Abstract
The purpose of this study was to investigate the association between the expression of programmed cell death ligand 1 (PD-L1) and the survival of patients with small cell lung cancer (SCLC) who had undergone complete resection. Formalin-fixed, paraffin-embedded tumor tissue samples from 61 patients with resected SCLC were stained with an anti-PD-L1 antibody (SP142) by immunohistochemistry (IHC) and scored according to staining intensity and the percentage of tumor cells staining positive for PD-L1. The PD-L1 positive threshold in tumor cells was defined as ≥ 5%. The percentage of positive PD-L1 staining in all SCLC specimens was 44.3% (27/61). The median survival time of patients with PD-L1-positive tumors was significantly longer than those with PD-L1-negative tumors (not reached vs. 34 months, P = 0.032). Multivariate analysis indicated that postoperative chemotherapy (HR = 0.322, P = 0.023) and PD-L1 expression ≥ 5% (HR = 0.253, P = 0.008) were independent prognostic factors for overall survival. The results suggest that PD-L1 expression is readily detectable in the tumor tissues of SCLC, and that PD-L1 expression can predict the survival of these patients.
Keywords: Small cell lung cancer, PD-L1, immunohistochemistry, overall survival, prognostic factor
Introduction
Small cell lung cancer (SCLC) is characterized by aggressive malignancy and high sensitivity to chemoradiotherapy, but half of all patients suffer a relapse within one year. The current clinical treatment modality for limited-stage small cell lung cancer (LS-SCLC) is conventional chemotherapy with platinum and etoposide, combined with sequential or concurrent thoracic irradiation, and the median survival time (MST) is 16-24 months, with a two-year survival rate of 25% [1]. No successful systemic therapeutics have been developed in recent years. Therefore, there is an urgent need to find new therapeutic strategies for treating SCLC.
Programmed cell death ligand 1 (PD-L1), one ligand of the programmed cell death 1 (PD-1) receptor, is expressed on the membrane of various tumor cells. The binding of PD-L1 to PD-1 suppresses the function of activated T cells; PD-L1 competes for the CD28 binding site on B7, which promotes immune evasion and intolerance [2-4]. PD-L1 is thus a negative regulator of the immune response during T-cell receptor activation. Recently, numerous clinical trials have demonstrated promising anti-tumor activity of PD-L1 and PD-1 antibody blockade in many malignant tumors such as non-small cell lung cancer and melanoma.
The influence of PD-L1 expression on the prognosis of SCLC and the anti-tumor activity of PD-L1 and PD-1 antibody blockade remain controversial. Only a few studies have focused on the prevalence of PD-L1 in SCLC and have investigated the correlation of PD-L1 expression with clinical outcomes. Several studies demonstrated that PD-L1 expression correlated with longer overall survival (OS) and disease-free survival of patients with SCLC [5-7]. Conversely, another study showed that PD-L1 expression correlated with advanced stage disease and poor survival [8].
The current retrospective study revealed that more than one-third of patients with resectable SCLC expressed PD-L1 at levels ≥ 5% PD-L1-positive threshold, and PD-L1 expression was a favorable prognostic factor for OS.
Materials and methods
Patients
We retrospectively collected a population of patients with SCLC who underwent radical surgery between January 2009 and December 2011 at Shandong Cancer Hospital. All patients underwent a standardized evaluation including thoracic and abdominal computed tomography (CT) scanning, brain magnetic resonance imaging (MRI) and radionuclide bone imaging. Surgical procedures included lobectomy, or pneumonectomy with ipsilateral hilar and mediastinal lymphadenectomy. Patients received either cisplatin/etoposide (PE: cisplatin, 30 mg/m2 on days 1-3 and etoposide, 100 mg/m2 on days 1-5 or 100 mg/m2 on days 1-3) or carboplatin/etoposide (CE: carboplatin AUC 5 or 300 mg/m2 on day 1 and etoposide, 100 mg/m2 on days 1-5 or 100 mg/m2 on days 1-3). Thoracic radiotherapy was conducted in patients with lymph node metastasis, and, radiotherapy was administered by the three-dimensional conformal radiotherapy technique. The clinical target volume (CTV) included the bronchial stump, the ipsilateral hilum, and the adjacent mediastinal lymph nodes and the planning target volume included the CTV with a 1-cm margin. Radiation was delivered by megavoltage linear accelerators. A total dose of 50-60 Gy was administered with 1.8-2.0 Gy per fraction for 5 days per week. Prophylactic cranial irradiation (PCI) was conducted in all patients; the dose fraction was 25 Gy in 10 fractions over 2 weeks.
