Abstract
Aim: To study the clinicopathologic characteristics of perivascular epithelioid cell tumor (PEComa) in the liver and to improve the understanding and diagnosis of this tumor. Methods and results: 13 cases of primary liver PEComa were retrieved from our hospital from January 2007 to September 2017. The clinicopathologic features and the computer tomographic (CT) or/and magnetic resonance imaging (MRI) tests of each case were retrospectively reviewed. All 13 patients were female, with ages ranging from 22 to 72 years (median, 35 years). The sizes of the tumor ranged from 1.0 to 19.8 cm. Histologically, the tumors were comprised of polygonal cells with clear to granular eosinophilic cytoplasm and were accompanied by positive immunohistochemical expression of HMB-45 and/or Melan-A. 6 cases showed moderate cytological atypia. Hemorrhage was present in 7 of 13 cases, and necrosis in 2 cases. Mitoses were scant, averaged from 0 to 1/50 high-power fields in every case. No vascular invasion was present in any case. Follow-up data were obtained from 9 of 13 cases, and none showed any evidence of tumor recurrence or metastasis. Conclusions: There are no specific symptoms of hepatic PEComa, and the preoperative imaging tests are also insensitive. In view of diverse histological growth patterns with atypical cell features in part of cases, the possibility of PEComa should be considered whenever unfamiliar hepatic tumors are encountered. Immunoreactivity for HMB-45, MelanA, and SMA, especially HMB-45, is very useful for the diagnosis of this tumor.
Keywords: PEComa, liver, HMB-45, TFE-3, differential diagnosis
Introduction
Perivascular epithelioid cell neoplasms (PEComas) are formed as a rare group of related mesenchymal tumors composed of histologically and immunohistochimically distinctive perivascular cells, and were first proposed by Bonetti et al. in 1992 [1]. As a new classification category established in the World Health Organization Classification of Tumors in 2002, the members of this family include angiomyolipoma (AML), lymphangioleiomyomatosis, pulmonary clear cell “sugar” tumors and PEComa-NOS [2]. Many anatomic sites can be affected and the uterus is the more common [3]. Cases that arise from the liver are extremely rare. Because of its rarity, little is known about these tumors, followed by further difficulties in the establishment of the diagnosis. Herein, we present 13 hepatic PEComas proven by pathology and review of the literature.
Materials and methods
Collection of cases
On retrieval of the pathology archives system in the Department of Pathology at the 1st Affiliated Hospital of Zhengzhou University, dated January 2007 to September 2017, 13 cases of hepatic PEComa were identified. Hepatectomy was performed on all patients. The histological sections were retrospectively reviewed by three pathologists. Written informed consent was obtained from the patients before the publication of this report and accompanying images.
Clinical data
Clinical data were retrospectively retrieved from patients’ records and included age, sex, location of tumor, clinical presentation, routine blood test, liver function tests, hepatitis B virus surface antigen (HbsAg), hepatitis C virus antigen, serum tumor markers such as alpha-fetoprotein (AFP), cancer antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA), imaging findings, and preoperative diagnosis. Follow-up data were obtained from the clinical record or by telephone.
Immunohistochemistry
Immunohistochemical stains including HMB-45, Melan-A, S-100, SMA, Desmin, CD34, HepPar-1, TEF-3, Syn, CgA, CD56 and pan-cytokeratin (CK) were applied for diagnosis and differential diagnosis. All immunohistochemical staining were performed by the PV-6000 system. The primary antibodies were all ready-to-use as follows: anti-HMB-45 (mouse monoclonal antibody), anti-Desmin (mouse monoclonal antibody), anti-CD34 (mouse monoclonal antibody), anti-TFE-3 (rabbit monoclonal antibody), anti-Syn (mouse monoclonal antibody), anti-panCK (mouse monoclonal antibody) were all purchased from Fuzhou Maixin Biotech. Co. Ltd., anti-MelanA (mouse monoclonal antibody), anti-S-100 (rabbit polyclonal antibody), anti-SMA (mouse monoclonal antibody) were purchased from Beijing Zhong Shan Biotechnology Co. Ltd., anti-CgA (mouse monoclonal antibody) and anti-CD56 (mouse monoclonal antibody) were purchased from Shanghai Jie Hao Biotechnology Co. Ltd. The peroxidase reaction was developed with 3,3’-diaminobenzidine (DAB). Secondary antibodies and DAB were obtained from Beijing Zhong Shan Biotechnology Co. Ltd. Sections that were stained without the primary antibodies served as negative controls.
