Abstract
It is well-known that decreased levels of NK cells are found in patients with systemic lupus erythematosus (SLE). However, the mechanism of deregulation of NK cells in SLE is largely unknown. In this study, expression of T-cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory domains (TIGIT) on NK cells was determined by flow cytometry and correlation with markers of autoimmune response, inflammation, disease activity and severity of SLE was further analyzed. Moreover, the function of TIGIT on NK cells in SLE was investigated. We have found that the frequency of TIGIT-expressing NK cells was significantly decreased in SLE patients. The frequency of TIGIT-expressing NK cells in patients with SLE was decreased significantly in subjects with low complement, positive anti-ribosomal RNP (anti-rRNP), and high SLE Disease Activity Index (SLEDAI) score. Furthermore, the frequency of TIGIT-expressing NK cells was significantly increased in SLE patients after regular treatment. In addition, the activation marker CD69, degranulation marker CD107a and cytokine IFN-γ production potential of TIGIT+ NK cells were significantly lower than those of TIGIT- NK cells. Blocking the TIGIT pathway by functional anti-TIGIT monoclonal antibody restored IFN-γ secretion of NK cells. In conclusion, TIGIT expression was significantly decreased on NK cells in patients with SLE and correlated negatively with disease activity and severity of SLE. Additionally, the functional potential of TIGIT+ NK cells was significantly decreased compared with TIGIT- NK cells. This study reveals that TIGIT is a powerful negative regulator of NK cells in SLE.
Keywords: Systemic lupus erythematosus, TIGIT, NK cells
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease characterized by overproduction of autoantibodies, which potentially cause immune-complex-related inflammation in various tissues and organs [1,2]. At present, the pathogenesis of SLE remains unclear. However, there is a large body of evidence that the development of SLE is attributed to disruptions in adaptive immunity. Adaptive immunological characteristics of SLE are hyperactive B cells and abnormal T cells, which lead to the production of autoantibodies against components of the cell nucleus.
However, evidence over the last few decades has clearly indicated disorder of innate immune responses in the development of SLE [3]. Natural killer (NK) cells are a subset of mononuclear cells, distinguished from B and T lymphocytes by virtue of their large granular morphology and belonging to the innate immune system [4]. NK cells affect immune responses by killing target cells directly or by producing pro-inflammatory and regulatory cytokines [5]. Recent evidence indicates that the levels of circulating NK cells are significantly lower in SLE patients and NK cells may play an important role in the pathogenesis of SLE [6,7]. Nevertheless, the role of NK cells in SLE pathogenesis and the mechanisms have not been well elucidated.
Substantial evidence indicates that NK cell function relies on receptor-ligand interactions [5]. It was recently shown that a new inhibitory receptor, named T-cell immunoglobulin and ITIM domain (TIGIT), is expressed mainly on activated T cells and NK cells [8]. CD155 has been identified as the physical ligand of TIGIT. The interaction of CD155, which is expressed mainly on antigen-presenting cells, and TIGIT could mediate inhibitory effects on TIGIT-expressing cells [9]. As expected, TIGIT was reported to inhibit activation of T cells and NK cells, manifested by downregulating cytokine secretion by T cells and the cytotoxicity of NK cells [10-12]. Previous studies have shown that genetic ablation or antibody blockade of TIGIT exacerbates the severity of autoimmune diseases in mouse model [13]. Furthermore, a previous study has shown that TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals [14]. Moreover, evidence from our previous study and others have indicated that the TIGIT pathway involving T-cell responses plays an important role in regulation of SLE [15,16]. However, whether TIGIT can regulate the function of NK cells in SLE has not been well elucidated.
In the present study, we detected expression of TIGIT on NK cells in patients with SLE. Correlation between expression of TIGIT on NK cells and activity of SLE was also investigated. Moreover, we explored the function of TIGIT on NK cells in SLE.
Subjects and methods
Subjects
A total of 44 patients fulfilling the revised American College of Rheumatology criteria for SLE [17] were recruited from the First Affiliated Hospital of Nanchang University. Among them, 8 patients were monitored before and after receiving regular treatment. Additionally, 5 patients were new-onset SLE with a first time diagnosis of SLE and no history of corticosteroid or immunosuppressive drug use before registration [18]. Disease activity was assessed by the SLE disease activity index (SLEDAI). Patients with SLE were classified into the inactive group (SLEDAI: 0-9) and the active group (SLEDAI ≥ 10) according to SLEDAI [19]. The patient characteristics of this group are shown in Table 1. As an autoimmune disease control, 21 rheumatoid arthritis (RA) patients were also enrolled from the First Affiliated Hospital of Nanchang University. In addition, this study included 27 healthy controls (HCs, female 85%, mean age 33.7 ± 10.8 years) who were unrelated to the patients and who did not have inflammatory or autoimmune diseases. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (052) and was carried out in compliance with the Helsinki Declaration. Informed consent was obtained from all participants before they entered the study.
