Abstract
Background
Health traits are of significant economic importance to the dairy industry due to their effects on milk production and associated treatment costs. Genome-wide association studies (GWAS) provide a means to identify associated genomic variants and thus reveal insights into the genetic architecture of complex traits and diseases. The objective of this study is to investigate the genetic basis of seven health traits in dairy cattle and to identify potential candidate genes associated with cattle health using GWAS, fine mapping, and analyses of multi-tissue transcriptome data.
Results
We studied cow livability and six direct disease traits, mastitis, ketosis, hypocalcemia, displaced abomasum, metritis, and retained placenta, using de-regressed breeding values and more than three million imputed DNA sequence variants. After data edits and filtering on reliability, the number of bulls included in the analyses ranged from 11,880 (hypocalcemia) to 24,699 (livability). GWAS was performed using a mixed-model association test, and a Bayesian fine-mapping procedure was conducted to calculate a posterior probability of causality to each variant and gene in the candidate regions. The GWAS detected a total of eight genome-wide significant associations for three traits, cow livability, ketosis, and hypocalcemia, including the bovine Major Histocompatibility Complex (MHC) region associated with livability. Our fine-mapping of associated regions reported 20 candidate genes with the highest posterior probabilities of causality for cattle health. Combined with transcriptome data across multiple tissues in cattle, we further exploited these candidate genes to identify specific expression patterns in disease-related tissues and relevant biological explanations such as the expression of Group-specific Component (GC) in the liver and association with mastitis as well as the Coiled-Coil Domain Containing 88C (CCDC88C) expression in CD8 cells and association with cow livability.
Conclusions
Collectively, our analyses report six significant associations and 20 candidate genes of cattle health. With the integration of multi-tissue transcriptome data, our results provide useful information for future functional studies and better understanding of the biological relationship between genetics and disease susceptibility in cattle.
Keywords: GWAS, Fine mapping, Health trait, Gene expression, Dairy cattle
Background
One of the fundamental goals of animal production is to profitably produce nutritious food for humans from healthy animals. Profitability of the dairy industry is influenced by many factors, including production, reproduction, and animal health [1]. Cattle diseases can cause substantial financial losses to producers as the result of decreased productivity, including milk that must be dumped, and increased costs for labor and veterinary care. Indirect costs associated with reduced fertility, reduced production after recovery, and increased risk of culling also can be substantial. For example, ketosis is a metabolic disease that occurs in cows during early lactation and hinders the cow’s energy intake, thus subsequently reduces milk yield and increases the risk of displaced abomasum, which is very costly [2]. Mastitis is a major endemic disease of dairy cattle that can lead to losses to dairy farmers due to contamination, veterinary care, and decreased milk production [3]. In addition, cows may develop milk fever, a metabolic disease that is related to a low blood calcium level known as hypocalcemia [4]. Another common disease in cattle is metritis, which is inflammation of the uterus and commonly seen following calving when cows have a suppressed immune system and are vulnerable to bacterial infection [5]. Complications during delivery can also result in a retained placenta [6]. Many of the postpartum diseases are caused by the energy imbalance due to onset of lactation, especially in high producing cows. These complex diseases are jointly affected by management, nutrition, and genetics. A better understanding of the underlying genetic components can help the management and genetic improvements of cattle health.
Genome-wide association studies (GWAS) have been successful at interrogating the genetic basis of complex traits and diseases in cattle [7–10]. Because complex traits are influenced by many genes, their interactions, and environment and due to the high level of linkage disequilibrium (LD) between genomic variants, pinpointing causal variants of complex traits has been challenging [11]. Fine-mapping is a common post-GWAS analysis, where posterior probabilities of causality are assigned to candidate variants and genes. In humans, fine-mapping of complex traits are currently on-going along or following GWAS studies. The utility of fine-mapping in cattle studies, however, has been limited by data availability and the high levels of LD present in cattle populations [12–14]. To circumvent this challenge, a recent study developed a fast Bayesian Fine-MAPping method (BFMAP), which performs fine-mapping by integrating various functional annotation data [10]. Additionally, this method can be exploited to identify biologically meaningful information from candidate genes to enhance the understanding of complex traits [15].
The U.S. dairy industry has been collecting and evaluating economically important traits in dairy cattle since the late 1800s, when the first dairy improvement programs were formed. Since then, a series of dairy traits have been evaluated, including production, body conformation, reproduction, and health traits. Cow livability was included in the national genomic evaluation system by the Council on Dairy Cattle Breeding (CDCB) in 2016 [16]. This trait reflects a cow’s overall ability to stay alive in a milking herd by measuring the percentage of on-farm deaths per lactation. Cow livability is partially attributable to health and can be selected to provide more milk revenue and less replacement of cows. In 2018, six direct health traits were introduced into the U.S. genomic evaluation, including ketosis, mastitis, hypocalcemia or milk fever, metritis, retained placenta, and displaced abomasum [17]. These phenotypic records along with genotype data collected from the U.S. dairy industry provide a unique opportunity to investigate the genetic basis of cattle health. The aim of our study is, therefore, to provide a powerful genetic investigation of seven health traits in cattle, to pinpoint the candidate disease genes and variants with relevant tissue-specific expression, and to provide insights into the biological relationship between candidate genes and the disease risk they may present on a broad scale.
Results
Genome-wide association study of livability and six direct health traits
We conducted genome-wide association analyses of seven health related traits in 27,214 Holstein bulls that have many daughter records and thus accurate phenotypes using imputed sequence data and de-regressed breeding values. After editing and filtering on reliability, we included 11,880 to 24,699 Holstein bulls across the seven traits (Table 1). Compared to the analysis using predicted transmitting ability (PTA) as phenotype (Additional file 1), GWAS on de-regressed PTA values produced more consistent and reliable results [18]. While different results between analyses of raw and de-regressed PTAs were obtained for the six health traits, little difference was observed for cow livability, which have more records and higher reliabilities (Table 1 and Additional file 2). Therefore, we only considered association results obtained with de-regressed PTAs in all subsequent analyses.
Table 1.
