Abstract
Candida auris is a rapidly emerging multidrug-resistant pathogenic yeast. In recent years, an increasing number of C. auris invasive infections and colonized patients have been reported, and C. auris has been associated with hospital outbreaks worldwide, mainly in intensive care units (ICUs). Here, we describe the first two cases of C. auris in The Netherlands. Both cases were treated in a healthcare facility in India prior to admission. The patients were routinely placed in contact precautions in a single room after admission, which is common practice in The Netherlands for patients with hospitalization outside The Netherlands. No transmission of C. auris was noticed in both hospitals. Routine admission screening both for multidrug-resistant (MDR) bacteria and MDR yeasts should be considered for patients admitted from foreign hospitals or countries with reported C. auris transmission.
Keywords: Candida auris, emergence, molecular typing, infection prevention
1. Introduction
Since the first report of Candida auris in 2009, an increasing number of C. auris invasive infections and colonized patients have been reported in at least 37 countries and territories, including the USA, Africa, Europe, and Asia [1,2,3,4,5,6,7,8]. C. auris has been associated with outbreaks in hospitals and other healthcare facilities. Ongoing transmission despite enhanced infection prevention and control measures during nosocomial outbreaks has been reported in several studies [4,5]. Resistance of C. auris to several antifungal classes of drugs with few having high minimum inhibitory concentrations (MICs) to all major drug classes has been described [9,10,11]. The nosocomial outbreaks and multidrug resistance of C. auris are worrisome and call for enhanced hospital infection prevention measures [12,13]. Previous C. auris outbreak investigations showed that co-colonization with multidrug-resistant (MDR) bacteria is common in critically ill patients [14]. In The Netherlands, admission screening, consisting of cultures from the nose, throat, and rectum, of all patients who were admitted to a foreign hospital for more than 24 hours is still only focused on MDR gram-negative bacteria and methicillin-resistant Staphylococcus aureus (MRSA). Concern is raised regarding the surveillance for MDR organisms after admission to foreign hospitals, since screening for MDR yeasts is not routinely included in The Netherlands. We report the first two cases of C. auris in The Netherlands, in patients who had healthcare exposures in hospitals in India.
2. Materials and Methods
Case 1 was a middle-aged man repatriated from a hospital in India to an intensive care unit (ICU) in The Netherlands. He had been hospitalized for five weeks in India for treatment of sepsis with multiple organ failure due to pneumonia. He was mechanically ventilated with a tracheostomy tube and continuous renal replacement therapy for which he had a central venous catheter. Since he was repatriated from a foreign healthcare facility, contact precautions and nursing in a private room were imposed according to standard practice in The Netherlands. Routine admission screening cultures for methicillin-resistant Staphylococcus aureus (MRSA) and MDR gram-negative bacteria taken from nose, throat, and rectum did not show growth of yeasts but demonstrated colonization with MDR Enterobacterales, producing OXA 48 and NDM. Although there were no signs of infection during admission in The Netherlands, the central venous catheter (CVC) was cultured after removal. Sheep blood agar (bioMérieux, Marcy l’Etoile, France) showed growth of yeast colonies that were identified with Matrix Assisted Laser Desorption Ionization-Time of Flight mass spectrometry (MALDI-TOF; Bruker, Bremen, Germany, database version MBT Compass 4.1) as C. auris. Since there were no signs and symptoms of infection, antifungal therapy was not initiated. Further screening during admission (axilla, groin, rectum, and oropharynx) of the patient for C. auris on CHROMOagar (BD) revealed the presence of C. auris in the groin. Cultures after discharge were negative for C. auris. No specific screening of the C. auris patient contacts or surveillance was performed since on hospital arrival the patient was placed in contact precautions in a single-patient room. But C. auris has not been cultured in clinical samples or routine selective digestive decontamination (SDD) screening samples since then.
