Abstract
Background: Non-small cell lung cancer (NSCLC) has been the leading cause of cancer death in recent years, its morbidity and mortality were increasing yearly. The presence of vasculogenic mimicry (VM) is associated with a high tumor grade, short survival, invasion, and metastasis. Slug is a key regulating factor in the process of EMT. Vimentin is one of the cytoskeleton proteins that plays an important role in EMT. However, associations among VM, Slug and vimentin and their clinicopathologic significance in NSCLC are unclear. In this study, we analyzed associations among VM, Slug and vimentin in NSCLC, and their respective associations with clinicopathologic characteristics and survival in NSCLC. Methods: Positive expression of VM, Slug and vimentin in 198 whole NSCLC tissue samples were detected by immunohistochemical staining. Patients’ clinical data were also collected. Results: Levels of VM, Slug and vimentin were significantly higher in NSCLC tissues than in normal lung tissues. Levels of VM, Slug and vimentin were positively associated with tumor grade, distant metastasis (DM), lymph node metastasis (LNM), and tumor-node metastasis (TNM) stage, and inversely with patients overall survival time (OST). In multivariate analysis, high expression of VM, Slug, vimentin, and tumor grade, DM, LNM, TNM stage, were potential to be independent prognostic factors for OST in patients with NSCLC. Conclusion: VM, Slug and vimentin affect NSCLC evolution; and the combined detection of VM, Slug and vimentin are valuable factors for metastasis and prognosis in NSCLC patients.
Keywords: Non-small cell lung cancer, VM, slug, vimentin, prognosis
Introduction
Lung cancer is one of the major malignant tumors that are most frequently diagnosed and the leading cause of cancer death in recent years [1], and its morbidity and mortality are increasing year by year. About 85% of these are non-small cell lung cancer (NSCLC). NSCLC represents a morphologically and clinically heterogeneous cancer type, with overall poor prognosis [2]. In the last decade, we have witnessed tremendous advances (improved molecular biology and targeted therapies) in the treatment of NSCLC, but the overall 5 year survival rate of NSCLC patients has not increased and remains below 20% [3].
Vasculogenic mimicry (VM) [4] is a blood supply system independent of endothelial vessels in tumor cells from different origins. During tumor progression, VM has availabile blood vessels for growth, and accesses to oxygen and nutrients [5]. VM typically exists in highly malignant tumor cells and not in normal cells [6]. VM refers to certain highly invasive tumor cells which can simulate the endothelial cells, form a pipeline structure and can be interlinked with the host microvascular system, and the red blood cells can be seen in some pipelines [7]. The presence of VM is associated with a high tumor grade, short survival, invasion, and metastasis [8,9]. The characteristics of VM can be summarized as follows: ① CD34 staining was negative, PAS (periodic acid-Schiff) staining was positive [10]; ② The inner wall of the tube is free of vascular endothelial cells [11]; ③ Microcirculation tubing defined by the extracellular matrix; ④ VM and tumor microvascular are interlinked, allowing blood flow to supply cell growth.
The Slug gene is a specific gene in vertebrates; it was first found in the development of nerve and mesoderm cells of the avian embryo [12]. The Slug gene is of crucial importance for the transformation of fibroblast-like phenotypes in malignant tumor cells and has the potential to invade epithelial markers that result in the production of mesenchymal markers [13]. The Slug gene is located on human chromosome 8q11.21. Slug belongs to a superfamily (also called Snail2); the members of the family have a similar structure which refers to the zinc-finger transcription factors. Slug has a highly conserved carboxyl terminal and variable amino terminal area which contains 4~6 zinc-fingers [14]. Overexpression of Slug messenger RNA was significantly associated with increased rate of cancer recurrence and decreased survival rate in NSCLC tumor specimens [15].
Vimentin, a major constituent of the intermediate filament family of proteins, is known to maintain cellular integrity and provide resistance against stress. It is not expressed in normal epithelial cells, but is widely distributed in a variety of endothelial cells, fiber cells, macrophages, lymphocytes and mesenchymal cells [16,17]. Vimentin is an important molecular marker of mesenchymal tissue, and its high expression in tumor cells is considered an important symbol of the transformation of tumor cells to the mesenchymal phenotype. It is reported that high vimentin protein expression is closely related to the infiltration and metastasis of tumor such as bladder cancer [18], breast cancer [19], and so on. These proved that vimentin can be used as a marker for cancer prognosis. However, the mechanisms underlying the functional contribution of vimentin to migration remain unclear.