Table 1.
Clinical characteristics of patients with SLE
| Categories | SLE (n = 44) |
|---|---|
| Females, n (%) | 40 (89.0) |
| Age, mean (S.D.), years | 39.0 ± 15.0 |
| SLEDAI score, mean (S.D.) | 9.2 ± 6.1 |
| ds-DNA (+, > 100 IU/mL), n (%) (43 patients) | 21 (48.8) |
| Anti-ENA (42 patients) | |
| Anti-Sm, n (%) | 11 (26.2) |
| Anti-Ro52, n (%) | 21 (50.0) |
| Anti-nRNP/Sm, n (%) | 15 (35.7) |
| Anti-rRNP, n (%) | 13 (31.0) |
| Anti-nucleosome, n (%) | 17 (40.5) |
| Anti-SSA, n (%) | 29 (69.0) |
| Anti-SSB, n (%) | 2 (4.8) |
| Decreased C3, n (%) | 31 (70.5) |
| Decreased C4, n (%) | 29 (65.9) |
| Increased IgG, n (%) | 21 (47.7) |
| Elevated ESR, n (%) | 27 (61.4) |
| Clinical features | |
| Fever, n (%) | 4 (9.1) |
| Cutaneous manifestations, n (%) | 20 (45.5) |
| Oral ulcer, n (%) | 3 (6.8) |
| Alopecia, n (%) | 8 (18.2) |
| Arthritis, n (%) | 16 (36.4) |
| Neuropathic lupus, n (%) | 3 (6.8) |
| Effusion, n (%) | 8 (18.2) |
| 24 hours proteinuria, n (%) | 10 (22.7) |
| Hematuresis, n (%) | 6 (13.6) |
| Pyuria, n (%) | 5 (11.4) |
| Leucopenia | 13 (29.5) |
| Erythrocytopenia | 21 (47.7) |
| Thrombocytopenia | 9 (20.5) |
| Anemia | 23 (52.3) |
Anti-dsDNA, anti double-stranded DNA; Anti-ENA, anti extractable nuclear antigen; C3, complement 3; C4, complement 4; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; RNP, ribonucleoprotein; rRNP, ribosomal RNP; SLE, Systemic lupus erythematosus; SLEDAI, SLE disease activity index; WBC, white blood cell.
Cell preparation and activation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of patients by using Ficoll-Hypaque density gradients (Sigma, USA). All cells were cultured in RPMI-1640 medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum and maintained at 37°C in a 5% CO2 humidified atmosphere. For cell stimulation studies, PBMCs were stimulated with IL-12 (100 U/ml, BioLegend), or LPS (10 µg/ml, Sigma) for 24 h. In some experiments, functional anti-human TIGIT antibody (5 µg/ml, ebioscience), or IgG control (ebioscience) were added to the culture medium for blocking TIGIT pathway.
Flow cytometry analysis
PBMCs were isolated from the fresh peripheral blood of SLE patients, RA patients and HCs on Ficoll-Paque gradient (Sigma, USA). The molecular phenotypes of NK cells were analyzed immediately using flow cytometry. The following monoclonal antibodies were used: ECD-conjugated anti-CD3, PC7-conjugated anti-CD56 (BECKMAN COULTER, USA), and PE-conjugated anti-TIGIT, FITC-conjugated anti-TIGIT, PE-conjugated anti-CD69, PE-conjugated anti-CD107a, PE-conjugated anti-IFN-γ (MIH clones, e Bioscience, San Diego, CA, USA). NK cells were identified as CD56+CD3- populations. Cells incubated with PE-conjugated mouse IgG or FITC-conjugated mouse IgG were used as isotype controls. All of these cell suspensions were incubated for 30 min on ice. For intracellular staining, cells were fixed and permeabilized, and stained with anti-IFN-γ. All flow samples were analyzed with a CYTOMICS FC 500 flow cytometer (BECKMAN COULTER) and associated software programs (CXP).