All procedures performed in studies involving human participants were in accordance with the ethical standards of Shandong Cancer Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients and/or their parents or legal guardian signed informed consent forms prior to inclusion in the present study.
Immunohistochemistry
Postoperative resected tumor tissue specimens were retrieved for determination of PD-L1 expression by IHC. PD-L1 IHC was performed with an anti-PD-L1 rabbit monoclonal antibody (SP142, Spring Bioscience, CA). Formalin-fixed, paraffin-embedded samples of 4 μm thickness were air-dried overnight at room temperature. After de-paraffinization, antigen retrieval was performed in a pressure cooker at high temperature. Then, the slides were quenched, and the tissues were incubated in 5% fetal bovine serum (Gibco) blocking buffer. The primary antibody or isotype control rabbit IgG antibody was applied, and slides were washed 3 times. The secondary antibody (EnVision + System-HRP Labelled Polymer Anti-Rabbit, K4003, Dako, CA) was then applied, and the slides were incubated and washed again. Next, the slides were incubated in DAB (Maixin, DAB-0031) for 5 minutes. Finally, the slides were stained with hematoxylin, dehydrated, and sealed with a coverslip. Positive control specimens for PD-L1 IHC were human placenta. An isotype-matched rabbit IgG antibody was used as a control to control for potential false positive staining in each specimen stained for PD-L1.
The immunostained slides were evaluated by at least two independent pathologists who were blinded to the clinical outcome. The staining intensity scores for the stained tumor cells were defined as follows: 0 (no staining), 1+ (weak), 2+ (moderate) and 3+ (strong). The color gradation associated with each positive score was light brown, brown and dark brown to black, respectively. The IHC score (histoscore) was calculated as follows: Histoscore = (% weak [1+] × 1) + (% moderate [2+] × 2) + (% strong [3+] × 3), where % weak [1+] refers to the percentage of tumor cells showing weak staining compared with all tumor cells. The percentage of tumor cells showing moderate [2+] and strong [3+] staining is similarly included in the formula. “PD-L1 positive” was defined as PD-L1 expression ≥ 5%.
Patient follow-up
Patient follow-up occurred every 3 months for the first 2 years, every 6 months for the following 3 years, and then annually. The follow-up evaluations consisted of obtaining a medical history, a physical examination, and a radiologic examination. The radiologic examination included a chest X-ray, chest CT, abdominal ultrasound/CT, and other necessary imaging as clinically indicated. Routine brain imaging was not performed during the follow-up period. Contrast-enhanced MRI/CT of the brain was performed if brain metastases were suspected.
Statistical analysis
The chi-square test (or Fisher’s exact test) was used to evaluate the associations between PD-L1 and patient characteristics. OS from any cause was measured from the date of diagnosis to the date of death, or to the last known date that the patient was alive. Progression-free survival (PFS) was measured from the date of diagnosis to the date of disease progression or the last known date that the patient was not suffering from disease progression. OS and PFS were calculated using the Kaplan-Meier method, and differences in survival curves between the groups were evaluated by log-rank test. Univariate survival analysis was performed using the Kaplan-Meier method. Multivariate survival analysis was performed using the Cox proportional hazards model with a stepwise variable selection method. Two-tailed P < 0.05 was considered statistically significant.