As we know, during the epithelial-mesenchymal transition (EMT) process, Slug is a key regulatory factor; existing research also suggests that EMT plays an important role in the formation of VM [20-22]. This raises a question: is there a connection between Slug and VM? So far the relationship between Slug and VM has barely been studied. In general, studies on VM, Slug and vimentin indicate that these indicators affect tumor progression, infiltration, and metastasis. However, there are few reports on the relationship between VM, Slug and vimentin in NSCLC. In this study, we detected the correlations between these indicators in NSCLC and the associations with metastasis and prognosis.
Materials and methods
Patients and tissue specimens
We collected 198 cases of NSCLC (median age: 56.8 years; range: 44-76 years) from the First Affiliated Hospital of Bengbu Medical College. All cases had complete clinical, pathologic and follow-up information. Time interval was January 2011 to December 2012. We selected 198 adjacent normal tissue samples as controls. Patients who had received preoperative chemotherapy or radiotherapy were excluded. Clinical data was derived from medical records. According to the World Health Organization (WHO) classification system, There were 38 grade I cases, 128 grade II cases and 32 grade III cases. According to the tumor-node-metastasis (TNM) staging system of the International Union Against Cancer (UICC) these specimens were divided into: stage I + stage II (81 cases), stage III + stage IV (117 cases). Mean clinical follow-up time was 34.7±20.4 months (n = 198), range 5-84 months. The control group was derived from 198 normal lung tissues of which the distance from the tumor site was greater than 10.0 cm. For specific characteristics see Table 1.
Table 1.
Patient characteristics
| Patient characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| <60 | 64 | 32.3 |
| ≥60 | 134 | 67.7 |
| Gender | ||
| Female | 52 | 26.3 |
| Male | 146 | 73.7 |
| Smoking | ||
| No | 80 | 40.4 |
| Yes | 118 | 59.6 |
| Size (cm) | ||
| <3.0 | 36 | 18.2 |
| ≥3.0, <7.0 | 130 | 65.7 |
| ≥7.0 | 32 | 16.1 |
| Gross Type | ||
| Central | 107 | 54.0 |
| Peripheral | 91 | 46.0 |
| Histologic Type | ||
| Squamous cell carcinoma | 105 | 53.0 |
| Adenocarcinoma | 93 | 47.0 |
| Grade | ||
| Well | 38 | 19.2 |
| Moderate | 128 | 64.6 |
| Poor | 32 | 16.2 |
| LNM | ||
| N0 | 84 | 42.4 |
| N1 | 86 | 43.4 |
| N2 | 28 | 14.2 |
| TNM stage | ||
| I + II | 81 | 40.9 |
| III + IV | 117 | 59.1 |
Immunohistochemistry
Immunohistochemical staining was performed using the ElivisionTM Plus detection kit instructions (Lab Vision, USA). All NSCLC and the corresponding normal lung tissues were fixed in 10% buffered formalin and embedded in paraffin. We cut the tissue into 4 micrometer (μm) thick for analysis. All sections were deparaffinized and dehydrated in a graded alcohol and xylene and then rinsed with phosphate buffered saline (PBS, pH 7.2) for 10 minutes. Subsequently, all slices were incubated in methanol containing 3% hydrogen peroxide for 10 minutes at room temperature to inhibit endogenous peroxidase activity. Then all sections were placed in citric acid buffer (pH 6.0) to repair antigen at 95°C for 30 minutes. After rinsing with PBS several times, all sections were blocked with goat serum for 30 minutes at room temperature, then incubated with mouse monoclonal antibody against human CD34 (Abcam, USA), Slug (Abcam, USA), and vimentin (Abcam, USA) at 37°C for one hour. All samples were conducted Periodic Acid-Schiff (PAS)-CD34 dual staining to identify vascular endothelial cells in the glycosylated basement membranes, as well as vascular-like (VM) structures [7,23]. Then all were counterstained with hematoxylin, then air-dried and mounted. Negative controls in the immunohistochemical procedure were processed by omitting primary antibodies. Slug mainly stained brown and yellow in nucleus as positive, and occasionally stained in the cytoplasm; vimentin was mainly expressed in cytoplasm. All steps were carried out according to the kit specification.