Case 2 was a middle-aged man referred by the Amsterdam airport medical services due to shortness of breath and an inability to stand without assistance. He was in the Amsterdam airport for a layover while travelling back to the USA from India. Among others, he had a history of diabetes mellitus type 2 and renal insufficiency for which he received hemodialysis. Up to two days prior to admission, he had received hemodialysis in a healthcare facility in India. Therefore, contact precautions in a private room were implemented and screening cultures for MRSA and MDR gram-negative bacteria were obtained. Cultures demonstrated colonization with several MDR gram-negative bacteria such as OXA and NDM-positive Escherichia coli and NDM positive Pseudomonas aeruginosa. Therefore, more stringent cleaning and disinfection in the single-patient room was implemented, which consisted of terminal cleaning and disinfection with hydrogen peroxide: incidin oxy (Ecolab, Monheim am Rhein, Germany) instead of cleaning alone.
Due to a low-grade fever, one day after admission, a urine sample was obtained. In the urine culture, 104–105 colony-forming units of smooth beige yeasts grew on Sabouraud dextrose agar supplemented with 50mg/L chloramphenicol (produced in-house). The colonies were identified as C. auris using MALDI-TOF (bioMérieux, database version 3.2). Antifungal therapy was not initiated since the patient did not have clinical signs of a urinary tract infection. On the ward where the patient had been admitted, we performed a surveillance screening for C. auris of patients using CHROMagar Candida (Becton Dickinson, Franklin Lakes, NJ, USA), including axilla, groin, rectum, oropharynx, and if applicable, catheter urine and wounds; no C. auris was detected during screening.
3. Results
Laboratory Investigations
C. auris in vitro susceptibility was performed according to the Clinical and Laboratory Standards Institute reference method for broth dilution of yeast [15]. Broth microdilution showed that both C. auris isolates had high fluconazole (>64 mg/L) and voriconazole (4 mg/L) minimum inhibitory concentrations (MICs). Amphotericin B (0.5–1 mg/L), anidulafungin (<0.016–0.063 mg/L), and micafungin (0.063 mg/L) had low MICs. Molecular typing with amplified fragment length polymorphism and microsatellite analysis showed that both isolates belonged to the South Asian C. auris Clade I (not shown).
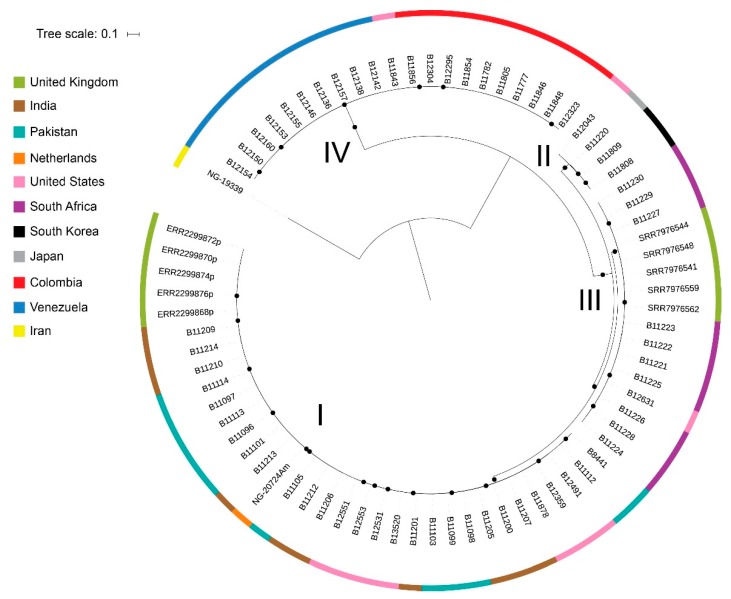
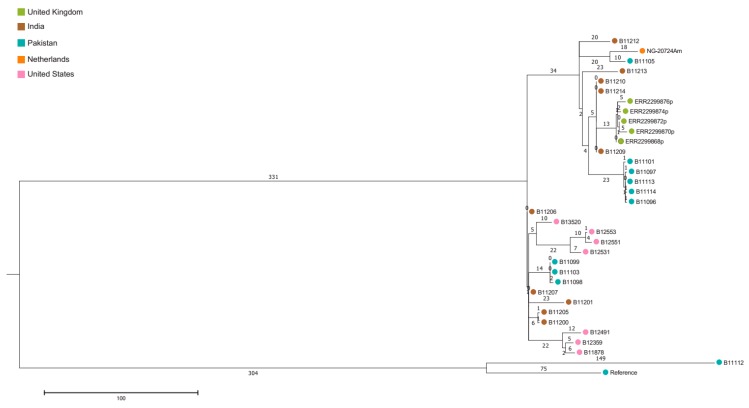
Since it was not clear whether case 2 had already colonized with C. auris contracted during his multiple stays in long-term care facilities in the New York area or had contracted the colonization during his hemodialysis in India, we compared whole genome sequencing (WGS) of this isolate (NG-20724Am) with published WGS sequences from the USA. Genomic DNA was extracted from 2-day-old colonies as described earlier [13]. Genomic libraries for WGS were constructed and sequenced using Illumina technology (Illumina, San Diego, CA, USA) at Eurofins Genomics (Ebersberg, Germany). Seventy-four C. auris WGS sequences retrieved from NCBI were added to the analysis. FastQC and PRINSEQ were used to assess the quality of read data and perform read filtering. Read data were aligned against a publicly available genome sequenced on PacBio RS II using BWA [7]. SNP variants were identified using SAMtools and filtered using the publicly