Results
Patients
Between January 2009 and December 2011, 85 patients with SCLC underwent resection in Shandong Cancer Hospital. Among these patients, 24 were excluded from the analysis for the following reasons: R1 resection (11 patients), missing follow-up (8 patients), and unusable specimen (5 patients). As a result, 61 patients who underwent complete resection were evaluated in this study, including 43 males and 18 females, with a median age at diagnosis 56 years (range, 30-74 years). The median follow-up period for all patients was 36.5 months (range, 5-75 months) and for the surviving patients was 44 months (range, 6-89 months). On the last date of follow-up, 37 (60.7%) patients were still alive. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Clinical characteristics of patients with resectable small cell lung cancer
| Characteristics | n = 61 | % |
|---|---|---|
| Sex | ||
| Male | 43 | 70.5 |
| Female | 18 | 29.5 |
| Age | ||
| > 60 | 19 | 31.1 |
| ≤ 60 | 42 | 68.9 |
| pT stage | ||
| T1 | 24 | 39.3 |
| T2 | 29 | 47.6 |
| T3 | 5 | 8.2 |
| T4 | 3 | 4.9 |
| pN stage | ||
| N0 | 24 | 39.3 |
| N1 | 18 | 29.5 |
| N2 | 19 | 31.1 |
| Pathologic stage | ||
| I | 20 | 32.8 |
| II | 17 | 27.9 |
| III | 24 | 39.3 |
| POCT | ||
| Yes | 51 | 83.6 |
| No | 10 | 16.4 |
| PORT | ||
| Yes | 14 | 23.0 |
| No | 47 | 77.0 |
Abbreviations: POCT, postoperative chemotherapy; PORT, postoperative radiotherapy; pT, pathological tumor; pN, pathological node.
Treatment
In addition to resection, 57 (93.4%) and 4 (6.6%) patients underwent lobectomy and pneumonectomy, respectively. Postoperative chemotherapy (POCT) was conducted in 51 patients; 10 patients did not receive POCT because of either a low Karnofsky performance status score or the patient’s refusal. Additionally, 14 patients were given postoperative radiotherapy (PORT); 12 of these patients were staged as N2 and the remaining 2 patients were staged as N1. Seven patients staged as N2 and 16 patients staged as N1 did not receive PORT because of either poor lung function or a low Karnofsky performance status score after surgery. Chemotherapy and radiotherapy were given sequentially after surgery. All patients were administered PCI.
PD-L1 protein expression
All of the 61 patient specimens were stained by IHC to investigate PD-L1 expression. As shown in Figure 1, PD-L1 was clearly stained in the cell membrane. A staining intensity of 1+ was only observed in 9 (14.8%) patients. Staining intensities of 2+ and 3+ were observed in 18 (29.5%) patients and 34 (55.7%) patients respectively. PD-L1 expression was as follows: < 1% in 26 (42.6%) patients, 1%-5% in 8 (13.1%) patients, 5%-10% in 14 (23.0%) patients and ≥ 10% in 13 (21.3%) patients. The PD-L1 positive threshold in tumor cells was defined as PD-L1 expression ≥ 5%. As a result, the percentage of PD-L1-positive tumor was 44.3% (27/61).
Figure 1.

Staining intensity and IHC score in FFPE sections. A. Staining intensity 3+ with IHC score 15%; B. Staining intensity 2+ with IHC score 10%; C. Staining intensity 2+ with IHC score less than 5%; D. Staining intensity 2+ with IHC score 1%.
Further analysis was performed to investigate the association between PD-L1 expression and clinical characteristics. No significant difference in PD-L1 expression with regard to gender, age, pathologic T stage, pathologic N stage was observed. The association between patient characteristics and PD-L1 expression is detailed in Table 2.
Table 2.
