Abstract
The lipophilic fungal pathogen Malassezia spp. must acquire long-chain fatty acids (LCFAs) from outside the cell. To clarify the mechanism of LCFA acquisition, we investigated fatty acid uptake by this fungus and identified the long-chain acyl-CoA synthetase (ACS) gene FAA1 in three Malassezia spp.: M. globosa, M. pachydermatis, and M. sympodialis. These FAA1 genes could compensate for the double mutation of FAA1 and FAA4 in Saccharomyces cerevisiae, suggesting that Malassezia Faa1 protein recognizes exogenous LCFAs. MgFaa1p and MpFaa1p utilized a medium-chain fatty acid, lauric acid (C12:0). Interestingly, the ACS inhibitor, triacsin C, affected the activity of the Malassezia Faa1 proteins but not that of S. cerevisiae. Triacsin C also reduced the growth of M. globosa, M. pachydermatis, and M. sympodialis. These results suggest that triacsin C and its derivatives are potential compounds for the development of new anti-Malassezia drugs.
Keywords: Malassezia, acyl-CoA synthetase, fatty acid uptake
1. Introduction
Malassezia spp. are basidiomycetous fungi that commonly inhabit the skin and scalp of humans and homothermic animals. Several Malassezia species can cause infectious diseases, such as atopic dermatitis, seborrheic dermatitis, and pityriasis versicolor in humans, particularly in immunocompromised individuals. By contrast, M. pachydermatis is found primarily in animals and causes otitis externa in dogs [1,2,3,4]. To date, 18 Malassezia species have been identified, all of which lack fatty acid synthetase (FAS) genes in their genome [5,6]. Because of their inability to synthesize fatty acids de novo, it is necessary for Malassezia species to obtain long-chain fatty acids (LCFAs) from outside the cell, such that most Malassezia spp. are lipid dependent. Although M. pachydermatis is capable of growth on rich media such as yeast peptone dextrose (YPD) and Sabouraud dextrose agar, and was previously thought to be lipid independent, it was recently reported that M. pachydermatis cannot grow on synthetic media lacking fatty acids. Because rich media contain a small amount of fatty acids, it is assumed that M. pachydermatis efficiently utilizes fatty acids [7].
To obtain exogenous fatty acids, Malassezia spp. produce and release many lipases [2,8,9,10]. Secretion of lipases to degrade exogenous lipids into fatty acids is a well-known pathogenic trait of Malassezia spp [9]. Although the acquired fatty acids are utilized for the growth and proliferation of the fungus, some of the unsaturated fatty acids can cause inflammation of the skin in animals and humans [9,11]. Thus, utilization of exogenous fatty acids is associated with both the pathogenicity and proliferation of Malassezia spp.
Long-chain acyl-CoA synthetases (ACSs) activate LCFAs into corresponding acyl-CoA esters via thioesterification of the fatty acids with coenzyme A. In the budding yeast Saccharomyces cerevisiae, six ACSs (Faa1p, Faa2p, Faa3p, Faa4p, Fat1p, and Fat2p) have been characterized, and both Faa1p and Faa4p are known to activate LCFAs [12,13]. Faa1p and Faa4p are paralogs that primarily recognize fatty acids containing 14–16 carbons [12]. Faa1p localizes in the endoplasmic reticulum, plasma membrane, lipid particles, and mitochondria, whereas Faa4p localizes in the cytosol and lipid particles. Another ACS, Faa2p, is involved in the import of short- and medium-chain fatty acids into the peroxisome in S. cerevisiae. Faa2p and Fat1p reportedly function with the half-size ABC transporter proteins Pxa1p-Pxa1p in transporting very-long-chain fatty acids into the yeast peroxisome [14,15]. The observation that faa1 and faa4 double-null mutants of S. cerevisiae cannot grow on medium supplemented with cerulenin (a FAS inhibitor) and LCFAs indicates that Faa1p and Faa4p are essential for the growth of this yeast. By contrast, FAA2, FAT1, and FAT2 null mutants are capable of growing on medium containing cerulenin and LCFAs [16].
For the clinical treatment of infectious diseases caused by Malassezia spp., azole drugs such as itraconazole, fluconazole, and ketoconazole, which interfere with sterol synthesis, are frequently used. However, a number of strains of other pathogenic fungi that are resistant to azole drugs have been isolated, like azole-resistant Candida parapsilosis and C. albicans [17,18], and azole-resistant Cryptococcus neoformans [19]. Various azole-resistant M. pachydermatis strains have also been isolated recently [20,21]. Therefore, as new drugs that inhibit other critical fungal metabolic pathways are needed, drugs that inhibit the activity of Faa proteins are potential alternatives. Triacsin C inhibits bacteria, mammals, and protozoan ACSs [22,23,24,25,26,27]. A previous report also indicated that triacsin C inhibits the activity of Faa4p (IC50 = 4.5 ± 0.5 μM), but not of Faa1p (IC50 > 500 μM) in S. cerevisiae [28].
Here, we identified homologs of the S. cerevisiae FAA1 and FAA4 genes in M. globosa, M. pachydermatis, and M. sympodialis. Introduction of these genes into an S. cerevisiae faa1faa4 mutant (SCFAA1-4) restored its growth in a medium containing cerulenin and LCFAs. The result suggested that the Malassezia genes MpFAA1, MgFAA1, and MsFAA1 encode an ACS in S. cerevisiae. The expression of the MpFAA1 gene in M. pachydermatis was induced in a medium containing C16:0 and C18:1 fatty acids. Interestingly, triacsin C inhibited the growth of S. cerevisiae faa1faa4 mutant harboring Malassezia FAA1 genes, but not the growth of inherent S. cerevisiae.