Number of Holstein bulls, reliability of PTA, and heritability (h2) for six disease traits and cow livability
| Trait | N | h2 | Average Reliability |
|---|---|---|---|
| Hypocalcemia | 11,880 | 0.006 | 0.228 |
| Displaced Abomasum | 13,229 | 0.011 | 0.269 |
| Ketosis | 12,468 | 0.012 | 0.260 |
| Mastitis | 14,382 | 0.031 | 0.338 |
| Metritis | 13,653 | 0.014 | 0.281 |
| Retained Placenta | 13,541 | 0.001 | 0.266 |
| Livability | 24,699 | 0.040 | 0.397 |
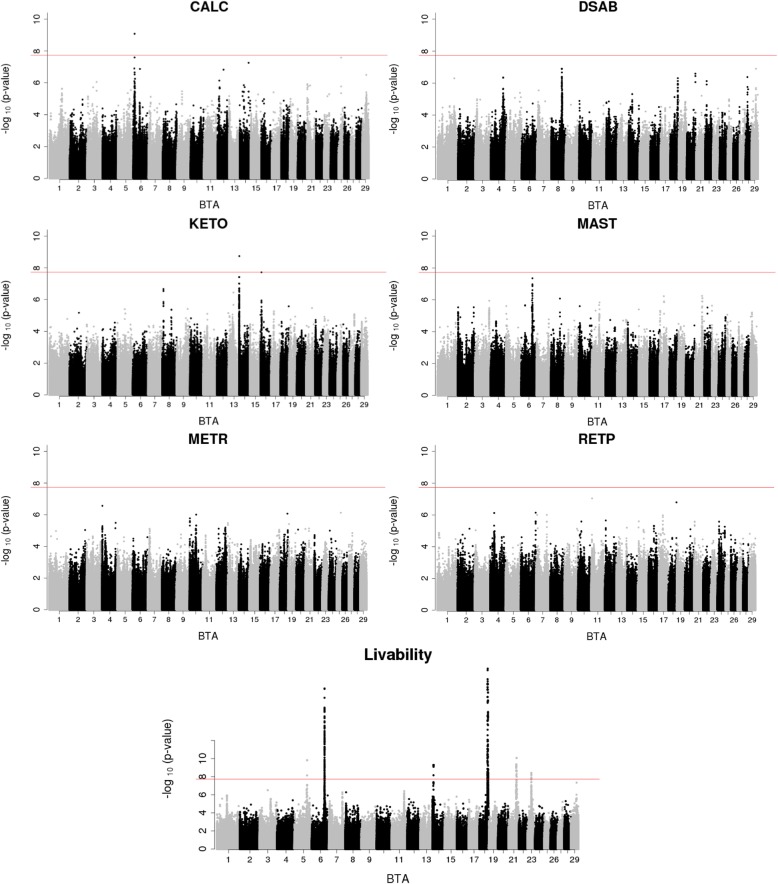
Out of the seven health traits, we detected significantly associated genomic regions only for three traits after Bonferroni correction, hypocalcemia, ketosis, and livability (Fig. 1). In total, we had one associated region on BTA 6 for hypocalcemia, one region on BTA 14 for ketosis, and six regions for cow livability on BTA 5, 6, 14, 18, 21, and 23, respectively (Table 2). Notably, the bovine Major Histocompatibility Complex (MHC) region on BTA 23 [20] is associated with cow livability. Additionally, association signals on BTA 16 for ketosis (P-value = 1.9 × 10− 8) and BTA 6 for mastitis (P-value = 4.2 × 10− 8) almost reached the Bonferroni significance level. Other traits had prominent signals, but their top associations were below the Bonferroni threshold. Since sequence data have the highest coverage of functional variants in our study, we included all these regions to query the Cattle QTLdb for a comparative analysis.
Fig. 1.
Manhattan plots for hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability. The genome-wide threshold (red line) corresponds to the Bonferroni correction
Table 2.
Top SNPs and candidate genes associated with hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability
| Trait | Chr | Position | MAF | P-value | Genes Nearby | Traits Previously Associatedb |
|---|---|---|---|---|---|---|
| CALC | 6 | 10,521,824 | 0.014 | 8.3 × 10−10a | TRAM1L1, NDST4 | Subcutaneous fat |
| DSAB | 4 | 97,101,981 | 0.021 | 4.4 × 10−7 | PLXNA4, CHCHD3 | Milk protein yield |
| DSAB | 8 | 83,052,202 | 0.109 | 1.3 × 10−7 | FANCC | Stature |
| DSAB | 29 | 35,977,236 | 0.073 | 1.3 × 10−7 | NTM | Milk kappa-casein percentage |
| KETO | 14 | 2,762,595 | 0.033 | 1.8 × 10− 9a | LY6K | Milk protein percentage |
| KETO | 16 | 7,048,452 | 0.019 | 1.9 × 10−8 | KCNT2 | Milk fat percentage |
| MAST | 6 | 88,868,886 | 0.460 | 4.2 × 10−8 | GC | Clinical mastitis |
| METR | 4 | 3,662,486 | 0.011 | 2.7 × 10−7 | RF00322 | Milk protein yield |
| RETP | 4 | 32,578,298 | 0.218 | 7.4 × 10−7 | RUNDC3B | Calving ease |
| RETP | 6 | 117,620,548 | 0.026 | 7.2 × 10−7 | QDPR | Milk kappa-casein percentage |
| RETP | 11 | 7,465,110 | 0.060 | 9.1 × 10− 8 | TMEM182 | Abomasum displacement |
| RETP | 18 | 64,492,219 | 0.012 | 1.6 × 10−7 | ZFP28 | Still birth |
| Livability | 5 | 88,823,164 | 0.472 | 1.5 × 10−10a | ABCC9 | Productive life |
| Livability | 6 | 88,801,999 | 0.454 | 1.7 × 10−18a | GC | Clinical mastitis |
| Livability | 14 | 8,536,538 | 0.020 | 5.3 × 10−10a | ZFAT | Productive life |
| Livability | 18 | 58,194,319 | 0.075 | 1.1 × 10−20a | ZNF614 | Bovine respiratory disease |
| Livability | 21 | 56,700,449 | 0.013 | 8.6 × 10−11a | CCDC88C | Type |
| Livability | 23 | 26,131,593 | 0.017 | 3.8 × 10−9a | BLA-DQB | Antibody-mediated immune response |
aGenome-wide significance after Bonferroni correction
bInformation obtained from the Animal QTLdb for cattle [19]
When compared to existing studies, many of these health related regions have been previously associated with milk production or disease related traits in cattle (Table 2) [19]. The top associated region for hypocalcemia is around 10,521,824 bp on BTA 6, where QTLs were reported for body/carcass weight and reproduction traits with nearby genes being Translocation Associated Membrane Protein 1 Like (TRAM1L1) and N-Deacetylase And N-Sulfotransferase (NDST4). The region around 2,762,595 bp on BTA 14 for ketosis is involved with milk and fat metabolism and the well-known Diacylglycerol O-Acyltransferase 1 (DGAT1) gene. The region around 7,048,452 bp on BTA 16 for ketosis was also previously associated with fat metabolism. The region around 88,868,886 bp on BTA 6 associated with mastitis is close to the GC gene with many reported QTLs associated with mastitis [10, 21–23]. This region was also associated with cow livability in this study with QTLs involved with the length of productive life [24]. For the six regions associated with cow livability (Table 2), we found reported QTLs related to productive life, somatic cell count, immune response, reproduction, and body conformation traits [24]. The top associated regions for displaced abomasum on BTA 4 and BTA 8 have been previously associated with cattle reproduction and body conformation traits [25–27]. For metritis, the top associated variant, 3,662,486 bp on BTA4, is close to Small nucleolar RNA MBI-161 (SNORA31), and around ±1 Mb upstream and downstream were QTLs associated with production, reproduction, and dystocia [28]. Genes RUN Domain Containing 3B (RUNDC3B; BTA 4), Quinoid Dihydropteridine Reductase (QDPR; BTA 6), Transmembrane Protein 182 (TMEM182; BTA 11), and Zinc Finger Protein (ZFP28; BTA 18) are the closest genes to the retained placenta signals with previous associations related to milk production, productive life, health and reproduction traits, including calving ease and stillbirth [8].
