Abstract
Purpose:
Stereotactic radiosurgery (SRS) is increasingly used in the management of patients with resected brain metastases (rBMs). A significant complication of this therapy can be radiation necrosis (RN). Despite radiation therapy dose de-escalation and the delivery of several rather than a single dose fraction, rates of RN after SRS for rBMs remain high. We evaluated the dosimetric parameters associated with radiographic RN for rBMs.
Methods and Materials:
From 2008 to 2016, 55 rBMs at a single institution that were treated postoperatively with 5-fraction linear acceleratorebased SRS (25–35 Gy) with minimum 3 months follow-up were evaluated. For each lesion, variables recorded included radiation therapy dose to normal brain, location and magnitude of hotspots, clinical target volume (CTV), and margin size. Hotspot location was stratified as within the tumor bed alone (CTV) or within the planning target volume (PTV) expansion margin volume (PTV minus CTV). Cumulative incidence with competing risks was used to estimate rates of RN and local recurrence. Optimal cut-points predicting for RN for hotspot magnitude based on location were identified via maximization of the log-rank test statistic.
Results:
Median age for all patients was 58.5 years. For all targets, the median CTV was 17.53 cm3, the median expansion margin to PTV was 2 mm, and the median max hotspot was 111%. At 1 year, cumulative incidence of radiographic RN was 18.2%. Univariate analysis showed that max hotspots with a hazard ratio of 3.28 (P = .045), hotspots within the PTV expansion margin with relative magnitudes of 105%, 110%, and 111%, and an absolute dose of 33.5 Gy predicted for RN (P = .029, P = .04, P = .038, and P = .0488, respectively), but hotspots within the CTV did not.
Conclusions:
To our knowledge, this is the first study that investigated dosimetric factors that predict for RN after 5-fraction hypofractionated SRS for rBM. Hotspot location and magnitude appear important for predicting RN risk, thus these parameters should be carefully considered during treatment planning.
Introduction
Brain metastases (BMs) are the most common intracranial tumors in adults and occur in 20% to 40% of patients with cancer.1 Several modalities exist for the treatment of BMs with surgery commonly used for symptomatic, accessible lesions and radiation employed in some manner for most patients.2,3 Randomized prospective trials have demonstrated a benefit for whole-brain radiation therapy (WBRT) after surgical resection of BMs, yet there are detrimental effects on cognitive function and quality of life associated with this treatment.2,4
Given the toxicity associated with WBRT, stereotactic radiosurgery (SRS) to the resection cavity has been used as a strategy to decrease local recurrence (LR) rates with less risk of cognitive toxicity than WBRT for patients after surgical resection.5,6 Although SRS can be highly effective at a tumor ablative dose, single-fraction SRS may not be suitable for all patients. For patients with larger lesions or cavities, single-fraction SRS may not be tolerable at the radiation therapy (RT) dose necessary for tumor control.7 In Radiation Therapy Oncology Group 90–05, a 15-Gy maximum tolerated dose was established for previously irradiated patients with new tumors 3.1–4.0 cm in diameter. The risk of unacceptable central nervous system toxicity was 16 times higher compared with patients with lesions ≤2 cm in this study of salvage single-fraction SRS.8
Because of this high rate of radiation necrosis (RN) associated with single-fraction SRS to targets larger than 3.0 cm in diameter, several groups have reported using hypofractionated SRS in this setting and have cited favorable outcomes with a lower rate of RN.9–11 Although the rate of RN after hypofractionated SRS appears lower than after single-fraction SRS, such a complication remains an issue. Dosimetric constraints predicting for RN have been reported for hypofractionated SRS for patients with resected BMs (rBMs) using 3 fractions; however, this information is not available for patients with rBMs using 5 fractions.10–12 The goal of this analysis is to evaluate how several key dosimetric parameters influence the RN risk for patients with rBMs.
Methods and Materials
The medical records of patients with rBMs treated with multifraction linear accelerator (LINAC)-based SRS between September 2008 and February 2017 at Emory University Hospital were reviewed. Eligibility criteria for this review included pathologic diagnosis of cancer, resection of BMs, completion of a 5-fraction SRS regimen, and at least 3 months of imaging follow-up. Patients were excluded if they had radiosensitive histologies including lymphoma, germ cell tumors, or small cell lung cancer. Patients who had received prior WBRT were eligible. Institutional review board approval was obtained for this study.
