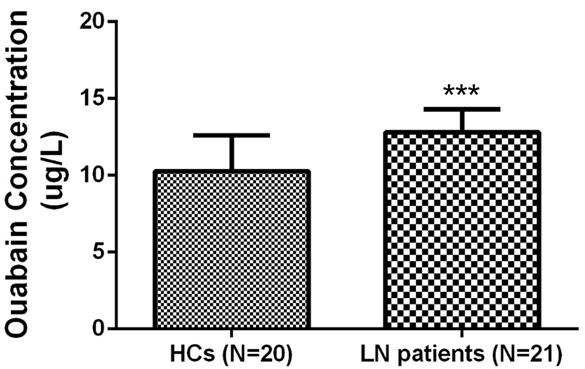
Serum ouabain concentration in lupus nephritis (LN) patients and healthy controls (HCs). Serum ouabain concentration was increased in LN patients (N=21) compared with HCs (N=20). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001. LN, lupus nephritis; HCs, healthy controls.
NKA expression in LN patients and controls
IHC assay disclosed that kidney tissue NKA protein expression was mainly present in the interstitial tubules (Figure 2A), and its expression was decreased in LN patients compared to controls (Figure 2B).
Figure 2.
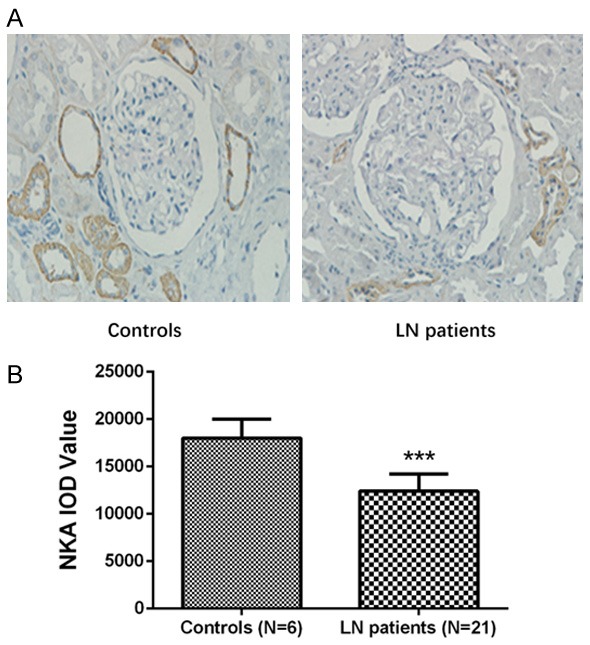
Kidney tissue NKA expression in LN patients and controls. NKA positive expression was not detected in the glomeruli, but in the renal tubule (A). NKA expression was decreased in LN patients (N=21) compared to controls (N=6) without LN who underwent nephrectomy (B). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001. LN, lupus nephritis; NKA, Na-K-ATPase α1.
Ouabain inhibited cells viability and promoted cells apoptosis in HK-2 cells
MTT assay revealed that cells viability was decreased at each time point (24 h, 48 h and 72 h) in ouabain 0.1 nM, 1 nM, 10 nM, 100 nM and 1000 nM groups compared with control group (Figure 3). AV/PI assay showed that cellapoptosis at 24 h was increased in ouabain 0.1 nM, 1 nM, 10 nM, 100 nM and 1000 nM groups compared to the control group (Figure 4A, 4B). These indicated ouabain inhibited cell viability and promoted cells apoptosis in a dose-dependent way.
Figure 3.

Cell viability of HK-2 cells after ouabain treatment. Cell viability was detected by MTT assay. Cell viability was inhibited at each time point (24 h, 48 h and 72 h) in ouabain 0.1 nM, 1 nM, 10 nM, 100 nM and 100 nM groups compared with control group (0 nM). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001.
Figure 4.

Apoptosis of HK-2 cells after ouabain treatment. Cell apoptosis was detected by AV/PI assay (A) which showed that the apoptosis rate was increased at each time point (24 h, 48 h and 72 h) in ouabain 0.1 nM, 1 nM, 10 nM, 100 nM and 100 nM groups compared with control group (0 nM) (B). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001.