Association of livability QTL with other disease traits
Cow livability is a health-related trait that measures the overall robustness of a cow. As the GWAS of cow livability was the most powerful among the seven traits and detected six QTL regions, we evaluated whether these livability QTLs were also associated with other disease traits. Out of the six livability QTLs, four of them were related to at least one disease trait at the nominal significance level (Table 3). All these overlapped associations exhibited consistent directions of effect: alleles related to longer productive life were more resistant to diseases. The most significant QTL of livability on BTA 18 is associated with displaced abomasum and metritis, both of which can occur after abnormal birth. This QTL has been associated with gestation length, calving traits, and other gestation and birth related traits [15]. The QTL on BTA 6 is associated with hypocalcemia, ketosis, and mastitis. The BTA 21 QTL is associated with hypocalcemia and mastitis. The BTA 5 QTL is related to displaced abomasum and ketosis. Interestingly, the bovine MHC region on BTA 23 is not associated with the immune-related disease traits, which suggests that those genes do not explain substantial variation for the presence or absence of a disease during a lactation and we have no enough power to detect the association.
Table 3.
Association results of the top SNPs associated with cow livability for hypocalcemia, displaced abomasum, ketosis, mastitis, and metritis. P-values larger than 0.05 and their Beta coefficients were excluded
| Chr | Position | Livability | Hypocalcemia | Displaced Abomasum | Ketosis | Mastitis | Metritis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | P-value | Beta | ||
| 5 | 88,823,164 | 1.5 × 10−10 | −0.43 | – | – | 0.04 | −0.14 | 0.04 | −0.21 | – | – | – | – |
| 6 | 88,801,999 | 1.7 × 10−18 | −0.66 | 5.0 × 10−3 | −0.2 | – | – | 2.1 × 10− 3 | − 0.35 | 4.2 × 10−7 | −0.75 | – | – |
| 14 | 8,536,538 | 5.3 × 10−10 | − 1.1 | – | – | – | – | – | – | – | – | – | – |
| 18 | 58,194,319 | 1.1 × 10−20 | −1.0 | – | – | 1.1 × 10−4 | −0.47 | – | – | – | – | 0.01 | −0.51 |
| 21 | 56,700,449 | 8.6 × 10−11 | − 1.5 | 0.03 | −0.58 | – | – | – | – | 9.1 × 10−3 | − 1.43 | – | – |
| 23 | 26,131,593 | 3.8 × 10−9 | 0.71 | – | – | – | – | – | – | – | – | – | – |
Fine-mapping analyses and validation from tissue-specific expression
Focusing on the candidate QTL regions in Table 2, the fine-mapping analysis calculated posterior probabilities of causalities (PPC) for individual variants and genes to identify candidates (Table 4), which were largely consistent with the GWAS results. A total of eight genes detected in GWAS signals were also successfully fine-mapped, including Plexin A4 (PLXNA4), FA Complementation Group C (FANCC), Neurotrimin (NTM) for displaced abomasum, GC for mastitis and livability, ATP Binding Cassette Subfamily C Member 9 (ABCC9) for livability, QDPR for retained placenta, Zinc Finger And AT-Hook Domain Containing (ZFAT) and CCDC88C for livability. In addition, fine-mapping identified new candidate genes, including Cordon-Bleu WH2 Repeat Protein (COBL) on BTA 4 for metritis, LOC783947 on BTA 16 for ketosis, LOC783493 on BTA 18 for retained placenta, and LOC618463 on BTA 18 and LOC101908667 on BTA 23 for livability. The genes LOC107133096 on BTA 14 and LOC100296627 on BTA 4 detected respectively for ketosis and retained placenta by fine mapping were close to two genes (DGAT1 and ABCB1 or ATP Binding Cassette Subfamily B Member 1) that have known biological association with milk production and other traits. In addition to the detected genes in these two cases, we further investigated genes with a potential biological link with disease, and genes with the highest PPC (PARP10 or PolyADP-ribose polymerase 10 and MALSU1 or Mitochondrial Assembly Of Ribosomal Large Subunit 1) that were located between these two references (Table 4). No genes were detected by fine-mapping in the signal on BTA 6 for hypocalcemia (Fig. 1), given that the nearest genes were beyond a 1 Mb window boundary.
Table 4.