Treatments
The decision for surgical resection was made by the treating neurosurgeon based on tumor size, location, associated symptoms, and need for tissue analysis. Neurosurgical resection was then performed using standard technique with the goal of gross total resection.
SRS was performed with multileaf collimatorebased planning using a frameless setup with earliest treatment in 2008. All SRS treatments were LINAC based. Details regarding SRS planning and delivery in postop settings have been previously published.13–16 For postop SRS, the clinical target volume (CTV) was defined as the magnetic resonance imaging (MRI)edefined cavity. The cavity CTV was then expanded to create a planning target volume (PTV). The PTV expansions were variable according to treating physician preference and typically ranged from 1.5 to 2 mm.
Dosimetric parameters
The hotspot was defined as the volume receiving a percentage of the prescription dose or a specified absolute dose. The hotspot locations were measured within the CTV and the PTV expansion margin. The PTV expansion margin was defined as the PTV minus the CTV. Dose to the entire brain was defined as whole-brain volume minus the CTV. Volumetric parameters were assessed for the entire brain volume for doses ranging from 1 to 40 Gy (V1-V40) using a 0.5 Gy step size.
Follow-up
Follow-up after treatment at both institutions consisted of clinical examination and MRI of the brain with and without contrast 4 to 8 weeks after SRS, followed by clinical examination and MRI brain imaging every 3 to 4 months thereafter, unless clinically indicated at an earlier time point. Protocols and equipment quality assurance are standardized between the 2 sites, with the same radiology group reading the studies.
Definition of RN
RN was defined primarily on the basis of 2 radiographic features: the development of a contrast-enhancing mass within previous radiation treatment fields and conventional imaging features including feathery enhancement and T2 hypterintensity.17,18 If there was a question of the enhancement representing LR or RN, additional advanced imaging (eg, MR perfusion, MR spectroscopy, or brain positron emission tomography) was obtained, and consensus was reached in a multidisciplinary neuro-oncology tumor board. Although certain types of enhancement, for example, “Swiss cheese,” are associated with lower sensitivities for the diagnosis of RN, the use of advanced imaging may bring the sensitivity to >90%.19 Additionally, the lesion was followed over time, and resolution with observation or glucocorticoids further assisted with the differentiation of RN from LR. Symptomatic RN was not used owing to small event size.
Statistical analysis
The primary endpoint of this study was radiographic RN between 2 groups determined by optimal dosimetric cut-points.14 RN was defined as time from SRS to RN, death without RN, or last imaging follow-up, where patients who did not have RN or did not die were censored at last follow-up. Death was considered a competing event. LR was defined as time from SRS to LR, death without LR, or last imaging follow-up, where patients who did not have LR or did not die were censored at last follow-up. Cumulative incidence was compared using Gray’s test, and univariate regression analyses using the semiparametric proportional hazards model in the presence of competing risks were performed.20,21 A cut-point analysis was also performed to identify statistically significant cut-points for variables with RN using an outcome-oriented approach22 after censoring patients who died without RN. The log-rank statistic was maximized, and the significance of the cut-points was assessed.22 Overall survival (OS) was defined as time from SRS to death or last follow-up, where patients who did not die were censored at last follow-up. OS was estimated using the Kaplan-Meier method and was compared using log-rank tests. Hazard ratios were estimated using univariate Cox proportional hazards regression models. All analyses were carried out using SAS version 9.4.0 statistical software (SAS Institute Inc, Cary, NC). All statistical analyses were 2-sided, with P values ≤.05 considered statistically significant.
Results
Patient and tumor characteristics
A total of 52 patients (33 female and 19 male) with 55 lesions were included for analysis. The median age for all patients was 58.5 years. Most of the lesions (n = 53; 96.4%) were treated with at least 30 Gy, and the remaining lesions (n = 2; 3.6%) were treated with 25 Gy. The majority of patients were in recursive partitioning analysis class 2 or 3 (n = 45; 86.5%); the remaining (n = 7; 13.5%) were in class 1 at time of consult. The median CTV was 17.53 cm3, and 23 of 55 lesions were located in the right hemisphere. Eighteen lesions (34.6%) were non-small cell lung cancer, with breast making up 14 lesions (26.9%). Baseline patient and tumor characteristics are seen in Tables 1 and 2, respectively.
Table 1.