2. Materials and Methods
2.1. Yeast Strains, Media, and Growth Conditions
Three Malassezia species were used in this study: M. pachydermatis CBS1879NT (purchased from Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands), M. globosa CBS7966T, and M. sympodialis CBS7222T (both kindly provided by Prof. Somay Yamagata Murayama, Kitasato University, Japan). The yeasts were revived and subcultured at 32 °C on MLNA (modified Leeming-Notman agar—1% BactoTM peptone (BD Difco), 1% glucose, 0.2% yeast extract, 0.8% desiccated ox bile, 1% glycerol, 0.05% glycerol monostearate, 0.5% Tween 60, 1.5% agar, and 2% olive oil) every 2–4 weeks for maintenance. Malassezia were typically cultured in a liquid medium using mDixon (3.6% malt extract (Kyokuto, Co. Ltd.), 0.6% BactoTM peptone (BD Difco), 2% desiccated ox bile, 0.1% Tween 40, 0.2% glycerol, and 0.2% oleic acid) at 32 °C.
Strains of S. cerevisiae used in this study are listed in Table S1. S. cerevisiae strains IFO10150 and faa1faa4 mutant (SCFAA1-4) were cultured in YPD medium (2% glucose, 2% peptone, and 1% yeast extract) and grown at 30 °C in CM ura− medium (0.67% yeast nitrogen base without amino acids (Difco), 0.12% uracil drop-out [29]) containing different fatty acids (C10:0, C12:0, C14:0, C16:0, C18:0, and C18:1) at 0.5 mM and 5 μg/mL cerulenin (Wako, Japan). Most chemicals used for media preparation were purchased from Nacalai Tesque Inc. (Kyoto, Japan).
2.2. Plasmids
Plasmids used in this study are listed in Table S2, and the primers used to construct the plasmids are listed in Table S3. General DNA manipulations were performed according to the protocols reported by Sambrook et al. (1989) [30].
Plasmid pTRS7 harboring the ADH1 promoter and terminator was used as an expression vector to insert the Malassezia FAA1 genes, ScFAA1 and ScFAA4, into SCFAA1-4. MpFAA1 was amplified from M. pachydermatis 1879NT genomic DNA extracted by PCR using primers MpFAA1-F and MpFAA1-R and then digested with SalI and SmaI. MgFAA1 was amplified from M. globosa 7966T genomic DNA by PCR using primers MgFAA1-F and MgFAA1-R and then cut with EcoRI and BamHI. MsFAA1 was amplified from M. sympodialis 7222T genomic DNA using primers MsFAA1-F and MsFAA1-R and then cut using SmaI and SpeI. For the control in this paper, a revertant strain containing one of the original S. cerevisiae FAA1 and FAA4 genes was constructed. ScFAA1 was amplified from S. cerevisiae IFO10150 genomic DNA using primers ScFAA1-F and ScFAA1-R, and then cut by SalI and NotI. On the other hand, ScFAA4 was amplified from the same S. cerevisiae strain using primers ScFAA4-F and ScFAA4-R, and then cut by BamHI and NotI. The amplified DNA fragments were cloned into digested sites in pTRS7, and the resulting plasmids were designated pTRS7-MpFAA1, pTRS7-MgFAA1, pTRS7-MsFAA1, pTRS7-ScFAA1, and pTRS7-ScFAA4, respectively. These plasmids were cloned into Escherichia coli DH5α (Agilent Technologies, Santa Clara, CA, USA), extracted, and transformed into the SCFAA1-4 strain.
2.3. Transformation of S. cerevisiae
S. cerevisiae was transformed using a previously described method [31]. URA3 was used as a marker to select transformants harboring the constructed plasmids encoding Malassezia genes. The successful transformants were confirmed by colony PCR using the same primers used for gene amplification.
2.4. Triacsin C Minimum Inhibitory Concentration (MIC) Analysis
Cells of mDixon-cultured Malassezia were collected, washed, and suspended in fresh mDixon liquid medium. Aliquots of cultures were then inoculated in wells of a 96-well plate and adjusted to a starting OD600 of 0.1 for each well. The wells were then treated with triacsin C (Cayman Chemical) at various doses and incubated at 32 °C in a shaking incubator. OD values were measured at indicated times using Varioskan Lux (Thermo Scientific).
2.5. Isolation of M. pachydermatis Total RNA
The mDixon liquid medium was inoculated with one loop of M. pachydermatis and pre-cultured for 5–7 days, then transferred to 100 mL of mDixon medium for a starting OD600 of 0.05. The cells were incubated in a shaker at 120 rpm at 32 °C until OD600 reached 1–2 and then collected by washing with the mYNB (modified Yeast Nitrogen Base) medium (0.67% yeast nitrogen base (Difco), 2% glycerol, 1% Tween 40, and 25 mM MOPS buffer (pH 6)). To induce gene expression, 10 mL of cell suspension (starting OD600 = 1) in mYNB containing 5 mM of each fatty acid (C12:0, C14:0, C16:0, and C18:1) or in mDixon containing 3 μM triacsin C were incubated in L-tubes at 32 °C for 6 h. Before the cells were collected, 0.05% Triton X-100 was added as a surfactant and mixed vigorously to facilitate pellet collection. The supernatants containing each fatty acid were carefully removed, and the pellets for RNA extraction were washed with DEPC water. Total RNA was extracted from each sample using the hot phenol method [32]. The concentration of each isolated RNA was determined using a GeneQuant100 spectrophotometer (GE Healthcare), and RNA quality was confirmed by agarose gel electrophoresis.
2.6. cDNA Synthesis and Transcription Level Analysis
Isolated mRNAs were reverse transcribed into cDNAs using ReverTra Ace qPCR Master Mix with gDNA Remover (Toyobo). The mRNA transcription level was analyzed using THUNDERBIRD SYBR qPCR Mix (Toyobo) with StepOne qualitative real-time PCR (qRT-PCR) from Applied Biosystems. The primers used for qRT-PCR are listed in Table S3.
