Abstract
Purpose:
We conducted a systematic review and meta-analysis to measure the extent to which race is associated with delayed initiation or receipt of inadequate chemotherapy among women with early stage breast cancer.
Methods:
We performed a systematic search of all articles published from January 1987 until June 2017 within four databases: PubMed/Medline, EMBASE, CINAHL, and Cochrane CENTRAL. Eligible studies were US-based and examined the influence of race on chemotherapy delays, cessation, or dose reductions among women with stage I, II, or III breast cancer. Data were pooled using a random effects model.
Results:
A total of twelve studies were included in the quantitative analysis. Blacks were significantly more likely than whites to have delays to initiation of adjuvant therapy of 90 days or more (OR 1.41, 95% CI: 1.06–1.87; X2 = 31.05, p < 0.00001; I2 = 90%). There was no significant association between race and chemotherapy dosing. Due to overlap between studies assessing the relationship between race and completion of chemotherapy, we conducted two separate analyses. Black patients were significantly more likely to discontinue chemotherapy, however, this was no longer statistically significant when larger numbers of patients with more advanced (stage III) breast cancer were included.
Conclusions:
These results suggest that black breast cancer patients experience clinically relevant delays in the initiation of adjuvant chemotherapy more often than white patients, which may in part explain the increased mortality observed among black patients.
Introduction
Early stage breast cancer is highly curable with multimodality treatment, including surgery with or without radiation, and often adjuvant chemotherapy. The chief reason for administration of chemotherapy in this setting is the prevention of distant metastases and, ultimately, breast cancer-related death. The quality of chemotherapy administration, and the relative benefit derived from this treatment, is affected by delays in the initiation of adjuvant therapy, as well as the dose of chemotherapy, schedule delays during treatment, and early discontinuation or incomplete therapy.
Prior literature has demonstrated improved survival with earlier initiation of adjuvant chemotherapy after definitive surgery for early stage breast cancer.1,2 In a 2016 study by Chavez-MacGregor, among over 40,000 patients with stage I–III breast cancer, those whose chemotherapy was initiated at least 91 days after definitive surgery had a 34% increased risk of death compared to patients whose treatment was initiated within 90 days.3 These results are consistent with two prior meta-analyses. Biagi and colleagues demonstrated a 6% increase in the risk of death for every four-week interval of time from surgery to adjuvant chemotherapy across four studies of a total of 15,237 patients.2 Another meta-analysis of seven studies published in 2013, demonstrated an even more pronounced impact on survival, with a decrease in overall survival of 15% for every 4-weeks interval between surgery and initiation of adjuvant chemotherapy.1
Another important metric that is associated with chemotherapy benefit is the relative dose intensity (RDI). RDI is defined as the ratio of actual to expected dose intensity per standard regimen and takes into account dose administered, number of cycles delivered, and the interval between cycles4. When applied to adjuvant chemotherapy for breast cancer, an RDI of <85% is associated with lower disease-free survival and overall survival.5–9 Inappropriate chemotherapy delays, dose-reductions, and discontinuation are, therefore, important yet modifiable risk factors for decreased survival in breast cancer patients.
Several studies have investigated the quality of chemotherapy administration in minority and/or low-income breast cancer patients. Despite success in the treatment of early stage breast cancer, racial and socioeconomic disparities in survival persist. For example, black race is associated with inferior survival, independent of tumor biology, disease stage, and socioeconomic factors.10 The extent to which differences in delays in initiation of chemotherapy and RDI or related metrics account for the observed disparities among black patients remains unclear. Treatment differences are a possible explanation for the inferior clinical outcomes observed among racial/ethnic minorities.
The existing literature suggests several reasons why minority patients may receive inadequate chemotherapy. For example, blacks may receive lower chemotherapy doses than whites or experience unnecessary delays due to the lower baseline white blood cell counts that have been observed in this group, despite the fact that they are not at increased risk of infection.11,12 Furthermore, physician biases or concerns regarding lack of social support, barriers to communication between black patients and white providers, and underlying distrust of the healthcare system among black patients all may compromise quality of care with regard to chemotherapy.13–15
We conducted a systematic review of the literature and meta-analysis to measure the extent to which race is associated with delayed initiation of chemotherapy or receipt of inadequate chemotherapy (based on dosing, on-treatment delays, or early cessation of therapy) among women with early stage breast cancer.
Methods
Data Sources
We conducted our systematic review following the Methodological Expectations of Cochrane Intervention Reviews and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses criteria. We performed a systematic search of all articles published from January 1987 until June 2017 within four databases: PubMed/Medline, EMBASE, CINAHL, and Cochrane Central Register of Controlled Trials. The final search strategy consisted of a combination of terms associated with these five concepts: 1) breast cancer; 2) chemotherapy; 3) treatment dosage; 4) compliance and adherence; and 5) socioeconomic, racial, health-related factors or disparities. Each component part of the search strategy was developed using a combination of Medical Subject Headings and keyword terms in Pubmed/Medline (Table 1) and was adapted for use in the other databases. Reference lists from eligible articles were also reviewed to identify additional publications.