Summary of patient characteristics, n = 52
| Age, y | |
| Median (range) | 58.5 (23–82) |
| Sex, n (%) | |
| Male | 19 (36.5) |
| Female | 33 (63.5) |
| ECOG, n (%) | |
| ≤1 | 32 (61.5) |
| >1 | 20 (38.5) |
| RPA at consult, n (%) | |
| 1 | 7 (13.5) |
| >1 | 45 (86.5) |
| Active system disease, n (%) | |
| No | 17 (32.7) |
| Yes | 30 (57.7) |
| Unknown | 5 (9.6) |
| Extracranial metastases, n (%) | |
| No | 20 (38.4) |
| Yes | 31 (59.6) |
| Unknown | 1 (1.9) |
| Extent of surgical resection, n (%) | |
| STR | 17 (32.7) |
| GTR | 35 (67.3) |
| Number of brain metastases, n (%) | |
| 1 | 34 (65.4) |
| >1 | 18 (34.6) |
| Age at SRS, y | |
| Median (range) | 58.29 (23–82) |
| Days from surgery to SRS | |
| Median (range) | 37.33 (20–63) |
| Prior whole-brain radiation, n (%) | 1 (1.9) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; GTR = gross total resection; RPA = recursive partitioning analysis; SRS = stereotactic radiosurgery; STR = subtotal resection.
Table 2.
Summary of lesion and treatment characteristics, n = 55
| CTV, cm3 | |
| Median (range) | 17.53 (2.7–54.6) |
| PTV, cm3 | |
| Median (range) | 30.2 (9.1–75.7) |
| Lesion laterality, n (%) | |
| Right | 23 (41.8) |
| Left | 31 (56.4) |
| Missing | 1 (1.8) |
| Lesion location, n (%) | |
| Frontal | 22 (40) |
| Parietal | 12 (21.8) |
| Temporal | 7 (12.7) |
| Occipital | 1 (1.8) |
| Cerebellum | 13 (23.6) |
| Lesion histology, n (%) | |
| NSCLC | 18 (32.7) |
| Breast | 14 (25.5) |
| Other | 23 (41.8) |
| Prescription IDL, %, n (%) | |
| <100 | 2 (3.6) |
| 100 | 52 (94.5) |
| Missing | 1 (1.8) |
| Prescription dose, Gy, n (%) | |
| 30 | 48 (87) |
| 35 | 4(9) |
| 25 | 2 (3.6) |
| 32.5 | 1 (1.8) |
| Margin, mm, n (%) | |
| ≤2 | 42 (76.4) |
| >2 | 13 (23.6) |
| Conformality index | |
| Median (range) | 1.11 (0.97–1.57) |
| Heterogeneity index | |
| Median (range) | 1.11 (1–1.28) |
Abbreviations: CTV = clinical target volume; IDL = isodose line; NSCLC = non-small cell lung cancer; PTV = planning target volume.
Lesion level outcomes
A total of 10 lesions demonstrated RN. In previous retrospective analyses of SRS in the postoperative setting, RN rates have ranged from 2.4% to 24%, and our current study, showing a cumulative incidence of 18.2%, falls in the range of the previous studies.10–12,23 Per Minniti et al, V24 > 16.8 cm3 in a 3-fraction regimen (biologically equivalent dose = 120 Gy with α/β = 2) correlated to the greatest risk of RN; thus, we performed an analysis of the isoequivalent dose of 30 Gy in 5 fractions (biologically equivalent dose = 120 Gy with α/β = 2).11 However, there was no association with RN at V30 (P = .764), and furthermore there was no association at V25 or V35 (P = .819 and P = .086, respectively). Univariate analysis demonstrated an association between the magnitude of the max hotspot and the risk of RN with a hazard ratio (HR) of 3.28 (P = .045). Figure 1a demonstrates a representative hotspot significantly overlapping the PTV expansion margin for a lesion that developed RN, and Figure 1b demonstrates a hotspot confined primarily to the CTV with no RN. When the magnitudes of different hotspots were calculated in the PTV expansion margin, the 105%, 110%, and 111% hotspots were found to be associated with RN, with HRs of 3.64, 8.47, and 6.90, respectively (P = .029, P = .04, and P = .038; Fig 2). Optimal volumetric cut-points for the 3 hotspots within the PTV margin that conferred an increased RN risk were identified (105%: 0.69 cm3, P = .024; 110%: 0.67 cm3, P = .0101; 111%: 0.04 cm3, P = .0124). Analysis was also performed using absolute dose, and the volume of 33.5 Gy in the PTV margin was found to be associated with radiographic RN (P = .0488) as demonstrated in Figure 3. The results demonstrate the importance of volumetric fractionation of the hotspot in the PTV.
