Abstract
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death worldwide. The role of GSK-3β in cancer progression is considered critical. However, the prognostic value of total GSK-3β protein levels in HCC remains undetermined. In this study, the expression and biologic significance of total GSK-3β in HCC were evaluated at mRNA and protein levels. We showed that GSK-3β mRNA levels were significantly upregulated in HCC tissues relative to the levels in the adjacent non-tumor tissues as recorded on the TCGA database (P < 0.001). Notably, GSK-3β protein levels were significantly downregulated in HCC tissues relative to those in the adjacent non-tumor tissues by immunohistochemistry (P < 0.001). We found that GSK-3β was negatively associated with the American Joint Committee on Cancer (AJCC) stage (P = 0.030) and positively correlated with good prognosis for HCC patients (P = 0.036). The data further indicated that GSK3β expression tended to be an independent prognostic marker for HCC after surgical resection (HR = 1.658, 95% CI 0.945-2.909, P = 0.078) and can potentially serve as a biomarker for the clinical diagnosis and prognosis of HCC.
Keywords: GSK3β, bioinformatic analysis, hepatocellular carcinoma, prognosis, immunohistochemistry
Introduction
Hepatocellular carcinoma (HCC) is currently the second most common cause of cancer-related death worldwide [1] and the third leading cause of cancer deaths among both men and women in China [2]. Although several strategies have been applied for the management of HCC, unsatisfactory clinical outcomes emphasize the need for novel indicators of survival and reliable therapeutic targets.
Numerous studies on GSK-3β in various cancers have been conducted, and most of them focus on the phosphorylated form of GSK-3β. Two phosphorylation sites were identified in GSK-3β, which is activated by phosphorylation at Tyr-216 and inactivated by phosphorylation at Ser-9. Accumulating evidence suggests that pGSK-3β (ser9) regulates Wnt signaling and is responsible for HCC progression [3-5]. Total GSK-3β has received increased interest from the research community in recent years. Although some reports demonstrated no relationship between total GSK-3β and pGSK-3β (ser9) levels [6,7], other studies indeed suggested a negative correlation between total GSK-3β and pGSK-3β (ser9) levels [8,9], indicating a potential prognostic value of total GSK-3β in HCC patients. However, the role of total GSK-3β protein levels in HCC is yet to be investigated and remains unclear.
In the present study, we investigated the expression and biologic significance of GSK-3β in HCC at the mRNA and protein levels. Bioinformatics analysis suggested an upregulation of GSK-3β mRNA levels in HCC tissues; regardless, GSK-3β was found to be significantly decreased in HCC tissues relative to that in peritumoral tissues at the protein level. We also demonstrated that GSK-3β protein levels were negatively associated with the American Joint Committee on Cancer (AJCC) stage and were confirmed as a good prognostic factor for patients with HCC.
Materials and methods
Bioinformatics analysis
RNA-seq data on liver hepatocellular carcinoma (LIHC) and stomach adenocarcinoma (STAD) were downloaded from the Cancer Genome Atlas (TCGA) Web site (http://cancergenome.nih.gov/). The data included 424 patients with HCC. Among these patients, 50 came with paired non-cancerous tissues, and 407 had gastric cancer, 32 of which had paired non-cancerous tissues.
Patients and tissues
Overall, 93 HCC and 87 non-tumor subjects with available clinical information and paraffin-embedded blocks participated in this study. All patients had undergone surgical resection as initial treatment. Cancer and adjacent non-cancerous tissues were collected from the patients during surgery. Each patient had been pathologically diagnosed with HCC. The protocols were approved by the Ethical Committee and Institutional Review Board of Traditional Chinese Medicine-Integrated Hospital of Southern Medical University, and written informed consent was obtained from each patient. All clinicopathological information was retrospectively collected from the medical records of the patients. The study was performed in accordance with the approved protocols.