Evaluation of immunostaining
Using the semi-quantitative scoring method, each specimen had 10 high-power-fields (400 magnification) areas selected. Then the percentage of positive cells was calculated in each field of view: 0 was negative, 1 (10% or less), 2 (11%~50%), 3 (51%~75%), 4 (>75%). The intensity of positive cells in each field was assessed: 0 was colorless, 1 was pale yellow, 2 was brown-yellow and 3 was strong staining. The combined score was calculated by multiplying the two scores. When the product of intensity and positive cell percentage was greater than or equal to 3, it was positive for immune reaction, otherwise it was deemed negative. The results were evaluated by two senior pathologists through a double-blind method.
Statistical analysis
The correlation between clinicopathologic features and the expression of VM, Slug, or vimentin was compared by Fisher’s exact test or Pearson Chi-square test. The correlation between VM, Slug, or vimentin was compared with the Spearman coefficient test. The effects of VM, Slug or vimentin on the overall survival time (OST) were determined by univariate and multivariate analysis. Survival analysis was made by Kaplan-Meier method with the log-rank test, to evaluate VM, Slug, vimentin staining results or the clinicopathologic characteristics. OST analysis was used to assess the relationship between the expression of VM, Slug and vimentin, using SPSS 24.0 software for Windows (New York, IBM, USA). P<0.05 was considered to be statistically significant.
Results
Associations between VM, Slug, and vimentin expressions and clinicopathological characteristics
To assess the contributions of VM, Slug and vimentin to NSCLC, the results thereof were immunohistochemically assessed for both NSCLC and corresponding normal lung tissue specimens. These data were compared to patient clinicopathologic characteristics.
The positive rate of VM (small vessel, which is like a lumen in NSCLC, the lumen was PAS-positive but CD34-negative). The VM structure pattern included tubular, linear, and network. In the NSCLC specimens VM (36.9%, 73/198) was significantly higher than that of the corresponding normal lung tissues (0%, 0/198; P<0.001; Figure 1A and 1B). There was a significant difference between the positive rate of VM and TNM stage, grade, LNM, and DM in NSCLC. However, the presence of VM was not associated with gender, age, smoking history, tumor localization, histological type or gross type (Table 2).
Figure 1.
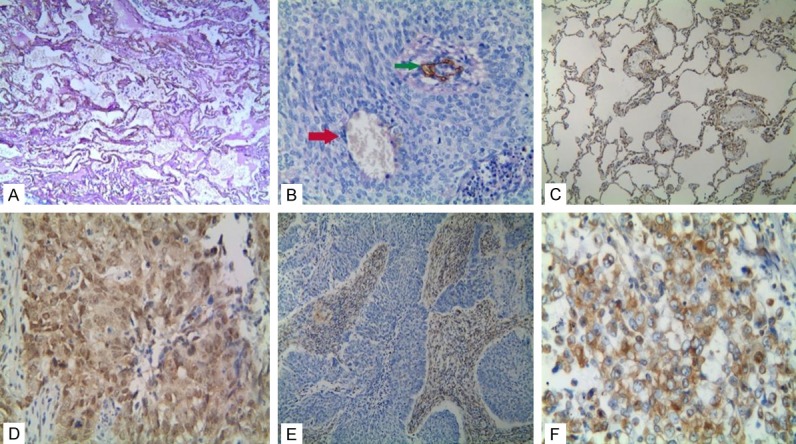
Immunostaining of VM, Slug, or vimentin in NSCLC or the control tissue. A: Negative staining of VM in the control tissue (100 magnification); B: Positive staining of VM in the NSCLC tissue (400 magnification, red arrow is VM structure, green arrow is vessels); C: Negative staining of Slug in the control tissue (100 magnification); D: Positive staining of Slug in the NSCLC tissue (400 magnification); E: Negative staining of vimentin in the NSCLC tissue (100 magnification); F: Positive staining of vimentin in the NSCLC tissue (400 magnification).
Table 2.