List of candidate genes with highest posterior probability of causality (PPC) and their minimum P-values for casualty (M_Causality) and GWAS (M_GWAS) associated with hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability and their tissue specific expression
| Trait | Gene | Chr | Start | End | M_GWAS P-value |
M_Casualty P-value |
PPC | Type | Tissue-Specific Expression |
|---|---|---|---|---|---|---|---|---|---|
| DSAB | PLXNA4 | 4 | 96,574,369 | 97,120,718 | 4.5 × 10−7 | 6.5 × 10−7 | 0.49 | protein_coding | Aorta, Liver, Trachea |
| DSAB | FANCC | 8 | 83,022,522 | 83,228,696 | 1.3 × 10−7 | 2.1 × 10−9 | 0.79 | protein_coding | Thyroid |
| DSAB | NTM | 29 | 35,153,012 | 36,117,726 | 1.3 × 10−7 | 1.7 × 10− 7 | 0.99 | protein_coding | Central Nervous System |
| LOC107133096 | 14 | 2,760,093 | 2,762,878 | 2.0 × 10−9 | 5.9 × 10−8 | 0.92 | IncRNA | – | |
| KETO | PARP10 | 14 | 2,024,509 | 2,031,477 | 7.0 × 10−7 | 1.7 × 10−5 | 0.16 | protein_coding | – |
| DGAT1 | 14 | 1,795,425 | 1,804,838 | 1.0 × 10−6 | 1.7 × 10−5 | 0.08 | protein_coding | Bone Marrow | |
| KETO | LOC783947 | 16 | 7,050,445 | 7,055,021 | 1.9 × 10−8 | 1.3 × 10−8 | 1.00 | lncRNA | – |
| MAST | GC | 6 | 88,687,845 | 88,739,292 | 2.0 × 10−7 | 1.2 × 10−7 | 0.15 | protein_coding | Kidney, Cortex, Liver |
| METR | COBL | 4 | 4,494,925 | 4,795,904 | 4.3 × 10−3 | 7.7 × 10−4 | 1.00 | protein_coding | – |
| LOC100296627 | 4 | 32,573,079 | 32,613,237 | 7.6 × 10−7 | 4.0 × 10−13 | 1.00 | protein_coding | – | |
| RETP | MALSU1 | 4 | 32,051,590 | 32,077,036 | 7.5 × 10−4 | 1,1 × 10−13 | 0.98 | protein_coding | – |
| ABCB1 | 4 | 33,013,208 | 33,095,708 | 6.3 × 10−1 | 8.4 × 10−3 | 0.28 | protein_coding | – | |
| RETP | TMEM182 | 11 | 7,449,519 | 7,492,871 | 9.0 × 10−8 | 9.9 × 10−8 | 0.96 | protein_coding | Heart, Muscle, Tongue |
| RETP | LOC783493 | 18 | 63,799,608 | 63,803,213 | 8.3 × 10−3 | 1.2 × 10−5 | 0.94 | Pseudogene | – |
| Livability | ABCC9 | 5 | 8,867,2047 | 88,834,491 | 1.5 × 10−10 | 1.5 × 10−10 | 1.00 | protein_coding |
Aorta, Atrium, Lung, Muscle Uterine myometrium, Ventricle |
| Livability | GC | 6 | 88,687,845 | 88,739,292 | 1.9 × 10−17 | 1.4 × 10− 19 | 0.03 | protein_coding | Kidney, Cortex, Liver |
| Livability | ZFAT | 14 | 8,144,774 | 8,305,775 | 2.1 × 10−5 | 3.2 × 10−5 | 0.23 | protein_coding | – |
| Livability | LOC618463 | 18 | 57,587,990 | 57,594,549 | 1.7 × 10−20 | 3.1 × 10−20 | 0.20 | protein_coding | – |
| Livability | CCDC88C | 21 | 56,645,629 | 56,773,438 | 8.6 × 10−11 | 8.9 × 10−11 | 0.95 | protein_coding | CD8_cell |
| Livability | LOC101908667 | 23 | 25,904,084 | 25,909,461 | 2.1 × 10−8 | 7.9 × 10−9 | 0.31 | lncRNA | – |
In addition, we investigated the expression levels of fine-mapped candidate genes across cattle tissues using existing RNA-Seq data from public databases. While many genes are ubiquitously expressed in multiple tissues, several fine-mapped genes were specifically expressed in a few tissues relevant to cattle health (Table 4). Interesting examples of tissue-specific expression and candidate genes included liver with mastitis and livability (GC), and CD8 cells with livability (CCDC88C). Although this analysis is preliminary, these results provide additional support for these candidate genes of cattle health and help the understanding of how and where their expression is related with dairy disease resistance.
Discussion
In this study, we performed powerful GWAS analyses of seven health and related traits in Holstein bulls. The resulting GWAS signals were further investigated by a Bayesian fine-mapping approach to identify candidate genes and variants. Additionally, we included tissue-specific expression data of candidate genes to reveal a potential biological relationship between genes, tissues and cattle diseases. Finally, we provide a list of candidate genes of cattle health with associated tissue-specific expression that can be readily tested in future functional validation studies.
In our GWAS analysis, we used de-regressed PTA as phenotype and incorporated the reliabilities of the de-regressed PTAs of livability and six disease traits. Three traits were found to have significant association signals, hypocalcemia, ketosis, and livability, which demonstrated the power of our GWAS study. For example, we also observed regions associated with livability, in particular, with the region around 58,194,319 on BTA 18 to possess a large effect on dairy and body traits. Our finding was corroborated by a BLAST analysis that identified a related molecule, Siglec-6, which is expressed in tissues such as the human placenta [29]. Further analyses can be performed to characterize the functional implications of these association regions for the seven health and related traits in cattle.
When using PTA values as phenotype in GWAS, we observed different regions to be associated, compared to the GWAS with de-regressed PTA (Fig. 1 and Additional file 2). For example, a genomic region larger than 4 Mb on BTA 12 was associated with most of the health traits (Additional file 2). Although these generally appeared as clear association signals, we observed only a few HD SNP markers to be associated, which may be due to poor imputation. Additionally, this region was reported by VanRaden et al. as having low imputation accuracy [30]. The lower imputation accuracy on BTA 12 was determined to be caused by a gap between the 72.4 and 75.2 Mb region where no SNPs were present on the HD SNP array [30]. Additional studies are needed to address this imputation issue in order to improve the accuracy and power of future analysis on this region. Since different family relationship will affect the GWAS results when using direct versus deregressed PTAs, these differences in relatedness can lead to false positive GWAS results, especially for low-quality imputed data. In sum, this comparison of GWAS using PTA and de-regressed PTA supports the use of de-regressed PTA values with reliabilities accounted for in future GWAS studies in cattle.
Application of BFMAP for fine-mapping allowed us to identify 20 promising candidate genes (Table 4) and a list of candidate variants (Additional file 3) for health traits in dairy cattle. We found that most of the genes possess tissue-specific expression, notably the detected gene LOC107133096 on BTA 14 for ketosis. This gene is located close to the DGAT1 gene that affects milk fat composition. A previous candidate gene association study by Tetens et al. proposed DGAT1 to be an indicator of ketosis [31]. In that study, the DGAT1 gene was determined to be involved in cholesterol metabolism, which is known to be an indicator of a ketogenic diet in humans [31]. This result highlights a potential pathway in the pathogenesis of ketosis that may be an area for future research. Additionally, ketosis is a multifactorial disease that is likely influenced by multiple loci. Therefore, implementation of a functional genomics approach would allow identification of more genetic markers, and in doing so, improve resistance to this disease. For displaced abomasum, the gene PLXNA4 was observed to have an association with the variant 97,101,981 bp on BTA 4 (Table 4 and Additional file 3). Our analysis also detected tissue-specific expression for PLXNA4 in the aorta. A previous study on atherosclerosis found that Plexin-A4 knockout mice exhibited incomplete aortic septation [32]. These findings provide some support for the potential association of PLXNA4 with cattle health.
Six signals were observed as clear association peaks for livability (Fig. 1). The associated variant at 8,144,774 – 8,305,775 bp on BTA 14 was close to the gene ZFAT, which is known to be expressed in the human placenta [33]. In particular, the expression of this gene is downregulated in placentas from complicated pregnancies. Additionally, a GWAS study performed in three French dairy cattle populations found the ZFAT gene to be the top variant associated with fertility [34]. Since calving and other fertility issues could be risk factors to cause animal death, these results lend support of this candidate gene with the livability. On BTA18, the associated variant at 57,587,990 – 57,594,549 bp was near the gene LOC618463, which has been previously identified as a candidate gene associated with calving difficulty in three different dairy populations [35]. For the associated variant at 56,645,629 – 56,773,438 bp on BTA21, it is located close to the CCDC88C gene (Table 4). In addition to our detection of tissue-specific expression with the CD8 cell, this gene has been associated with traits such as dairy form and days to first breeding in cattle [10].