3. Results
3.1. Identification of Malassezia orthologs of S. cerevisiae FAA1 Genes
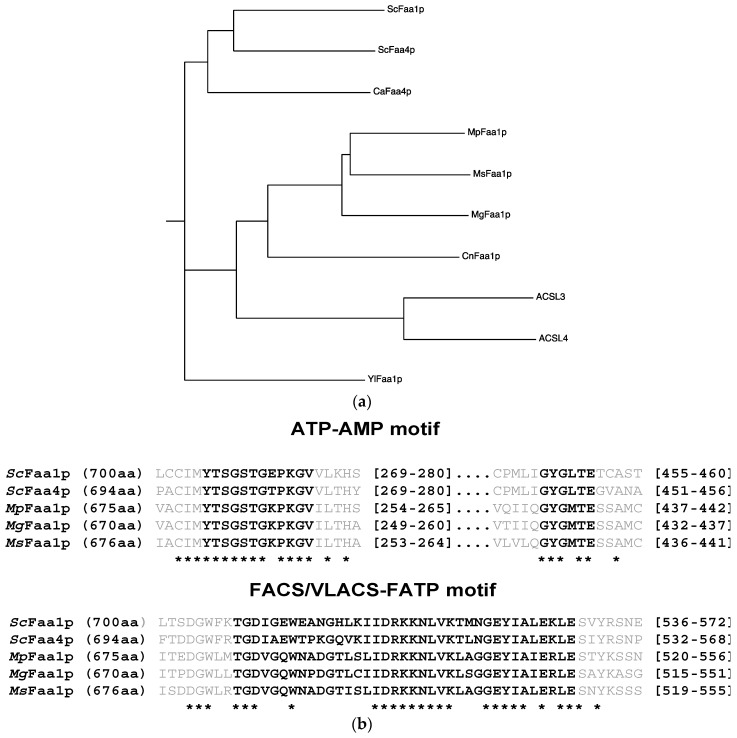
To identify orthologs of the ScFAA1 gene in Malassezia spp., M. pachydermatis, M. globose, and M. sympodialis genomic data were searched against the NCBI database using blastp analysis. Faa orthologs of these Malassezia species exhibited 36–38% identity with ScFaa1p (Table 1, Figure 1). MGL_3626 of M. globosa (MgFAA1) and Malapachy_0054 of M. pachydermatis (MpFAA1) encode 670- and 675-amino acid residue proteins, respectively. For M. sympodialis (MsFAA1), we identified two highly homologous orthologs: MSY001_1358 (662 amino acids) and MSYG_3835 (676 amino acids). However, the nucleic acid sequences of these orthologs were almost identical. A 14-amino acid sequence spanning residues 438–451 was missing in MSY001_1358, compared with MSYG_3835. We hypothesized that MSY001_1358 and MSYG_3835 are the same gene in the M. sympodialis genome. In addition, the amino acid sequences of the three Faa1p orthologs exhibiting the highest identity were found to contain a conserved ATP–AMP motif and FACS/VLACS–FATP motif (Figure 1b). The orthologs from these three species with the second-highest degree of similarity exhibited a lower degree of identity with ScFaa1p (Malapachy_0976: 29%, MGL_0129: 27%, and MSY001_0514: 25%). However, as they exhibited higher identity with ScFaa2p than ScFaa1p, it was concluded that these three genes were not good matches for Faa1p of Malassezia.
Table 1.
Acyl-CoA synthetase orthologs identified in the Malassezia spp. genome using the National Center for Biotechnology Information (NCBI) conserved domain database.
| Gene Name | Accession No. | Malassezia Gene ID | Protein Size | Description | % Identity to | Query Cover to | ||
|---|---|---|---|---|---|---|---|---|
| ScFAA1 | ScFAA4 | ScFAA1 | ScFAA4 | |||||
| MpFAA1 | XP_017991190.1 | Malapachy_0054 | 675 aa | long-chain fatty acid ligase | 38 | 37 | 87 | 99 |
| MgFAA1 | XP_001729159.1 | MGL_3626 | 670 aa | hypothetical protein | 37 | 36 | 97 | 98 |
| MsFAA1 | SHO79486.1 | MSYG_3835 | 676 aa | similar to S. cerevisiae FAA4 | 37 | 39 | 99 | 98 |
| MsFAA1 | XP_018739945.1 | MSY001_1358 | 662 aa | hypothetical protein | 36 | 38 | 99 | 98 |
Figure 1.
Phylogenetic tree of ACS enzymes in S. cerevisiae and Malassezia spp. (a) Phylogenetic tree of Faa1 proteins in various fungi, constructed using ClustalW and drawn using Phylodendron’s phenogram (http://iubio.bio.indiana.edu/treeapp/treeprint-form.html). MpFaa1p: Malapachy_0054; MgFaa1p: MGL_3626; MsFaa1p: MSYG_3835; CaFaa4p: Candida albicans Faa4; CnFaa1p: Cryptococcus neoformans Faa1; YlFaa1p: Yarrowia lipolytica Faa1; ACSL3: human long-chain fatty acid–CoA ligase 3; ACSL4: human long-chain fatty acid–CoA ligase 4. Scale bar denotes 0.1 substitutions per site. (b) Amino acid sequence alignment of Faa1p in S. cerevisiae and M. globosa, M. pachydermatis, and M. sympodialis, showing the ATP–AMP motif and FACS/VLACS motif in bold letters. * indicating similar amino acid in all protein aligned in this study. Multiple sequences were aligned using T-Coffee (http://tcoffee.crg.cat/apps/tcoffee/do:regular). Asterisks denote amino acids conserved in all proteins aligned.