Immunohistochemistry (IHC)
Tissue sections (4 μm thick) were dewaxed in xylene and rehydrated in an alcohol bath solution. Heat-induced antigen retrieval was performed by incubating the slides in 0.01 M citrate buffer S (pH 6.0). The slides were then blocked in 3% hydrogen peroxide for 10 min to inhibit endogenous peroxidase activity. Subsequently, the slides were incubated for 60 min at room temperature, with primary antibodies for GSK3β (1:100, Proteintech, USA, 22104-1-AP). After rinsing with phosphate-buffered saline, the slides were incubated with secondary antibody for 30 min and counterstained using 3, 3’-diaminobenzidine and hematoxylin.
Evaluation of immunohistochemical staining
The IHC sections were scored by 2 experienced pathologists who were blinded to the clinicopathological data. Immunohistochemical staining was scored according to the intensity and the percentage of positively-stained cells. Staining intensity was estimated on a scale of 0 to 4, as follows: 0 (no staining), 1 (weakly positive staining), 2 (moderately positive staining), and 3 (strong staining). The percentages of cells were scored as 0 (0%), 1 (≤ 25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%). The final staining scores were calculated by multiplying the 2 scores.
Statistical analysis
All data were analyzed with SPSS ver. 21.0 (SPSS Inc., USA). For comparison, differential expression analysis was performed using the Wilcoxon rank sum test. Relationships between gene expression and clinicopathological characteristics were investigated using the Chi-square test and Fisher’s exact test. Log-rank tests were performed on Kaplan-Meier survival curves to elucidate any significant correlation between gene expression and overall patient survival. Univariate and multivariate survival analyses were conducted using the Cox proportional-hazards regression model. The hazard ratio (HR) and corresponding 95% confidence intervals (95% CI) were calculated for each factor. All tests were 2-sided and considered statistically significant when P < 0.05.
Results
Clinicopathological characteristics
The median age of 93 patients with HCC was 54 y (range: 25-73 y). Follow-up ranged from 4 y to 6.7 y. According to the criteria set by the AJCC Cancer Staging Manual, 8th Edition, 12 patients with HCC were in stage I, 31 in stage II, 40 in stage III, and only 2 in stage IV. The available medical records of 93 patients showed that only 1 patient had lymph node metastasis, and 1 case had distant metastasis. The clinicopathological characteristics of 93 patients with HCC are listed in Table 1.
Table 1.
Correlations between GSK3β expression and the clinicopathologic features of hepatocellular carcinoma patients
| Characteristic | Total | GSK3β expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low, n (%) | High, n (%) | |||
| Age (years) | ||||
| ≤ Median | 49 | 39 (79.6%) | 10 (20.4%) | 0.828 |
| > Median | 43 | 35 (81.4%) | 8 (18.6%) | |
| Gender | ||||
| Male | 83 | 67 (80.7%) | 16 (19.3%) | 0.956 |
| Female | 10 | 8 (80.0%) | 2 (20.0%) | |
| AJCC stage | ||||
| I-II | 43 | 31 (72.1%) | 12 (27.9%) | 0.030 |
| III-IV | 42 | 38 (90.5%) | 4 (9.5%) | |