It is notable that our GWAS signal for livability at 25,904,084 – 25,909,461 bp on BTA 23 is located in the bovine MHC region (Table 4). The gene we detected was LOC101908667, which is one of the immune genes of MHC. This is of considerable interest because MHC genes have a role in immune regulation. The MHC complex of cattle located on BTA 23 is called the bovine leukocyte antigen (BoLA) region. This complex of genes has been extensively studied, such as in research investigating the polymorphism of genes in BoLA and their association with disease resistance [36]. Therefore, our research highlights a gene of considerable interest that should be further explored to understand its importance in breeding programs and its potential role in resistance to infectious diseases.
Additionally, we identified an associated variant for livability at 88,687,845 - 88,739,292 bp on BTA6 was close to the gene GC, which was specifically expressed in tissues such as the liver (Table 4). This gene has been previously studied in an association analysis that investigated the role of GC on milk production [21]. It found that the gene expression of GC in cattle is predominantly expressed in the liver. Moreover, affected animals displayed decreased levels of the vitamin D-binding protein (DBP) encoded by GC, highlighting the importance of GC for a cow’s production. Additionally, liver-specific GC expression has been identified in humans, specifically regulated through binding sites for the liver-specific factor HNF1 [37]. Collectively, these results offer evidence for GC expression in the liver, which may be an important factor for determining cow livability.
Interestingly, the GC gene was also detected to have tissue-specific expression in the liver for mastitis (Table 4). This is corroborated by a study on cattle infected with mastitis to possess limited DBP concentration [21]. Vitamin D plays a key part in maintaining serum levels of calcium when it is secreted into the milk [38]. Since GC encodes DBP, it was suggested that the GC gene has a role in regulating milk production and the incidence of mastitis infection in dairy cattle. It is important to note that bovine mastitis pathogens, such as Staphylococcus aureus and Escherichia coli, also commonly occur as pathogens of humans. Therefore, development of molecular methods to contain these pathogens is of considerable interest for use in human medicine to prevent the spread of illness and disease. For instance, the use of enterobacterial repetitive intergenic consensus typing enables trace back of clinical episodes of E. coli mastitis, thus allowing for an evaluation of antimicrobial products for the prevention of mastitis [39]. Continued investigation using molecular methods are needed to understand the pathogenesis of mastitis and its comparative relevance to human medicine. Based on the fine mapping for metritis, the new gene assigned was COBL on BTA 6 (Table 4). However, this candidate gene was found to have variants only passing the nominal significance level for causality and for GWAS. Further exploration of this candidate gene is needed to contribute to our understanding of its function and potential tissue-specific expression.
For retained placenta, the gene TMEM182 was observed to have an association with a variant between 7,449,519 – 7,492,871 bp on BTA11 (Table 4). Our tissue-specific analysis identified TMEM182 to have an association in muscle tissues. A study performed in Canchim beef cattle investigated genes for male and female reproductive traits and identified TMEM182 on BTA 11 as a candidate gene that could act on fertility [40]. Additionally, the gene TMEM182 has been found to be up-regulated in brown adipose tissue in mice during adipogenesis, which suggests a role in the development of muscle tissue [41]. One important factor that causes retention of fetal membranes in cattle is the impaired muscular tone of organs such as the uterus and abdomen [42]. This suggests the importance of the TMEM182 gene and the need for future studies to better understand its role in the cattle breeding program.
Conclusions
In this study, we reported eight significant associations for seven health and related traits in dairy cattle. In total, we identified 20 candidate genes of cattle health with the highest posterior probability, which are readily testable in future functional studies. Several candidate genes exhibited tissue-specific expression related to immune function, muscle growth and development, and neurological pathways. The identification of a novel association for cow livability in the bovine MHC region also represented an insight into the biology of disease resistance. Overall, our study offers a promising resource of candidate genes associated with complex diseases in cattle that can be applied to breeding programs and future studies of disease genes for clinical utility.
Methods
Ethics statement
This study didn’t require the approval of the ethics committee, as no biological materials were collected.
Genotype data
Using 444 ancestor Holstein bulls from the 1000 Bull Genomes Project as reference, we previously imputed sequence variants for 27,214 progeny-tested Holstein bulls that have highly reliable phenotypes via FindHap version 3 [43]. We applied stringent quality-control procedures before and after imputation to ensure the data quality. The original 777,962 HD SNPs were reduced to 312,614 by removing highly correlated SNP markers with a |r| value higher than 0.95 and by prior editing. Variants with a minor allelic frequency (MAF) lower than 0.01, incorrect map locations (UMD3.1 bovine reference assembly), an excess of heterozygotes, or low correlations (|r| < 0.95) between sequence and HD genotypes for the same variant were removed. The final imputed data was composed of 3,148,506 sequence variants for 27,214 Holstein bulls. Details about the genomic data and imputation procedure are described by VanRaden et al. [30]. After imputation, we only retained autosomal variants with MAF ≥0.01 and P-value of Hardy-Weinberg equilibrium test > 10− 6.
Phenotype data
The data used were part of the 2018 U.S. genomic evaluations from the Council on Dairy Cattle Breeding (CDCB), consisting of 1,922,996 Holstein cattle from the national dairy cattle database. Genomic predicted transmitting ability (PTA) values were routinely calculated for these animals and were included in this study. De-regressed PTA values according to Garrick et al. [18] were analyzed in GWAS for livability, hypocalcemia, displaced abomasum, ketosis, mastitis, metritis, and retained placenta. We restricted the de-regression procedure to those bulls with PTA reliability greater than parent average reliability, thus reducing the total number of animals from 27,214 to 11,880, 13,229, 12,468, 14,382, 13,653, 13,541, and 24,699 for the seven traits, respectively (Table 1).
Genome-wide association study (GWAS)
A mixed-model GWAS was performed using MMAP, a comprehensive mixed model program for analysis of pedigree and population data [44]. The additive effect was divided into a random polygenic effect and a fixed effect of the candidate SNP. The variance components for the polygenic effect and random residuals were estimated using the restricted maximum likelihood (REML) approach. MMAP has been widely used in human and cattle GWAS studies [45–47]. The model can be generally presented as:
where y is a vector with de-regressed PTAs; μ is the global mean; m is the candidate SNP genotype (allelic dosage coded as 0, 1 or 2) for each animal; b is the solution effect of the candidate SNP; a is a solution vector of polygenic effect accounting for the population structure assuming , where G is a relationship matrix; and e is a vector of residuals assuming , where R is a diagonal matrix with diagonal elements weighted by the individual de-regressed reliability (). For each candidate variant, a Wald test was applied to evaluate the alternative hypothesis, H1: b ≠ 0, against the null hypothesis H0: b = 0. Bonferroni correction for multiple comparisons was applied to control the type-I error rate. Gene coordinates in the UMD v3.1 assembly [48] were obtained from the Ensembl Genes 90 database using the BioMart tool. The cattle QTLdb database [19] was examined to check if any associated genomic region was previously reported as a cattle quantitative trait locus (QTL).