Serum IgG, C3, C4, autoantibody, ESR, urine routine, blood routine measurement
The concentrations of serum Immunoglobulin G (IgG), Complement 3 (C3) and Complement 4 (C4) were determined by nephelometry methods according to the instructions described by the manufacturer (IMMUNE800, BeckMan, American). Anti-extractable nuclear antigens (ENAs) antibodies including anti-SSA, anti-SSB, anti-Ro52, anti-Sm, anti-nRNP/Sm, anti-rRNP and anti-nucleosome antibody were determined using immunoenzyme dot assay (Euroimmun, Germany) according to the manufacturer’s instructions. The results of anti-ENAs detection were shown to be negative (-) and positive (+, ++, +++) manner by EuroBlot One. Anti-dsDNA of IgG in serum was measured using commercially available ELISA kits (Kexin, Shanghai, China). Erythrocyte sedimentation rate (ESR), urine routine and blood routine were determined according to the instructions described by the manufacturer.
Statistical analysis
Statistical analysis and graphic presentation were carried out with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). A t-test was used where the normality test passed; otherwise, the nonparametric Mann-Whitney test was used to analyze the data. For evaluation of changes with treatment in the group of 8 patients and blocking the TIGIT pathway on NK cells, paired t tests or Wilcoxon matched pairs test was used. Likewise, the Pearson method or the nonparametric Spearman method was used for correlation analysis. Value of P < 0.05 is considered as significant difference.
Results
Levels of circulating natural killer (NK) cells in SLE patients
According to the evidence that the levels of circulating immune effector cells may be significantly altered in autoimmune diseases, the levels of circulating NK cells in the peripheral blood were determined by flow cytometry. As shown in Figure 1, while SLE patients showed a significantly lower proportion of circulating NK cells and NK cells counts than HC (P < 0.0001; P < 0.0001), the proportion of circulating NK cells and NK cells counts were higher in RA patients than SLE patients (P = 0.0210; P = 0.0365). RA patients also showed a significantly lower proportion of circulating NK cells and NK cells counts than HC (P = 0.0160; P = 0.0011).
Figure 1.
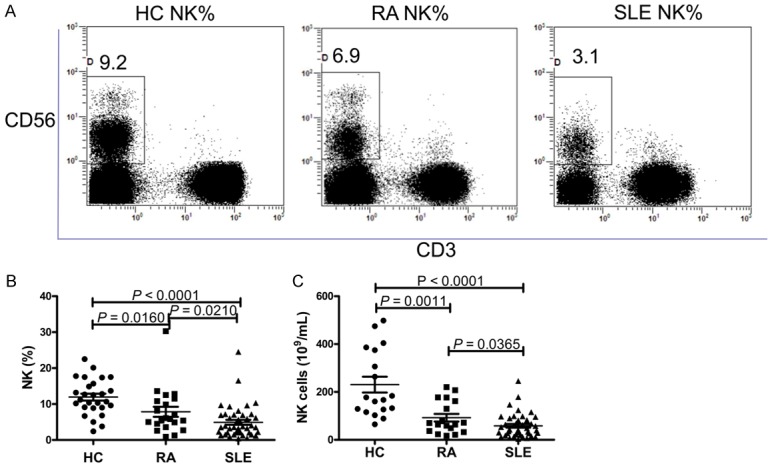
Levels of natural killer (NK) cells in systemic lupus erythematosus (SLE) patients. A. Representative dot plots of population gating and NK cells from healthy controls (HC), rheumatoid arthritis (RA) and SLE patients. Proportion of NK cells among HC, RA patients and SLE patients are shown. B. Summary data of NK cells proportion in HC, RA patients and SLE patients. C. Summary data of NK cells in HC, RA patients and SLE patients.