3.2. Malassezia Faa1 Proteins Complement the Function of S. cerevisiae Faa1p and Faa4p
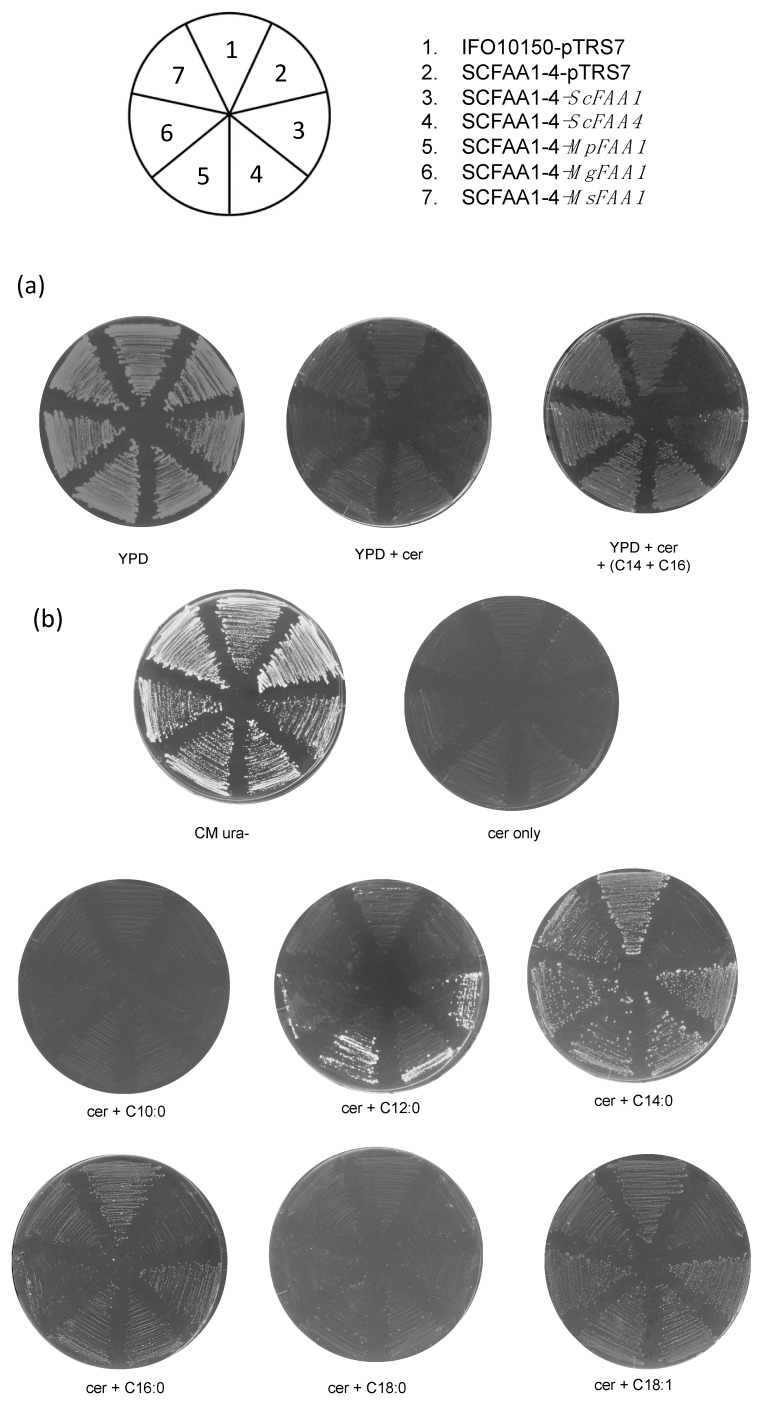
ScFAA1 and ScFAA4 are primary LCFA activators and play important roles in facilitating the utilization of exogenous LCFAs. The deletion of both of these genes caused a growth defect in S. cerevisiae in media containing cerulenin and LCFAs [16]. The introduction of MpFAA1, MgFAA1, and MsFAA1 into SCFAA1-4 rescued the growth defect in the S. cerevisiae mutant in YPD medium containing cerulenin, myristic acid (C14:0), and palmitic acid (C16:0) (Figure 2a). These data indicated that the three Malassezia FAA1 genes and ScFAA1 and ScFAA4 are functionally complementary to loss of ScFAA1 and ScFAA4.
Figure 2.
(a) Complementation analysis and substrate specificity determination for MpFAA1, MgFAA1, and MsFAA1. S. cerevisiae IFO10150-pTRS7 (FAA1FAA4) and its FAA1 and FAA4 deletion mutant (SCFAA1-4--pTRS7) as well as SCFAA1-4-ScFAA1, SCFAA1-4-ScFAA4, SCFAA1-4-MpFAA1, SCFAA1-4-MgFAA1, and SCFAA1-4-MsFAA1 were streaked on YPD agar plates containing cerulenin and C14:0 and C16:0 fatty acids. (b) Growth on CM ura− agar plates containing C10:0, C12:0, C14:0, C16:0, C18:0, and C18:1 (0.5 mM) and supplemented with 5 μg/mL cerulenin and cultured at 30 °C for 5–10 days. CM ura− agar plates containing cerulenin without fatty acids were used as controls. (c) Levels of MpFAA1 transcripts in mYNB medium containing various fatty acids. Malassezia pachydermatis 1879NT was grown in mDixon liquid medium until early/mid-log phase, transferred to mYNB medium containing 5 mM fatty acid (C10:0, C12:0, C14:0, C16:0, C18:0, and C18:1), and incubated for 6 h at 32 °C. RNAs were extracted, reverse transcribed to cDNA, and then analyzed to determine gene expression level by qRT-PCR relative to the expression of MpACT1 (n = 3 experiments, each done in duplicate; * p < 0.05).
To investigate the substrate specificity of the Malassezia FAA1 genes, we analyzed the growth of strains SCFAA1-4 harboring MpFAA1, MgFAA1, and MsFAA1 in a synthetic medium (CM ura−) supplemented with cerulenin and fatty acids with varying numbers of carbons: Capric acid (C10:0), lauric acid (C12:0), C14:0, C16:0, stearic acid (C18:0), and oleic acid (C18:1). On media containing C14:0, C16:0, and C18:1, all the strains except SCFAA1-4-pTRS7 could grow, although the growth on media with C16:0 and C18:1 was slower than that observed in C14:0. SCFAA1-4 strains expressing MpFAA1, MgFAA1, ScFAA1, and ScFAA4 showed a slight growth in the C12-supplemented medium, while IFO10150-pTRS7 and SCFAA1-4 expressing MsFaa1p were unable to grow in this medium (Figure 2b).