available SNP analysis pipeline NASP to remove positions that had less than 10x coverage, less than 90% variant allele calls, or that were identified by Nucmer as being within duplicated regions in the reference. Phylogenetic analysis and bootstrapping with 500 iterations was performed on SNP matrices. The raw sequence read files were uploaded to the NCBI Sequence Read Archive and are publicly available under BioProject ID: PRJNA560710 (Submission ID: SUB6189371). Figure 1 and Figure 2 show the isolate from case 2 clustering with isolates from India and those from the USA, which have been argued to be a result of C. auris introductions from South Asia followed by local transmission [7].
Figure 1.
Description of C. auris major clades. Maximum likelihood phylogenetic tree of isolates from C. auris cases from 11 countries. Circles at nodes indicate separations with a bootstrap value ≥99%. I: South Asian (Clade I), II: East Asian (Clade II), III: African Clade (Clade III), and IV: South American (Clade IV).
Figure 2.
Description of South Asian Clade I. Maximum parsimony phylogenetic tree of isolates from The Netherlands, India, Pakistan, UK, and the USA.
4. Discussion
Here we present the first two imported cases of C. auris in The Netherlands and the first whole-genome sequences of a C. auris isolate from The Netherlands. Both patients were placed in contact precautions and admission screening samples were taken from the nose, oropharynx, and rectum since both patients had recently been admitted to a healthcare facility in India. During initial admission screening in The Netherlands, no specific effort was taken to detect C. auris. However, clinical samples taken during hospitalization showed growth of C. auris from a central venous line tip and from urine cultures. No additional cases of C. auris in clinical samples have been identified at both medical centers in over a year since the detection of C. auris in case 1 and 2. Since not all cultured yeasts are identified, there is a possibility that transmission has occurred. However, we believe that since a large proportion of cultures with yeast are identified to species level, a nosocomial outbreak would not remain unnoticed. Screening of axilla and groins after the clinical samples became positive identified skin colonization in case 1, but fortunately the implementation of admission contact precautions for MDR may have prevented hospital transmission of C. auris in both cases. Similar scenarios were recently reported from the USA [14,16]. This suggests that routine screening for C. auris should be strongly considered in the general admission workup when a patient is admitted to a hospital in The Netherlands from a foreign healthcare facility, especially from endemic regions such as South Asia, the Middle East, and South Africa but also transfers from facilities in Europe with C. auris endemic transmission as seen in some hospitals in Spain and the UK.
Both C. auris isolates exhibited high azole MICs, which is in accordance with the reported literature [3,17,18,19]. Reduced susceptibility to amphotericin B has also been demonstrated [18,19,20], and therefore, echinocandins are the recommended empirical treatment for C. auris [21,22]. However, although rare, reduced susceptibility of echinocandins to C. auris has been reported [6]. Fortunately, in the absence of an infection no antifungal treatment was initiated in our cases.