Differential expression of TIGIT on NK cells in patients with SLE
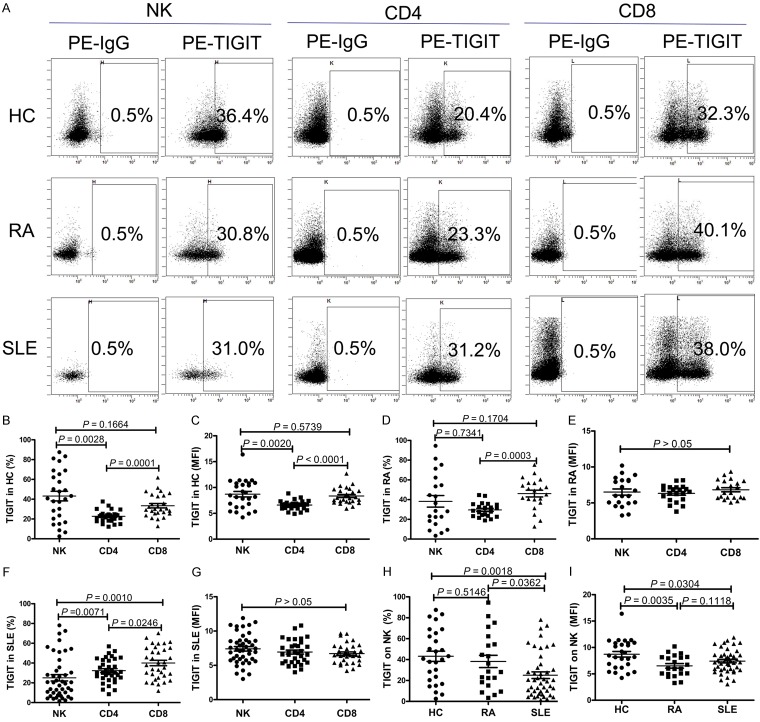
To determine the expression profile of TIGIT in SLE patients and HCs, we used flow cytometry to assess the expression of TIGIT on CD4+ T lymphocytes, CD8+ T lymphocytes and NK cells. We observed that the frequency of TIGIT-expressing NK cells and the mean fluorescence intensity (MFI) of TIGIT on NK cells were significantly elevated compared to CD4+ T lymphocytes in HC (P < 0.01) (Figure 2B and 2C). Furthermore, the frequency of TIGIT-expressing CD8+ T lymphocytes and the MFI of TIGIT on CD8+ T lymphocytes were significantly elevated compared to CD4+ T lymphocytes in HC (P < 0.01) (Figure 2B and 2C). But no significant difference was found between the expression of TIGIT on NK cells and CD8+ T lymphocytes in HC (P > 0.05) (Figure 2B and 2C). In RA patients, we only found that the frequency of TIGIT-expressing CD8+ T lymphocytes was significantly elevated compared to CD4+ T lymphocytes (P = 0.0003) (Figure 2D and 2E). As shown in Figure 2F, the frequency of TIGIT-expressing NK cells and CD4+ T lymphocytes were significantly decreased compared to CD8+ T lymphocytes, and the frequency of TIGIT-expressing NK cells was significantly decreased compared to CD4+ T lymphocytes in patients with SLE. The MFI of TIGIT on CD4+ T lymphocytes, CD8+ T lymphocytes, and NK cells from SLE patients were also determined, but no significant difference was found (P > 0.05) (Figure 2G).
Figure 2.
TIGIT expression on natural killer (NK) cells is decreased in patients with systemic lupus erythematosus (SLE). A. Representative dot plots of population gating and TIGIT expressing cells from healthy controls (HC), rheumatoid arthritis (RA) and SLE patients. Percentages of TIGIT expressing cells among NK, CD4+, CD8+ cells are shown. B. Summary data of the positive cell frequency in gated NK, CD4+, CD8+ cells from HC. C. Summary data of the mean fluorescence intensity (MFI) of TIGIT on NK, CD4+, CD8+ cells from HC. D. Summary data of the positive cell frequency in gated NK, CD4+, CD8+ cells from RA patients. E. Summary data of the mean fluorescence intensity (MFI) of TIGIT on NK, CD4+, CD8+ cells from RA patients. F. Summary data of the positive cell frequency in gated NK, CD4+, CD8+ cells from SLE patients. G. Summary data of the mean fluorescence intensity (MFI) of TIGIT on NK, CD4+, CD8+ cells from SLE patients. H. Summary data of the frequency of TIGIT-expressing NK cells from HC, RA and SLE patients. I. Summary data of the MFI of TIGIT on NK cells from HC, RA and SLE patients.
Then, we compared the expression of TIGIT on CD4+ T lymphocytes, CD8+ T lymphocytes, NK cells, between SLE patients, RA patients and HC. Our previous research has indicated that expression of TIGIT on T lymphocytes was significantly elevated in patients with SLE and RA compared to HC [15,20]. As shown in Figure 2H and 2I, when the frequency of TIGIT-expressing NK cells and the MFI of TIGIT on NK cells were significantly decreased in SLE patients compared to HC (P < 0.05), the frequency of TIGIT-expressing NK cells was significantly decreased in SLE patients compared to RA patients (P = 0.0362), but no significant difference was observed in the MFI of TIGIT on NK cells between SLE and RA patients (P > 0.05). Moreover, we found that the MFI of TIGIT on NK cells was significantly decreased in RA patients compared to HCs (P = 0.0035), but no significant difference was observed in the frequency of TIGIT-expressing NK cells between RA patients and HC (P > 0.05).
