Abstract
Rotavirus commonly causes diarrhea but can also cause seizures. Analysis of insurance claims for 1,773,295 US children with 2950 recorded seizures found that, compared to rotavirus-unvaccinated children, seizure hospitalization risk was reduced by 24% (95% confidence interval [CI], 13% – 33%) and 14% (95%CI, 0% – 26%) among fully and partially rotavirus-vaccinated children, respectively.
Keywords: Rotavirus, Rotavirus vaccine, Immunizations, Seizures, Pediatric gastroenteritis
Background
Before vaccine implementation, rotavirus was associated with an estimated 55,000 – 70,000 hospitalizations annually among children in the United States [1]. Following the implementation of rotavirus vaccine in 2006, the burden of rotavirus hospitalizations in the U.S. has declined by ~70%−80% [2]. Two rotavirus vaccines are currently recommended by the Advisory Committee on Immunization Practices (ACIP): pentavalent RotaTeq® (RV5; Merck and Company), given at 2, 4, and 6 months of age; and monovalent Rotarix®(RV1; GlaxoSmithKline Biologicals), given at 2 and 4 months of age [3].
Rotavirus disease has also been associated with extraintestinal symptoms. Seizures, particularly benign afebrile convulsions, are the most commonly described [4]. This observation has led to the hypothesis that rotavirus vaccination, by preventing natural rotavirus infection, may have the added benefit of reducing seizure risk. A prior study found a significant protective effect of rotavirus vaccination on the one-year seizure risk (18 – 21% decrease) in a retrospective cohort of U.S. children [5]. In Australia, rotavirus vaccination status was significantly lower in children hospitalized for seizure as compared to the overall catchment population [6]. Further, recent ecological analyses from the U.S. and Spain have both demonstrated decreases in annual seizure hospitalization rates (decreases of 1 – 8% in the U.S. and 16 – 34% in Spain) among children < 5 years of age following implementation of rotavirus vaccination [7, 8], although a separate ecological analysis in Spain’s Valencia region did not find a significant effect [9]. However, ecological analyses are prone to several biases, and data are lacking regarding the longer-term impact of rotavirus vaccination on individual seizure risk. We utilized administrative claims data to determine differences in seizure hospitalization risk by rotavirus vaccination status among commercially insured U.S. children < 5 years of age.
Methods
Data for 2006 – 2014 were abstracted from the Truven Health MarketScan Commercial Claims and Encounters database, which captures de-identified individual-level claims and encounter data from individuals < 65 years of age enrolled in employer-sponsored commercial health insurance plans [10]. This database contains information on enrollment and medical claims for approximately 30 – 40 million employees and their beneficiaries from all 50 states. Medicaid recipients are not included. This analysis was not considered human subjects research.
For this analysis, the following eligibility criteria were applied: 1) Child born on or after January 1, 2006 (birthdate proxied using the date of the earliest delivery-related claim identified by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes: V30-V39), to ensure eligibility for rotavirus vaccination, which was recommended for use in the U.S. in February, 2006 with the first dose given at 2 months of age [3]; 2) Child continuously enrolled in the insurance plan since the month of birth, to ensure that all vaccination doses and all seizure events could be captured. Children were followed beginning from the month of birth. Rotavirus vaccination status and timing were calculated using the following Current Procedural Terminology (CPT) codes: 90680 (RV5) and 90681 (RV1). Children were excluded from analysis if rotavirus vaccine dose 1 was given at < 4 weeks, dose 2 given < 6 weeks, or dose 3 < 8 weeks, or if the date of any previous dose was missing. The remaining children were classified as “unvaccinated” as long as they had not received any dose of any rotavirus vaccine. Once a child received a single dose of either RV1 or RV5, they were reclassified as “partially vaccinated.” Children were reclassified as “fully vaccinated” once they had received two doses of RV1 or three doses of RV5. Seizures were defined based on hospitalization discharge ICD-9-CM codes 333.2*, 345*, 779.0*, 780.3*; only seizure codes in the first diagnostic position were included in the analysis, and if multiple seizure-related hospitalizations occurred over the study period, only the first was analyzed. In the absence of a seizure event, children were censored at 60 months or when no longer enrolled, whichever came first. For the primary analysis, only children surviving until at least 27 weeks of age without a seizure hospitalization were included. Further, because completely unvaccinated children may differ from those who receive routine immunizations, we excluded children who had not received any doses of vaccines containing diphtheria, pertussis, and tetanus antigens (DTaP) by the age of 6 months.