Associations between VM and expression of Slug and vimentin and clinicopathologic characteristics of NSCLC
| Variables | VM | P | Slug | P | Vimentin | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| - | + | - | + | - | + | ||||
| Age (years) | 0.078 | 0.402 | 0.135 | ||||||
| <60 | 46 | 18 | 15 | 49 | 47 | 17 | |||
| ≥60 | 79 | 55 | 39 | 95 | 84 | 50 | |||
| Gender | 0.163 | 0.668 | 0.376 | ||||||
| Female | 37 | 15 | 13 | 39 | 37 | 15 | |||
| Male | 88 | 58 | 41 | 105 | 94 | 52 | |||
| Smoking | 0.294 | 0.020 | 0.347 | ||||||
| No | 54 | 26 | 29 | 51 | 56 | 24 | |||
| Yes | 71 | 47 | 25 | 93 | 75 | 43 | |||
| Size (cm) | <0.001 | 0.004 | <0.001 | ||||||
| <3.0 | 31 | 5 | 16 | 20 | 34 | 2 | |||
| ≥3.0, <7.0 | 86 | 44 | 35 | 95 | 89 | 41 | |||
| ≥7.0 | 8 | 24 | 3 | 29 | 8 | 24 | |||
| Gross Type | 0.451 | 0.222 | 0.400 | ||||||
| Central | 65 | 42 | 33 | 74 | 68 | 39 | |||
| Peripheral | 60 | 31 | 21 | 70 | 63 | 28 | |||
| Histological Type | 0.206 | 0.839 | 0.658 | ||||||
| Squamous cell carcinoma | 62 | 43 | 28 | 77 | 68 | 37 | |||
| Adenocarcinoma | 63 | 30 | 26 | 67 | 63 | 30 | |||
| Grade | <0.001 | <0.001 | <0.001 | ||||||
| Well | 32 | 6 | 21 | 17 | 33 | 5 | |||
| Moderate | 83 | 45 | 30 | 98 | 87 | 41 | |||
| Poor | 10 | 22 | 3 | 29 | 11 | 21 | |||
| LNM | <0.001 | <0.001 | <0.001 | ||||||
| N0 | 72 | 12 | 42 | 42 | 74 | 10 | |||
| N1 | 49 | 37 | 10 | 76 | 51 | 35 | |||
| N2 | 4 | 24 | 2 | 26 | 6 | 22 | |||
| TNM stage | <0.001 | <0.001 | <0.001 | ||||||
| I + II | 74 | 7 | 42 | 39 | 76 | 5 | |||
| III + IV | 51 | 66 | 12 | 105 | 55 | 62 | |||
The positive rate of Slug expression was significantly higher in NSCLC tissues (72.7%, 144/198) than that in the control tissues (25.7%, 51/198; P<0.001; Figure 1C and 1D) similar to VM. The positive rate of Slug expression in NSCLC was related to tumor grade, DM, LNM, TNM stage, but not with gender, age, smoking history, tumor localization, histologic type or gross findings (Table 2).
The positive rate of vimentin expression was significantly higher in NSCLC tissues (33.8%, 67/198) than that in the control tissues (18.2%, 36/198; P<0.001; Figure 1E and 1F). The positive rate of vimentin expression was inversely associated with tumor grade, DM, LNM, and TNM stage. No correlation was found between vimentin expression and patient gender, age, smoking history, tumor localization, histologic type, or gross type (Table 2).
Correlations among VM, expression of Slug and vimentin in NSCLC
In the case of the VM positive group, the Slug positive rate was 90.4% (66/73); it was significantly higher than the non-VM group (62.4%, 78/125). In the VM positive group, there were 61 cases of vimentin positive expression (65.8%, 48/73); the difference was statistically significant. Among Slug positive and negative groups, the expression rates of vimentin were 42.4% and 11.1% respectively. According to the Spearman correlation coefficient analysis, the expression of the VM in NSCLC group was positively correlated with the expression of Slug (r = 0.303, P<0.001) and vimentin (r = 0.294, P<0.001); also the expression of Slug was positively correlated with the expression of vimentin (r = 0.515, P<0.001; Table 3).
Table 3.
Correlation among VM, expression of Slug and vimentin in NSCLC
| Variables | VM | r | p | Slug | r | p | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| - | + | - | + | |||||
| VM | 0.303 | <0.001@ | ||||||
| - | 47 | 78 | ||||||
| + | 7 | 66 | ||||||
| Vimentin | 0.515 | <0.001@ | 0.294 | <0.001@ | ||||
| - | 106 | 25 | 48 | 83 | ||||
| + | 19 | 48 | 6 | 61 | ||||
Positive association.