The aforementioned results and other research [7,21] demonstrated SLE and RA patients showed a significantly lower proportion of circulating NK cells than HCs. Our results also showed that expression of TIGIT on NK cells was significantly decreased in SLE and RA patients compared to HC. Thus, the correlation between the proportion of circulating NK cells and the frequency of TIGIT-expressing NK cells in HC, RA and SLE patients was investigated. As shown in Figure 3, positive correlations between the proportion of circulating NK cells and the frequency of TIGIT-expressing NK cells in RA and SLE patients were found (r2 = 0.3733, P = 0.0033; r2 = 0.2189, P = 0.0014). No obvious correlation was observed in HC (r2 = 0.1042, P = 0.1005).
Figure 3.
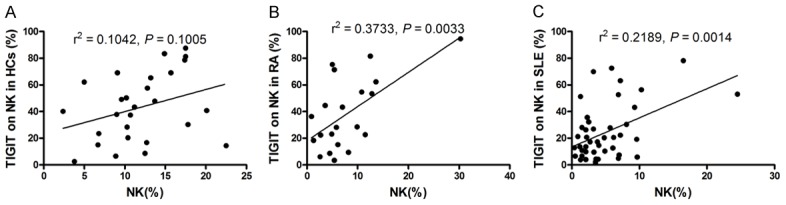
Correlation of frequency of TIGIT-expressing natural killer (NK) cells with proportion of circulating NK cells. A. No obvious correlation was observed between frequency of TIGIT-expressing NK cells and proportion of circulating NK cells in healthy controls (HC). B. The frequency of TIGIT-expressing NK cells in rheumatoid arthritis (RA) patients correlated significantly with proportion of circulating NK cells. C. The frequency of TIGIT-expressing NK cells in systemic lupus erythematosus (SLE) patients correlated significantly with proportion of circulating NK cells.
The frequency of TIGIT-expressing NK cells correlated negatively with disease activity and severity of SLE
We further observed that the frequency of TIGIT-expressing NK cells in patients with inactive patients was significantly higher than that in those with active patients (P = 0.0209) (Figure 4A). Further correlation analysis showed that the frequency of TIGIT-expressing NK cells was negatively correlated with the SLEDAI (r2 = 0.0899, P = 0.0479) (Figure 4B). Subsequently, we performed at least one week follow-up evaluation in 8 SLE patients received regular treatment with corticosteroids and immunosuppressive drugs. The frequency of TIGIT-expressing NK cells was significantly increased in SLE patients that received a one week regular treatment with corticosteroids and immunosuppressive drugs (P = 0.0469) (Figure 4C). In addition, our results showed that the frequency of TIGIT-expressing NK cells was significantly decreased in patients with positive anti-rRNP compared to patients with negative anti-rRNP (P = 0.0327) (Figure 4D). The frequency of TIGIT-expressing NK cells was significantly decreased in decreased C3 and C4 group compared to normal C3 and C4 group, respectively (P < 0.05) (Figure 4E, 4F). These results thus demonstrate that the frequency of TIGIT-expressing NK cells is correlated negatively with disease activity and disease severity of SLE.
Figure 4.

Correlation of frequency of TIGIT-expressing natural killer (NK) cells with disease activity and severity of SLE. A. The frequency of TIGIT-expressing NK cells in systemic lupus erythematosus (SLE) patients was significantly decreased in active SLE patients compared to inactive SLE patients. B. The frequency of TIGIT-expressing NK cells in SLE patients correlated negatively with SLE disease activity index (SLEADI). C. The frequency of TIGIT-expressing NK cells in SLE patients was significantly increased after treatment. D. The frequency of TIGIT-expressing NK cells was significantly decreased in patients with positive anti ribosomal RNP (anti-rRNP) compared to patients with negative anti-rRNP. E. The frequency of TIGIT-expressing NK cells was significantly decreased in SLE patients with decreased complement 3 (C3). F. The frequency of TIGIT-expressing NK cells was significantly decreased in SLE patients with decreased complement 4 (C4).
We also compared the frequency of TIGIT-expressing NK cells between new-onset and re-visiting SLE patients. Data showed that the frequency of TIGIT-expressing NK cells tends to be decreased in new-onset patients, but a significant difference was not reached (data not shown).