Due to its antifungal resistance, association with nosocomial outbreaks, and for the potential prevention of outbreaks when identified early, there is a rationale for screening for C. auris. However, there is limited evidence and experience with screening for C. auris. The Centers for Disease Control and Prevention (CDC) states that screening could be considered for close healthcare contacts with newly identified C. auris infection or colonization and patients who have had an overnight stay in a healthcare facility outside the USA in the previous year, especially in countries with documented C. auris cases [23]. The European Centre for Disease Prevention and Control (ECDC) has similar recommendations [24]. However, little is known of the prevalence of C. auris patient colonization in various countries and hospital settings (e.g., ICU, ICU with a nosocomial outbreak, or long-term care facilities). Most studies reporting colonization are during nosocomial outbreaks. During a nosocomial outbreak in an ICU in the UK, 63 out of 900 (7%) screened patients were colonized, and 7 patients were infected (0.7%) [5]. In addition, New York State health officials are considering mandatory pre-admission screening for C. auris since in various healthcare facilities in New York there is an ongoing C. auris outbreak [2]. A study investigating this outbreak reported C. auris colonization in 61 of 581 patients screened (10.4%) [2]. In a recent study in ICUs in India, C. auris represented 5.2% of all candidemia cases [25], and C. auris has become the second most isolated pathogen at a large trauma center [26]. Therefore, C. auris colonization of patients in ICUs in India is expected to be substantially higher. Based on the above described prevalence, there is a rationale for routine admission screening for C. auris in patients from healthcare facilities (especially ICUs) in India, from healthcare facilities in which nosocomial transmission has been reported, and other high prevalence areas (although there is limited information about what the other high prevalence areas are).
When screening for C. auris, it is not yet defined which body sites should be included. The CDC advises screening patients’ bilateral axilla and groin. In addition to the axilla and groin, the ECDC suggests that other sites (urine, wounds, catheter exit sites, throat, and other locations) can be sampled if indicated. During a large C. auris outbreak in an ICU, screening of patients involved the nose, axilla, groin, urine culture, and if present, tracheostomy and wounds [5]. In patients colonized with C. auris, the first positive screening result was from the axilla in 22 of 60 patients (37%), from another site (groin or urine) in 21 of 60 (35%), and from both the axilla and one or more other sites in 17 of 60 (28%). No other studies describe the colonization sites of C. auris specifically. The high percentage of patients first colonized in the axilla is probably due to the fact that the hospital outbreak was found to be linked to reusable axillary temperature probes, and therefore, the generalizability of this result is probably limited [5].
Only one study investigated the sensitivity of a single screen for C. auris [5]. Patients underwent screening twice within two days: 62 of 79 screening samples (78%) after a first positive screen were positive again. The authors concluded that a single screen was not sensitive enough to detect colonization with C. auris. More studies are needed before a recommendation about the number of screenings necessary to adequately detect C. auris can be made. In endemic regions, the implementation of a rapid and automated molecular surveillance admission screening for C. auris may be considered [27,28]. The advantage in endemic regions would be that these sensitive tests allow rapid diagnosis and may therefore prevent spread. In non-endemic areas, most tests will be negative and thus automated molecular surveillance admission screening will probably be not cost-effective.
C. auris has been isolated in numerous countries on five continents, and WGS have revealed 5 distinct clades that represent the following geographical regions: South Asia (India/Pakistan), South Africa, South America, East Asia, and the Middle East [6,29]. Both our cases recently returned from India, and molecular typing showed that the isolates belonged to the South Asian C. auris Clade I. Two recent studies from the USA demonstrated that the majority of detected C. auris clustered with the South Asian clade [2,7]. Interestingly, our second case was living in the USA and therefore could have been colonized with C. auris clones from healthcare facilities in the USA (mainly in the New York area). By using a maximum parsimony phylogenetic tree of isolates, WGS has the potential to identify the origin of imported cases and could thereby identify populations relevant for targeted screening. With WGS, this isolate clustered with isolates from both the Indian subcontinent and isolates from the USA.