Survival analysis was employed using time-to-first-seizure as the outcome, and age (in weeks) as the time scale. The time-varying nature of the exposure (vaccination status) was accounted for by analyzing data in the counting process format and using extended Cox regression. The model included rotavirus vaccination status as the (time-varying) exposure and controlled for year of birth (to account for secular changes in rotavirus vaccine coverage and rotavirus circulation) and receipt of DTaP vaccine doses (also time-varying); robust standard errors were calculated and used to generate 95% confidence intervals (CI). Multiple sensitivity analyses were done. These are described in Supplementary Material. Data were cleaned and analyzed using SAS version 9.4 (Cary, NC) and the R Environment for Statistical Computing.
Results
Overall, 1,773,295 children were eligible for analysis, among whom 2,950 seizures were recorded (654 of these were before 6 months of age and were not included in the primary analysis); the estimated 5-year risk was 0.35% (95% CI: 0.34 – 0.36). Among the 1,193,425 children followed until at least 6 months of age without a seizure hospitalization and receiving at least one dose of DTaP, 848,869 (71.1%) were fully vaccinated, 176,350 (14.8%) were partially vaccinated, and 168,206 (14.1%) were unvaccinated against rotavirus. Most vaccinated children (887,251 [86.5%]) had received RV5.
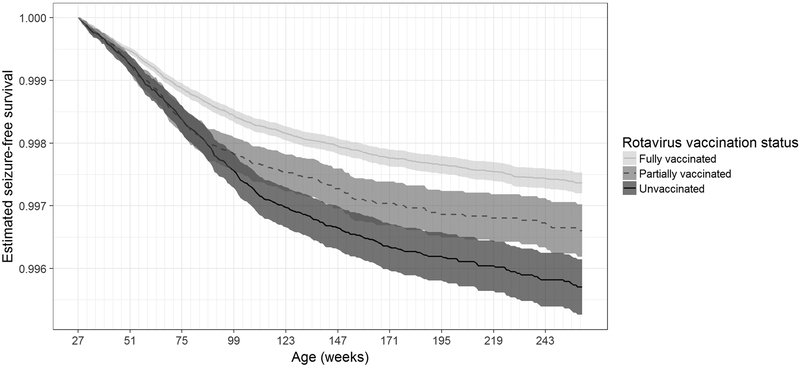
Examination of the unadjusted survival curve showed a reduced risk of seizure among fully vaccinated children, compared to unvaccinated or partially vaccinated children (Figure).
Figure 1: Unadjusted seizure hospitalization survival curve, by vaccination status.
Seizure survival was highest for children fully vaccinated against rotavirus (lightest gray), as compared to partially vaccinated (middle gray) or unvaccinated (darkest gray) children.
Extended Cox regression models gave a crude hazard ratio of 0.66, 95% CI (0.60 – 0.72) comparing fully vaccinated to unvaccinated children, and 0.88 (0.78, 1.01) comparing partially vaccinated to unvaccinated children. HRs were slightly attenuated after adjusting for year of birth and receipt of DTaP vaccine doses: 0.76 (0.67 – 0.87) for full vaccination and 0.86 (0.74 – 1.00) for partial vaccination, compared to unvaccinated children. Results were very similar across all sensitivity analyses, though the effect of partial rotavirus vaccination was significant in analyses using any-position seizure diagnostic codes (Supplementary Table 1).
Discussion
We found that full rotavirus vaccination, compared to no rotavirus vaccination, was associated with a 24% reduction in seizure hospitalization hazard among commercially insured U.S. children under 5 years of age. This observation is consistent with a prior analysis using the Vaccine Safety Datalink (VSD) dataset, which found an 18 – 21% risk reduction in one-year seizure risk among fully rotavirus vaccinated U.S. children, compared to unvaccinated children followed up to 1 year after vaccination [5].
Our analysis, demonstrating the beneficial impact of rotavirus vaccination on seizure hospitalization risk in the first 5 years of life, is the first to address this association in a cohort of children followed for more than 1 year after rotavirus vaccination. The large amount of data in the MarketScan database enhanced the power of this analysis, enabling us to detect significant differences in seizure hospitalization risk despite the rarity of this event. Further, the similarity of results from multiple sensitivity analyses lends confidence to our findings. The fact that results were nearly identical when excluding children born in universal vaccination states is reassuring regarding the potential impact of misclassifying vaccination status in these states. Further, even if additional misclassification of vaccination status occurred (for instance, if rotavirus immunizations were received outside the child’s insurance network), it is most likely that vaccinated children would have been misclassified as unvaccinated; this would bias results towards the null. Similarly, results excluding or controlling for preterm children show that confounding by gestational age is likely minimal or towards the null. Sensitivity analyses where we lagged the time of vaccination by 4 weeks also provided additional evidence regarding biological plausibility. First, the introduced lag time allowed for an immunologic response to rotavirus vaccination. Second, the lag time helped to mitigate spurious associations between vaccination and seizure, for instance febrile seizures related to concurrent vaccination with other vaccines (e.g., pneumococcal vaccine). The sensitivity analyses using seizure diagnostic codes in any position also helped to account for a potential lack of sensitivity of first-position seizure diagnostic codes. Restricting to children surviving until at least 6 months of age without a seizure hospitalization also may have helped to maximize the specificity and positive predictive value of the ICD-9-CM codes used to identify seizures, though an analysis including children from birth gave similar results.