NKA protein expression after ouabain treatment
IF assay showed that NKA protein expression was increased at 1 h and 6 h compared to 0 h, while dramatically decreased at 12 h and 24 h compared to 0 h after ouabain treatment (Figure 5A, 5B). WB also disclosed that NKA protein expression was initially increased at 0.5 h, 1 h and 6 h than 0 h, while subsequently decreased at 12 h and 24 h compared with 0 h after ouabain treatment (Figure 5C, 5D).
Figure 5.

NKA expression in HK-2 cells after ouabain treatment. NKA expression was detected by immunofluorescence assay and western blot at 0 h, 0.5 h, 1 h, 6 h, 12 h and 24 h after 100 nM ouabain treatment. NKA expression was elevated at 1 h and 6 h while reduced greatly at 12 h and 24 h compared to 0 h after ouabain treatment by immunofluorescence assay (A, B). Western blot assay also revealed that NKA expression was increased at 0.5 h, 1 h and 6 h while critically decreased at 12 h and 24 h compared with 0 h after ouabain treatment (C, D). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001. NKA, Na-K-ATPase α1.
pSrc, pERK, pAkt, pS6K and caspase 3 expressions after ouabain treatment
WB (Figure 6A) showed that pSrc protein expression was increased at 1 h, 6 h and 12 h compared with 0 h (Figure 6B); pS6K protein was elevated at 1 h and 6 h compared to 0 h (Figure 6E); and pERK protein (Figure 6C), pAkt protein (Figure 6D) and caspase 3 protein (Figure 6F) were all increased at 0.5 h, 1 h, 6 h, 12 h and 24 h compared to 0 h. These indicated ouabain might regulate HK-2 cells viability and apoptosis through modulating NKA, pSrc, pERK, pAkt, pS6K and caspase 3 expressions.
Figure 6.
pSrc, pERK, pAkt, pS6K, and caspase 3 expression in HK-2 cells after ouabain treatment. pSrc, pERK, pAkt, pS6K and caspase 3 expressions were determined by western blot, which showed that they were all activated after ouabain treatment (A-F). Comparisons were determined by t-test. *P<0.05, **P<0.01, ***P<0.001.
pSrc, pERK, pAkt, pS6K and caspase 3 expression after ouabain treatment with PP2 or rapamycin
WB (Figure 7A) illustrated that PP2 decreased pSrc (Figure 7B), pERK (Figure 7C), pAkt (Figure 7D), pS6K (Figure 7E) and caspase 3 (Figure 7F) protein expressions in ouabain treated HK-2 cells (p+o group vs oua group). However, rapamycin only decreased pS6K (Figure 7E) and caspase 3 (Figure 7F) protein expressions in ouabain-treated HK-2 cells (r+o group vs oua group), but not pSrc (Figure 7B), pERK (Figure 7C), or pAkt (Figure 7D) protein expressions.
Figure 7.
pSrc, pERK, pAkt, pS6K, and caspase 3 expression in HK-2 cells after ouabain treatment with PP2 or rapamycin. Src kinase inhibitor (PP2) and mTOR inhibitor (Rapamycin) were used to treat HK-2 cells with or without ouabain, and then pSrc, pERK, pAkt, pS6K, and caspase 3 expressions at 24 h were detected by western blot (A). We observed that PP2 decreased pSrc (B), pERK (C), pAkt (D), pS6K (E) and caspase 3 (F) expression in ouabain-treated HK-2 cells (p+o group vs oua group). Rapamycin only decreased pS6K (E) and caspase 3 (F) expression in ouabain-treated HK-2 cells (r+o group vs oua group). Comparisons were determined by t test. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In this present study, we observed that: (1) Circulating ouabain was elevated in LN patients compared with HCs, and NKA expression in kidney tissue of LN patients was decreased versus controls. (2) Ouabain promoted HK-2 cell apoptosis while inhibiting proliferation in a dose-dependent way. (3) Ouabain decreased NKA expression and activated pSrc, pERK, pAkt, pS6K, and caspase 3 expressions.
SLE is a multisystem autoimmune disorder disease which presents with a broad spectrum of effects on multiple organs including the kidney, and it is estimated that 28-70% of patients with SLE will develop LN [4,11]. A recent comprehensive review discloses that the prevalence of LN in United States ranges from 50-70 per 100,000 persons in different socioeconomic groups [3], which not only increases patients’ functional burden but also economic burden [12]. Thus, investigation of the underlying mechanism of LN pathogenesis is of great importance.