| T classification | ||||
| T1-T2 | 43 | 31 (72.1%) | 12 (27.9%) | 0.030 |
| T3-T4 | 42 | 38 (90.5%) | 4 (9.5%) | |
| N classification | ||||
| N0 | 83 | 67 (80.7%) | 16 (19.3%) | 0.810 |
| N1 | 1 | 1 (100.0%) | 0 (0.0%) | |
| Distant metastasis | ||||
| No | 84 | 67 (79.8%) | 17 (20.2%) | 0.800 |
| Yes | 1 | 1 (100.0%) | 0 (0.0%) | |
| Hepatic cirrhosis | ||||
| No | 52 | 45 (86.5%) | 7 (13.5%) | 0.105 |
| Yes | 41 | 30 (73.2%) | 11 (26.8%) | |
| Edmondson-Steiner grade | ||||
| I-II | 61 | 47 (77.0%) | 14 (23.0%) | 0.226 |
| III-IV | 32 | 28 (87.5%) | 4 (12.5%) | |
| Tumor number | ||||
| Single | 24 | 21 (87.5%) | 3 (12.5%) | 0.325 |
| Multiple | 45 | 35 (77.8%) | 10 (22.2%) | |
| Tumor size (cm) | ||||
| ≤ 5 | 42 | 31 (73.8%) | 11 (26.2%) | 0.142 |
| >5 | 50 | 43 (80.4%) | 7 (19.6%) | |
Bioinformatics analysis and immunohistochemistry of GSK3β in HCC and non-cancerous tissues
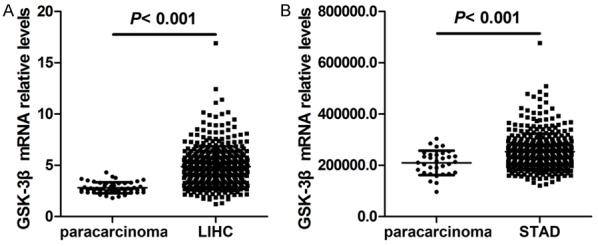
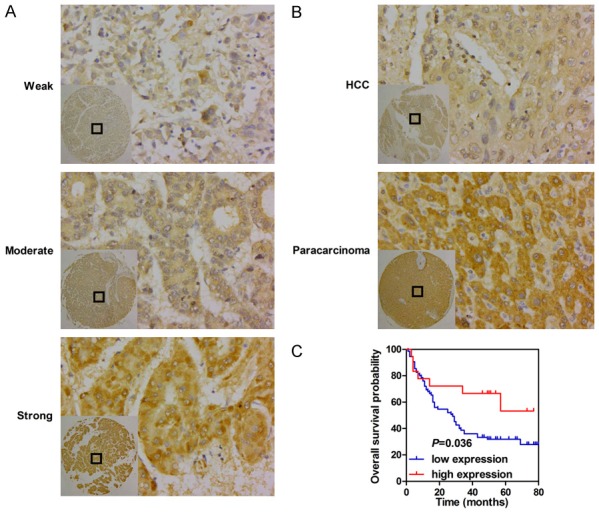
To explore the role of GSK3β in HCC, we initially investigated its mRNA expression in HCC on the basis of the TCGA LIHC dataset. RNA-seq data from HCC tissues and para-carcinoma tissues showed that GSK3β expression in HCC tissues was significantly elevated relative to that in matched non-tumor tissues (P < 0.001, Figure 1A). We then detected GSK3β expression in 93 HCC tissues and 87 adjacent non-tumor tissues by immunohistochemistry. The analysis indicated that patients exhibited different GSK3β levels, as determined by the strength of staining from weak to strong (Figure 2A). Notably, GSK3β protein levels were significantly downregulated in HCC samples relative to those in matched para-carcinoma tissues (P < 0.001, Table 2; Figure 2B).
Figure 1.
GSK-3β is downregulated in HCC tissues and confers good prognosis for HCC patients. A. Representative images of GSK-3β staining in HCC. B. Representative images of GSK-3β staining in paired cancerous tissues and non-cancerous tissues.
Figure 2.
The bioinformatics analysis of GSK-3β expression in HCC and gastric cancer tissues, and peritumoral tissues. A. The comparison of GSK-3β expression between HCC tissues and non-cancerous tissues. B. Comparison of GSK-3β expression between gastric tissues and non-cancerous tissues. C. Kaplan-Meier survival analysis based on GSK-3β expression.
Table 2.
GSK3β expression in HCC tissues and adjacent non-tumor tissues
| Group | Cases (n) | GSK3β expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Hepatocellular carcinoma | 93 | 75 (80.6%) | 18 (19.4%) | < 0.001 |
| Para-carcinoma tissue | 87 | 28 (32.3%) | 59 (67.8%) | |
Correlations between GSK3β expression and the clinicopathological characteristics of patients with HCC
The correlations between the GSK3β levels and the clinicopathological features of patients with HCC are presented in Table 1. High GSK3β expression was negatively correlated with AJCC staging (I-II vs. III-IV) and T classification (T1-2 vs. T3-4) (P = 0.030, Table 1); however, no relationship was found between GSK3β expression and other parameters, such as age, gender, N classification, distant metastasis, hepatic cirrhosis, Edmondson-Steiner grade, number of tumors, and size of tumors.