Table 1.
PubMed/MEDLINE Search Strategy
| 1 | (((((“Breast Neoplasms” [Mesh] OR ((“breast” OR “mammary”) AND (“neoplasms” OR “neoplasm” OR “neoplastic” OR “cancer” OR “cancers” OR “cancerous” OR “carcinoma” OR “carcinomas” OR “tumor” OR “tumors” OR “tumour” OR “tumours” OR “malignant” OR “malignancy” OR “malignancies” OR “metastatic” OR “metastasis” OR “metastases”)))) |
| 2 | ((“Chemotherapy, Adjuvant” [Mesh] OR “Antineoplastic Agents” [Mesh] OR “Adjuvant Drug Therapy” OR “chemotherapy” OR “Antineoplastic Combined Chemotherapy Protocols”[Mesh]))) |
| 3 | (“relative dose intensity” OR “RDI” OR “Drug Administration Schedule” [Mesh] OR ((“dose” OR “doses” OR “dosage” OR “dosages” OR “treatment” OR “therapy” OR “schedule” OR “regimen” OR “regimens”) AND (“intensity” OR “density” OR “dense” OR “interval” OR “reduction” OR “reductions” OR “reduced” OR “delay” OR “delays” OR “delayed” OR “alternate” OR “alternative” OR “interruptions” OR “interruption” OR “interrupted” OR “discontinuation” OR “discontinuations” OR “decreases” OR “decreased” OR “suboptimal” OR “variation” OR “variations”)))) |
| 4 | (“Practice Patterns, Physicians’“[Mesh] OR “Patient Compliance”[Mesh] OR “Patient Non Compliance” OR “patient non-adherence” OR “nonadherence” OR “adherent” OR “noncompliance” OR “non-persistence” OR “compliance” OR “adherence” OR “Drug Utilization Review”[Mesh] OR “Drug-Use Review” OR “Drug Utilization Evaluation” OR “Guideline Adherence” [Mesh] OR “Medication Adherence” [MeSH Terms] OR “Withholding Treatment” [MeSH Terms] OR “treatment refusal” OR “variation” OR “variations”)) |
| 5 | ((“Socioeconomic” OR “socio-economic” OR “Social Class”[MeSH Terms] OR “Socioeconomic Factors”[MeSH Terms] OR “race” OR “races” OR “racial” OR “ethnicity” OR “ethnic” OR “minority” OR “insurance status” OR “Insurance Coverage” [MeSH Terms] OR “Healthcare Disparities” [MeSH Terms] OR “Disparities” OR “disparity”OR “inequality” OR “inequalities” OR “unequal” OR “African Continental Ancestry Group” [MeSH Terms] OR “African-American” OR “African-Americans” OR “Black” OR “Blacks” OR “White” OR “Whites” OR “Caucasian” OR “Non-white” OR “Non-whites” OR “Continental Population Groups” [MeSH Terms] OR “European Continental Ancestry Group” [MeSH Terms] OR “Hispanic Americans” [MeSH Terms] OR “Overweight” OR “obese” OR “Obesity” OR “Body Mass Index” [MeSH Terms] OR “BMI” OR “Obesity” [MeSH Terms] OR “Feeding Behavior” [MeSH Terms] OR “Employment” [Mesh] OR “Return to Work” [Mesh] OR “Work” [Mesh] OR “Unemployment” [Mesh] OR “Workplace” [Mesh] OR “Employment” OR “labor” OR “labour” OR “underemployment” OR “unemployment” OR “work” OR “working” OR “population”)) |
| 6 | 1 AND 2 AND 3 AND 4 AND 5 |
Abbreviation: Mesh = Medical Subject Heading
Study Selection
Citations from all search results were downloaded and merged by using a reference management software package (EndNote X8; Thomson Reuters, Philadelphia, PA). One author (AKG) screened study titles and abstracts for potential inclusion and two authors (AKG, EMA) reviewed full-text articles, including reference lists, to determine their eligibility. Studies were eligible for inclusion if they were US-based and examined the influence of race, socioeconomic status, or employment status on delays in initiation of chemotherapy, dose delays or dose reductions among women with stage I, II, or III breast cancer. Published abstracts, conference proceedings, and unpublished studies were excluded.
Data Extraction
A standardized spreadsheet was used to extract data on first author, publication year, study setting and design, sample characteristics, and metrics used to characterize chemotherapy administration. Studies were included in the meta-analyses if they included comparable outcomes and units of measure. All studies examined the role of race. We extracted data regarding delays to initiation of chemotherapy, chemotherapy dosing, and completion of recommended cycles from each study and, when necessary, contacted study authors to obtain data not included in the published manuscripts.