Figure 1.
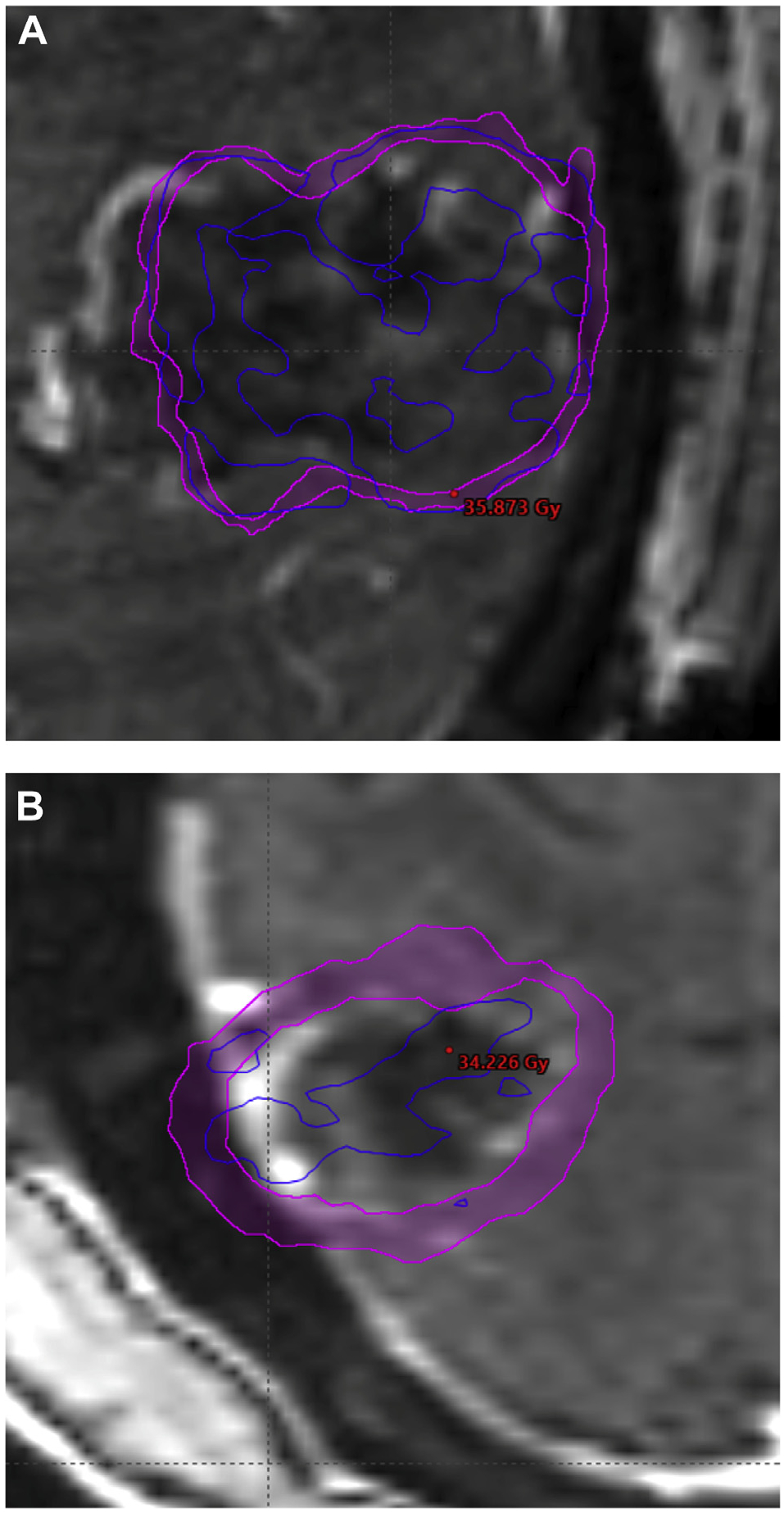
Resection cavities with 33.5 Gy isodose lines shown (dark blue) and max hotspot. (A) A lesion that developed radiation necrosis with the isodose line overlapping the planning target volume margin. (B) The isodose line has more limited overlap with the planning target volume margin, and no radiation necrosis developed.
Figure 2.