Ouabain belongs to a group of compounds (the cardiotonic steroids), which are composed of a steroidal backbone, the aglycone, or genin; a five-membered unsaturated lactone ring; and a sugar moiety, which varies depending on the type of cardenolide [13]. It is reported to have a critical role in several kidney diseases [13-15]. In a previous animal experiment, ouabain has been observed to induce kidney damage in renal ischemia-reperfusion injury rat model [9]; and ouabain preoperative plasma level could be used as a convincing biomarker for AKI risk in patients undergoing cardiac surgery; in addition, ouabain is observed to be upregulated in chronic kidney disease patients [10,11,16]. These findings indicate ouabain contributes to the development and/or progression of kidney disease. In this present study, we found serum ouabain was increased in LN patients compared with HCs, which might result from ouabain causing kidney injuries by multiple mechanisms; thus it was upregulated in LN patients.
Some studies show that ouabain induces cell apoptosis and suppresses cell proliferation by regulating multiple pathways such as in Burkitt’s lymphoma Raji cells, glioblastoma cells, cochlear hair cells and lung cancer cells [7,8,17,18]. Other studies reveal that ouabain inhibits cell apoptosis and improves cell proliferation, as in autosomal dominant polycystic kidney disease cells, leukemia cells and vascular smooth muscle cells [19,20]. This implies that ouabain may present with dual effects on cell proliferation and apoptosis. Further studies in several cell lines support this inference. A recent experiment illustrates that ouabain presents with dual effects on regulating human umbilical vein endothelial cells proliferation and apoptosis involving NKA and NF-κB pathways [21]. Another experiment shows a dual effect of ouabain on cell apoptosis in human fibroblasts [22]. These studies disclose that a low dose of ouabain promotes cells proliferation and inhibits cell apoptosis, while middle orhigh dose of ouabain supresses cell proliferation and induces cell apoptosis, which depends on the expression of NKA after ouabain intervention [21,22]. However, there is little data on the role of ouabain and NKA in LN. In this study, we found kidney tissue NKA expression was decreased in LN patients compared with controls, which indicated NKA downregulation might be involved in the etiology of LN. We found ouabain promoted HK-2 cells apoptosis while it inhibits proliferation in a dose-dependent way; moreover, ouabain decreased NKA expression and activated pSrc, pERK, pAkt, pS6K and caspase 3 expression. These findings implied ouabain may contribute to LN development and progression by modulating NKA, pSrc, pERK, pAkt, pS6K and caspase 3.
In conclusion, ouabain may contribute to the etiology of LN by inhibiting human proximal tubular cell viability and promoting cell apoptosis by regulating NKA, pSrc, pERK, pAkt, pS6K and caspase 3.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81400734) and the Natural Science Foundation of Hubei Province of China (No. 2015CFB241).
Disclosure of conflict of interest
None.
References
- 1.Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13:483–495. doi: 10.1038/nrneph.2017.85. [DOI] [PubMed] [Google Scholar]
- 2.Zampeli E, Klinman DM, Gershwin ME, Moutsopoulos HM. A comprehensive evaluation for the treatment of lupus nephritis. J Autoimmun. 2017;78:1–10. doi: 10.1016/j.jaut.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Hoover PJ, Costenbader KH. Insights into the epidemiology and management of lupus nephritis from the US rheumatologist’s perspective. Kidney Int. 2016;90:487–492. doi: 10.1016/j.kint.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat Rev Nephrol. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
- 5.Blanco G, Wallace DP. Novel role of ouabain as a cystogenic factor in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2013;305:F797–812. doi: 10.1152/ajprenal.00248.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, Wen Y, Zhou M, Li J, Wang T, Xu P, Ouyang J. Ouabain induces apoptosis and autophagy in Burkitt’s lymphoma Raji cells. Biomed Pharmacother. 2016;84:1841–1848. doi: 10.1016/j.biopha.2016.10.114. [DOI] [PubMed] [Google Scholar]