Results
Clinical findings
The age of the patients ranged from 28 to 72 years (middle age = 35 years), with 12 of the 13 patients being female. Four patients presented with right upper abdomen intermittent pain or discomfort, 9 patients were incidentally found to have a mass of the liver on physical examination. One patient was positive for HbsAg, and the remaining 12 patients were negative for HbsAg and hepatitis C virus antigen. Only 1 patient showed the increased level of the carbohydrate antigen 125 (CA125). None of these patients was associated with tuberous sclerosis complex, while 5 patients had cysts in the liver or kidney.
All the patients were examined by computer tomographic (CT) or magnetic resonance imaging (MRI), and the common radiologic feature on CT and/or scan was arterial phase enhancement and portovenous phase washout. The patients were preliminarily diagnosed as hemangioma (4 cases), hepatocellular adenoma (HCA, 1 case), angiomyolipoma (1 case), hepatocellular carcinoma (HCC, 4 case), focal nodular hyperplasia (FNH, 1 case), and hepatic tumors of uncertain nature (3 cases). All patients had primary liver tumors and none presented with extrahepatic metastasis at diagnosis. All the data are summarized in Table 1.
Table 1.
Clinicopathologic data of the 13 cases
| No. | Sex | Age | Symptom | Clinical diagnosis | Viral hepatitis history | Liver function (cirrhosis) | Associated diseases | Tumoral markers | Enhanced CT/MR | Treatment | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Arterial phase enhancement | Portovenous phase washout | |||||||||||
| 1 | F | 28 | Poor appetite for 1 year, pain in the right upper abdomen for 1 month | Hemangioma | Negative | ALT↑ | None | Negative | + | + | Partial hepatectomy | Loss |
| 2 | F | 54 | No symptom | AML | Positive for hepatitis B surface antigen | ALT↑ | Multiple cysts in liver and kidney | Negative | Inhomogeneous | + | Partial hepatectomy | Disease-free survival (1 year) |
| 3 | F | 48 | No symptom | HCA | Negative | Normal | Multiple cysts in the liver | Negative | + | + | Partial hepatectomy | Loss |
| 4 | F | 35 | No symptom | FNH | Negative | Normal | None | CA125↑ | + | + | Partial hepatectomy | Disease-free survival (1 year) |
| 5 | F | 33 | Pain in the right upper abdomen for 2 year | Hemangioma | Negative | ALT↑, GGT↑, ALP↑ | None | Negative | + | + | Partial hepatectomy | Disease-free survival (half 1 year) |
| 6 | F | 72 | No symptom | Uncertain | Negative | Normal | None | Negative | Inhomogeneous | + | Partial hepatectomy | Disease-free survival (5 year) |
| 7 | F | 32 | No symptom | Uncertain | Negative | Normal | None | Negative | + | + | Partial hepatectomy | Loss |
| 8 | F | 33 | No symptom | Uncertain | Negative | Normal | None | Negative | + | + | Partial hepatectomy | Loss |
| 9 | F | 33 | Right upper abdomen for 4 days, | HCC | Negative | GGT↑ | Multiple cysts in the kidney | Negative | + | + | Partial hepatectomy | Disease-free survival (half 1 year) |
| 10 | F | 47 | No symptom | HCC | Negative | Normal | One cyst in the kidney | Negative | + | + | Partial hepatectomy | Disease-free survival (3 month) |
| 11 | F | 65 | No symptom | HCC | Negative | Normal | None | Negative | + | + | Partial hepatectomy | Disease-free survival (15 month) |
| 12 | F | 26 | Pain in the right upper abdomen for 8 days | Hemangioma | Negative | GGT↑ | Multiple cysts in the kidney and liver | Negative | + | + | Partial hepatectomy | Disease-free survival (2 month) |
| 13 | M | 49 | No symptom | Hemangioma or HCC | Negative | Normal | None | Negative | + | + | Partial hepatectomy | Disease-free survival (2 month) |
CT = computer tomographic, MRI = magnetic resonance imaging, F = female, M = male, HCC = hepatocellular carcinoma, AML = angiomyolipoma, HCA = hepatocellular adenoma, HCC = hepatocellular carcinoma, FNH = focal nodular hyperplasia, ALT = alanine aminotransferase, GGT = glutamyl transpeptadase, ALP = alkaline phosphatase.