Next, the clinical features of patients with SLE including fever, cutaneous manifestations, oral ulcer, alopecia, arthritis, effusion, Neuropathic lupus, 24 hour proteinuria, hematuresis, pyuria, leucopenia, erythrocytopenia, thrombocytopenia, and anemia were analyzed and correlated with the expression of TIGIT on NK cells, but no significant difference was found (data not shown).
TIGIT as a negative regulator of NK cells function in patients with SLE
We further evaluated the relationship between TIGIT and the function of NK cells. After LPS stimulation, there was a significantly decreased expression of activation markers CD69 on TIGIT+ NK cells from patients with SLE than that in TIGIT- NK cells in patients with SLE (P = 0.0189) (Figure 5A). Moreover, the percentage of CD69+ NK cells was significantly inversely correlated with the percentage of TIGIT+ NK cells among patients with SLE (r2 = 0.4127, P = 0.0330) (Figure 5B). We similarly observed that after LPS stimulation, there was a significant decrease in expression of CD107a on TIGIT+ NK cells from patients with SLE compared to that in TIGIT- NK cells in patients with SLE (P = 0.0435) (Figure 5C). After IL-12 stimulation, the production of IFN-γ in TIGIT- NK cells was significantly higher than that in TIGIT+ NK cells in patients with SLE (P = 0.0135) (Figure 5E). Furthermore, the percentage of TIGIT+ NK cells was significantly inversely correlated with their IFN-γ-producing capacity between different patients with SLE (rs = 0.6101, P = 0.0205) (Figure 5F). In addition, we observed that the functional anti-TIGIT monoclonal antibody could increase the IL-12-stimulated IFN-γ production by high level TIGIT expression NK cells in SLE patients (P = 0.0075) (Figure 5G). This result indicates that TIGIT functions as a negative regulator of human NK cells in SLE.
Figure 5.
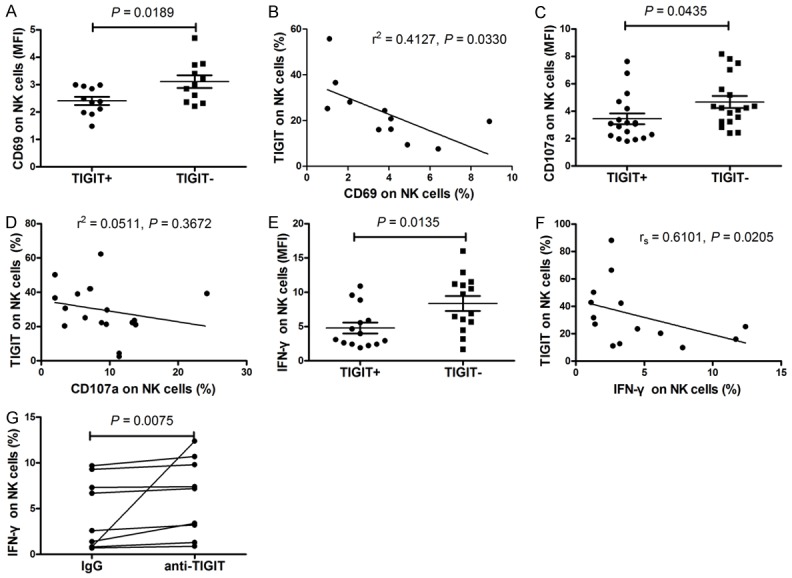
The relationship between the frequency of TIGIT-expressing natural killer (NK) cells, NK-cell phenotype, and cytokine secretion. A. There was significantly decreased expression of activation markers CD69 on TIGIT+ NK cells from patients with systemic lupus erythematosus (SLE) compared with that in TIGIT- NK cells in patients with SLE. B. The percentage of CD69+ NK cells was significantly inversely correlated with the percentage of TIGIT+ NK cells among patients with SLE. C. There was a significantly decreased expression of CD107a on TIGIT+ NK cells from patients with SLE compared with that in TIGIT- NK cells in patients with SLE. D. No obvious correlation was observed between the percentage of CD107a+ NK cells and the percentage of TIGIT-expressing NK cells in SLE patients. E. The production of IFN-γ in TIGIT- NK cells was significantly higher than that in TIGIT+ NK cells in patients with SLE. F. The percentage of TIGIT+ NK cells was significantly inversely correlated with their IFN-γ-producing capacity in patients with SLE. G. The functional anti-TIGIT monoclonal antibody could increase the IL-12-stimulated IFN-γ production by high level TIGIT expression NK cells in SLE patients.