Data Synthesis and Statistical Analysis
We pooled the association between race and time from definitive surgery to initiation of adjuvant therapy for greater than 60 days and 90 days, respectively, and presented odds ratios and associated 95% confidence intervals (CI). We combined the odds of first-cycle dose reductions,16,17 as well as reductions in dose proportion18 and RDI19 among black versus white patients to obtain a pooled odds ratio for the association between race and chemotherapy dosing. Dose proportion is the ratio of administered chemotherapy dose for the entire regimen to the expected chemotherapy dose for the entire regimen. First-cycle dose reductions (those that are implemented prior to the administration of any chemotherapy) have been correlated with reduced dose and reduced RDI for the entire regimen and are (by definition) independent of treatment toxicity, such as neutropenia.20
In a separate analysis, we calculated the pooled odds ratio and the associated 95% CI for the association between race and completion of recommended chemotherapy cycles. Two studies utilized the same cancer registry with overlapping timeframes. Therefore, we conducted this analysis twice including only one of these studies in each analysis.21,22
We used Review Manager 5.3.5 to test for heterogeneity and conducted pooled analyses using a fixed effects model versus a random effects model according to the absence or presence of between-study heterogeneity. Heterogeneity among articles was estimated using the I2 statistic, which indicates the percentage of total variability explained by heterogeneity. Values of 25%, 50%, and 75% are considered as indicative of low, moderate, and high heterogeneity, respectively.
Risk of Bias Assessment
We examined the quality of evidence across the 16 included studies based on the Newcastle Ottawa Scale. The scale provides an overall methodological rating based on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Quality assessments were performed independently by two authors (AKG and EMA) with differences resolved through discussion and consensus.
Results
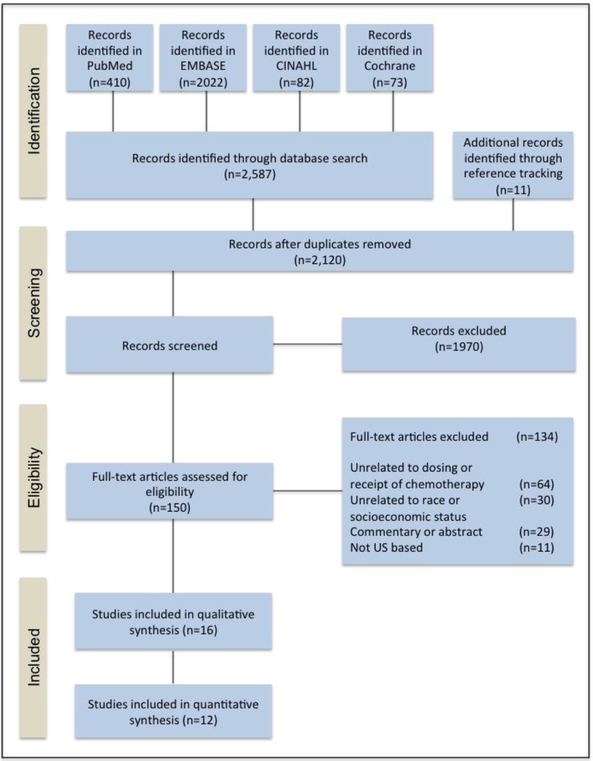
Figure 1 shows the study identification, screening, and selection of studies. A total of 2587 articles were identified through four database searches, and an additional 11 articles were identified from a review of reference lists. After removing duplicate publications, 2120 titles and abstracts were screened for eligibility; 1970 articles did not meet eligibility criteria and were excluded. After full-text review, 134 additional articles were excluded. Sixteen publications were eligible for inclusion in our analysis.
Figure 1.
Diagram of study identification and selection process (PRISMA).
All studies were retrospective analyses, including 2 that utilized the Henry Ford Health System based in Detroit, MI; 3 that used SEER-Medicare; and one study that used data from the Southwest Oncology Group adjuvant clinical trials. Three studies that evaluated treatment delays after diagnosis included patients with stage IV breast cancer, while all other studies included in this analysis were limited exclusively to early stage breast cancer. Most studies focused on disparities on the basis of race/ethnicity, rather than on other social determinants of health, such as income, which were included as covariates but not specifically investigated.
Treatment Delays
Ten studies in total evaluated the impact of race on delays in the initiation of treatment (Table 2). Five of these studies examined time from definitive surgery to adjuvant chemotherapy (four studies included delays > 60 days and four studies included delays > 90 days) among patients with stage I–III breast cancer and were included in the meta-analysis. Three studies examined time from diagnosis to any therapy, including surgery, chemotherapy, radiation, or hormonal therapy among patients with stage I–IV breast cancer. Two separate studies evaluated time from diagnosis to adjuvant chemotherapy among patients with stage I–III breast cancer.
Table 2.