The present analysis is subject to the following limitations. Although the MarketScan database is representative of the U.S. population who receives health insurance through employer-sponsored plans, the database does not include individuals covered by Medicaid or non-employer-sponsored insurance plans. The study population may therefore not be representative of all U.S. children, and may exhibit differences in immunization-related factors. Second, we did not have sufficient data to control for all possible confounders, and so our results may be subject to residual confounding. However, the apparent dose-response pattern lends biological plausibility to our findings. Third, because of the rare nature of seizure discharges, we were not able to stratify analyses based on seizure type, so differential effects on febrile versus afebrile seizures cannot be determined. Lastly, seizure ICD-9-CM codes may not be fully sensitive or specific. However, they have been shown to have a high positive predictive value in the inpatient setting, particularly for first-time seizures [11], and any misclassification of seizure status would likely be non-differential, thus biasing results towards the null.
Despite its limitations, the present study provides compelling data to suggest that rotavirus vaccination, in addition to preventing diarrheal morbidity, also has significant benefits on seizure risk in children. A seizure hospitalization presents not only substantial cost and morbidity [12, 13], but also causes important emotional trauma to the child and family. Reduction in seizure hospitalization risk is an important added benefit of rotavirus vaccination and supports continued universal rotavirus vaccination in the U.S.
Supplementary Material
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
CONFLICT OF INTEREST
No authors have any conflicts of interest to declare.
REFERENCES
- 1.Fischer TK, Viboud C, Parashar U, et al. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003. J Infect Dis 2007; 195(8): 1117–25. [DOI] [PubMed] [Google Scholar]
- 2.Aliabadi N, Tate JE, Haynes AK, Parashar UD, CDC. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2015; 64(13): 337–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Cortese MM, Parashar UD, CDC. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58(RR-2): 1–25. [PubMed] [Google Scholar]
- 4.Rivero-Calle I, Gomez-Rial J, Martinon-Torres F. Systemic features of rotavirus infection. J Infect 2016; 72 Suppl: S98–S105. [DOI] [PubMed] [Google Scholar]
- 5.Payne DC, Baggs J, Zerr DM, et al. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in US children. Clin Infect Dis 2014; 58(2): 173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Febrile Seizures in the Era of Rotavirus Vaccine. J Pediatric Infect Dis Soc 2016; 5(2): 206–9. [DOI] [PubMed] [Google Scholar]
- 7.Pardo-Seco J, Cebey-Lopez M, Martinon-Torres N, et al. Impact of Rotavirus Vaccination on Childhood Hospitalization for Seizures. Pediatr Infect Dis J 2015; 34(7): 769–73. [DOI] [PubMed] [Google Scholar]
- 8.Pringle KD, Burke RM, Steiner CA, Parashar UD, Tate JE. Trends in Rate of Seizure-Associated Hospitalizations Among Children <5 Years Old Before and After Rotavirus Vaccine Introduction in the United Sates, 2000–2013. J Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orrico-Sanchez A, Lopez-Lacort M, Munoz-Quiles C, Diez-Domingo J. Lack of impact of rotavirus vaccines on seizure-related hospitalizations in children under 5 years old in Spain. Hum Vaccin Immunother 2018: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HL G, Stella C Health Research Data for the Real World: The MarketScan Databases (white paper).
- 11.Shui IM, Shi P, Dutta-Linn MM, et al. Predictive value of seizure ICD-9 codes for vaccine safety research. Vaccine 2009; 27(39): 5307–12. [DOI] [PubMed] [Google Scholar]
- 12.Moreau JF, Fink EL, Hartman ME, et al. Hospitalizations of children with neurologic disorders in the United States. Pediatr Crit Care Med 2013; 14(8): 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standridge SM, Horn PS. Variations in hospitalization outcomes in children admitted with seizures between 2003 and 2006. J Child Neurol 2012; 27(7): 898–906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.