Fine-mapping association study
In order to identify potential candidate genes and their causal variants, GWAS signals were investigated through a fine-mapping procedure using a Bayesian approach with the software BFMAP v.1 (https://github.com/jiang18/bfmap) [10]. BFMAP is a software tool for genomic analysis of quantitative traits, with a focus on fine-mapping, SNP-set association, and functional enrichment. It can handle samples with population structure and relatedness and calculate posterior probability of causality (PPC) to each variant and its causality p-value for independent association signals within candidate QTL regions. The minimal region covered by each lead variant was determined as ±1 Mb upstream and downstream (candidate region ≥2 Mb). This extension allowed the region to cover most variants that have an LD r2 of > 0.3 with the lead variants. The employed fine-mapping approach included three steps: forward selection to add independent signals in the additive Bayesian model, repositioning signals, and generating credible variant sets for each signal. Details about the BFMAP algorithm and its procedure are described by Jiang et al. [10].
Tissue-specific expression of candidate genes
From public available resources including the NCBI GEO database, we have assembled RNA-seq data of 723 samples that involves 91 tissues and cell types in Holstein cattle. We processed all the 732 RNA-seq data uniformly using a rigorous bioinformatics pipeline with stringent quality control procedures. After data cleaning and processing, we fitted all data into one model to estimate the tissue specificity of gene expression. We then calculated the t-statistics for differential expression for each gene in a tissue using a previous method [49]. Specifically, the log2-transformed expression (i.e., log2FPKM) of genes was standardized with mean of 0 and variance of 1 within each tissue or cell type,
where yi is the standardized log2-transformed expression level (i.e., log2FPKM) of ith gene; μi is the overall mean of the ith gene; xis is the tissue effect, where samples of the tested tissue were denoted as ‘1’, while other samples as ‘-1’; xiage, xisex, xistudy were age, sex, and study effects for the ith gene, respectively; ei is residual effect. We fitted this model for each gene in each tissue using the ordinary least-square approach and then obtained the t-statistics for the tissue effect to measure the expression specificity of this gene in the corresponding tissue. Using this approach, we evaluated the expression levels for each of the candidate genes that were fine-mapped in this study across the 91 tissues and cell types and identified the most relevant tissue or cell type for a disease trait of interest.
Supplementary information
Additional file 1. Boxplot with PTA reliability for hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability.
Additional file 2. Manhattan plots using the PTA as phenotype for hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability. The genome-wide threshold (red line) corresponds to the Bonferroni correction for a nominal P-value = 0.05.
Additional file 3. List of variants into genes with highest posterior probability of causality mostly associated with displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability.
Acknowledgements
We thank the 1000 Bull Genomes Project for providing reference sequence data for imputation. The Council on Dairy Cattle Breeding (CDCB; Bowie, MD), Cooperative Dairy DNA Repository (Verona, WI), and dairy industry contributors are thanked for providing phenotypic, pedigree, and genomic data. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The USDA is an equal opportunity provider and employer.
Abbreviations
- BTA
Bos taurus chromosome
- CALC
Hypocalcemia
- GWAS
Genome-wide association study
- HO
Holstein
- LD
Linkage disequilibrium
- MAF
Minor allelic frequency
- PPC
Probability of causality
- PTA
Predicted transmitting ability
- QTL
Quantitative trait locus
- SNP
Single nucleotide polymorphism
Authors’ contributions
LM, JC, and CM conceived the study. EF, DS, LF, JJ analyzed and interpreted data. EF, DS, CM and LM wrote the manuscript. GEL, KPG, JC, PMV contributed tools and materials. All authors read and approved the final manuscript.
Funding
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2016–67015-24886 and 2018–67015-28128 from the USDA National Institute of Food and Agriculture, MAES Competitive Grants from the Maryland Experimental Station 2017 and 2019, and the BARD Grant US-4997-17 from the US-Israel Binational Agricultural Research and Development Fund. JBC and PMV was supported by appropriated project 8042–31000–002-00-D, “Improving Dairy Animals by Increasing Accuracy of Genomic Prediction, Evaluating New Traits, and Redefining Selection Goals”, and GEL was supported by appropriated project 8042–31000–001-00-D, “Enhancing Genetic Merit of Ruminants Through Improved Genome Assembly, Annotation, and Selection”, of the Agricultural Research Service (ARS) of the United States Department of Agriculture. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The original performance and pedigree data are owned by CDCB. A request to CDCB to access the data may be sent to: João Dürr, CDCB Chief Executive Officer (joao.durr@cdcb.us). Bull genotypes are controlled by the Collaborative Dairy DNA Repository (CDDR; Verona, WI), and a request to access those data must be made to Jay Weiker, CDDR Administrator (jweiker@naab-css.org). The bovine transcriptome data can be directly downloaded from NCBI GEO database with accession numbers SRP042639, PRJNA177791, PRJNA379574, PRJNA416150, PRJNA305942, SRP111067, PRJNA392196, PRJNA428884, PRJNA298914, PRJEB27455, PRJNA268096, and PRJNA446068. All other data and results are included in the published article.
Ethics approval and consent to participate
The Council on Dairy Cattle Breeding (CDCB) approved data access and the research, which was conducted on USDA-AGIL computers using the national database shared jointly by CDCB and AGIL under a non-funded cooperative agreement: http://aipl.arsusda.gov/reference/CDCB_NFCA.pdf.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ellen Freebern and Daniel J. A. Santos contributed equally to this work.
Contributor Information
Ellen Freebern, Email: efreeber@terpmail.umd.edu.
Daniel J. A. Santos, Email: djordand@umd.edu
Lingzhao Fang, Email: lingzhaofang@gmail.com.
Jicai Jiang, Email: jicai.jiang@gmail.com.
Kristen L. Parker Gaddis, Email: kristen.gaddis@uscdcb.com
George E. Liu, Email: George.Liu@usda.gov
Paul M. VanRaden, Email: Paul.Vanraden@usda.gov
Christian Maltecca, Email: cmaltec@ncsu.edu.
John B. Cole, Email: john.cole@usda.gov
Li Ma, Email: lima@umd.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12864-020-6461-z.