Pathology findings
By gross examination, the maximal size of the tumors ranged from 1.0 to 19.8 cm, and the mean and median sizes were 3.6 and 3.8 cm, respectively. All the cases showed unencapsulated lesion, 10 were sharply demarcated from the adjacent hepatic parenchyma while the other 3 were ill-defined. The cut surfaces were tan-colored or grey brown with tough texture, and local hemorrhage were observed in 7 cases.
Microscopically, 2 cases were diagnosed as the classical epithelioid angiomyolipoma, which showed a mixture of thick-walled blood vessels, epithelioid smooth muscle, and mature adipose tissue. In the 2 cases, smooth muscle cells were arranged in whorled and interlacing fascicles, and surrounded by the tortuous vessels with the mature lipocytes being scattered (Figure 1A). Another 3 cases were PEComas with a small account of lipocytes less than 5% in total. The remaining 8 cases were PEComas comprised solely of perivascular cells. The epithelioid tumor cells of PEComas were polygonal or spheroidal, characterized by abundant cytoplasm that varied from eosinophilic granular to clear, with distinct cell border (Figure 1B).
Figure 1.
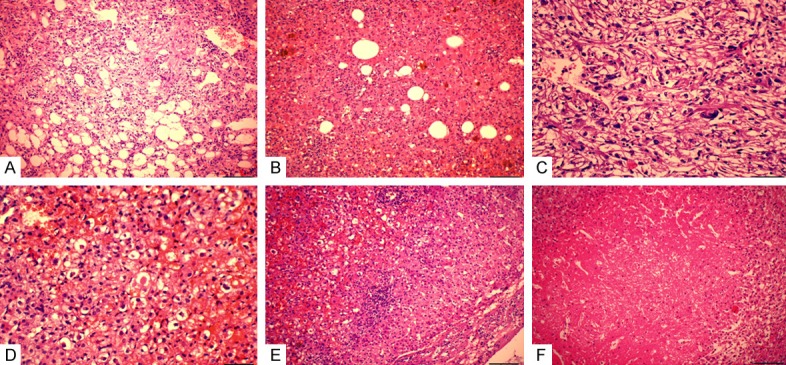
Histology of PEComas. A. Epithelial smooth muscle cells were arranged in whorled and interlacing fascicles, surrounded by the tortuous vessels with the mature lipocytes being scattered. B. The epithelioid tumor cells of PEComas are polygonal or spheroidal, characterized by abundant cytoplasm that varied from eosinophilic granular to clear, with distinct cell border. C. Moderate cytologic atypia with enlarged vesicular nucleus and notable nucleolus, bizarre pleomorphic multinucleated giant cells were present. D. Extracellular hyaline globules were seen singly or in clusters. E. Epithelioid neoplastic cell invaded into the surrounding hepatic parenchyma without clear boundary. F. Local necrosis was present.
In most cases, they were arranged in solid nests or sheets, separated by a rich sinusoidal vascular network. The tumor cell had vesicular nuclei with a small or notable nucleoli. Six cases showed mild cytological atypia with small nucleus and invisible nucleolus. The remaining 7 cases showed moderate cytological atypia with enlarged vesicular nucleus and notable nucleolus. Bizarre pleomorphic multinucleated giant cells were present in 3 cases (Figure 1C). Extracellular hyaline globules were identified singly or in clusters in 2 cases (Figure 1D). In 3 cases, epithelioid neoplastic cell invaded into the surrounding hepatic parenchyma (Figure 1E), and the remaining 10 cases had distinct boundary with 6 cases showing partial fibrous capsule. No vascular invasion was present in any case. In all of the cases, obvious small lymphocytes invasion can be easily seen with lymphoid follicle even formed in 3 cases. In 4 cases, mast cells invasion can be discovered simultaneously. Hemorrhage was present in 9 of 13 cases, and necrosis in 2 cases (Figure 1F). Mitoses were scant, averaged from 0 to 1/50 high-power fields in every case.