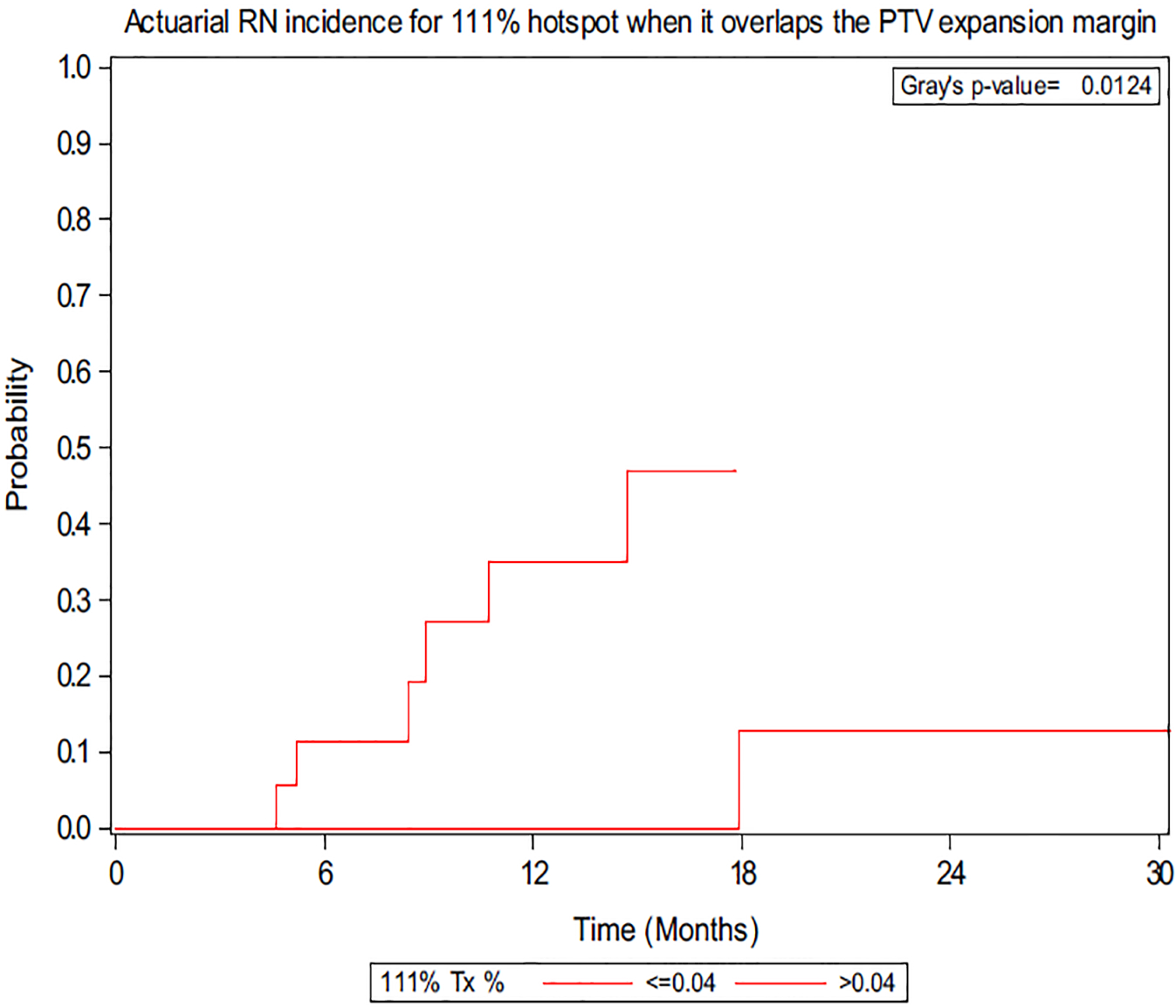
Actuarial radiation necrosis incidence for 111% hotspot when it overlaps the planning target volume expansion margin. Optimal cut-point for volume (cm3) is shown.
Figure 3.