Studies examining association between race and delays to treatment initiation
| First Author | Population | Baseline Timepoint | Outcome Event | Sample Characteristics | Unit of measurement | Insurance Status | Covariates | Results |
|---|---|---|---|---|---|---|---|---|
| McLaughlin 201225 | North Carolina Medicaid System linked to the North Carolina Central Cancer Registry–Medicaid Claims database | Diagnosis | Any treatment | N=1786 Any Stage Age ≥18 Jan 2000– Dec 2002 |
≥ 60 days versus < 60 days | Medicaid | Age Race Marital status Year at diagnosis County-level metropolitan and HPSA status Stage Tumor size Hormone receptor status CCI Disability status Assisted living and home health Surgery Type Treatment Type |
Unadjusted: Treatment delay ≥6 months (nonwhite v white): OR: 1.37, 95% CI 1.01–1.86 |
| Gwyn 200426 | Residents of metropolitan area of Atlanta, Georgia, SEER for case ascertainment with data abstracted from records and surveys | Diagnosis | Any treatment: Definitive surgery, initial neoadjuvant chemotherapy, or the initiation of chemotherapy or hormonal therapy for metastatic disease. | N=950 Any Stage Age 20–54 May 1990– Dec 1992 |
< 1 month v ≥ 1 month | Whites: 95.6% Private 2.1% Medicare or Medicaid 2.3% None Blacks: 79.4% Private 9.8% Medicare or Medicaid 10.7% None |
Age Insurance status Poverty Index Marital Status BMI Education level Insurance type Mammography history Method of detection Number of comorbidities Smoking status Breast self-exam Bra cup size LN status Tumor size Disease stage |
Treatment delay ≥1 month (black v white) OR 2.34; 95% CI: 1.25–4.38 |
| Gorin 200627 | SEER-Medicare | Diagnosis | Any treatment: Definitive surgery, neoadjuvant chemotherapy, radiation, chemotherapy or hormonal therapy for metastatic disease. | N=49,865 Any Stage Age ≥65 Jan 1992–Dec 1999 |
<1 month, 1–2 months, > 2 months | Medicare | Age Marital status Population of city of residency Stage Hormone receptor status Tumor size Lymph node involvement Cormorbid conditions Method of cancer detection Member of HMO Year of diagnosis Physician visits per year Census tract percentage in poverty |
Treatment delays ≥1 month (black v white) OR 1.64; 95% CI: 1.40–1.94 |
| Simon 201222 | Henry Ford Health System, SEER | Diagnosis | Adjuvant chemotherapy | N=2,234 Stage I–III Any Age Jan 1996-Dec2005 |
0–60 days, >60 days | Whites: 95.6% Private 2.1% Medicare or Medicaid 2.3% None Blacks 79.4% Private 9.8% Medicare of Medicaid 10.7% None Total: 61.3% Private 34.9% Medicare 3.8% Other |
Age Insurance status CCI Deprivation index |
Treatment delay > 60 days v ≤ 60 days (black v white) OR 1.18, 95% CI: 0.80–1.74 |
| Vandergrift 201328 | National Comprehensive Cancer Network Outcomes Database | Diagnosis | Adjuvant chemotherapy | N=6222 Stages I–III Any Age January 2003–December 2009 |
Weeks | Total: 79% Commercial 11% Medicare 8% Medicaid 3% Other |
CCI BMI Age Stage Lymph node status Hormone receptor status HER2 status Grade LVI Pathologic upstage Number of excisional procedures Reconstruction before adjuvant therapy Received ALND Diagnostic breast US Diagnostic breast MRI 21 gene RT PCR assay Type of Diagnostic biopsy Treating institution Type of initial surgery |
Time to chemotherapy (black v white) +1.5 weeks, P < .001 |
| Chavez-Macgregor 20163 | California Cancer Registry | Definitive Surgery | Adjuvant chemotherapy | Stage I–III Any Age January 1,2005–December31,2010 |
91 days or more | Total: 24,843 Private: 17772 Medicare: 1651 Military: 223 Medicaid: 4259 Not insured/self pay: 208 Unknown: 70 |
Age Sex Year of diagnosis SES, breast cancer stage, Breast cancer subtype, Marital status Type of breast surgery, whether reconstructive surgery was performed, Primary payer, Treatment at an NCI–designated cancer center |
Time to chemotherapy 91 days or more (black v white) OR, 1.38; 95% CI, 1.19–1.60 |
| Hershman 200629 | SEER-Medicare | Definitive Surgery | Adjuvant chemotherapy | Stage I, II Age ≥65 1992–1999 |
<1 month, 1–2 months, 2–3 months, > 3 months | Medicare | Age Live in metropolitan area Stage Hormone receptor status Tumor Grade Comorbid conditions SES score Marital status Teaching hospital Surgery performed Radiation received |
Treatment delay ≥3 months v < 3 months (black v white) OR 1.2, 95% CI: 0.7 to 1.8 |