In summary, since C. auris is potentially multidrug resistant, is associated with nosocomial outbreaks, and outbreaks can potentially be prevented when identified early by implementing contact precautions, the performance of routine admission screening both for MDR bacteria and yeasts should be considered for patients admitted from foreign hospitals or countries with reported C. auris transmission.
Author Contributions
Conceptualization, E.H.V., J.F.M., and K.v.D.; laboratory investigations, J.F.M. and N.A.C.; writing—original draft preparation, E.H.V. and K.v.D.; writing—review and editing, E.H.V., A.J.L.W., R.v.M., N.A.C., J.F.M., and K.v.D.; supervision, K.v.D.
Funding
The Canisius Wilhelmina Hospital Nijmegen provided funding for the WGS.
Conflicts of Interest
The authors declare no conflict of interest. The use of product names in this manuscript does not imply their endorsement by the U.S. Department of Health and Human Services. The finding and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Saris K., Meis J.F., Voss A. Candida auris. Curr. Opin. Infect. Dis. 2018;31:334–340. doi: 10.1097/QCO.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 2.Adams E., Quinn M., Tsay S., Poirot E., Chaturvedi S., Southwick K., Greenko J., Fernandez R., Kallen A., Vallabhaneni S., et al. Candida auris in Healthcare Facilities, New York, USA, 2013–2017. Emerg. Infect. Dis. 2018;24:1816–1824. doi: 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magobo R.E., Corcoran C., Seetharam S., Govender N.P. Candida auris-associated candidemia, South Africa. Emerg. Infect. Dis. 2014;20:1250–1251. doi: 10.3201/eid2007.131765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Gaitan A., Moret A.M., Tasias-Pitarch M., Aleixandre-Lopez A.I., Martinez-Morel H., Calabuig E., Salavert-Lleti M., Ramirez P., Lopez-Hontangas J.L., Hagen F., et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781. [DOI] [PubMed] [Google Scholar]
- 5.Eyre D.W., Sheppard A.E., Madder H., Moir I., Moroney R., Quan T.P., Griffiths D., George S., Butcher L., Morgan M., et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018;379:1322–1331. doi: 10.1056/NEJMoa1714373. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., Colombo A.L., Calvo B., Cuomo C.A., Desjardins C.A., et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow N.A., Gade L., Tsay S.V., Forsberg K., Greenko J.A., Southwick K.L., Barrett P.M., Kerins J.L., Lockhart S.R., Chiller T.M., et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: A molecular epidemiological survey. Lancet Infect. Dis. 2018;18:1377–1384. doi: 10.1016/S1473-3099(18)30597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govender N.P., Magobo R.E., Mpembe R., Mhlanga M., Matlapeng P., Corcoran C., Govind C., Lowman W., Senekal M., Thomas J. Candida auris in South Africa, 2012–2016. Emerg. Infect. Dis. 2018;24:2036–2040. doi: 10.3201/eid2411.180368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kathuria S., Singh P.K., Sharma C., Prakash A., Masih A., Kumar A., Meis J.F., Chowdhary A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015;53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhary A., Prakash A., Sharma C., Kordalewska M., Kumar A., Sarma S., Tarai B., Singh A., Upadhyaya G., Upadhyay S., et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018;73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 11.Arikan-Akdagli S., Ghannoum M., Meis J.F. Antifungal Resistance: Specific Focus on Multidrug Resistance in Candida auris and Secondary Azole Resistance in Aspergillus fumigatus. J. Fungi. 2018;4:129. doi: 10.3390/jof4040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsay S., Kallen A., Jackson B.R., Chiller T.M., Vallabhaneni S. Approach to the Investigation and Management of Patients With Candida auris, an Emerging Multidrug-Resistant Yeast. Clin. Infect. Dis. 2018;66:306–311. doi: 10.1093/cid/cix744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenters N., Kiernan M., Chowdhary A., Denning D.W., Peman J., Saris K., Schelenz S., Tartari E., Widmer A., Meis J.F., et al. Control of Candida auris in healthcare institutions. Outcome of an ISAC expert meeting. Int. J. Antimicrob. Agents. 