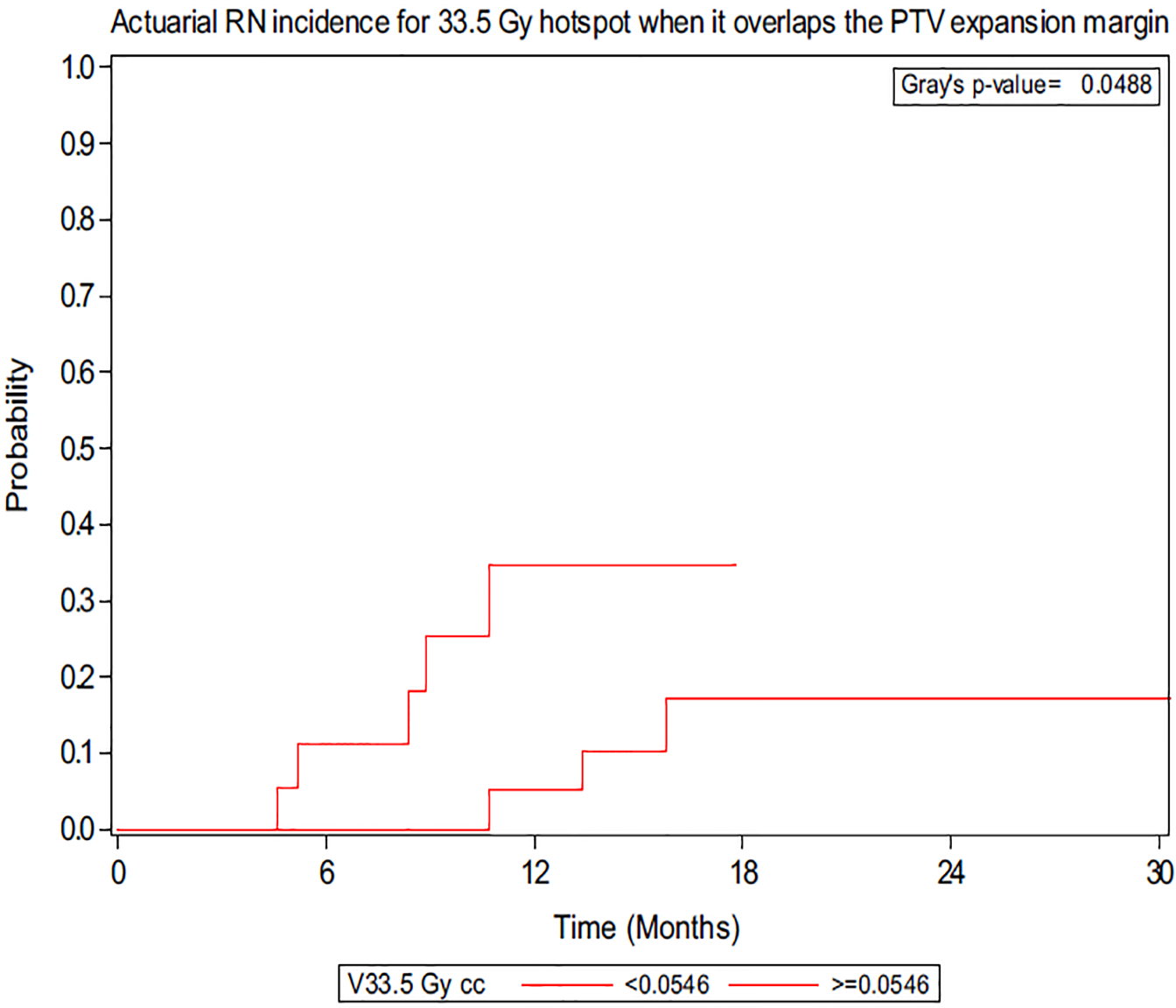
Actuarial radiation necrosis incidence for 33.5 Gy hotspot when it overlaps the planning target volume expansion margin. Optimal cut-point for volume (cm3) is shown.
Importantly, there were no associations between hotspots of any magnitude or volume occurring within the CTV and the incidence of RN. In terms of absolute dose, 80% of patients who developed RN were prescribed 30 Gy to the 99% or 100% isodose line, with remaining patients prescribed 32.5 and 35 Gy.
Lesion-level outcomes are detailed in Table 3. Margin size, stratified by >2 mm versus ≤2 mm, isodose line, and conformality index were not associated with RN. The overall local recurrence rate was 13.1%, and the rate of death without local recurrence was 33.1% at 1 year. Importantly, none of the volumetric data evaluated (V10: P = .4405; V12: P = .9873; V15: P = .5535) altered the incidence of local recurrence, nor did the magnitude or location of the hotspot (within the PTV expansion margin, 105%: P = .9941; 110%: P = .1968; and 111%: P = .8826; within the CTV, 105%: P = .1515; 110%: P = .1515; and 111%: P = .1517).
Table 3.