High GSK3β as an indicator of good prognosis in HCC tissues
The relationship between GSK3β levels and the overall survival of patients with HCC was elucidated by survival analysis. As shown in Figure 2C, high GSK3β expression correlated with good prognosis in patients with HCC (median survival 50.5 months vs. 28 months) (Log-rank, P = 0.036). Moreover, univariate Cox proportional-hazard analysis was performed to evaluate the potential value of GSK3β in the prediction of HCC prognosis. High GSK3β expression, AJCC staging (I-II), T classification (T1-2), absence of distant metastasis, and small tumor size (≤ 5 cm in diameter) conferred longer overall survival time in patients with HCC (Table 3).
Table 3.
Univariate and multivariate survival analysis of clinicpathologic variables of hepatocellular carcinoma patients
| Clinical parameters | Overall survival | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| GSK3β expression | 1.743 | (1.037-2.932) | 0.036 | 1.658 | (0.945-2.909) | 0.078 |
| Low | ||||||
| High | ||||||
| Age (years) | 1.260 | (0.751-2.113) | 0.382 | |||
| ≤ Median | ||||||
| > Median | ||||||
| Gender | 1.393 | (0.626-3.097) | 0.416 | |||
| Male | ||||||
| Female | ||||||
| AJCC stage | 0.340 | (0.192-0.602) | < 0.001 | 0.399 | (0.189-0.840) | 0.016 |
| I-II | ||||||
| III-IV | ||||||
| T classification | 0.340 | (0.192-0.602) | < 0.001 | |||
| T1-T2 | ||||||
| T3-T4 | ||||||
| N classification | 0.121 | (0.004-3.930) | 0.235 | |||
| N0 | ||||||
| N1 | ||||||
| Distant metastasis | 0.000 | (0.000-0.000) | < 0.001 | |||
| No | ||||||
| Yes | ||||||
| Hepatic cirrhosis | 0.858 | (0.508-1.450) | 0.568 | |||
| No | ||||||
| Yes | ||||||
| Edmondson-Steiner grade | 0.784 | (0.451-1.363) | 0.388 | |||
| I-II | ||||||
| III-IV | ||||||
| Tumor number | 1.796 | (0.912-3.540) | 0.101 | |||
| Single | ||||||
| Multiple | ||||||
| Tumor size (cm) | 0.490 | (0.289-0.831) | 0.008 | 0.861 | (0.411-1.805) | 0.693 |
| ≤ 5 | ||||||
| > 5 | ||||||
Subsequently, multivariate Cox proportional-hazard analysis was conducted to investigate meaningful parameters identified by univariate Cox analysis. T classification was consistent with the AJCC stage; only 1 patient had lymph node metastasis, and 1 case had distant metastasis; thus, we included the characteristics of GSK3β expression, AJCC stage, and tumor size in the multivariate Cox analysis for patients with HCC. As summarized in Table 3, only the AJCC stage (HR = 0.424, 95% CI 0.206-0.873, P = 0.020) was an independent prognostic factor for patients with HCC. However, GSK3β expression tended to be an independent prognostic marker for patients with HCC (HR = 1.658, 95% CI 0.945-2.909, P = 0.078).
Discussion
GSK3β participates in the regulation of tumorigenesis and cancer progression and may function as a “tumor suppressor” or “tumor promoter” for certain types of tumors [10]. In the present study, GSK3β expression and its biologic significance in HCC were investigated. GSK-3β mRNA levels were upregulated; however, GSK3β protein levels in HCC were downregulated. High GSK3β expression, which was negatively correlated with the AJCC stage, was indicative of good prognosis for patients with HCC. Moreover, we showed that GSK3β expression tended to be an independent prognostic marker for patients with HCC.