References
- 1.Liang D, Arnold L, Stowe C, Harmon R, Bewley J. Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci. 2017;100(2):1472–1486. doi: 10.3168/jds.2016-11565. [DOI] [PubMed] [Google Scholar]
- 2.Duffield T. Subclinical ketosis in lactating dairy cattle. Vet Clin N Am Food Anim Pract. 2000;16(2):231–253. doi: 10.1016/S0749-0720(15)30103-1. [DOI] [PubMed] [Google Scholar]
- 3.Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003;34(5):475–491. doi: 10.1051/vetres:2003027. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt TA, Lippolis JD, McCluskey BJ, Goff JP, Horst RL. Prevalence of subclinical hypocalcemia in dairy herds. Vet J. 2011;188(1):122–124. doi: 10.1016/j.tvjl.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett PC, Kirk JH, Wilke MA, Kaneene JB, Mather EC. Metritis complex in Michigan Holstein-Friesian cattle: incidence, descriptive epidemiology and estimated economic impact. Prev Vet Med. 1986;4(3):235–248. doi: 10.1016/0167-5877(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 6.Laven R, Peters A. Bovine retained placenta: aetiology, pathogenesis and economic loss. Vet Rec. 1996;139(19):465–471. doi: 10.1136/vr.139.19.465. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Cole J, Da Y, VanRaden P. Symposium review: genetics, genome-wide association study, and genetic improvement of dairy fertility traits. J Dairy Sci. 2019;102(4):3735–43. [DOI] [PubMed]
- 8.Cole JB, Wiggans GR, Ma L, Sonstegard TS, Lawlor TJ, Crooker BA, Van Tassell CP, Yang J, Wang S, Matukumalli LK. Genome-wide association analysis of thirty one production, health, reproduction and body conformation traits in contemporary US Holstein cows. BMC Genomics. 2011;12(1):408. doi: 10.1186/1471-2164-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaddis KP, Megonigal J, Jr, Clay J, Wolfe C. Genome-wide association study for ketosis in US jerseys using producer-recorded data. J Dairy Sci. 2018;101(1):413–424. doi: 10.3168/jds.2017-13383. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Cole JB, Freebern E, Da Y, VanRaden PM, Ma L. Functional annotation and Bayesian fine-mapping reveals candidate genes for important agronomic traits in Holstein bulls. Commun Biol. 2019;2(1):212. doi: 10.1038/s42003-019-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaid DJ, Chen W, Larson NB. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat Rev Genet. 2018;19(8):491–504. doi: 10.1038/s41576-018-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargolzaei M, Schenkel F, Jansen G, Schaeffer L. Extent of linkage disequilibrium in Holstein cattle in North America. J Dairy Sci. 2008;91(5):2106–2117. doi: 10.3168/jds.2007-0553. [DOI] [PubMed] [Google Scholar]
- 13.Khatkar MS, Nicholas FW, Collins AR, Zenger KR, Cavanagh JA, Barris W, Schnabel RD, Taylor JF, Raadsma HW. Extent of genome-wide linkage disequilibrium in Australian Holstein-Friesian cattle based on a high-density SNP panel. BMC Genomics. 2008;9(1):187. doi: 10.1186/1471-2164-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKay SD, Schnabel RD, Murdoch BM, Matukumalli LK, Aerts J, Coppieters W, Crews D, Neto ED, Gill CA, Gao C. Whole genome linkage disequilibrium maps in cattle. BMC Genet. 2007;8(1):74. doi: 10.1186/1471-2156-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang L, Jiang J, Li B, Zhou Y, Freebern E, VanRaden PM, Cole JB, Liu GE, Ma L. Genetic and epigenetic architecture of paternal origin contribute to gestation length in cattle. Commun Biol. 2019;2(1):100. doi: 10.1038/s42003-019-0341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright J, VanRaden P. Genetic evaluation of dairy cow livability. J Anim Sci. 2016;94:178. doi: 10.2527/jam2016-0368. [DOI] [Google Scholar]
- 17.Parker Gaddis K, Tooker M, Wright J, Megonigal J, Clay J, Cole J, VanRaden P: Development of national genomic evaluations for health traits in U.S. Holsteins. Proc 11th World Congr Genet Appl Livest Prod, Auckland, New Zealand, Feb 11–16 2018, Vol. Biol. & Species–Bovine (dairy) 1, p. 594.
- 18.Garrick DJ, Taylor JF, Fernando RL. Deregressing estimated breeding values and weighting information for genomic regression analyses. Genet Sel Evol. 2009;41(1):55. doi: 10.1186/1297-9686-41-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Z-L, Park CA, Wu X-L, Reecy JM. Animal QTLdb: an improved database tool for livestock animal QTL/association data dissemination in the post-genome era. Nucleic Acids Res. 2012;41(D1):D871–D879. doi: 10.1093/nar/gks1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TAKESHIMA SN, AIDA Y. Structure, function and disease susceptibility of the bovine major histocompatibility complex. Anim Sci J. 2006;77(2):138–150. doi: 10.1111/j.1740-0929.2006.00332.x. [DOI] [Google Scholar]
- 21.Olsen HG, Knutsen TM, Lewandowska-Sabat AM, Grove H, Nome T, Svendsen M, Arnyasi M, Sodeland M, Sundsaasen KK, Dahl SR. Fine mapping of a QTL on bovine chromosome 6 using imputed full sequence data suggests a key role for the group-specific component (GC) gene in clinical mastitis and milk production. Genet Sel Evol. 2016;48(1):79. doi: 10.1186/s12711-016-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahana G, Guldbrandtsen B, Thomsen B, Lund MS. Confirmation and fine-mapping of clinical mastitis and somatic cell score QTL in N ordic H olstein cattle. Anim Genet. 2013;44(6):620–626. doi: 10.1111/age.12053. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Lund MS, Sahana G, Guldbrandtsen B, Sun D, Zhang Q, Su G. Association analysis for udder health based on SNP-panel and sequence data in Danish Holsteins. Genet Sel Evol. 2015;47(1):50. doi: 10.1186/s12711-015-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayeri S, Sargolzaei M, Abo-Ismail M, Miller S, Schenkel F, Moore S, Stothard P. Genome-wide association study for lactation persistency, female fertility, longevity, and lifetime profit index traits in Holstein dairy cattle. J Dairy Sci. 2017;100(2):1246–1258. doi: 10.3168/jds.2016-11770. [DOI] [PubMed] [Google Scholar]
- 25.Snelling W, Allan M, Keele J, Kuehn L, Mcdaneld T, Smith T, Sonstegard T, Thallman R, Bennett G. Genome-wide association study of growth in crossbred beef cattle. J Anim Sci. 2010;88(3):837–848. doi: 10.2527/jas.2009-2257. [DOI] [PubMed] [Google Scholar]
- 26.Pryce JE, Hayes BJ, Bolormaa S, Goddard ME. Polymorphic regions affecting human height also control stature in cattle. Genetics. 2011;187(3):981–984. doi: 10.1534/genetics.110.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nalaila S, Stothard P, Moore S, Li C, Wang Z. Whole-genome QTL scan for ultrasound and carcass merit traits in beef cattle using Bayesian shrinkage method. J Anim Breed Genet. 2012;129(2):107–119. doi: 10.1111/j.1439-0388.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 28.Olsen H, Hayes B, Kent M, Nome T, Svendsen M, Lien S. A genome wide association study for QTL affecting direct and maternal effects of stillbirth and dystocia in cattle. Anim Genet. 2010;41(3):273–280. doi: 10.1111/j.1365-2052.2009.01998.x. [DOI] [PubMed] [Google Scholar]