Immunohistochemical study showed a strong and diffuse expression of HMB45 in all the cases, and SMA in 9 cases, while Melan-A was just local positive in parts of cases (Figure 2A, 2B). In case No. 2, only the smooth muscle of the thick vascular wall was positive for SMA. The neoplastic cells were negative for S-100 protein, CD34 and panCK. Only 2 of 13 cases showed the weak positive expression of TFE-3 in the cell nuclei (Figure 2C). The proliferation indexes were also low with less than 5% in all the cases (Figure 2D). All the data were summarized in Table 2.
Figure 2.
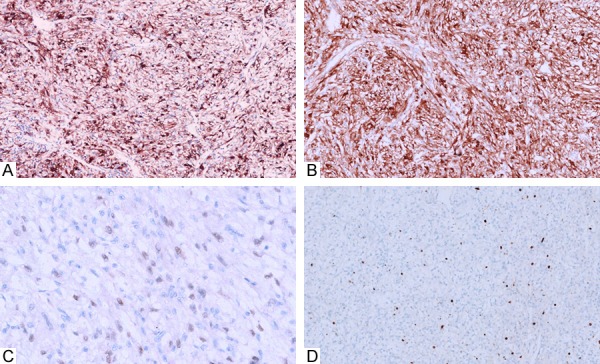
Immunohistochemical study showed a strong and diffuse expression of HMB45 (A) in all the cases and Melan-A (B) in part of cases. Only 1 case showed the weak positive expression of TFE-3 (C) in the nucleus. The proliferation indexes were also low with less than 5% in all the cases (D).
Table 2.
Histopathological characters of the 13 cases
| No. | Location, lobe | Numbers of nodules | Size (cm) | Boundary | Histology | Hemorrhage | Necrosis | Nuclear morphology | Mitotic count (/50HPF) | Immunohistochemistry | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| HMB45 | S-100 | MelanA | SMA | TFE-3 | Ki-67 (%) | ||||||||||
| 1 | Right lobe | 1 | 10 | Ambiguous | Mainly PECs | + | - | Mild to moderate heteromorphosis, tumor giant cell could be seen | 1 | + | - | + | + | - | 5 |
| 2 | Right lobe | 1 | 15 | Distinct, part fibrous capsule | E-AML | - | - | Mild heteromorphosis | 0~1 | + | - | + | - | - | 5 |
| 3 | Right lobe | 1 | 3 | Distinct, part fibrous capsule | Mainly PECs | - | Local + | Mild to moderate heteromorphosis | 0~1 | + | - | + | + | - | 5 |
| 4 | Hepatic portal | 1 | 3 | Distinct, no fibrous capsule | Mainly PECs | + | - | Mild heteromorphosis | 0~1 | + | - | + | + | - | 5 |
| 5 | Right lobe | 1 | 19.8 | Distinct, part fibrous capsule | Mainly PECs | Local + | Local + | Mild heteromorphosis, tumor giant cell could be seen | 0~1 | + | - | + | + | - | 3 |
| 6 | Right lobe | 1 | 4 | Distinct, part fibrous capsule | E-AML | Local + | - | Mild to moderate heteromorphosis, tumor giant cell could be seen | 0~1 | + | - | + | + | - | 5 |
| 7 | Right lobe | 1 | 5 | Ambiguous | Mainly PECs | + | - | Mild to moderate heteromorphosis | 0~1 | + | - | + | + | - | 5 |
| 8 | Left lobe | 1 | 1.2 | Distinct, no part fibrous capsule | Mainly PECs | - | - | Mild heteromorphosis | 0~1 | + | - | + | + | - | 2 |
| 9 | Left lobe | 1 | 6 | Ambiguous | Mainly PECs | Local + | - | Mild to moderate heteromorphosis | 0~1 | + | - | + | + | Weak+ | 5 |
| 10 | Right lobe | 1 | 5 | Distinct, part fibrous capsule | Mainly PECs | Local + | - | Mild heteromorphosis | 0~1 | + | + | + | + | - | 1 |
| 11 | Left lobe | 1 | 3.8 | Distinct, part fibrous capsule | Mainly PECs | - | - | Mild to moderate heteromorphosis | 0~1 | + | - | + | + | - | 5 |