Association between patient characteristics and PD-L1 expression
| n = 61 | PD-L1 < 0.05 | PD-L1 ≥ 0.05 | P | |
|---|---|---|---|---|
| Sex | ||||
| Male | 43 | 28 (65.1) | 15 (34.9) | 0.48 |
| Female | 18 | 10 (55.6) | 8 (44.4) | |
| Age | ||||
| > 60 | 19 | 10 (52.6) | 9 (47.4) | 0.29 |
| ≤ 60 | 42 | 28 (66.7) | 14 (33.3) | |
| pT stage | ||||
| T1 | 24 | 15 (62.5) | 9 (37.5) | 0.99 |
| T2 | 29 | 18 (62.1) | 11 (37.9) | |
| T3-4 | 8 | 5 (62.5) | 3 (37.5) | |
| pN stage | ||||
| N0 | 24 | 14 (58.3) | 10 (41.7) | 0.59 |
| N1 | 18 | 13 (72.2) | 5 (27.8) | |
| N2 | 19 | 11 (57.9) | 8 (42.1) | |
| Pathologic stage | ||||
| I-II | 37 | 25 (67.6) | 12 (32.4) | 0.29 |
| III | 24 | 13 (54.2) | 11 (45.8) |
Abbreviations: PD-L1, programmed cell death ligand-1; pT, pathological tumor; pN, pathological node.
Survival
Among the entire study cohort, the median PFS was 30.1 months (95% CI, 16.98-43.22). The MST was not reached (range, 6-89 months), and the 1-, 3-, and 5-year survival rates were 90.0%, 57.3% and 54.2%, respectively. The survival curves for the entire cohort are detailed in Figure 2.
Figure 2.

Progression-free survival (PFS) and overall survival (OS) of all patients with resectable small cell lung cancer included in this study.
The associations between the level of PD-L1 expression and survival were evaluated. Survival of patients with PD-L1-positive tumors was significantly longer than that of patients with PD-L1-negative tumors (MST: not reached vs. 34 months). In patients with PD-L1-positive tumors, the 1- and 3-year OS rates were 95.7% and 72.3%, respectively; in patients with PD-L1-negative tumors, these rates were 86.5% and 47.5% (P = 0.032, Figure 3).
Figure 3.
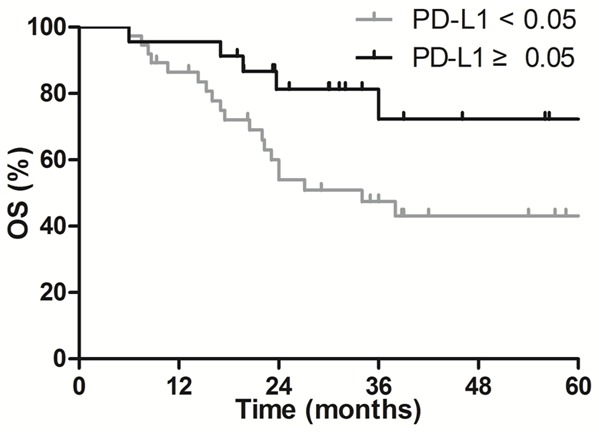
Comparison of survival curves in patients with PD-L1-negative and PD-L1 positive tumors, P = 0.032.
Prognostic factors
The prognostic value of PD-L1 expression and clinical characteristics for survival are evaluated in Table 3. Univariate analysis indicated that N stage (P = 0.022), POCT (P = 0.048) and PD-L1 positive staining (P = 0.032) were independent prognostic factors for OS, while gender, age, T stage, pathologic stage and PORT were not. Multivariate analysis revealed that POCT (HR = 0.322, P = 0.023), and PD-L1-positive staining (HR = 0.253, P = 0.008) were independent prognostic factors for OS.
Table 3.