We also analyzed the transcription of MpFAA1 in M. pachydermatis cultured in mYNB medium with several different fatty acids. Expression of the MpFAA1 gene in medium containing C16:0 and C18:1 was enhanced approximately 2-fold compared with cells grown in mYNB medium only. These data indicated that MpFAA1 expression was induced by addition of at least C16:0 and C18:1 (Figure 2c).
3.3. Inhibition of Malassezia Faa1p by Triacsin C
Triacsin C is known as an inhibitor of bacterial and mammal ACSs [23,24]. To investigate the effect of triacsin C on Malassezia Faa1p, SCFAA1-4 strains expressing MpFAA1, MgFAA1, and MsFAA1 were cultivated in a medium containing cerulenin, LCFAs, and triacsin C. Triacsin C blocked the rescue of Malassezia Faa1 proteins in the SCFAA1-4 mutants. In contrast, the growth of S. cerevisiae IFO10150 and SCFAA1-4 strains expressing ScFAA1 and ScFAA4 was not affected by triacsin C (Figure 3). These data suggested that triacsin C inhibits the activity of Malassezia Faa1 proteins, not ScFaa1 and ScFaa4 proteins.
Figure 3.
Triacsin C blocks the rescue of Malassezia FAA1 in S. cerevisiae faa1 and faa4 mutant. A CM ura− plate containing cerulenin (5 μg/mL), C14:0 + C16:0 (each 0.5 mM), and triacsin C (3 μM) was prepared. An agar plate containing C14:0 + C16:0 and triacsin C without cerulenin, and an agar plate containing cerulenin and triacsin C were used as positive and negative controls, respectively. S. cerevisiae strains were grown at 30 °C for 3–5 days.
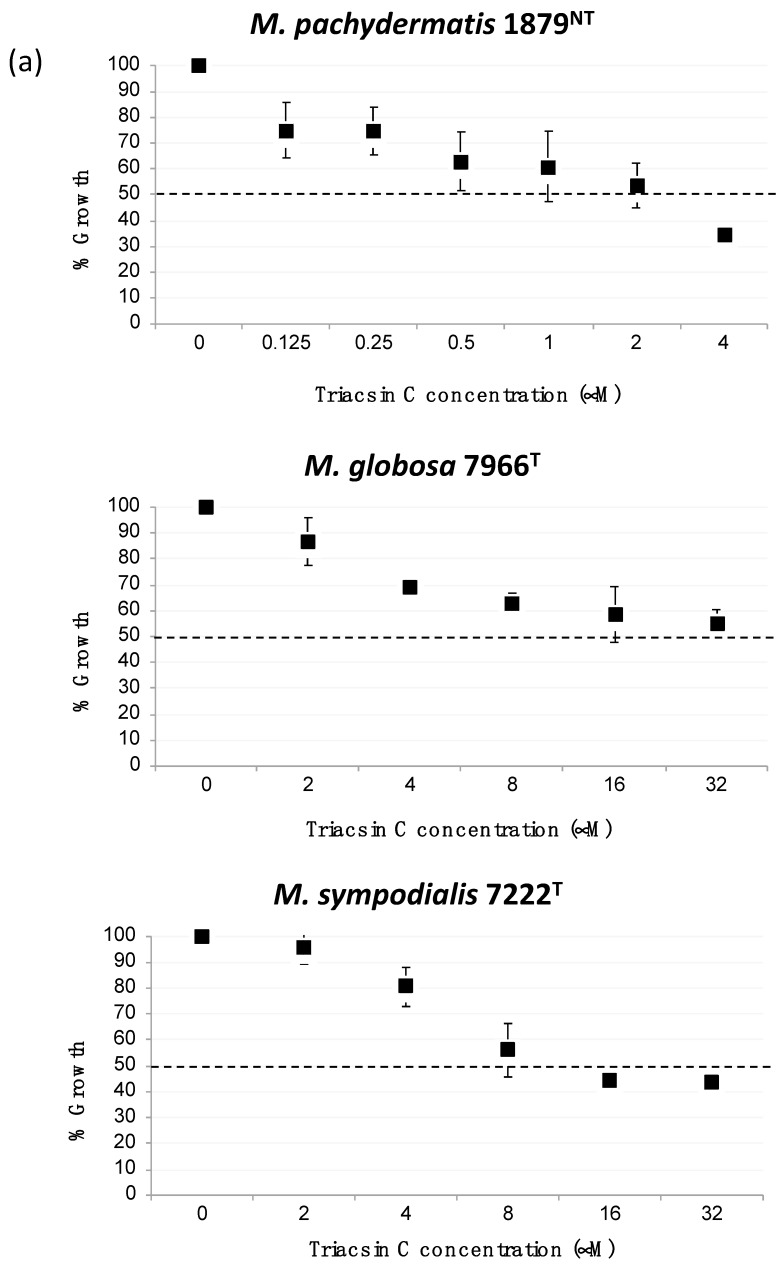
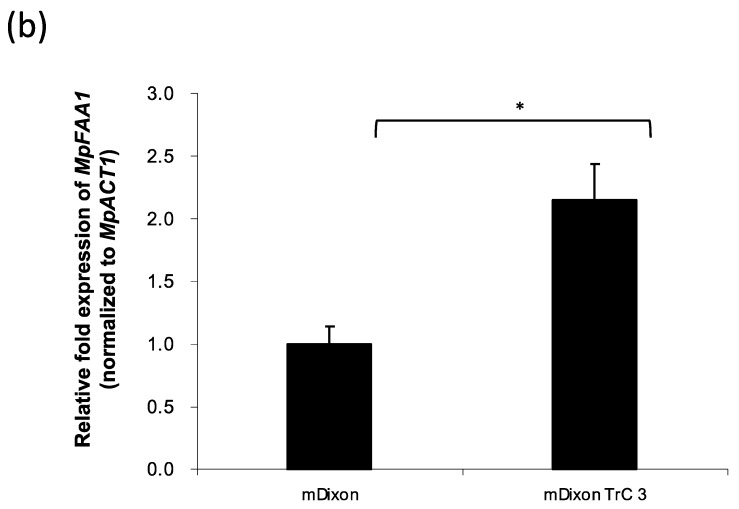
To assess the inhibitory effect of triacsin C on the growth of Malassezia spp., we cultured the three species of this yeast in mDixon medium with LCFAs and triacsin C. The growth of M. pachydermatis, M. globosa, and M. sympodialis decreased approximately 50% in the presence of triacsin C at 2 μM, 32 μM, and 8 μM (MIC50), respectively (Figure 4a). We hypothesized that the suppressive effect of triacsin C on the growth of the Malassezia species was due to interference with fatty acid activation. We also analyzed transcription of MpFAA1 in the presence of triacsin C and found that the expression of MpFAA1 was induced by the inhibitor (Figure 4b). These data suggested that triacsin C-mediated blockade of fatty acid activation induced an increase in the production of Faa1p, thus upregulating the expression of MpFAA1.