| Fedewa 201030 | National Cancer Database | Definitive surgery | Adjuvant chemotherapy | N=107,587 Stage I–III Any Age Jan 2004 – Dec 2006 |
60 days, 90 days | Whites: 63.2% Private 33.6% Medicare 3.3% Other Blacks: 57.6% Private 37.7% Medicare 4.8% Other |
Age CCI Population without high school diploma Treatment facility type Volume of patients with breast cancer at facility Census region Insurance status Stage Hormone receptor status Year diagnosis Year diagnosis |
Treatment delay > 90 days v ≤ 90 days (black v white) RR: 1.56; 95% CI: 1.44–1.89 |
| Nurgalieva 201331 | SEER-Medicare | Definitive surgery | Adjuvant chemotherapy | N= 14,380 Stages I–III January 1, 1992–Dec 31,2005 |
> 90 days | Medicare | Age Marriage status Tumor stage, size, grade Hormone receptor status Comorbidity Year of diagnosis SEER region Primary surgery and radiotherapy, and chemotherapy |
Unadjusted: Treatment delay > 60 days v ≤ 60 days (black v white) 1.07; 95% CI
0.89–1.29 Treatment delay > 90 days v ≤ 90 days (black v white) 1.05; 95% CI 0.81–1.36 |
| Gagliato201432 | Breast Medical Oncology Institutional database at The University of Texas MD Anderson Cancer Center | Definitive surgery | Adjuvant chemotherapy | N=6827 Stages I–III 1997–2011 |
≤ 30 days, 31 to 60 days, and ≥ 61 days | Unknown | Age Race/ethnicity Pathologic tumor size according to TNM classification Pathologic nodal status according to TNM classification Histologic grade LVI Type of surgery Number of comorbidities (0, 1 to 2, 3 to 4, or 5+) |
Unadjusted: Treatment delay > 60 days v ≤ 60 days (black v white) 0.79; 95% CI 0.62–1.00 |
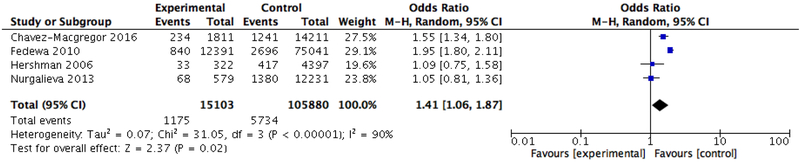
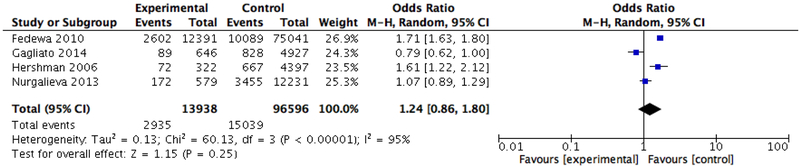
In our pooled analysis of treatment delays, black patients were significantly more likely than whites to have a delay to initiation of adjuvant therapy of 90 days or more (OR 1.41, 95% CI: 1.06–1.87) using a random effects model to account for heterogeneity (Tau2 = 0.07; Chi2 = 31.05, df = 3 (P < 0.00001); I2 = 90%) (Figure 2). However, the odds of experiencing a delay in starting adjuvant therapy of 60 days or more was not significantly associated with race (OR 1.24, 95% CI: 0.86–1.80) (Figure 3).
Figure 2.
Meta-analysis of the association between race and delays in initiation of chemotherapy (90 days)
Figure 3.
Meta-analysis of the association between race and delays in initiation of chemotherapy (60 days)
Chemotherapy Dosing
Four studies assessed the impact of race on chemotherapy dosing (Online Resource Table 1). Three studies included first-cycle dose reduction as an outcome measure, two studies included RDI, and one study included dose proportion. Available data by race from each study (dose proportion18, first cycle dose reduction16,17 and RDI19) were combined to calculate a pooled estimate of the odds of receiving lower chemotherapy dosing. Overall, there was no significant difference in dosing of chemotherapy among blacks versus whites (OR: OR 1.31, 95% CI: 0.90 to 1.90 with random effects model; X2=5.94, p=0.11, I2=50%).
Completion of Prescribed Chemotherapy
Five studies assessed the association between race and completion of all planned chemotherapy cycles (Online Resource Table 2). Two studies (Hershman 2005, Simon 2012) used the Henry Ford Health System tumor registry during overlapping timeframes and were included separately in the meta-analysis. Simon and colleagues included chemotherapy records from 593 patients with stage I–III breast cancer and found no significant difference in completion of chemotherapy cycles among blacks versus whites. In contrast, Hershman and colleagues found that blacks had 1.56 times the odds (p=0.03) of not completing recommended chemotherapy cycles compared to whites among 472 patients with stage I–II breast cancer.
In the meta-analysis that included the Simon study, we found no significant association between race and the completion of recommended chemotherapy cycles. The combined OR was 1.28 (95% CI: 0.89, 1.84). There was significant heterogeneity among studies, thus a random effects model was used (X2=7.46, p=0.02, I2=73). However, when we included the Hershman study instead of the Simon study we found a significant difference in the odds of completing all chemotherapy cycles between races, with blacks having higher odds of incomplete therapy than whites (OR 1.51, 95% CI: 1.00–2.29).