GSK3β is a multifunctional serine/threonine kinase and serves as a critical mediator in glycogen metabolism and signaling pathways involved in the regulation of cell fate, cell mobility, survival, proliferation, and protein synthesis [11]. The role of GSK3β in the development and progression of cancer remains inconclusive. STAT3 and β-catenin signaling pathways contribute to cancer progression, and downregulation of STAT3 or β-catenin may increase GSK-3β expression, indicating a tumor suppressor role for GSK-3β in HCC [12]. In HCC xenografted nude mice, increased GSK-3β expression downregulated the nuclear and cytosolic β-catenin levels by facilitating the proteosomal degradation of β-catenin [8]. In addition, GSK-3β knockdown enhanced cell survival and proliferation in HCC [13], increased cisplatin resistance via activation of Wnt/β-catenin signaling in lung cancer [14], and resulted in radioresistance of pancreatic cancer [15]. Moreover, the repressive function of GSK-3β in rRNA biogenesis, Wnt, and TGF-β pathways supported its role as a tumor suppressor [16,17]. However, GSK-3β knockdown inhibited tumor growth and angiogenesis in pancreatic cancer [18], reduced cell proliferation and survival in non-small cell lung cancer [19], activated p53-dependent apoptosis in colorectal cancer [20], promoted hydrogen peroxide-induced cell death in HCC cells [21], and enhanced chemosensitivity in cervical carcinoma [22]. In addition, GSK-3β overexpression contributed to chemoresistance in ovarian carcinoma [23], related to aggressive clinicopathological features in prostate cancer [24], and conferred poor prognosis in endometrial carcinoma [25]. Given these findings, we found that although the role of GSK3β in cancer progression remains inconclusive, in most cases, GSK-3β seems to be a tumor suppressor in HCC. Consistently, in the present study, we determined that GSK-3β functions as a negative regulator of HCC progression. GSK-3β mRNA levels were increased in HCC and stomach cancer tissues relative to those in non-tumor tissues, according to the TCGA database (Figure 1). Interestingly, GSK-3β protein levels were decreased in HCC and gastric cancer [26] tissues relative to those in non-tumor tissues. In the current study, we consider that post-transcriptional modification of GSK3β may partly explain the obtained results. We also demonstrated that high GSK3β expression was negatively correlated with the AJCC stage, and the findings might partly explain its role in HCC. The previous study suggested that reduced expression of GSK-3β was associated with good clinicopathological prognostic markers at the mRNA level in HCC [27]; however, low GSK3β protein level was identified as a poor prognostic factor for HCC in the study. Similarly, reduced GSK3β confers poor prognosis in squamous cell carcinoma of the tongue and malignant glioma [28,29]. Although further analysis revealed that GSK3β expression tended to be an independent prognostic marker for patients with HCC, we believe that significant results could be obtained by increasing the sample volume.
In addition, the locations of GSK3β also have distinct functions. Nuclear GSK3β was correlated with shorter overall survival in colon carcinoma [30]; conversely, another study suggested that GSK3β formed a complex with β-catenin in the nucleus to inhibit the canonical Wnt signaling pathway [31]. In human bladder cancer, nuclear accumulation of GSK-3β was also identified as a novel prognostic marker contributing to urothelial cancer cell proliferation and survival [32]. By contrast, we demonstrated in the present study that GSK-3β is mainly located in the cytoplasm of HCC cells.
In conclusion, our results aid in supporting a tumor suppressor role of GSK-3β in HCC. We also show that GSK-3β can potentially serve as a biomarker for the clinical diagnosis and prognosis of HCC. Lastly, the targeted inhibition of GSK-3β might be an alternative strategy for the treatment of HCC.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (NSFC) (No. 81773151), and the Guangzhou Science and Technology Plan Projects (No. 201604020009).
Disclosure of conflict of interest
None.