| 12 | Left lobe and right lobe | 3 | 1.0 | Distinct, no part fibrous capsule | Mainly PECs | - | - | Mild to moderate heteromorphosis | 0~1 | + | - | + | + | - | 1-5 |
| 2.8 | |||||||||||||||
| 3.4 | |||||||||||||||
| 13 | Left lobe | 1 | 2.0 | Distinct, no part fibrous capsule | Mainly PECs | - | - | Mild heteromorphosis | 0~1 | + | - | + | + | - | 3 |
Cm = centimeter, HPF = high power field, PECs = perivascular cells, E-AML = epithelioid angiomyolipoma.
Treatment and follow-up
In our study, all the patients were treated by partial hepatectomy and were discharged from the hospital without postoperative complications. The follow-up study was completed for 9 of them. The longest follow-up time was 5 years for No. 5, and the shortest was 2 months for No. 11 and No. 12. No tumor recurrence or metastasis was found during the follow-up period. All the data are summarized in Table 2.
Discussion
PEComa is a rare tumor which arises from mesenchymal tissues. In 2002, the World Health Organization defined PEComa as a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells. The “PEComa family” includes many distinct clinicopathologic entities, such as AML, lymphangiomyomatosis and clear cell “sugar” tumors of the lung. Some of which have been linked to tuberous sclerosis complex, especially AML of the kidney. The group comprising solely perivascular epithelioid cells is rare, and it has been discriminated from classic angiomyolipoma by various appellations including monotypic epithelioid AML, clear cell myomelanocytic tumor, primary extrapulmonary sugar tumor, and PEComas-NOS or simple PEComa [4]. Until now, the etiology and histogenesis of PEComas has been incompletely unclear. However, perivascular epithelioid cells show evidence of both melanocytic and smooth muscle differentiation and do not recapitulate the differentiation of any known cell type in normal tissues [5]. In our study, 2 cases were diagnosed as the epithelioid AML, which showed a mixture of blood vessels, epithelioid smooth muscle and mature adipose tissue, while the other 11 cases were PEComas mainly comprised PECs.
Hepatic PEComa occurs most commonly in females, and usually show no specific symptoms. It was reported that the majority of patients had solitary lesions in the hepatic right lobe and were always found incidentally in health examinations. With increasing size of tumor, some patients may show symptoms caused by tumor compression. Compared with the PEComas in the renal, no association with the tuberous sclerosis complex was found [6-8]. In our study, 12 of 13 patients were female, and the tumors of 7 patients were located in the right lobes. Five patients had intermittent non-specific discomfort in the upper abdomen, and the remaining 8 ones were asymptomatic and discovered by accident. Tuberous sclerosis complex was not found in any case, but 5 patients were presented with multiple cysts in liver or kidney, which had not been reported in previous reports.