Quality of Evidence
Table 3 summarizes the quality of evidence assessments conducted on the 16 studies based on the Newcastle Ottawa Scale.
Table 3.
Review of bias for 16 included studies based on the Newcastle Ottawa Scale
| Criteria | *Hershman 200919 | *Griggs 201416 | *Griggs 200317 | *Griggs 200721 | *Simon 201222 | *Hershman 200521 | *Reyes 201633 | Gwyn 200426 | Gorin 200627 | *Fedewa 201033 | Vander grift 201328 | *Hershman 200629 | McLaughlin 2012 | *Chavez-MacGregor 2016 | *Nurgalieva 2013 | *Gagliato 2014 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | 0 – clinical trial participants in SWOG | 1 – low income, multi-ethnic women in Breast and Cervical Cancer Prevention Treatment Program | 1- treatment sites from Monroe County (New York) and Henry Ford Health System (Michigan) tumor registries | 1- Multicentr observational study of cancer patients starting chemotherapy for nonmyeloid malignancies | 1- Treatment sites from Henry Ford Health System | 1- Treatment sites from Henry Ford Health System | 0 – Multicenter clinical trial participants among 8 inner city hospitals evaluating patient assistance plan usage | 0 – derived from prior case-control study of breast cancer patients residing in Atlanta, GA | 1 – SEER Medicare | 1 – Patients from the National Cancer Data Base, hospital based cancer registry | 1 – NCCN outcome database | 1 – SEER-Medicare | 0 – North Carolina Cancer Registry and Medicaid linked database | 1 – California Cancer Registry (CCR) | 1 – SEER Medicare | 0 – Single center study of MD Anderson Cancer Center patients |
| Selection of the non-exposed Cohort | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source | 1- uses same source |
| Ascertainment of Exposure | 1 – self-reported race according to NCI reporting criteria at time of enrollment | 1 – self-reported race obtained through telephone interview | 0 – obtained from medical record, not stated if self-reported | 0 – obtained from medical record or patient interview, unknown if self-reported | 0 – obtained from medical record, unknown if self-reported | 0 – obtained from administrative databases, unknown if self-reported | 1- self reported race | 1 – self-reported race | 0 – obtained from SEER data | 0 – obtained from database, unknown if self reported | 0 – obtained from database, unknown if self reported | 0 – obtained from SEER data | 0 – obtained from database, unknown if self-reported | 0 – obtained from CCR, unknown if self-reported | 0 – obtained from SEER data | 0 – obtained from medical record, unknown if self-reported |
| Adjustment for confounding | 1- HR status Tumor size Axillary nodal status Menopausa1 status Baseline ANC
BSA Did not include comorbidities, socioeconomic factors |
1-Age CC1 Years of education Social Support Patient self efficacy BMI Academic v non-academic
treatment site Hormone receptor status Did not include baseline ANC |
2- Age Insurance Type Census block group Per Capita Income CCI BMI Chemotherapy regimen Treatment site Reasons for changes in chemotherapy Menopausal status Tumor size LN involvement HR status Initial WBC count | 0 - Age CCI Marital status Occupation Employment status BMI HR status Did not include tumor size, nodal status, baseline ANC |
0 – Age Insurance status CCI Deprivation index Did not include disease characteristics |
0- Age Stage CCI Hormone receptor status Chemotherapy with or without
doxorubicin Did not include LN status, tumor size, socioeconomic factors |
1- Age CCI Level of education Insurance status Employment status Marital status Hormone receptor status Tumor size Mastectomy Mental/Physical Health Status Perceptions/treatment beliefs Side effects | 2 - Age Insurance status Poverty Index Marital Status BMI Education level Insurance type Mammography history Method of detection Number of comorbidities Smoking status Breast self-exam Bra cup size LN status Tumor size Disease stage | 2 - Age Marital status Population of city of residency Stage Hormone receptor status Tumor size Lymph node involvement Comorbid conditions Method of cancer detection Member of HMO Year of diagnosis Physician visits per year Census tract percentage in poverty | 2 - Age CCI Population without high school diploma Treatment facility type Volume of patients with breast cancer at facility Census region Insurance Stage Hormone receptor status Year diagnosis | 2 – Age CCI BMI Stage Lymph node status Hormone receptor status HER2 status Grade LVI Pathologic upstage Number of excisional procedures Reconstruction before adjuvant therapy Received ALND Diagnostic breast US Diagnostic breast MRI 21 gene RT PCR assay Type of Diagnostic biopsy Treating institution Type of initial surgery | 2 - Age Live in metropolitan area Stage Hormone receptor status Tumor Grade Comorbid conditions SES score Marital status Teaching hospital Surgery performed Radiation Received | 2- Age Race Marital status Year at diagnosis County-level metropolitan and HPSA status Stage Tumor size Hormone receptor status CCI Disability status Assisted living and home health Surgery Type Treatment Type | 2 - Age Sex Year of diagnosis SES, breast cancer stage, Breast cancer subtype, Marital status Type of breast surgery, whether reconstructive surgery was performed, Primary payer, Treatment at an NCI-designated cancer center | 1- Age Marriage status Tumor stage, size, grade Hormone receptor status Comorbidity Year of
diagnosis SEER region Primary surgery and radiotherapy, and chemotherapy No information on socioeconomic status or comorbidities |
1- Age Race/ethnicity Pathologic tumor size according to TNM classification (T1, T2,
T3–4) Pathologic nodal status according to TNM classification (N1, N2, N3) Histologic grade LVI Type of surgery