Figure 4.
(a) Inhibition of the growth of M. pachydermatis 1879NT, M. globosa 7966T, and M. sympodialis 7222T by triacsin C. All the three Malassezia strains were grown at 32 °C in mDixon liquid medium supplemented with triacsin C in a 96-well plate with a starting OD600 of 0.1. After 48 h, the OD was measured at 600 nm using a microplate reader (n > 3 experiments, each done in triplicate). (b) M. pachydermatis 1879NT was grown in mDixon liquid medium until early/mid-log phase, transferred to a fresh liquid mDixon medium containing 3 μM triacsin C, and incubated for 6 h at 32 °C. RNAs were extracted, reverse transcribed to cDNAs, and then assayed to determine gene expression levels relative to the expression of MpACT1 using qRT-PCR analysis (n = 3 independent experiments, each done in duplicate; * p < 0.05).
4. Discussion
Fatty acid uptake and activation are thought to be essential processes in Malassezia spp. The absence of genes encoding FASs results in the absolute requirement that Malassezia cells obtain LCFAs from the skin of the hosts in order to grow. Internalized LCFAs are activated to long-chain fatty acyl–CoA by ACSs by various metabolic pathways, including membrane lipid synthesis and other metabolic pathways that could be crucial for pathogenicity.
The details of fatty acid uptake and activation in Malassezia spp. remain unclear. In the present study, we identified FAA1 genes encoding ACSs in three Malassezia species (Figure 1a), and we introduced these genes into an S. cerevisiae faa1-faa4 double mutant SCFAA1-4. The results of these experiments suggested that MpFAA1, MgFAA1, and MsFAA1 function similar to ScFAA1 and ScFAA4 in the activation of exogenous LCFAs (Figure 2). In addition, although other Malassezia orthologs (Malapachy_0976, MGL_0129, and MSY001_0514) that exhibited the second-highest similarity to ScFaa1p were introduced into SCFAA1-4, these genes could not restore the growth of this mutant in media containing cerulenin and fatty acids (data not shown). The disruption of FAA4 in the pathogenic fungus Candida albicans is lethal in a medium containing 14:0 and 16:0 fatty acids in the presence of cerulenin [33]. Similar to C. albicans, the Malassezia spp. genome may contain only one gene encoding an exogenous LCFA-activating enzyme.
Unexpectedly, S. cerevisiae strains SCFAA1-4 expressing MpFAA1 and MgFAA1 were able to grow slightly in medium containing cerulenin and C12:0 fatty acid, although the IFO10150--pTRS7 (FAA1FAA4) strain could not (Figure 2b). SCFAA1-4 strains expressing ScFAA1 and ScFAA4 also showed the ability to grow a little in C12-supplemented medium. Thus, the overexpression of these genes may lead to the activation of C12:0 for the minimal growth although the C12:0-substrate affinity of these LACSs are quite low. Nevertheless, since SCFAA1-4 strain expressing MsFAA1 is unable to grow in C12:0, this result assumed that MsFaa1 protein could not recognize C12:0 fatty acid as a substrate.
A comparison of the amino acid sequences of MpFaa1, MgFaa1, and MsFaa1 proteins revealed no remarkable differences. In this experiment, we did not perform codon optimization of Malassezia FAA1 genes for expression in S. cerevisiae. This could be one reason why these genes could not fully restore the FAA1 and FAA4 mutations in SCFAA1-4. To explain this discrepancy, further experiments investigating whether the expression level of Malassezia Faa1 proteins is sufficient to activate LCFAs in S. cerevisiae will be necessary.
As triacsin C is known to affect mammalian cells, this compound was used to block the function of Malassezia Faa1 proteins in S. cerevisiae. We hypothesized that Faa1p and Faa4p are potential drug targets for therapies against Malassezia spp., which are incapable of synthesizing fatty acids endogenously. Triacsin C prevented the rescue of SCFAA1-4 expression of MpFAA1, MgFAA1, and MsFAA1 in a medium containing cerulenin and exogenous LCFAs. As expected, triacsin C did not suppress the growth of S. cerevisiae IFO10150 as well as SCFAA1-4 strains harboring ScFAA1 and ScFAA4, even when de novo fatty acid synthesis pathways were inactive. A previous study reported that 10 μM of triacsin C did not affect the growth of S. cerevisiae strains harboring the FAA1 and FAA4 genes in a synthetic medium containing cerulenin and C14:0 fatty acid [28]. Thus, our results provide evidence that Malassezia Faa1 proteins are targets of triacsin C, but S. cerevisiae Faa1p and Faa4p are not.