2019;54:400–406. doi: 10.1016/j.ijantimicag.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Brooks R.B., Walters M., Forsberg K., Vaeth E., Woodworth K., Vallabhaneni S. Candida auris in a U.S. Patient with Carbapenemase-Producing Organisms and Recent Hospitalization in Kenya. MMWR Morb. Mortal. Wkly. Rep. 2019;68:664–666. doi: 10.15585/mmwr.mm6830a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard—3rd ed. CLSI; Wayne, PA, USA: 2008. [Google Scholar]
- 16.Woodworth M.H., Dynerman D., Crawford E.D., Doernberg S.B., Ramirez-Avila L., Serpa P.H., Nichols A., Li L.M., Lyden A., Tato C.M., et al. Sentinel Case of Candida auris in the Western United States Following Prolonged Occult Colonization in a Returned Traveler from India. Microb. Drug Resist. 2019;25:677–680. doi: 10.1089/mdr.2018.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schelenz S., Hagen F., Rhodes J.L., Abdolrasouli A., Chowdhary A., Hall A., Ryan L., Shackleton J., Trimlett R., Meis J.F., et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016;5:35. doi: 10.1186/s13756-016-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendrup M.C., Prakash A., Meletiadis J., Sharma C., Chowdhary A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhary A., Anil Kumar V., Sharma C., Prakash A., Agarwal K., Babu R., Dinesh K.R., Karim S., Singh S.K., Hagen F., et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 20.Prakash A., Sharma C., Singh A., Kumar Singh P., Kumar A., Hagen F., Govender N.P., Colombo A.L., Meis J.F., Chowdhary A. Evidence of genotypic diversity among Candida auris isolates by multilocus sequence typing, matrix-assisted laser desorption ionization time-of-flight mass spectrometry and amplified fragment length polymorphism. Clin. Microbiol. Infect. 2016;22:277.e1-9. doi: 10.1016/j.cmi.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhary A., Voss A., Meis J.F. Multidrug-resistant Candida auris: “new kid on the block” in hospital-associated infections? J. Hosp. Infect. 2016;94:209–212. doi: 10.1016/j.jhin.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Centres for Disease Control and Prevention Recommendations for Treatment of Candida auris Infections. [(accessed on 14 June 2019)]; Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-treatment.html.
- 23.Centres for Disease Control and Prevention Candida auris Screening. [(accessed on 14 June 2019)]; Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-screening.html.
- 24.European Centre for Disease Prevention and Control Candida auris in Healthcare Settings–Europe. [(accessed on 14 June 2019)]; Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Candida-in-healthcare-settings_19-Dec-2016.pdf.
- 25.Chakrabarti A., Sood P., Rudramurthy S.M., Chen S., Kaur H., Capoor M., Chhina D., Rao R., Eshwara V.K., Xess I., et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med. 2015;41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 26.Mathur P., Hasan F., Singh P.K., Malhotra R., Walia K., Chowdhary A. Five-year profile of candidaemia at an Indian trauma centre: High rates of Candida auris blood stream infections. Mycoses. 2018;61:674–680. doi: 10.1111/myc.12790. [DOI] [PubMed] [Google Scholar]
- 27.Kordalewska M., Perlin D.S. Molecular Diagnostics in the Times of Surveillance for Candida auris. J. Fungi. 2019;5:77. doi: 10.3390/jof5030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leach L., Russell A., Zhu Y., Chaturvedi S., Chaturvedi V. A rapid and automated sample-to-result Candida auris real-time PCR assay for high-throughput testing of surveillance samples with BD MAX open system. J. Clin. Microbiol. 2019 doi: 10.1128/JCM.00630-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow N.A., de Groot T., Badali H., Abastabar M., Chiller T.M., Meis J.F. Potential Fifth Clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019;25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]