Univariate and multivariate analyses of the effect of prognostic factors on the overall survival of patients with resectable small cell lung cancer
| Characteristic | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| MST (mo) | 3-y OS (%) | χ2 | P | HR | 95% CI | P | |
| Sex | 1.260 | 0.262 | |||||
| Male | 36 | 49.6 | |||||
| Female | NR | 66.3 | |||||
| Age | 2.029 | 0.154 | |||||
| > 60 | 36 | 73.7 | |||||
| ≤ 60 | NR | 50.0 | |||||
| pT stage | 1.821 | 0.402 | |||||
| T1 | NR | 65.5 | |||||
| T2 | 38 | 51.7 | |||||
| T3 | 19.7 | 50.0 | |||||
| pN stage | 7.616 | 0.022 | |||||
| N0 | NR | 76.5 | |||||
| N1 | 28.9 | 58.1 | |||||
| N2 | 22.3 | 28.4 | |||||
| Pathologic stage | 1.806 | 0.405 | |||||
| I | NR | 72.4 | |||||
| II | 24 | 53.9 | |||||
| III | 18 | 40.7 | |||||
| POCT | 3.922 | 0.048 | 0.322 | 0.121-0.856 | 0.023 | ||
| Yes | NR | 61.1 | |||||
| No | 17 | 40.0 | |||||
| PORT | 2.674 | 0.102 | |||||
| Yes | 38 | 51.2 | |||||
| No | 22 | 35.7 | |||||
| PD-L1 expression | 4.592 | 0.032 | 0.253 | 0.092-0.697 | 0.008 | ||
| < 0.05 | 43.2 | 34.0 | |||||
| ≥ 0.05 | 72.3 | NR | |||||
Abbreviations: MST, median survival time; 3/5-y, 3/5 year; mo, months; NR, not reached; OS, overall survival; POCT, postoperative chemotherapy; PORT, postoperative radiotherapy; PD-L1, programmed cell death ligand 1.
Discussion
Much progress in the development of targeted therapies for the treatment of malignant tumors has been made in the last 10 years. However, the etiology and molecular pathogenesis of SCLC with poor prognosis are not well understood. In addition, no data are available that establish an optimal therapeutic target in SCLC. We investigated the prevalence of PD-L1 expression and explored its predictive value for survival in patients with resectable SCLC. Among the 61 SCLC patients who underwent complete resection and pathologic TNM stage classification in the present study, a PD-L1 expression level of ≥ 1% was observed in 35 patients (57.4%, 35/61), and ≥ 5% in 23 patients (37.7%, 23/61). Our data indicated that more than half of the SCLC patients with limited-stage disease scored positive for PD-L1 expression, and the survival of patients with PD-L1-positive (≥ 5%) tumors was longer than that of patients with PD-L1-negative tumors. Multivariate analysis demonstrated that PD-L1-positive staining was an effective predictive biomarker for survival in patients with resectable SCLC.
SCLC is a rapidly progressive disease and is sensitive to chemoradiotherapy, but frequently relapses. No remarkable breakthroughs in chemotherapy have been developed in the past several years. Additionally, because SCLC lacks EGFR mutation, ALK rearrangement and other gene mutations common in lung cancer, no effective targeted therapy is available for clinical use. Although surgery and PCI can improve patient survival, the survival of SCLC patients as a whole remains poor [9-11]. The identification of optimal treatments for patients with SCLC is urgently needed to further prolong survival.
PD-L1 is primarily expressed on the membrane of tumor cells, where it interacts with PD-1 to inhibit the function of activated T-cells and promotes immune evasion of tumor cells. Previous studies have reported that the prevalence of PD-L1 expression and its correlation with clinical features were significantly different. In a retrospective study including 83 patients with SCLC, PD-L1 was detected with Clone SP66 by IHC and a positive value was defined as PD-L1 expression ≥ 5% in tumor cells. The results showed PD-L1-positive staining in 51.8% of patients, particularly in patients with LS-SCLC (67.4%) [6]. Another study including 102 specimens indicated that 71.6% (73/102) of patients with SCLC had PD-L1-positive tumor staining, and the frequency of PD-L1-positive staining in LS-SCLC was higher than that in extensive-stage (ES-) SCLC (85.4% vs. 62.3%, P = 0.011) [5]. A retrospective study including 186 cases showed that the overall frequency of PD-L1 overexpression in tumor cells and tumor infiltrating lymphocytes (TILs) were 78.0% and 54.3%, respectively. High PD-L1 expression was associated with advanced age (≥ 60 years) (P < 0.001) and PD-L1 expression in TILs (P = 0.001) [8]. Additionally, PD-L1 was highly expressed, at levels of up to 75.0% (24/32), in SCLC tissue specimens obtained from brain metastases [12]. Furthermore, the present study showed that PD-L1-positive tumors were detected in 37.7% (23/61) of specimens from patients with resectable SCLC. These data all indicated that anti-PD-L1/PD-1 therapy might play an important role in the treatment of SCLC.