To confirm that triacsin C inhibits the growth of Malassezia spp., the drug was added to cultures of three Malassezia species. Triacsin C reduced the growth of all three species in a dose-dependent manner in the mDixon medium. However, triacsin C was not totally lethal to the Malassezia species examined. This suggests that Malassezia species have an alternative mechanism for LCFA activation involving triacsin C-resistant ACSs. It is also possible that triacsin C does not completely inhibit the activity of Malassezia Faa1 proteins due to low binding affinity. To address this possibility, it will be necessary to conduct in vitro enzyme assays using cell-free lysate and purified Malassezia Faa1 proteins. Nonetheless, in this work, we set the scope to Malassezia FAA1 gene complementation in S. cerevisiae and Malassezia growth reduction by triacsin C. Further experiments will determine the target proteins of triacsin C in Malassezia spp.
ACSs are conserved in all organisms, including humans. Triacsin C is often used in studies of lipid metabolism in various human diseases. A previous report showed that triacsin C inhibits the incorporation of oleic acid into lipids and glycerolipids in human fibroblasts [24]. Triacsin C is also a potent inhibitor of microsomal and mitochondrial long-chain ACSs in human hepatocytes [27]. However, to date, there are no other reports describing triacsin C toxicity in humans (particularly skin toxicity) or therapeutic applications for human or animal skin. Research in mice demonstrated that at low doses, triacsin C can be used to control the growth of cancer cells, with no negative side effects [34]. We demonstrated that triacsin C partially inhibits the activity of Faa1 proteins in Malassezia spp. Therefore, changes to the structure of this compound will be required to develop a more effective anti-Malassezia drug for treating skin diseases. In conclusion, our data demonstrate that Malassezia Faa1 proteins are important targets in efforts to develop novel anti-Malassezia drugs. Our results indicate that triacsin C and its derivatives are potentially ideal candidates.
Acknowledgments
We would like to thank Professor Somay Yamagata Murayama (Nihon University, Japan) who kindly provided M. globosa CBS7966T, and M. sympodialis CBS7222T.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/5/4/88/s1, Table S1: Saccharomyces cerevisiae strains used in this study, Table S2: Plasmids used in this study, Table S3: Primers used in this study.
Author Contributions
Conceptualization, T. and S.K.; methodology, T., K.T. and S.I.; formal analysis, T.; investigation, T. and X.C.; writing—original draft preparation, T.; writing—review and editing, T. and S.K.; supervision and funding acquisition, S.K.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sugita T., Suto H., Unno T. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J. Clin. Microbiol. 2001;39:3486–3490. doi: 10.1128/JCM.39.10.3486-3490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J., Saunders C.W., Hu P., Grant R.A., Boekhout T., Kuramae E.E., Kronstad J.W., DeAngelis Y.M., Reeder N.L., Johnstone K.R., et al. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc. Natl. Acad. Sci. USA. 2007;104:18730–18735. doi: 10.1073/pnas.0706756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harada K., Saito M., Sugita T., Tsuboi R. Malassezia species and their associated skin diseases. J. Dermatol. 2015;42:250–257. doi: 10.1111/1346-8138.12700. [DOI] [PubMed] [Google Scholar]
- 4.Yurayart C., Chindamporn A., Suradhat S., Tummaruk P., Kajiwara S., Prapasarakul N. Comparative analysis of the frequency, distribution and population sizes of yeasts associated with canine seborrheic dermatitis and healthy skin. Vet. Microbiol. 2011;148:356–362. doi: 10.1016/j.vetmic.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Triana S., Ohm R.A., De Cock H., Restrepo S., Celis A. Draft Genome Sequence of the Animal and Human Pathogen Malassezia pachydermatis Strain CBS 1879. Genome Announc. 2015;3:e01197-15. doi: 10.1128/genomeA.01197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorch J.M., Palmer J.M., Vanderwolf K.J., Schmidt K.Z., Verant M.L., Weller T.J., Blehert D.S. A new cold-tolerant species of yeast isolated from bats. Persoonia. 2018;41:56–70. doi: 10.3767/persoonia.2018.41.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu G., Zhao H., Li C., Rajapakse M.P., Wong W.C., Xu J., Saunders C.W., Reeder N.L., Reilman R.A., Scheynius A., et al. Genus-Wide Comparative Genomics of Malassezia Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin. PLoS Genet. 2015;11:1–26. doi: 10.1371/journal.pgen.1005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juntachai W., Oura T., Murayama S.Y., Kajiwara S. The lipolytic enzymes activities of Malassezia species. Med. Mycol. 2009;47:477–484. doi: 10.1080/13693780802314825. [DOI] [PubMed] [Google Scholar]
- 9.Deangelis Y.M., Saunders C.W., Johnstone K.R., Reeder N.L., Coleman C.G., Kaczvinsky J.R., Gale C., Walter R., Mekel M., Lacey M.P., et al. Isolation and expression of a Malassezia globosa lipase gene, LIP1. J. Investig. Dermatol. 2007;127:2138–2146. doi: 10.1038/sj.jid.5700844. [DOI] [PubMed] [Google Scholar]
- 10.Sommer B., Overy D.P., Kerr R.G. Identification and characterization of lipases from Malassezia restricta, a causative agent of dandruff. FEMS Yeast Res. 2015;15:fov078. doi: 10.1093/femsyr/fov078. [DOI] [PubMed] [Google Scholar]
- 11.Dawson T.L. Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J. Investig. Dermatol. Symp. Proc. 2007;12:15–19. doi: 10.1038/sj.jidsymp.5650049. [DOI] [PubMed] [Google Scholar]