References
- 1.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Yang WJ, Chang CJ, Yeh SH, Lin WH, Wang SH, Tsai TF, Chen DS, Chen PJ. Hepatitis B virus X protein enhances the transcriptional activity of the androgen receptor through c-Src and glycogen synthase kinase-3beta kinase pathways. Hepatology. 2009;49:1515–1524. doi: 10.1002/hep.22833. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H. Axin and hepatocellular carcinomas. Nat Genet. 2000;24:206–208. doi: 10.1038/73396. [DOI] [PubMed] [Google Scholar]
- 5.Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003;199:201–208. doi: 10.1016/s0304-3835(03)00421-x. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell SR, Hwang SR, Basrur V, Conlon KP, Fermin D, Wey E, Murga-Zamalloa C, Zeng Z, Zu Y, Elenitoba-Johnson KS, Lim MS. NPM-ALK signals through glycogen synthase kinase 3beta to promote oncogenesis. Oncogene. 2012;31:3733–3740. doi: 10.1038/onc.2011.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao P, Li Q, Shi Z, Li C, Wang L, Liu X, Jiang C, Qian X, You Y, Liu N, Liu LZ, Ding L, Jiang BH. GSK-3beta regulates tumor growth and angiogenesis in human glioma cells. Oncotarget. 2015;6:31901–31915. doi: 10.18632/oncotarget.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu HC, Lay IS, Shibu MA, Ho TJ, Cheng SM, Lin CH, Dung TD, Jeng LB, Viswanadha VP, Huang CY. Zanthoxylum avicennae extract enhances GSK-3beta to attenuate beta-catenin via phosphatase 2A to block metastatic effects of HA22T cells and hepatocellular carcinoma xenografted nude mice. Environ Toxicol. 2017;32:2133–2143. doi: 10.1002/tox.22426. [DOI] [PubMed] [Google Scholar]
- 9.Fishman P, Madi L, Bar-Yehuda S, Barer F, Del Valle L, Khalili K. Evidence for involvement of Wnt signaling pathway in IB-MECA mediated suppression of melanoma cells. Oncogene. 2002;21:4060–4064. doi: 10.1038/sj.onc.1205531. [DOI] [PubMed] [Google Scholar]
- 10.Luo J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Ro JY. Differential expression of GSK3beta and pS9GSK3beta in normal human tissues: can pS9GSK3beta be an epithelial marker? Int J Clin Exp Pathol. 2015;8:4064–4073. [PMC free article] [PubMed] [Google Scholar]
- 12.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang XH, Meng XW, Xing H, Qu B, Han MZ, Chen J, Fan YJ, Lu CQ, Lu ZW. STAT3 and beta-catenin signaling pathway may affect GSK-3beta expression in hepatocellular carcinoma. Hepatogastroenterology. 2011;58:487–491. [PubMed] [Google Scholar]
- 14.Gao Y, Liu Z, Zhang X, He J, Pan Y, Hao F, Xie L, Li Q, Qiu X, Wang E. Inhibition of cytoplasmic GSK-3beta increases cisplatin resistance through activation of Wnt/beta-catenin signaling in A549/DDP cells. Cancer Lett. 2013;336:231–239. doi: 10.1016/j.canlet.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Watson RL, Spalding AC, Zielske SP, Morgan M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER, Hammer GD, Lawrence TS, Ben-Josef E. GSK3beta and beta-catenin modulate radiation cytotoxicity in pancreatic cancer. Neoplasia. 2010;12:357–365. doi: 10.1593/neo.92112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent T, Kukalev A, Andang M, Pettersson R, Percipalle P. The glycogen synthase kinase (GSK) 3beta represses RNA polymerase I transcription. Oncogene. 2008;27:5254–5259. doi: 10.1038/onc.2008.152. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Li Z, Chen G, Li J, Li H, Yu M, Zhang W, Guo W, Tian W. GSK3beta regulates ameloblast differentiation via Wnt and TGF-beta pathways. J Cell Physiol. 2018;233:5322–5333. doi: 10.1002/jcp.26344. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Wang L, Gou SM, Wang TL, Zhang M, Liu T, Wang CY. ShRNA silencing glycogen synthase kinase-3 beta inhibits tumor growth and angiogenesis in pancreatic cancer. Cancer Lett. 2012;316:178–186. doi: 10.1016/j.canlet.