At present, there is no definite conclusion regarding the correlation of PD-L1 expression with the survival of SCLC patients. In a study by Miao et al., SCLC patients with PD-L1-positive tumors had a significantly longer OS than patients with PD-L1-negative tumors (median OS, 17.0 vs. 9.0, P = 0.018), but sub-analysis of the LS-SCLC patients and ES-SCLC patients showed no significant difference in OS between the PD-L1-positive and PD-L1-negative groups [6]. The data from Ishii et al. showed that the MST of the PD-L1-positive group was longer than that of the PD-L1-negative group in the entire cohort (16.3 months vs. 7.3 months, P < 0.001), whereas no significant difference was found between the positive PD-L1 group and negative PD-L1 group in patients with LS-SCLC (25.5 months vs. 21.8 months, P = 0.146) [5]. Another study revealed that disease-free survival in patients with positive PD-L1 expression in TILs and tumor cells and/or TILs was longer in comparison to patients negative for PD-L1 expression (MST, 83.7 vs. 9.3 months, P = 0.003; and 83.7 vs. 9.0 months, P = 0.005, respectively), while no significant survival benefits were observed in patients with positive PD-L1 expression in tumor cells alone [7]. In a study by Chang et al., high PD-L1 expression in tumors was significantly correlated with poor survival; the 5-year survival in the PD-L1-positive tumor group and PD-L1-negative tumor group was 8.2% and 79.6%, respectively (P = 0.001), and multivariate analysis revealed that high PD-L1 expression in tumors was an independent risk factor for poor survival. The results of our present study indicated that the 5-year survival rate in patients with PD-L1-positive tumors was significantly higher than in those with PD-L1-negative tumors (72.3% vs. 43.2%, P = 0.032) [8].
Several reasons exist that might explain the discrepancies, but none of those reasons are definite. First, the location from which the sample is collected, i.e., from biopsy or from resection, may result in sample heterogeneity. Obviously, samples obtained from resection provide more material for analysis than those from biopsy. Furthermore, the information obtained from resected specimens is more precise. Second, different antibodies for detection of PD-L1 expression may result in various staining outcomes even when the same detection method (IHC) is used. In addition, PD-L1 is expressed not only on the membrane of tumor cells but also on the surface of TILs. PD-L1 expression was detected using two antibodies in surgically resectable LS-SCLC, and the results showed that 14.7% (14/95) of patients expressed PD-L1 in tumor cells with a cut-off of ≥ 1% when SP142 was used, whereas the percentage was 19.4% (13/67) when clone 28-8 was used [13]. Moreover, different cut-off values and other factors such as delays in fixation and inadequate fixatives may alter immunoreactivity in formalin-fixed, paraffin embedded specimens, which can also contribute to differences in staining outcomes [14].
Our study features several advantages that improve reliability: all specimens were obtained from surgical resection, therefore ruling out heterogeneity; all patients were staged accurately as pathologic stage I-III; and all patients underwent standard postoperative chemotherapy and/or radiotherapy, which can eliminate differences caused by dissimilar postoperative treatments.
However, our study still has some limitations. First, this is a retrospective analysis, with a small sample size, in a single cancer center. Second, only PD-L1 expression was studied; other factors such as PD-1, TIL and CD8+ T cells in the tumor microenvironment were not currently evaluated. Thus, the potential mechanisms underlying our results were not revealed in the present study. Third, IHC was used to detect PD-L1 expression even though the cut-off value is indefinite. However, no standard detection methods were prescribed, and the cut-off value of ≥ 5% performed well; therefore, the credibility of this study is still high. On the other hand, as the SP142 antibody was used in the present study, which was different from other studies. Therefore, the final results might be slightly different. According to a prospective study assessing 4 unique PD-L1 antibodies on 2 separate staining platforms, the SP142 antibody is an outlier that detected significantly less PD-L1 expression [15]. Other antibodies approved for the detection of PD-L1 expression are being evaluated further in our cancer center. In that ongoing study, larger samples, more antibodies and additional tested factors are being considered.