Univariate association with radiation necrosis
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| Patient characteristics | ||
| CTV | 0.98 (0.94–1.02) | .28 |
| PTV | 0.99 (0.95–1.02) | .331 |
| Use of angioblockade | 1.73 (0.46–6.57) | .420 |
| Yes | ||
| No | ||
| Use of immunotherapy | 0.45 (0.07–3.06) | .417 |
| Yes | ||
| No | ||
| Use of systemic therapy | 0.69 (0.21–2.26) | .543 |
| Yes | ||
| No | ||
| Treatment characteristics | ||
| Prescription IDL, % | 0.38 (0.03–4.81) | .452 |
| 100 | ||
| <100 | ||
| Margin size, mm | 0.84 (0.21–3.33) | .804 |
| ≤2 | ||
| >2 | ||
| Conformality index | 0.97 (0.00–307.29) | .992 |
| Brain minus CTV V25 Gy, cm3 | 1.15 (0.35–3.82) | .819 |
| >21.49 | ||
| ≤21.49 | ||
| Brain minus CTV 30 Gy, cm3 | 0.83 (0.25–2.75) | .764 |
| >10.47 | ||
| ≤10.47 | ||
| Brain minus CTV 35 Gy, cm3 | 0.35 (0.11–1.16) | .086 |
| >0.13 | ||
| ≤0.13 | ||
| Mean max hotspot, % | 3.28 (1.03–10.49) | .045 |
| >114 | ||
| ≤114 | ||
| 105% PTV exp margin, % | 3.64 (1.14–11.62) | .029 |
| >3.05 | ||
| ≤3.05 | ||
| 110% PTV exp margin, cm3 | 8.47 (1.11–64.87) | .040 |
| >0.67 | ||
| ≤0.67 | ||
| 111% PTV exp margin, % | 6.90 (1.12–42.61) | .038 |
| >0.04 | ||
| ≤0.04 |
Abbreviations: CI = confidence interval; CTV = clinical target volume; exp = expansion; IDL = isodose line; PTV = planning target volume.
Cohort survival outcomes
For all patients, median OS was 16.2 months. On univariate analysis, the presence of extracranial metastases, extent of surgical resection, and age at SRS predicted for shorter OS with respective HRs of 3.41, 2.20, and 0.96 (P = .016, P = .050, and P = .006, respectively).
Discussion
In this study of hypofractionated, LINAC-based SRS for rBM, we demonstrate that magnitude and location of hot sports are important for determining the risk of radiographic RN. Hotspots that occurred in the PTV outside of the CTV of 105%, 110%, and 111% were significantly associated with radiographic RN in our data set. However, there was no association between RN and the brain volume receiving at least 25 Gy (V25), 30 Gy (V30), and 35 Gy (V35). Additionally, there was no association between the size of the PTV expansion margin and RN.
Previously, the brain volume receiving 12 Gy was established as a predictor for RN in the setting of single-fraction SRS.24,25 Prior retrospective studies evaluating the occurrence of RN in 3-fraction regimens for postoperative SRS demonstrated an association with V14 and infratentorial location.12,26 The occurrence of RN has been previously associated with the amount of normal brain tissue exposed to different doses of radiation in both single-fraction and hypofractionated regimens.23–25 Similarly, the volume contained in the PTV that is outside of the CTV is normal brain tissue and is consistent with the hotspot location associated with radiographic RN in our study. Some institutions constrain hotspots to within the CTV cavity, however, that is not a practice at our institution.
To our knowledge, no previous study has investigated the dosimetric characteristics associated with RN in 5-fraction regimens for postoperative SRS, and this is the first study demonstrating the importance of the location of the hotspots. The data for single-fraction SRS are not standardized or easily translatable to hypofractionated SRS owing to the preferential sparing of normal tissue with fractionation.27,28 Increased heterogeneity with hotspots inside of the gross tumor volume may be desirable when prescribing high doses with 5-fraction stereotactic body radiation therapy for lung cancer.29 However, postoperative SRS cases are distinct as the treatment volume is a resection bed. The nearby tissue is affected by postsurgery remodeling with a common volume reduction in the postoperative cavity yet typically has a larger PTV margin than the intact BMs.30 The α/β ratio for BMs is higher than that of the surrounding tissue; thus, fractionating the SRS regimen has less effect on repair in BMs but allows for more repair of sublethal damage to the surrounding normal brain tissue.31 Therefore, the dosimetric variables need to be standardized for each hypofractionated regimen.