- 12.Knoll L.J., Johnson D.R., Gordon J.I. Biochemical Studies of Three Saccharomyces cerevisiae Acyl-CoA Synthetases, Faa1p, Faa2p, and Faa3p. J. Biol. Chem. 1994;269:16348–16356. [PubMed] [Google Scholar]
- 13.Black P.N., Dirusso C.C. Transmembrane Movement of Exogenous Long-Chain Fatty Acids: Proteins, Enzymes, and Vectorial Esterification Transmembrane Movement of Exogenous Long-Chain Fatty Acids: Proteins, Enzymes, and Vectorial Esterification. Microbiol. Mol. Biol. Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hettema E.H., van Roermund C.W., Distel B., van den Berg M., Vilela C., Rodrigues-Pousada C., Wanders R.J., Tabak H.F. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 1996;15:3813–3822. doi: 10.1002/j.1460-2075.1996.tb00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Roermund C.W.T., Ijlst L., Majczak W., Waterham H.R., Folkerts H., Wanders R.J.A., Hellingwerf K.J. Peroxisomal fatty acid uptake mechanism in saccharomyces cerevisiae. J. Biol. Chem. 2012;287:20144–20153. doi: 10.1074/jbc.M111.332833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson D.R., Knoll J., Rowleyo N., Gordon J.I. Genetic Analysis of the Role of Saccharomyces cerevisiae Acyl-CoA Synthetase Genes in Regulating Protein N-Myristoylation. J. Biol. Chem. 1994;269:18037–18046. [PubMed] [Google Scholar]
- 17.Thomaz D.Y., de Almeida J.N., Lima G.M.E., Nunes M.D.O., Camargo C.H., Grenfell R.D.C., Benard G., Del Negro G.M.B. An Azole-Resistant Candida parapsilosis Outbreak: Clonal Persistence in the Intensive Care Unit of a Brazilian Teaching Hospital. Front. Microbiol. 2018;9:2997. doi: 10.3389/fmicb.2018.02997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popp C., Ramírez-Zavala B., Schwanfelder S., Krüger I., Morschhäuser J. Evolution of Fluconazole-Resistant Candida albicans Strains by Drug-Induced Mating Competence and Parasexual Recombination. mBio. 2019;10:e02740-18. doi: 10.1128/mBio.02740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith K.D., Achan B., Hullsiek K.H., McDonald T.R., Okagaki L.H., Alhadab A.A., Akampurira A., Rhein J.R., Meya D.B., Boulware D.R., et al. Increased Antifungal Drug Resistance in Clinical Isolates of Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 2015;59:7197–7204. doi: 10.1128/AAC.01299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijima M., Kano R., Nagata M., Hasegawa A., Kamata H. An azole-resistant isolate of Malassezia pachydermatis. Vet. Microbiol. 2011;149:288–290. doi: 10.1016/j.vetmic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Kano R., Yokoi S., Kariya N., Oshimo K., Kamata H. Multi-azole-resistant strain of Malassezia pachydermatis isolated from a canine Malassezia dermatitis. Med. Mycol. 2018;57:346–350. doi: 10.1093/mmy/myy035. [DOI] [PubMed] [Google Scholar]
- 22.Omura S., Tomoda H., Xu Q.M., Takahashi Y., Iwai Y. Triacsins, New Inhibitore of Acyl-CoA Synthetase produced by Streptomyces sp. J. Antibiot. 1986;39:1211–1218. doi: 10.7164/antibiotics.39.1211. [DOI] [PubMed] [Google Scholar]
- 23.Tomoda H., Igarashi K., Omura S. Inhibition of acyl-CoA synthetase by triacsins. Biochim. Biophys. Acta. 1987;921:595–598. [PubMed] [Google Scholar]
- 24.Igal R.A., Wang P., Coleman R.A. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: Evidence for functionally separate pools of acyl-CoA. Pt 2Biochem. J. 1997;324:529–534. doi: 10.1042/bj3240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo F., Zhang H., Fritzler J.M., Rider S.D., Jr., Xiang L., Mcnair N.N., Mead J.R. Amelioration of Cryptosporidium parvum Infection In Vitro and In Vivo by Targeting Parasite Fatty Acyl-Coenzyme A Synthetases. J. Infect. Dis. 2014;209:1279–1287. doi: 10.1093/infdis/jit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo F., Ortega-Pierres G., Argüello-García R., Zhang H., Zhu G. Giardia fatty acyl-CoA synthetases as potential drug targets. Front. Microbiol. 2015;6:753. doi: 10.3389/fmicb.2015.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vessey D.A., Kelley M., Warren R.S. Characterization of Triacsin C Inhibition of Short-, Medium-, and Long-Chain Fatty Acid: CoA Ligases of Human Liver. J. Biochem. Mol. Toxicol. 2004;18:100–106. doi: 10.1002/jbt.20009. [DOI] [PubMed] [Google Scholar]
- 28.Knoll L.J., Schall O.F., Suzuki I., Gokel G.W., Gordon J.I. Comparison of the Reactivity of Tetradecanoic Acids, a Triacsin, and Unsaturated Oximes with Four Purified Saccharomyces cerevisiae Fatty Acid Activation Proteins. J. Biol. Chem. 1995;270:20090–20097. doi: 10.1074/jbc.270.34.20090. [DOI] [PubMed] [Google Scholar]
- 29.Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K., Wiley C.J., Ausubel F.M., Brent R., et al. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; New York, NY, USA: 2003. [Google Scholar]
- 30.Sambrook J., Fritsch E.F., Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York, NY, USA: 1989. [Google Scholar]
- 31.Boeke J.D., Lacroute F., Fink G.R. A positive selection for mutants lacking orotidine-5’-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 32.Collart M.A., Oliviero S. Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; New York, USA: 2001. Preparation of Yeast RNA; pp. 13.12.1–13.12.5. [DOI] [PubMed] [Google Scholar]
- 33.Tejima K., Ishiai M., Murayama S.O., Iwatani S. Candida albicans fatty acyl-CoA synthetase, Ca Faa4p, is involved in the uptake of exogenous long-chain fatty acids and cell activity in the biofilm. Curr. Genet. 2017;64:429–441. doi: 10.1007/s00294-017-0751-2. [DOI] [PubMed] [Google Scholar]
- 34.Mashima T., Sato S., Okabe S., Miyata S., Matsuura M., Sugimoto Y., Tsuruo T., Seimiya H. Acyl-CoA synthetase as a cancer survival factor: Its inhibition enhances the efficacy of etoposide. Cancer Sci. 2009;100:1561. doi: 10.1111/j.1349-7006.2009.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.