Number of comorbidities (0, 1 to 2, 3 to 4, or 5+) No information on socioeconomic status or comorbidities |
| Assessment of Outcome | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure, | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 1 – objective measure | 0 – date of administration of chemotherapy defined as date of first chemotherapy claim |
| Was follow-up long enough for outcomes to occur | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes | 1 - yes |
| Adequacy of follow-up of cohorts | 1- Adequate | 1 – Adequate | 1 – Adequate 3% with missing covariate data | 1- Adequate 7% did not have information on actual chemotherapy dose received, 4% were missing covariate information | 1- Adequate | 1- Adequate | 1 – Adequate 2% with missing data on chemotherapy cycles planned or completed |
1 – Adequate Response rate of 88% in original case-control study, possible source of bias |
1 – Adequate Missing billing data for delay outcomes, possible source of bias |
1 – Adequate Reason for chemotherapy nonadministration unknown for 5.6% |
1 – Adequate | 1 – Adequate | 1 – Adequate 8% excluded due to incomplete treatment information, treatment delay > months or refusal of treatment |
1 – Adequate 20% excluded due to incomplete treatment information available |
1 – Adequate | 1 – Adequate |
Included in meta-analysis
Discussion
In this systematic review, we evaluated the available research on the association between race and the quality of chemotherapy administration, including treatment delays, incomplete receipt of prescribed chemotherapy, and reduced RDI. We found that black breast cancer patients appear to be at greater risk than whites of experiencing clinically significant delays of 90 days or longer in the initiation of adjuvant chemotherapy. Thus, based on our findings, racial differences in the time to treatment initiation may play a role in the higher mortality observed among black versus white breast cancer patients.
In contrast, we found no difference in the dosing of chemotherapy between black and white patients. Pooled results for the completion of chemotherapy differed depending on whether we included the Simon or Hershman study. When we included Hershman’s study, blacks had modestly higher odds of not completing recommended chemotherapy compared to whites. This finding may be driven by the lack of stage III patients in Hershman’s analysis. It is possible that in more advanced cases, referrals and initiation of chemotherapy may be expedited regardless of race, whereas in earlier stages recommendations may be relaxed to disproportionately affect black patients. However, prior research has shown a wide variability in prognosis within stage based on tumor biology.23 Therefore, it is important to ensure both timely initiation and completion of therapy among all breast cancer patients deemed to need adjuvant chemotherapy, regardless of their cancer stage.
Our analyses suggest that racial disparities in chemotherapy initiation may be more salient than disparities in its administration (either in dosing or in completion). Additional research is necessary to understand the factors driving this disparity. The extent to which these factors are provider-related, patient-related, or health system-related remains unknown. On a provider level, delays in both the referral process to chemotherapy after surgery as well as scheduling delays after the initial consult may unnecessarily prolong the time to chemotherapy. In addition, patients may not be adequately educated regarding the significance and timing of chemotherapy post-surgery. Social factors such as lack of social support or work-related constraints may contribute to the observed delays in care among black patients. In the analysis using SWOG trials by Hershman in 2009, missed appointments were more common among black patients compared to white patients, 19% versus 9%19. On a health systems level, lack of insurance coverage may result in delays in initiation of chemotherapy among black patients due to difficulties in accessing medical oncology care. Prior research has shown that differences in health insurance status may account for nearly 40% of the total excess risk of death in black patients with stage I–III breast cancer compared to whites24. Delays in initiating chemotherapy could help explain the mechanism behind this dramatic disparity.
Several of the limitations inherent to systematic reviews and meta-analyses are relevant to our own study. In particular, we found that outcome measures used for chemotherapy dosing were inconsistent in these retrospective analyses and included RDI, first cycle dose proportion, and first cycle dose reductions. Only two studies used RDI, the gold standard for measuring adherence to recommended chemotherapy. Two other studies used first cycle dose reductions as a surrogate for RDI to represent planned or intentional dose reductions. First cycle dose reduction has been demonstrated in prior studies to predict reductions in RDI, but its validity as a measure of the complete treatment experience is unknown. To facilitate future cross-study comparisons, we recommend that prospective studies should report RDI in a standardized way regarding both dosing and interval between cycles. In addition, due to the retrospective nature of the studies included in this systematic review, there was statistical heterogeneity between the study results when calculating pooled estimates. The degree to which other covariates may explain differences in the observed effects between studies remains unclear, and lack of available data precluded additional subgroup analyses.