- 8.Yan X, Liang F, Li D, Zheng J. Ouabain elicits human glioblastoma cells apoptosis by generating reactive oxygen species in ERK-p66SHC-dependent pathway. Mol Cell Biochem. 2015;398:95–104. doi: 10.1007/s11010-014-2208-y. [DOI] [PubMed] [Google Scholar]
- 9.Villa L, Buono R, Ferrandi M, Molinari I, Benigni F, Bettiga A, Colciago G, Ikehata M, Messaggio E, Rastaldi MP, Montorsi F, Salonia A, Manunta P. Ouabain contributes to kidney damage in a rat model of renal ischemia-reperfusion injury. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonini M, Lanzani C, Bignami E, Casamassima N, Frati E, Meroni R, Messaggio E, Alfieri O, Hamlyn J, Body SC, Collard CD, Zangrillo A, Manunta P CABG Genomics Investigators. A new clinical multivariable model that predicts postoperative acute kidney injury: impact of endogenous ouabain. Nephrol Dial Transplant. 2014;29:1696–1701. doi: 10.1093/ndt/gfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 12.Barber MRW, Hanly JG, Su L, Urowitz MB, Pierre YS, Romero-Diaz J, Gordon C, Bae SC, Bernatsky S, Wallace DJ, Isenberg DA, Rahman A, Ginzler EM, Petri M, Bruce IN, Fortin PR, Gladman DD, Sanchez-Guerrero J, Ramsey-Goldman R, Khamashta MA, Aranow C, Mackay M, Alarcon GS, Manzi S, Nived O, Jonsen A, Zoma AA, van Vollenhoven RF, Ramos-Casals M, Ruiz-Irastorza G, Sam Lim S, Kalunian KC, Inanc M, Kamen DL, Peschken CA, Jacobsen S, Askanase A, Theriault C, Farewell V, Clarke AE. Economic evaluation of lupus nephritis in the Systemic Lupus International Collaborating Clinics inception cohort using a multistate model approach. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23480. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrueto F Jr, Kirrane BM, Cotter BW, Hoffman RS, Nelson LS. Cardioactive steroid poisoning: a comparison of plant- and animal-derived compounds. J Med Toxicol. 2006;2:152–155. doi: 10.1007/BF03161183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Khodus GR, Kruusmagi M, Kamali-Zare P, Liu XL, Eklof AC, Zelenin S, Brismar H, Aperia A. Ouabain protects against adverse developmental programming of the kidney. Nat Commun. 2010;1:42. doi: 10.1038/ncomms1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venugopal J, Blanco G. On the many actions of ouabain: pro-cystogenic effects in autosomal dominant polycystic kidney disease. Molecules. 2017:22. doi: 10.3390/molecules22050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bignami E, Casamassima N, Frati E, Lanzani C, Corno L, Alfieri O, Gottlieb S, Simonini M, Shah KB, Mizzi A, Messaggio E, Zangrillo A, Ferrandi M, Ferrari P, Bianchi G, Hamlyn JM, Manunta P. Preoperative endogenous ouabain predicts acute kidney injury in cardiac surgery patients. Crit Care Med. 2013;41:744–755. doi: 10.1097/CCM.0b013e3182741599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, Ding D, Wei L, Jiang H, Salvi R. Ouabain-induced apoptosis in cochlear hair cells and spiral ganglion neurons in vitro. Biomed Res Int. 2013;2013:628064. doi: 10.1155/2013/628064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanvorachote P, Pongrakhananon V. Ouabain downregulates Mcl-1 and sensitizes lung cancer cells to TRAIL-induced apoptosis. Am J Physiol Cell Physiol. 2013;304:C263–272. doi: 10.1152/ajpcell.00225.2012. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AN, Jansson K, Sanchez G, Sharma M, Reif GA, Wallace DP, Blanco G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am J Physiol Renal Physiol. 2011;301:F897–906. doi: 10.1152/ajprenal.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Wang Q, Guan L. Effects of ouabain on proliferation, intracellular free calcium and c-myc mRNA expression in vascular smooth muscle cells. J Comp Physiol B. 2007;177:589–595. doi: 10.1007/s00360-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 21.Ren YP, Zhang MJ, Zhang T, Huang RW. Dual effects of ouabain on the regulation of proliferation and apoptosis in human umbilical vein endothelial cells: involvement of Na(+)-K(+)-ATPase alpha-subunits and NF-kappaB. Int J Clin Exp Med. 2014;7:1214–1222. [PMC free article] [PubMed] [Google Scholar]
- 22.Winnicka K, Bielawski K, Bielawska A, Miltyk W. Dual effects of ouabain, digoxin and proscillaridin a on the regulation of apoptosis in human fibroblasts. Nat Prod Res. 2010;24:274–285. doi: 10.1080/14786410902991878. [DOI] [PubMed] [Google Scholar]