Univariate and multivariate analyzes
Follow-up data showed that OST was significantly lower in NSCLC patients with VM+ specimens (22.3±13.3 months) compared with those with VM- (41.9±20.4 months; log-rank = 56.100, P<0.001; Figure 2A). Similarly, OST of Slug + patients (30.0±18.3 months) was significantly lower than that of Slug-patients (47.2±20.6 months; log-rank = 25.376, P<0.001; Figure 2B). The OST of vimentin + patients (21.6±14.4 months) was significantly lower than for those who were Vimentin- (41.4±19.8 months; log-rank = 61.383, P<0.001; Figure 2C). In univariate analysis, OST was significantly related to clinicopathologic characteristics, including tumor size (log-rank = 24.638, P<0.001, Figure 2D), LNM (log-rank = 79.942, P<0.001, Figure 2E), and TNM stage (log-rank = 82.953, P<0.001, Figure 2F; Table 4).
Figure 2.
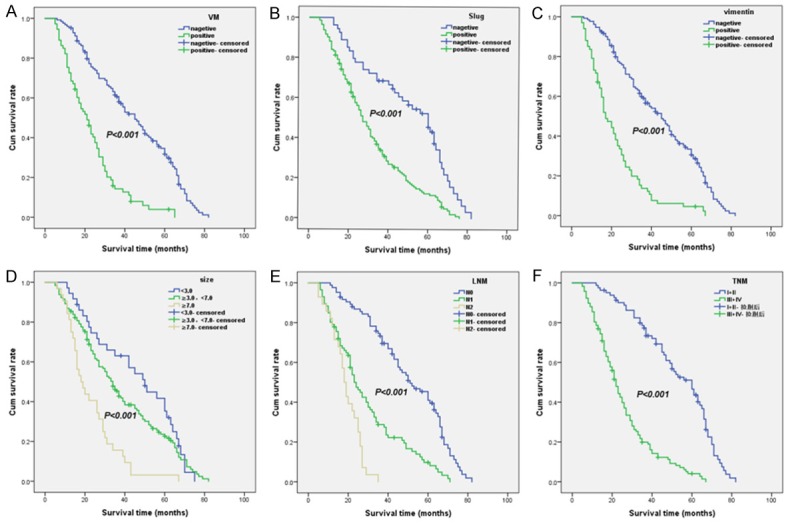
Kaplan-Meier analysis of the survival rate of patients with NSCLC. The y-axis represents the percentage of patients; the x-axis, their survival in months. (A) Overall survival of all patients in relation to VM (log-rank = 56.100, P<0.001); (B) Overall survival of all patients in relation to Slug expression (log-rank = 25.376, P<0.001;); (C) Overall survival of all patients in relation to vimentin expression (log-rank = 61.383, P<0.001). In (A-C) analyses, the green line represents patients with positive VM, or Slug, or vimentin and the blue line representing the negative VM, or Slug, or vimentin group. In (D) analyses, the blue line represents patients in tumor size <3.0 cm group, the green line represents patients in 3.0 cm ≤ tumor size <7.0 cm group, and the brown line represents patients in tumor size ≥7.0 cm group. In (E) analyses, the blue line represents patients in N0 group, the green line represents patients in N1 group, and the brown line represents patients in N2 group. In (F) analyses, the blue line represents patient in I + II stage group, and the green line represents patients in stage III + IV group.
Table 4.
Univariate analyses of overall survival (OS) time
| Variable | n | Mean OS (months) | Log-rank | P value |
|---|---|---|---|---|
| VM | 56.100 | <0.001 | ||
| Negative | 125 | 41.9±20.4 | ||
| Positive | 73 | 22.3±13.3 | ||
| Slug | 25.376 | <0.001 | ||
| Negative | 54 | 47.2±20.6 | ||
| Positive | 144 | 30.0±18.3 | ||
| Vimentin | 61.383 | <0.001 | ||
| Negative | 131 | 41.4±19.8 | ||
| Positive | 67 | 21.6±14.4 | ||
| Age (years) | 11.645 | 0.001 | ||
| <60 | 64 | 40.9±23.4 | ||
| ≥60 | 134 | 31.7±18.2 | ||
| Gender | 0.994 | 0.319 | ||
| Female | 52 | 37.2±21.4 | ||
| Male | 146 | 33.8±20.1 | ||
| Smoking | 1.658 | 0.198 | ||
| No | 80 | 37.8±20.7 | ||
| Yes | 118 | 32.5±20.0 | ||
| Size (cm) | 24.638 | <0.001 | ||
| <3.0 | 36 | 43.5±20.6 | ||
| ≥3.0, <7.0 | 130 | 35.1±20.6 | ||
| ≥7.0 | 32 | 22.9±13.2 | ||
| Gross Type | 0.001 | 0.975 | ||
| Central | 107 | 33.9±19.8 | ||
| Peripheral | 91 | 35.5±21.1 | ||
| Histological Type | 0.066 | 0.797 | ||
| Squamous cell carcinoma | 105 | 35.4±20.5 | ||
| Adenocarcinoma | 93 | 33.9±20.4 | ||
| Grade | 11.513 | 0.003 | ||
| Well | 38 | 42.2±20.4 | ||
| Moderate | 128 | 34.6±20.5 | ||
| Poor | 32 | 26.3±17.1 | ||
| LNM | 79.942 | <0.001 | ||
| N0 | 84 | 47.2±19.0 | ||
| N1 | 86 | 27.6±17.5 | ||
| N2 | 28 | 18.8±7.7 | ||
| TNM stage | 82.953 | <0.001 | ||
| I + II | 81 | 49.5±18.5 | ||
| III + IV | 117 | 24.4±14.6 |
Multivariate analysis indicated that VM, Slug, vimentin, DM, LNM, as well as TNM stage, were independent prognostic factors for NSCLC (Table 5).