Discussion
Systemic lupus erythematosus (SLE) is a systemic autoimmune syndrome with unclear etiology. Despite advances in the treatment of SLE in recent years, including the introduction of biologic therapies and new therapeutic strategies, remission rates remain suboptimal. It may be because the real cause of the disease is still unknown. NK cells, as components of the innate immune system, are best known for their direct killing effects on infected and tumor cells. However, recent studies on the interaction between NK cells and other immune cells has provided strong evidence that NK cells play roles in a number of autoimmune diseases by interacting with both immune and non-immune cells [22]. More and more evidence indicates NK cells play an important role in the pathogenesis of SLE [6,7]. Although patients with SLE have a higher proportion of activated NK cell subsets and lower proportions of inhibitory NK cell subsets, it actually results in the pathogenesis of SLE [23,24]. Further elucidation of the mechanism of NK cells deregulation in SLE will contribute to finding new therapeutic targets for the disease. The present study revealed another mechanism by which TIGIT regulates NK cells function in SLE. We showed that the frequency of TIGIT-expressing NK cells was significantly decreased in patients with SLE compared with HCs. Moreover, our research revealed that the frequency of TIGIT-expressing NK cells was associated negatively with disease activity of SLE. These data suggest that the TIGIT signaling pathway may be used as a new therapeutic target for the treatment of SLE.
Consistent with previous studies [7,24], our study found a lower proportion of NK cells in SLE patients. Additionally, we found that the frequency of TIGIT-expressing CD8+ T lymphocytes was significantly elevated compared to CD4+ T lymphocytes and the frequency of TIGIT-expressing CD4+ T lymphocytes was significantly elevated compared to NK cells in SLE. The frequency of TIGIT-expressing NK cells was significantly decreased in patients with SLE compared to HCs. Moreover, we found that the proportion of circulating NK cells was associated with the frequency of TIGIT-expressing NK cells. This supports the observations that SLE involves an imbalance of NK cells and that the abnormal expression of key signaling molecules on NK cells plays an important role in SLE pathogenesis [7,23-25].
In addition to imbalance of NK cells, another characteristic of SLE is dysfunction of NK cells. Evidence has indicated that NK cell involvement in the direct killing of tissue cells and in IFN-γ secretion accelerates progression of autoimmune diseases [26]. To further investigate the function of TIGIT on NK cells in SLE, activation markers CD69, and degranulation markers CD107a and cytokine IFN-γ were used in this study. We observed that the percentage of CD69+ NK cells and IFN-γ+ NK cells was significantly inversely correlated with the percentage of TIGIT+ NK cells among patients with SLE. This result indicates that TIGIT functions as a negative regulator of human NK cells in SLE.
Recently, more and more published data indicate that TIGIT acts as a checkpoint inhibitor of the immune system. For instance, TIGIT overexpression down-regulates the function of CD4+ T cells and reduces the severity of rheumatoid arthritis in mice [27]. Furthermore, increased expression of TIGIT can inhibit the function of CD4+ T cells, which contributes to ameliorate the severity of SLE [16]. Additionally, a recent study has shown that TIGIT acts as a inhibitory receptor and the expression levels on human NK cells correlate with functional heterogeneity among healthy individuals, which results in individuals have different susceptibilities to infection, autoimmune disease, and cancer [14]. These studies and our data suggest that activation of the TIGIT pathway may offer a new therapeutic strategy for the treatment of autoimmune diseases.
There are however some limitations in the present study. First is the relatively small sample size, especially the sample of new-onset SLE. Thus these data should be confirmed in large-scale studies. Second, we did not show the TIGIT functions as a negative regulator of NK cells in MRL/lpr mice.
In conclusion, we found that TIGIT expression was significantly decreased on NK cells in patients with SLE and correlated negatively with disease activity and severity of SLE. Additionally, the functional potential of TIGIT+ NK cells was significantly decreased compared with TIGIT- NK cells. Blocking the TIGIT pathway by functional anti-TIGIT monoclonal antibody restored IFN-γ secretion of NK cells. This study reveals that TIGIT is a powerful negative regulator of NK cells in SLE, which suggests that the TIGIT signaling pathway may be used as a potential therapeutic target for treating this disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81360459, 81660277), Jiangxi Provincial Natural Science Foundation of China (20151BAB215031, 20171BAB205113), the Science and Technology Project of Health and Family Planning Commission of Jiangxi Province of China (20165094) and the Foundation for Distinguished Young Scientists of Jiangxi Province of china (20171BCB23087), the Science and Technology Plan Project of the Education Department of Jiangxi Province (GJ170008).