- 29.Cole J, VanRaden P, O’Connell J, Van Tassell C, Sonstegard T, Schnabel R, Taylor J, Wiggans G. Distribution and location of genetic effects for dairy traits. J Dairy Sci. 2009;92(6):2931–2946. doi: 10.3168/jds.2008-1762. [DOI] [PubMed] [Google Scholar]
- 30.VanRaden PM, Tooker ME, O'Connell JR, Cole JB, Bickhart DM. Selecting sequence variants to improve genomic predictions for dairy cattle. Genet Sel Evol. 2017;49(1):32. doi: 10.1186/s12711-017-0307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tetens J, Seidenspinner T, Buttchereit N, Thaller G. Whole-genome association study for energy balance and fat/protein ratio in G erman H olstein bull dams. Anim Genet. 2013;44(1):1–8. doi: 10.1111/j.1365-2052.2012.02357.x. [DOI] [PubMed] [Google Scholar]
- 32.Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol. 2008;321(1):251–262. doi: 10.1016/j.ydbio.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Barbaux S, Gascoin-Lachambre G, Buffat C, Monnier P, Mondon F, Tonanny M-B, Pinard A, Auer J, Bessières B, Barlier A. A genome-wide approach reveals novel imprinted genes expressed in the human placenta. Epigenetics. 2012;7(9):1079–1090. doi: 10.4161/epi.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marete AG, Guldbrandtsen B, Lund MS, Fritz S, Sahana G, Boichard D. A meta-analysis including pre-selected sequence variants associated with seven traits in three French dairy cattle populations. Front Genet. 2018;9:522. doi: 10.3389/fgene.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purfield DC, Bradley DG, Evans RD, Kearney FJ, Berry DP. Genome-wide association study for calving performance using high-density genotypes in dairy and beef cattle. Genet Sel Evol. 2015;47(1):47. doi: 10.1186/s12711-015-0126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gowane G, Vandre R, Nangre M, Sharma A. Major histocompatibility complex (MHC) of bovines: an insight into infectious disease resistance. Livestock Res Int. 2013;1(2):46–57. [Google Scholar]
- 37.Hiroki T, Liebhaber SA, Cooke NE. An intronic locus control region plays an essential role in the establishment of an autonomous hepatic chromatin domain for the human vitamin D-binding protein gene. Mol Cell Biol. 2007;27(21):7365–7380. doi: 10.1128/MCB.00331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horst R, Goff J, Reinhardt T. Role of vitamin D in calcium homeostasis and its use in prevention of bovine periparturient paresis. Acta Vet Scand Suppl. 2003;97:35–50. [PubMed] [Google Scholar]
- 39.Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mammary Gland Biol Neoplasia. 2011;16(4):357–372. doi: 10.1007/s10911-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzanskas ME, do Amaral Grossi D, Ventura RV, Schenkel FS, TCS C, Stafuzza NB, Rola LD, SLC M, Mokry FB, de Alvarenga Mudadu M. Candidate genes for male and female reproductive traits in Canchim beef cattle. J Anim Sci Biotechnol. 2017;8(1):67. doi: 10.1186/s40104-017-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Smas CM. Expression and regulation of transcript for the novel transmembrane protein Tmem182 in the adipocyte and muscle lineage. BMC Res Notes. 2008;1(1):85. doi: 10.1186/1756-0500-1-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlafer D, Fisher P, Davies C. The bovine placenta before and after birth: placental development and function in health and disease. Anim Reprod Sci. 2000;60:145–160. doi: 10.1016/S0378-4320(00)00132-9. [DOI] [PubMed] [Google Scholar]
- 43.VanRaden PM, Sun C. Fast Imputation Using Medium- or Low-Coverage Sequence Data. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connell JR. MMAP User Guide. 2015. [Google Scholar]
- 45.Backman JD, O’Connell JR, Tanner K, Peer CJ, Figg WD, Spencer SD, Mitchell BD, Shuldiner AR, Yerges-Armstrong LM, Horenstein RB. Genome-wide analysis of clopidogrel active metabolite levels identifies novel variants that influence antiplatelet response. Pharmacogenet Genomics. 2017;27(4):159. doi: 10.1097/FPC.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos D, Cole J, Null D, Byrem T, Ma L. Genetic and nongenetic profiling of milk pregnancy-associated glycoproteins in Holstein cattle. J Dairy Sci. 2018;101(11):9987–10000. doi: 10.3168/jds.2018-14682. [DOI] [PubMed] [Google Scholar]
- 47.Ma L, O'Connell JR, VanRaden PM, Shen B, Padhi A, Sun C, Bickhart DM, Cole JB, Null DJ, Liu GE. Cattle sex-specific recombination and genetic control from a large pedigree analysis. PLoS Genet. 2015;11(11):e1005387. doi: 10.1371/journal.pgen.1005387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimin AV, Delcher AL, Florea L, Kelley DR, Schatz MC, Puiu D, Hanrahan F, Pertea G, Van Tassell CP, Sonstegard TS. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009;10(4):R42. doi: 10.1186/gb-2009-10-4-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finucane HK, Reshef YA, Anttila V, Slowikowski K, Gusev A, Byrnes A, Gazal S, Loh P-R, Lareau C, Shoresh N. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nat Genet. 2018;50(4):621. doi: 10.1038/s41588-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Boxplot with PTA reliability for hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability.
Additional file 2. Manhattan plots using the PTA as phenotype for hypocalcemia (CALC), displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability. The genome-wide threshold (red line) corresponds to the Bonferroni correction for a nominal P-value = 0.05.
Additional file 3. List of variants into genes with highest posterior probability of causality mostly associated with displaced abomasum (DSAB), ketosis (KETO), mastitis (MAST), metritis (METR), retained placenta (RETP) and cow livability.
Data Availability Statement
The original performance and pedigree data are owned by CDCB. A request to CDCB to access the data may be sent to: João Dürr, CDCB Chief Executive Officer (joao.durr@cdcb.us). Bull genotypes are controlled by the Collaborative Dairy DNA Repository (CDDR; Verona, WI), and a request to access those data must be made to Jay Weiker, CDDR Administrator (jweiker@naab-css.org). The bovine transcriptome data can be directly downloaded from NCBI GEO database with accession numbers SRP042639, PRJNA177791, PRJNA379574, PRJNA416150, PRJNA305942, SRP111067, PRJNA392196, PRJNA428884, PRJNA298914, PRJEB27455, PRJNA268096, and PRJNA446068. All other data and results are included in the published article.