Table 5.
Multivariate analysis of overall survival time (OST)
| Variable | B | SE | P | RR | 95% Cl |
|---|---|---|---|---|---|
| VM | 0.433 | 0.208 | 0.038 | 1.542 | 1.025-2.320 |
| Slug | 0.548 | 0.225 | 0.009 | 1.793 | 1.154-2.786 |
| Vimentin | 0.607 | 0.210 | 0.004 | 1.834 | 1.215-2.770 |
| LNM | 0.399 | 0.187 | 0.033 | 1.491 | 1.033-2.150 |
| TNM | 0.778 | 0.296 | 0.009 | 2.178 | 1.154-3.893 |
| DM | 2.493 | 0.407 | <0.001 | 12.100 | 5.451-26.860 |
Discussion
NSCLC is a highly heterogeneous carcinoma, whose prognosis is influenced by many factors. Because of the similarities between VM formation and EMT [22], we hypothesized that epithelial-derived tumor cells may differentiate into mesenchymal-derived endothelial cells via EMT to mimic the morphology and function of endothelial cells and thus form VM. Therefore, the study of the relationship between Slug and vimentin (an important factor inducing the formation of EMT and the key molecules formed by VM), contributes to reveal the mechanism of VM formation in epithelial-derived tumors.
Inthis study we found 73 examples that showed a structure that conformed to VM, demonstrating the existence of VM in 198 cases of NSCLC. We found that VM was significantly higher in NSCLC tissues than in the control group. It was also positively correlated with tumor grade, DM, LNM and TNM stage. In addition, the Kaplan-Meier survival analysis showed that the OST of patients with VM+ was significantly lower than for VM-patients in NSCLC. These findings suggest that VM not only offers blood supply for tumor invasion, but also has a close relationship with development, progression and metastasis of NSCLC, which leads to poor prognosis. These results can be associated with other malignant tumors in the previous literature reports [24-27]. These all proved that VM should serve as a useful clinical biomarker for NSCLC.
At present, it is believed that EMT-inducing cancer cells invade surrounding tissues and enter the bloodstream, leading primary tumors to metastasize other organs, which are necessary steps for cancer migration, progression and metastasis [28,29]. Slug promotes EMT formation by inhibiting the expression of E-cadherin and binding to the E-box structure of the target gene (5’-CAGGTG-3’) [30] to increase the invasive ability [31]. As discussed in detail below, several studies had investigated the role of Slug in cancers, such as pancreatic cancer [32], breast cancer [33], gallbladder carcinoma [34], ovarian cancer [35], gastric cancer [36] etc. Our results were similar to prior results, and also demonstrated that in NSCLC, the expression of Slug was significantly higher than that of the control group, and was positively correlated with TNM stage, tumor grade, DM and LNM. In addition, the graphs of Kaplan-Meier survival analysis indicated that the survival rate of Slug-positive patients was significantly lower than the rate for the Slug- negative group. These results indicated that the overexpression of Slug promoted the progression and metastasis of NSCLC. Slug may be a potential therapeutic target for NSCLC.