Conclusion
In summary, the expression of PD-L1 is readily detectable, and PD-L1 expression is associated with favorable survival in patients with resectable SCLC. Therefore, the PD-L1/PD-1 pathway is one potential target in the precise treatment of SCLC.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province under Grant ZR2015HM040 (to Hui Zhu), and the Key Research and Development Program of Shandong Province under Grant 2016GSF201148 (to Hui Zhu), and the Key Research and Development Program of Shandong Province under Grant ZR2015HZ004 (to Jinming Yu).
Disclosure of conflict of interest
None.
References
- 1.Metro G, Duranti S, Fischer MJ, Cappuzzo F, Crino L. Emerging drugs for small cell lung cancer--an update. Expert Opin Emerg Drugs. 2012;17:31–36. doi: 10.1517/14728214.2012.656588. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 3.Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–8850. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, Kinoshita T, Kage M, Hoshino T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 6.Miao L, Lu Y, Xu Y, Zhang G, Huang Z, Gong L, Fan Y. PD-L1 and c-MET expression and survival in patients with small cell lung cancer. Oncotarget. 2016;8:53978–53988. doi: 10.18632/oncotarget.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyokawa G, Takada K, Haratake N, Takamori S, Akamine T, Katsura M, Fujishita T, Shoji F, Okamoto T, Oda Y, Maehara Y. Favorable disease-free survival associated with programmed death ligand 1 expression in patients with surgically resected small-cell lung cancer. Anticancer Res. 2016;36:4329–4336. [PubMed] [Google Scholar]
- 8.Chang YL, Yang CY, Huang YL, Wu CT, Yang PC. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8:18021–18030. doi: 10.18632/oncotarget.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein SD, Yang SC. Role of surgery in small cell lung cancer. Surg Oncol Clin N Am. 2011;20:769–777. doi: 10.1016/j.soc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka T, Takenoyama M, Inamasu E, Yoshida T, Toyokawa G, Nosaki K, Hirai F, Yamaguchi M, Shimokawa M, Seto T, Ichinose Y. Role of surgical resection for patients with limited disease-small cell lung cancer. Lung Cancer. 2015;88:52–56. doi: 10.1016/j.lungcan.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhu H, Guo H, Shi F, Zhu K, Luo J, Liu X, Kong L, Yu J. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer. 2014;86:334–338. doi: 10.1016/j.lungcan.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Berghoff AS, Ricken G, Wilhelm D, Rajky O, Widhalm G, Dieckmann K, Birner P, Bartsch R, Preusser M. Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC) J Neurooncol. 2016;130:19–29. doi: 10.1007/s11060-016-2216-8. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Batenchuk C, Badzio A, Boyle TA, Czapiewski P, Chan DC, Lu X, Gao D, Ellison K, Kowalewski AA, Rivard CJ, Dziadziuszko R, Zhou C, Hussein M, Richards D, Wilks S, Monte M, Edenfield W, Goldschmidt J, Page R, Ulrich B, Waterhouse D, Close S, Jassem J, Kulig K, Hirsch FR. PD-L1 expression by two complementary diagnostic assays and mrna in situ hybridization in small cell lung cancer. J Thorac Oncol. 2017;12:110–120. doi: 10.1016/j.jtho.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apple S, Pucci R, Lowe AC, Shintaku I, Shapourifar-Tehrani S, Moatamed N. The effect of delay in fixation, different fixatives, and duration of fixation in estrogen and progesterone receptor results in breast carcinoma. Am J Clin Pathol. 2011;135:592–598. doi: 10.1309/AJCPB1RIT5YXMRIS. [DOI] [PubMed] [Google Scholar]
- 15.Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, Fujimoto J, Yu H, Anders R, Kowalewski A, Rivard C, Rehman J, Batenchuk C, Burns V, Hirsch FR, Wistuba II. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–1058. doi: 10.1001/jamaoncol.2017.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]