The imaging characteristics of PEComas are correlated with its histological components. The fat content of classical PEComas produces a characteristic imaging appearance, which enables PEComas differentiation from other hepatic tumors clinically [9]. As shown in our study, only 1 patient was accurately diagnosed as AML before surgical resection just because of the abundant fat component. In fact, imaging studies could not add any valuable preoperative diagnostic clues, especially for tumors with a minimal fat component [10]. On the other hand, PEComas are euangiotic lesions, which show abundant blood vessels or blood sinus in the tumor mesenchyme. Contrast enhancement in arterial phase and hypoattenuation in portal phase on dynamic CT or MRI examination were a common finding on imaging for PEComas, and hence it is understood that PEComas are prone to be misdiagnosed as hemangioma [11]. Consistent with these studies, all the tumors in our study were markedly enhanced in the arterial phase and decreased obviously in the portal venous phase. Some studies on PEComas imaging features reported that central abundant vessels, especially thickly distorted vessels could be observed in PEComas compared with other hypervascular hepatic tumors including hemangioma or HCC. Alternatively, a part of PEComas with small or no vessels maybe showed prolonged enhancement in the portal venous, and this manifestation was supposed to differentiate PEComas from HCCs and cavernous hemangiomas to some extent [12]. However, all of these details were not observed in our study, and it remains to be discussed further. In fact, in our series only 1 patient was accurately diagnosed as AML before surgical resection, whereas the remaining were given preoperative diagnosis of HCC, HCA, hemangioma or undetermined lesions. This was probably attributed to the nonspecific clinical and radiologic features of PEComas.
Clinically, this entity tends to occur in middle age females, with the tumor nodules more frequently involving the right hepatic lobe. These data coincide with those of HCA [13]. Considering the histological features, including the epithelioid appearance, clear to eosinophilic cytoplasm, a trabecular, sinusoidal or alveolar pattern structures, and the invasive growth pattern in some circumstances, the most important differential diagnoses should involve HCC (especially HCC with local fatty degeneration) [14] or metastatic renal cell carcinoma [15]. Other differential diagnosis should include epithelioid smooth muscle tumors, epithelioid gastrointestinal stromal tumor, melanoma, clear cell sarcoma of soft parts, and alveolar soft part sarcoma. These tumors can show epithelioid cell morphology, sometimes show striking nuclear pleomorphism such as prominent nucleoli and multinucleated tumor cells, and sometimes prominent mitoses and necrosis could be seen. However, these lesions do not generally show any characteristic perivascular orientation to some degree. In addition, just as discovered in this study, obvious small lymphocytes and mast cells invasion can be easily seen and lymphoid follicles were even formed in these lesions, together with local hemorrhage, inflammatory pseudo-tumor should be considered as another differential [16].
Despite the difficulties in making a correct diagnosis of PEComa by clinical findings and morphologic features, immunohistochemical staining is a favorable means to distinguish the entity from most of the differentials. Markers such as HMB-45, MelanA, and SMA are routinely expressed in PEComa, while panCK, EMA, HepPar-1, AFP, CD10, CD34, CD117 and DOG-1 are clearly negative [17,18].
Recently, it has been reported that a small subset of PEComas harbor gene fusions involving the TFE3 gene, a member of the microphthalmia transcription factor-transcription factor E gene family (MiTF-TFE), located at chromosome Xp11.2 [19]. This site is also notable for its involvement in translocations in a subgroup of renal cell carcinomas [20]. In these renal cell carcinomas, translocations result in gene fusions between TFE3 and one of several fusion partners. The TFE3 gene is also involved in a balanced translocation with alveolar soft part sarcoma chromosome region 1 (ASPSCR1) in alveolar soft part sarcomas (ASPS) [21]. PEComas harboring TFE3 gene fusion have been thus far found to preferentially exhibit “clear cells” morphology [22]. In our study, we performed immunohistochemical staining of TFE-3 in all of the 13 cases, and only 2 cases showed weakly positive, and the expression of TFE-3 did not seem to be associated with “clear cells” morphology. Maybe this is because of the small sample size, and we will explore more cases in the future work [23].