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Zeng J, Liu D, Qiu Z, Huang Y, Chen B, Wang L, Xu H, Huang N, Liu L, Li W. GSK3beta overexpression indicates poor prognosis and its inhibition reduces cell proliferation and survival of non-small cell lung cancer cells. PLoS One. 2014;9:e91231. doi: 10.1371/journal.pone.0091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh JC, Altieri DC. Activation of p53-dependent apoptosis by acute ablation of glycogen synthase kinase-3beta in colorectal cancer cells. Clin Cancer Res. 2005;11:4580–4588. doi: 10.1158/1078-0432.CCR-04-2624. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Liu L, Dou Y, Song D, Deng H. Glycogen synthase kinase-3beta antagonizes ROS-induced hepatocellular carcinoma cell death through suppression of the apoptosis signal-regulating kinase 1. Med Oncol. 2016;33:60. doi: 10.1007/s12032-016-0776-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Shu YM, Wang SF, Da BH, Wang ZH, Li HB. Stabilization of mismatch repair gene PMS2 by glycogen synthase kinase 3beta is implicated in the treatment of cervical carcinoma. BMC Cancer. 2010;10:58. doi: 10.1186/1471-2407-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Y, Hu D, Qiu J, Xie X, Ye F, Lu WG. Overexpression of glycogen synthase kinase-3 in ovarian carcinoma cells with acquired paclitaxel resistance. Int J Gynecol Cancer. 2011;21:439–444. doi: 10.1097/IGC.0b013e31820d7366. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Erdamar S, Dai H, Sayeeduddin M, Frolov A, Wheeler TM, Ayala GE. Cytoplasmic accumulation of glycogen synthase kinase-3beta is associated with aggressive clinicopathological features in human prostate cancer. Anticancer Res. 2009;29:2077–2081. [PubMed] [Google Scholar]
- 25.Chen S, Sun KX, Liu BL, Zong ZH, Zhao Y. The role of glycogen synthase kinase-3beta (GSK-3beta) in endometrial carcinoma: a carcinogenesis, progression, prognosis, and target therapy marker. Oncotarget. 2016;7:27538–27551. doi: 10.18632/oncotarget.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Zheng D, Hu P, Zeng Z, Li M, Tucker L, Monahan R, Resnick MB, Liu M, Ramratnam B. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the beta-Catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Res. 2014;42:2988–2998. doi: 10.1093/nar/gkt1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zekri AR, Bahnassy AA, Abdel-Wahab SA, Khafagy MM, Loutfy SA, Radwan H, Shaarawy SM. Expression of pro- and anti-inflammatory cytokines in relation to apoptotic genes in Egyptian liver disease patients associated with HCV-genotype-4. J Gastroenterol Hepatol. 2009;24:416–428. doi: 10.1111/j.1440-1746.2008.05699.x. [DOI] [PubMed] [Google Scholar]
- 28.Goto H, Kawano K, Kobayashi I, Sakai H, Yanagisawa S. Expression of cyclin D1 and GSK-3beta and their predictive value of prognosis in squamous cell carcinomas of the tongue. Oral Oncol. 2002;38:549–556. doi: 10.1016/s1368-8375(01)00121-x. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Lu H, Huang Y, Xiao R, Cai X, He S, Yan G. Glycogen synthase kinases-3beta controls differentiation of malignant glioma cells. Int J Cancer. 2010;127:1271–1282. doi: 10.1002/ijc.25020. [DOI] [PubMed] [Google Scholar]
- 30.Salim T, Sjolander A, Sand-Dejmek J. Nuclear expression of glycogen synthase kinase-3beta and lack of membranous beta-catenin is correlated with poor survival in colon cancer. Int J Cancer. 2013;133:807–815. doi: 10.1002/ijc.28074. [DOI] [PubMed] [Google Scholar]
- 31.Caspi M, Zilberberg A, Eldar-Finkelman H, Rosin-Arbesfeld R. Nuclear GSK-3beta inhibits the canonical Wnt signalling pathway in a beta-catenin phosphorylation-independent manner. Oncogene. 2008;27:3546–3555. doi: 10.1038/sj.onc.1211026. [DOI] [PubMed] [Google Scholar]
- 32.Naito S, Bilim V, Yuuki K, Ugolkov A, Motoyama T, Nagaoka A, Kato T, Tomita Y. Glycogen synthase kinase-3beta: a prognostic marker and a potential therapeutic target in human bladder cancer. Clin Cancer Res. 2010;16:5124–5132. doi: 10.1158/1078-0432.CCR-10-0275. [DOI] [PubMed] [Google Scholar]