Disclosure of conflict of interest
None.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of immunosuppressants in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69:20–8. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 3.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, Lee KD, Gavalchin J, Kaplan MJ. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176:2095–104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 4.Johansson S, Berg L, Hall H, Höglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–8. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 6.Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, Shin MH, Park JJ, Kim TJ, Lee SS, Yoo DH, Kang HS. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–63. doi: 10.1002/art.24556. [DOI] [PubMed] [Google Scholar]
- 7.Huang Z, Fu B, Zheng SG, Li X, Sun R, Tian Z, Wei H. Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:3421–31. doi: 10.4049/jimmunol.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, Cella M, Colonna M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–63. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43:2138–50. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, Li P, Yang X, Fan Z. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrest in 2-mediated negative signaling. J Biol Chem. 2014;289:17647–57. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, Sharpe AH, Quintana FJ, Mathis D, Benoist C, Hafler DA, Kuchroo VK. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–81. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–42. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, Yin B, Huang J, Mao L, Lu Y, Sun Z. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45:2886–97. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 15.Luo Q, Ye J, Zeng L, Li X, Fang L, Ju B, Huang Z, Li J. Elevated expression of TIGIT on CD3+CD4+ T cells correlates with disease activity in systemic lupus erythematosus. Allergy Asthma Clin Immunol. 2017;13:15. doi: 10.1186/s13223-017-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L, Hou H, Wu S, Zhou Y, Wang J, Yu J, Wu X, Lu Y, Mao L, Bosco MJ, Wang F, Sun Z. TIGIT signalling pathway negatively regulates CD4+ T-cell responses in systemic lupus erythematosus. Immunology. 2017;151:280–90. doi: 10.1111/imm.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Erkeller-Yüsel F, Hannet I, Isenberg D, Lydyard P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993;2:227–31. doi: 10.1177/096120339300200404. [DOI] [PubMed] [Google Scholar]
- 19.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 20.Luo Q, Deng Z, Xu C, Zeng L, Ye J, Li X, Guo Y, Huang Z, Li J. Elevated expression of immunoreceptor tyrosine-based inhibitory motif (TIGIT) on T lymphocytes is correlated with disease activity in rheumatoid arthritis. Med Sci Monit. 2017;23:1232–41. doi: 10.12659/MSM.902454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagihara Y, Shiozawa K, Takai M, Kyogoku M, Shiozawa S. Natural killer (NK) T cells are significantly decreased in the peripheral blood of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1999;118:131–6. doi: 10.1046/j.1365-2249.1999.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000;1:245–51. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- 23.Ye Z, Ma N, Zhao L, Jiang ZY, Jiang YF. Differential expression of natural killer activating and inhibitory receptors in patients with newly diagnosed systemic lupus erythematosus. Int J Rheum Dis. 2016;19:613–21. doi: 10.1111/1756-185X.12289. [DOI] [PubMed] [Google Scholar]
- 24.Li WX, Pan HF, Hu JL, Wang CZ, Zhang N, Li J, Li XP, Xu JH, Ye DQ. Assay of T- and NK-cell subsets and the expression of NKG2A and NKG2D in patients with new-onset systemic lupus erythematosus. Clin Rheumatol. 2010;29:315–23. doi: 10.1007/s10067-009-1322-9. [DOI] [PubMed] [Google Scholar]
- 25.Lin YL, Lin SC. Analysis of the CD161-expressing cell quantities and CD161 expression levels in peripheral blood natural killer and T cells of systemic lupus erythematosus patients. Clin Exp Med. 2017;17:101–9. doi: 10.1007/s10238-015-0402-1. [DOI] [PubMed] [Google Scholar]
- 26.Johansson S, Hall H, Berg L, Höglund P. NK cells in autoimmune disease. Curr Top Microbiol Immunol. 2006;298:259–77. doi: 10.1007/3-540-27743-9_13. [DOI] [PubMed] [Google Scholar]
- 27.Zhao W, Dong Y, Wu C, Ma Y, Jin Y, Ji Y. TIGIT overexpression diminishes the function of CD4 T cells and ameliorates the severity of rheumatoid arthritis in mouse models. Exp Cell Res. 2016;340:132–8. doi: 10.1016/j.yexcr.2015.12.002. [DOI] [PubMed] [Google Scholar]