Although classical AMLs are universally regarded as benign tumors and often grow slowly, it is becoming increasingly clear that some types of PEComas should be regarded as tumors of uncertain malignant potential. Indeed, PEComa encompasses a wide range of biological behavior including benign, uncertain malignant potential, and malignant [24]. Folpe et al. performed statistical analysis, and found that behavior of PEComa in gynecologic tract and soft tissue correlated with ① tumor size greater than 5 cm, ② infiltrative, ③ high nuclear grade and cellularity, ④ mitotic rate greater than 1/50 high-power fields (HPF), ⑤ necrosis, and ⑥ vascular invasion. PEComa showing 2 or more worrisome histological features should be classified as “malignant” [25]. The course of the disease in liver is unpredictable mainly due to the rarity of the tumor, especially for malignant ones. Several reported cases of malignant PEComa in the liver indicate that the malignant histological features includes coagulative necrosis, nuclear polymorphisms or multinucleated giant cells and > 5 cm size [26-28]. Although not considered to be ideal criteria, it appears to be the best approach at present. In our study, comprehensive analysis showed that 6 cases had tumor greater than 5 cm. Two cases showed tumor tissues infiltrated into adjacent nonneoplastic hepatic parenchyma, and no vascular invasion was seen in all the cases. Six cases showed moderate cytological atypia with increasing vesicular nucleus and notable nucleolus. In 3 cases, multinucleated tumor giant cells were observed. Mitoses were scant, and averaged from 0 to 1/50 high-power fields in every case. Proliferation indexes were also low with less than 5% in all the cases. As a result, only one case (No. 5) possessed 2 worrisome histological features, which indicated the malignant potency. Altogether, most PEComa inclined to be benign, and malignant cases are relatively rare. In the follow-up, survival data of 9 cases were obtained, and none had shown any evidence of tumor recurrence or metastasis including No. 5 followed by 1 year. The subsequent follow-up will continue.
Conclusion
In conclusion, there are no specific symptoms of hepatic PEComa, and the preoperative imaging tests are also insensitive. In view of diverse histological growth patterns with occasional atypical cell features, the possibility of PEComa should be considered whenever unfamiliar hepatic tumors are encountered. Immunoreactivity for HMB-45, MelanA, and SMA, especially HMB-45, is very useful for the diagnosis of AML.
Acknowledgements
Supported by the National Natural Science Youth Foundation of China (81401936); The Youth Foundation of the 1st Affiliated Hospital, Zhengzhou University.
Disclosure of conflict of interest
None.
References
- 1.Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD, Unni KK, Mertens F. World health organization classification of tumors of pathology and genetics of soft tissue and bone. Lyon, France: IARC Press; 2002. pp. 221–222. [Google Scholar]
- 3.Reyes MC, Cooper K. Recently described entities in the gynaecological tract. Pathology. 2015;47:414–422. doi: 10.1097/PAT.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–415. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Yang CY, Ho MC, Jeng YM, Hu RH, Wu YM, Lee PH. Management of hepatic angiomyolipoma. J Gastrointest Surg. 2007;11:452–457. doi: 10.1007/s11605-006-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Z, Zhang JM, Ying JQ, Ge YP. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis. 2011;20:65–69. doi: 10.1007/s11749-010-0230-2. [DOI] [PubMed] [Google Scholar]
- 7.Nonomura A, Enomoto Y, Takeda M, Takano M, Morita K, Kasai T. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology. 2012;61:863–880. doi: 10.1111/j.1365-2559.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- 8.Lo RC. Epithelioid angiomyolipoma of the liver: a clinicopathologic study of 5 cases. Ann Diagn Pathol. 2013;17:412–415. doi: 10.1016/j.anndiagpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Hekimoglu K, Haberal M. Liver perivascular epithelioid cell tumor with an unusual location: diagnostic characteristics with multidetector computed tomography and magnetic resonance imaging. J Clin Imaging Sci. 2017;7:36. doi: 10.4103/jcis.JCIS_43_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics--analysis of 32 cases and review of the literature. Clin Radiol. 2013;68:555–561. doi: 10.1016/j.crad.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Phillips CH, Keraliya AR, Shinagare AB, Ramaiya NH, Tirumani SH. Update on the imaging of malignant perivascular epithelioid cell tumors (PEComas) Abdom Radiol (NY) 2016;41:368–376. doi: 10.1007/s00261-015-0568-8. [DOI] [PubMed] [Google Scholar]