In summary, the results of this review suggest that black breast cancer patients experience clinically relevant delays in the initiation of adjuvant chemotherapy more often than white patients. This may in part explain the increased mortality observed among black patients. Additional research is needed to identify the factors that drive disparities in treatment delays. Further efforts are needed to ensure that black patients initiate adjuvant chemotherapy within the recommended three-month timeframe after definitive breast cancer surgery. The development of interventions to facilitate timely referral to medical oncology and initiation of chemotherapy could mitigate the impact of this important disparity in quality of care, potentially with important downstream effects of improved clinical outcomes, including overall survival.
Supplementary Material
Acknowledgements:
Nina A. Bickell MD MPH, Dawn L. Hershman MD MS, and Joseph Unger, PhD MS for provision of data used in the meta-analysis.
Footnotes
Conflict of Interest: Author Angela K. Green declares that she has no conflict of interest. Author Emeline M. Aviki declares that he/she has no conflict of interest. Author Emeline M. Aviki declares that she has no conflict of interest. Author Konstantina Matsoukas declares that she has no conflict of interest. Author Sujata Patil declares that she has no conflict of interest. Author Deborah Korenstein declares that she has no conflict of interest. Author Victoria Blinder declares that she has no conflict of interest.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracycline-based chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biagi JJ RM, King WD, Kong W, Booth CM, Mackillop WJ. . The effect of delay in time to adjuvant chemotherapy (TTAC) on survival in breast cancer (BC): A systematic review and meta-analysis. Journal of Clinical Oncology. May 2011;29(15):1128–1128. [Google Scholar]
- 3.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol. 2016;2(3):322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2(11):1281–1288. [DOI] [PubMed] [Google Scholar]
- 5.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253–1259. [DOI] [PubMed] [Google Scholar]
- 6.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. Journal of the National Cancer Institute. 1998;90(16):1205–1211. [DOI] [PubMed] [Google Scholar]
- 7.Pronzato P, Campora E, Amoroso D, et al. Impact of administration-related factors on outcome of adjuvant chemotherapy for primary breast cancer. Am J Clin Oncol. 1989;12(6):481–485. [DOI] [PubMed] [Google Scholar]
- 8.Hryniuk W, Levine MN. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J Clin Oncol. 1986;4(8):1162–1170. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332(14):901–906. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. Journal of the National Cancer Institute. 2003;95(20):1545–1548. [DOI] [PubMed] [Google Scholar]
- 12.Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol. 1996;49(8):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penner LA, Eggly S, Griggs JJ, Underwood W 3rd, Orom H, Albrecht TL. Life-Threatening Disparities: The Treatment of Black and White Cancer Patients. J Soc Issues. 2012;68(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22(9):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med. 2004;38(6):777–785. [DOI] [PubMed] [Google Scholar]
- 16.Griggs JJ, Liu Y, Sorbero ME, Jagielski CH, Maly RC. Adjuvant chemotherapy dosing in low-income women: The impact of Hispanic ethnicity and patient self-efficacy. Breast Cancer Research and Treatment. 2014;144(3):665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griggs JJ, Culakova E, Sorbero ME, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007;25(3):277–284. [DOI] [PubMed] [Google Scholar]
- 18.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31. [DOI] [PubMed] [Google Scholar]
- 19.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: Retrospective analysis of southwest oncology studies S8814/S8897. Journal of Clinical Oncology. 2009;27(13):2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–4531. [DOI] [PubMed] [Google Scholar]
- 21.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. [DOI] [PubMed] [Google Scholar]
- 22.Simon MS, Lamerato L, Krajenta R, et al. Racial differences in the use of adjuvant chemotherapy for breast cancer in a large urban integrated health system. International journal of breast cancer. 2012;2012:453985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(35):4654–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jemal A, Robbins AS, Lin CC, et al. Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. J Clin Oncol. 2018;36(1):14–24. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30(36):4493–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. [DOI] [PubMed] [Google Scholar]
- 27.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–2252. [DOI] [PubMed] [Google Scholar]
- 28.Vandergrift JL, Niland JC, Theriault RL, et al. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. Journal of the National Cancer Institute. 2013;105(2):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. 2006;99(3):313–321. [DOI] [PubMed] [Google Scholar]
- 30.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–4141. [DOI] [PubMed] [Google Scholar]
- 31.Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Med Oncol. 2013;30(1):419. [DOI] [PubMed] [Google Scholar]
- 32.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes SA, King TA, Fei K, Franco R, Bickell NA. Factors Affecting the Completion of Adjuvant Chemotherapy in Early-Stage Breast Cancer. Annals of surgical oncology. 2016;23(5):1537–1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.