The limitations of the present study include its retrospective design, different chemotherapy schedules, the limited number of events precluding multivariable analysis, and heterogeneous primary histologies. Additionally, radiographic rather than symptomatic RN had to be used owing to the small sample size and limited follow-up, which is a less clinically applicable and potentially less objective and more equivocal metric. The small cohort size with a limited number of events is also more susceptible to confounding effects, and it is likely too small to observe statistical trends among the magnitude of the HRs. Nevertheless, we believe our study may provide useful information for the dosimetric planning of SRS for postoperative resection cavities.
Conclusions
In conclusion, this is the first study that investigated dosimetric factors that predict for radiographic RN after 5-fraction LINAC-based SRS to rBMs. We demonstrated that the magnitude and location of the hotspot are important for predicting radiographic RN risk, with the volumetric fraction of the hotspot in the PTV margin demonstrating a significant association. The results represent another possible constraint for the dosimetric evaluation of postoperative SRS plans.
Sources of support:
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and National Institutes of Health and the National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: none.
References
- 1.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. [DOI] [PubMed] [Google Scholar]
- 9.Eaton BR, La Riviere MJ, Kim S, et al. Hypofractionated radiosurgery has a better safety profile than single fraction radiosurgery for large resected brain metastases. J Neurooncol. 2015;123:103–111. [DOI] [PubMed] [Google Scholar]
- 10.Wang CC, Floyd SR, Chang C-H, et al. CyberKnife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: Efficacy and safety of an 800 cGy x 3 daily fractions regimen. J Neurooncol. 2012;106:106–110. [DOI] [PubMed] [Google Scholar]
- 11.Minniti G, Esposito V, Clarke E, et al. Multidose stereotactic radiosurgery (9 Gy × 3) of the postoperative resection cavity for treatment of large brain metastases. Int J Radiat Oncol Biol Phys. 2013;86:623–629. [DOI] [PubMed] [Google Scholar]
- 12.Keller A, Dore M, Antoni D, et al. [Risk of radionecrosis after hypofractionated stereotactic radiotherapy targeting the postoperative resection cavity of brain metastases]. Cancer Radiother. 2017;21:377–388 [in French]. [DOI] [PubMed] [Google Scholar]
- 13.Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. [DOI] [PubMed] [Google Scholar]
- 14.Patel KR, Burri SH, Asher AL, et al. Comparing preoperative with postoperative stereotactic radiosurgery for resectable brain metastases: A multi-institutional analysis. Neurosurgery. 2016;79: 279–285. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu R, Shu HK, Hadjipanayis C, et al. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys. 2012;83:e61–e66. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu RS, Dhabaan A, Hall WA, et al. Clinical outcomes for a novel 6 degrees of freedom image guided localization method for frameless radiosurgery for intracranial brain metastases. J Neurooncol. 2013;113:93–99. [DOI] [PubMed] [Google Scholar]
- 17.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. [DOI] [PubMed] [Google Scholar]
- 18.Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013;87:449–457. [DOI] [PubMed] [Google Scholar]
- 19.Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8, 395–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 22.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:250–270. [Google Scholar]
- 23.Eaton BR, Gebhardt B, Prabhu R, Shu HK, Curran WJ Jr, Crocker I. Hypofractionated radiosurgery for intact or resected brain metastases: Defining the optimal dose and fractionation. Radiat Oncol. 2013;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. [DOI] [PubMed] [Google Scholar]
- 25.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. [DOI] [PubMed] [Google Scholar]
- 26.Inoue HK, Seto K-I, Nozaki A, et al. Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: Referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res. 2013;54:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner DJ, Martel MK, Hall EJ. Fractionated regimens for stereotactic radiotherapy of recurrent tumors in the brain. Int J Radiat Oncol Biol Phys. 1991;21:819–824. [DOI] [PubMed] [Google Scholar]
- 28.Aoyama H, Shirato H, Onimaru R, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56: 793–800. [DOI] [PubMed] [Google Scholar]
- 29.Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8:145–152. [DOI] [PubMed] [Google Scholar]
- 30.Atalar B, Choi CY, Harsh GR 4th, et al. Cavity volume dy-namics after resection of brain metastases and timing of post-resection cavity stereotactic radiosurgery. Neurosurgery. 2013; 72:180–185. [DOI] [PubMed] [Google Scholar]
- 31.Eaton BR, Press RH, Zhong J, Shu H-KG, Olson JJ, Oyesiku NM. The use of hypofractionated radiosurgery for the treatment of intracranial lesions unsuitable for single-fraction radiosurgery. Neurosurgery. 2018;83:850–857. [DOI] [PubMed] [Google Scholar]