- 12.Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features--analysis of 7 cases and review of the literature. Abdom Imaging. 2012;37:781–787. doi: 10.1007/s00261-012-9850-1. [DOI] [PubMed] [Google Scholar]
- 13.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, Balabaud C, Zucman-Rossi J. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 14.Wang SY, Kuai XP, Meng XX, Jia NY, Dong H. Comparison of MRI features for the differentiation of hepatic angiomyolipoma from fat-containing hepatocellular carcinoma. Abdom Imaging. 2014;39:323–333. doi: 10.1007/s00261-013-0070-0. [DOI] [PubMed] [Google Scholar]
- 15.Jhaveri KS, Elmi A, Hosseini-Nik H, Hedgire S, Evans A, Jewett M, Harisinghani M. Predictive value of chemical-shift mri in distinguishing clear cell renal cell carcinoma from non-clear cell renal cell carcinoma and minimal-fat angiomyolipoma. AJR Am J Roentgenol. 2015;205:W79–86. doi: 10.2214/AJR.14.13245. [DOI] [PubMed] [Google Scholar]
- 16.Belghiti J, Cauchy F, Paradis V, Vilgrain V. Diagnosis and management of solid benign liver lesions. Nat Rev Gastroenterol Hepatol. 2014;11:737–749. doi: 10.1038/nrgastro.2014.151. [DOI] [PubMed] [Google Scholar]
- 17.Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38:176–188. doi: 10.1097/PAS.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 18.Nonomura A, Enomoto Y, Takeda M, Takano M, Morita K, Kasai T. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology. 2012;61:863–880. doi: 10.1111/j.1365-2559.2012.04306.x. [DOI] [PubMed] [Google Scholar]
- 19.Henthorn PS, Stewart CC, Kadesch T, Puck JM. The gene encoding human TFE3, a transcription factor that binds the immunoglobulin heavy-chain enhancer, maps to Xp11.22. Genomics. 1991;11:374–378. doi: 10.1016/0888-7543(91)90145-5. [DOI] [PubMed] [Google Scholar]
- 20.Aydin H, Chen L, Cheng L, Vaziri S, He H, Ganapathi R, Delahunt B, Magi-Galluzzi C, Zhou M. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010;34:1608–1621. doi: 10.1097/PAS.0b013e3181f2ee0b. [DOI] [PubMed] [Google Scholar]
- 21.Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H, Meloni-Ehrig A, Sorensen PH, Mertens F, Mandahl N, van den Berghe H, Sciot R, Dal Cin P, Bridge J. The der(17) t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20:48–57. doi: 10.1038/sj.onc.1204074. [DOI] [PubMed] [Google Scholar]
- 22.Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol. 2016;29(Suppl 1):S104–120. doi: 10.1038/modpathol.2015.139. [DOI] [PubMed] [Google Scholar]
- 23.Jimbo N, Nishigami T, Noguchi M, Iijima H, Hirota S, Tajiri T, Inoue T, Hirose T, Itoh T, Zen Y. Hepatic angiomyolipomas may overexpress TFE3, but have no relevant genetic alterations. Hum Pathol. 2016;61:41–48. doi: 10.1016/j.humpath.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Liu Y, Zhuang Y, Zhang S. Hepatic perivascular epithelioid cell neoplasm: a clinical and pathological experience in diagnosis and treatment. Mol Clin Oncol. 2017;6:487–493. doi: 10.3892/mco.2017.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasm of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the lliterature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 26.Abhirup B, Kaushal K, Sanket M, Ganesh N. Malignant hepatic perivascular epithelioid cell tumor (PEComa)-case report and a brief review. J Egypt Natl Canc Inst. 2015;27:239–242. doi: 10.1016/j.jnci.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Maebayashi T, Abe K, Aizawa T, Sakaguchi M, Ishibashi N, Abe O, Takayama T, Nakayama H, Matsuoka S, Nirei K, Nakamura H, Ogawa M, Sugitani M. Improving recognition of hepatic perivascular epithelioid cell tumor: case report and literature review. World J Gastroenterol. 2015;21:5432–5341. doi: 10.3748/wjg.v21.i17.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patra S, Vij M, Kota V, Kancherla R, Rela M. Pigmented perivascular epithelioid cell tumor of the liver: report of a rare case with brief review of literature. J Cancer Res Ther. 2013;9:305–307. doi: 10.4103/0973-1482.113401. [DOI] [PubMed] [Google Scholar]