EMT was characterized by a deletion of epithelial cell markers such as E-cadherin and increased expression of interstitial cells such as vimentin [16,20]. As an important factor in the EMT, vimentin was an important signal and transcription factor to participate in the rapid growth of tumor cells, infiltration and metastasis [37,38], the research and intervention of vimentin was of great significance to the treatment of cancer. The experimental results showed that in NSCLC tissues, as with VM and Slug mentioned above, vimentin was positively related to TNM stage, tumor grade and LNM. We concluded that vimentin was associated with the metastasis and invasion of NSCLC. Furthermore, Kaplan-Meier survival analysis showed that NSCLC patients with positive vimentin expression had obviously reduced OST by comparison to those were negative of vimentin. According to the results, vimentin was also related to the prognosis of NSCLC. Moreover, in Slug positive NSCLC tissues, the expression of vimentin had been increased, which demonstrated that Slug could increase the expression of vimentin and maybe induce EMT in NSCLC.
This study also found that in the absence of lymph node metastasis of NSCLC tissues, the probability of VM occurrence was 14.3%, the expression of vimentin rate was 11.9%; whereas in the tissues with lymph node metastasis of NSCLC, the rates were 53.5% and 50.0% respectively. Thus, we speculated that patients with EMT had more frequent metastasis to lymph nodes, and this will be help decide whether lymph node dissection for NSCLC surgery should be performed.
Conclusions
Our results imply that VM, Slug, and vimentin are associated with tumor metastasis and poor prognosis in NSCLC; and the combined detection of VM, Slug, and vimentin has potential for predicting metastasis and prognosis in NSCLC. We hope the results can provide a new direction for studying the metastasis and prognosis of NSCLC.
Acknowledgements
This work was supported by the Nature Science Foundation of Anhui Province (No. 1708085MH230).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Sakashita S, Sakashita M, Sound Tsao M. Genes and pathology of non-small cell lung carcinoma. Semin Oncol. 2014;41:28–39. doi: 10.1053/j.seminoncol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delgado-Bellido D, Serrano-Saenz S, Fernandez-Cortes M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer. 2017;16:65. doi: 10.1186/s12943-017-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiliopoulos K, Peschos D, Batistatou A, Ntountas I, Papoudou-Bai A, Zioga A, Agnantis N, Kitsos G. Immunohistochemical study of vasculogenic mimicry and angiogenesis in melanocytic tumors of the eye and the periocular area. Anticancer Res. 2017;37:1113–1120. doi: 10.21873/anticanres.11424. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Gu R, Han X, Wu G, Liu J. Cyclin-dependent kinase 5 controls vasculogenic mimicry formation in non-small cell lung cancer via the FAK-AKT signaling pathway. Biochem Biophys Res Commun. 2017;492:447–452. doi: 10.1016/j.bbrc.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 9.Zhu B, Zhou L, Yu L, Wu S, Song W, Gong X, Wang D. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg. 2017;17:47. doi: 10.1186/s12893-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun W, Shen ZY, Zhang H, Fan YZ, Zhang WZ, Zhang JT, Lu XS, Ye C. Overexpression of HIF-1α in primary gallbladder carcinoma and its relation to vasculogenic mimicry and unfavourable prognosis. Oncol Rep. 2012;27:1990–2002. doi: 10.3892/or.2012.1746. [DOI] [PubMed] [Google Scholar]
- 11.Frenkel S, Barzel I, Levy J, Lin AY, Bartsch DU, Majumdar D, Folberg R, Pe’er J. Demonstrating circulation in vasculogenic mimicry patterns of uveal melanoma by confocal indocyanine green angiography. Eye (Lond) 2008;22:948–952. doi: 10.1038/sj.eye.6702783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 13.Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–4422. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Jang K, Miller P, Picon-Ruiz M, Yeasky TM, El-Ashry D, Slingerland JM. VEGFA links self-renewal and metastasis by inducing Sox2 to repress miR-452, driving slug. Oncogene. 2017;36:5199–5211. doi: 10.1038/onc.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299–1304. doi: 10.1093/carcin/bgr110. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AM, Havel LS, Koyen AE, Konen JM, Shupe J, Wiles WG 4th, Martin WD, Grossniklaus HE, Sica G, Gilbert-Ross M, Marcus AI. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24:420–432. doi: 10.1158/1078-0432.CCR-17-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahmani AH, Babiker AY, AlWanian WM, Elsiddig SA, Faragalla HE, Aly SM. Association of cytokeratin and vimentin protein in the genesis of transitional cell carcinoma of urinary bladder patients. Dis Markers. 2015;2015:204759. doi: 10.1155/2015/204759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollong MJ, Pietila M, Pearson AD, Sarkar TR, Ahmad I, Soundararajan R, Lyssiotis CA, Mani SA, Schultz PG, Lairson LL. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc Natl Acad Sci U S A. 2017;114:E9903–E9912. doi: 10.1073/pnas.1716009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 21.Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016:5. doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Qiao L, Liang N, Xie J, Zhang J, Deng G, Luo H, Zhang J. The relationship between vasculogenic mimicry and epithelial-mesenchymal transitions. J Cell Mol Med. 2016;20:1761–1769. doi: 10.1111/jcmm.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson SC, Metcalf RL, Trapani F, Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N, Polanski R, Nonaka D, Priest L, Fusi A, Carlsson F, Carlsson A, Hendrix MJ, Seftor RE, Seftor EA, Rothwell DG, Hughes A, Hicks J, Miller C, Kuhn P, Brady G, Simpson KL, Blackhall FH, Dive C. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Q, Yuan Y, Jin Z, Xu T, Gao Y, Wei H, Li C, Hou W, Hua B. Association between tumor vasculogenic mimicry and the poor prognosis of gastric cancer in china: an updated systematic review and meta-analysis. Biomed Res Int. 2016;2016:2408645. doi: 10.1155/2016/2408645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Sun B, Zhao X, Liu Z, Wang X, Yao X, Dong X, Chi J. Clinical significances and prognostic value of cancer stem-like cells markers and vasculogenic mimicry in renal cell carcinoma. J Surg Oncol. 2013;108:414–419. doi: 10.1002/jso.23402. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JT, Sun W, Zhang WZ, Ge CY, Liu ZY, Zhao ZM, Lu XS, Fan YZ. Norcantharidin inhibits tumor growth and vasculogenic mimicry of human gallbladder carcinomas by suppression of the PI3-K/MMPs/Ln-5γ2 signaling pathway. BMC Cancer. 2014;14:193. doi: 10.1186/1471-2407-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 29.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 30.Nassour M, Idoux-Gillet Y, Selmi A, Come C, Faraldo ML, Deugnier MA, Savagner P. Slug controls stem/progenitor cell growth dynamics during mammary gland morphogenesis. PLoS One. 2012;7:e53498. doi: 10.1371/journal.pone.0053498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun D, Sun B, Liu T, Zhao X, Che N, Gu Q, Dong X, Yao Z, Li R, Li J, Chi J, Sun R. Slug promoted vasculogenic mimicry in hepatocellular carcinoma. J Cell Mol Med. 2013;17:1038–1047. doi: 10.1111/jcmm.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsukasa K, Ding Q, Yoshimitsu M, Miyazaki Y, Matsubara S, Takao S. Slug contributes to gemcitabine resistance through epithelial-mesenchymal transition in CD133 (+) pancreatic cancer cells. Hum Cell. 2015;28:167–174. doi: 10.1007/s13577-015-0117-3. [DOI] [PubMed] [Google Scholar]
- 33.Dhasarathy A, Phadke D, Mav D, Shah RR, Wade PA. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS One. 2011;6:e26514. doi: 10.1371/journal.pone.0026514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DG, Lee SH, Kim JS, Park J, Cho YL, Kim KS, Jo DY, Song IC, Kim N, Yun HJ, Park YJ, Lee SJ, Lee HG, Bae KH, Lee SC, Shim S, Kim YM, Kwon YG, Kim JM, Lee HJ, Min JK. Loss of NDRG2 promotes epithelial-mesenchymal transition of gallbladder carcinoma cells through MMP-19-mediated Slug expression. J Hepatol. 2015;63:1429–1439. doi: 10.1016/j.jhep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Gu A, Jie Y, Yao Q, Zhang Y, Mingyan E. Slug is associated with tumor metastasis and angiogenesis in ovarian cancer. Reprod Sci. 2017;24:291–299. doi: 10.1177/1933719116654989. [DOI] [PubMed] [Google Scholar]
- 36.Castro Alves C, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. J Pathol. 2007;211:507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 37.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahat G, Zhu QS, Huang KL, Wang S, Bolshakov S, Liu J, Torres K, Langley RR, Lazar AJ, Hung MC, Lev D. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS One. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


