Abstract
Understanding and effectively treating anxiety disorders are a challenge for both scientists and clinicians. Despite a variety of available therapies, the efficacy of current treatments is still not optimal and adverse side effects can result in non-compliance. Animal models have been useful for studying the underlying biology of anxiety and assessing the anxiolytic properties of potential therapeutics. The open field (OF) is a commonly used assay of anxiety-like behavior. The OF was developed and validated in rats and then transferred to use in the mouse with only limited validation. The present study tests the efficacy of prototypical benzodiazepine anxiolytics, chlordiazepoxide (CDP) and diazepam (DZ), for increasing center time in the OF in C57BL/6J (B6) mice. Multiple doses of CDP and DZ did not change time spent in the center of the OF. Increasing illumination in the OF did not alter these results. The non-benzodiazepine anxiolytic, buspirone (BUSP) also failed to increase center time in the OF while the anxiogenic meta-chlorophenylpiperazine (mCPP) increased center time. Additional inbred mouse strains, BALB/cJ (BALB) and DBA/2J (D2) did not show any change in center time in response to CDP. Moreover, evaluation of CDP in B6 mice in the elevated plus maze (EPM), elevated zero maze (EZM) and light dark assay (LD) did not reveal changes in anxiety-like behavior while stress-induced hyperthermia (SIH) was decreased by DZ. Pharmacokinetic (PK) studies suggest that adequate CDP is present to induce anxiolysis. We conclude that the measure of center time in the OF does not show predictive validity for anxiolysis in these inbred mouse strains.
Keywords: Mice, Anxiety, Open field, Benzodiazepine, C57BL/6J
1. Introduction
Anxiety disorders continue to plague society and the healthcare system as nearly one in five individuals worldwide suffer each year and only about one third receive treatment (Demyttenaere et al., 2004; Kessler et al., 2009). Moreover, the most commonly prescribed psychoactive drugs for anxiety, benzodiazepines and selective serotonin reuptake inhibitors, are flawed. Fast-acting benzodiazepines sedate patients and are prone to result in physical and psychological dependence (Woods et al., 1992). In light of these features, selective serotonin reuptake inhibitors have risen in prominence for treating anxiety but take weeks of consistent therapy to achieve equivalent anxiolytic effects and may exacerbate symptoms in the interim (Altamura et al., 2013; Lader, 1987). Research into more effective treatments with a better side effect profile is necessary to advance treatment of these debilitating disorders.
Animal models of anxiety-like behavior have been useful for identifying compounds with anxiolytic properties. The open field (OF) assay, developed by Hall in 1932, is by far the most commonly reported rodent anxiety assay (Hall, 1934; Hall and Ballechey, 1932). The OF takes advantage of the tendency of rodents to avoid brightly lit open spaces that might expose them to predation (Grossen and Kelley, 1972; Post et al., 2011). Rodents in the wild tend to stay in contact with objects or perimeters in the environment (Barnett, 1963) and in the OF, rodents also spend much of their time near the walls and corners of the arena. This behavior, called thigmotaxis, is reduced in rats upon the administration of commonly used anxiolytics such as diazepam (DZ) and chlordiazepoxide (CDP) (Bruhwyler, 1990; Gentsch et al., 1987; Nichols and Schreur, 1987). Thus, thigmotaxis in the OF as a model of anxiety in rats has been shown to have predictive validity in many, but not all cases, (for a review see Carola et al., 2002; Prut and Belzung, 2003) and the amount of time a rodent spends in the center of the OF arena is often cited as the primary measure of anxiety-like behavior.
A review of the literature describing validation studies of anxiolytics in the OF, however, reveals a significant dearth of evidence for the validity of center time as a measure of anxiety in mice (Prut and Belzung, 2003). Studies of classical anxiolytics in mice in the OF typically report drug-induced locomotor and rearing effects but often do not report or observe an increase in time in the center of the arena (Birkett et al., 2011; Crabbe et al., 1998; Crawley, 1981; Fahey et al., 1999; Heredia et al., 2013a; Lalonde and Strazielle, 2010; Lopez et al., 1988; Novas et al., 1988; Seredenin et al., 1990). The lack of evidence for changes in center time in response to anxiolytics suggests that this measure may not be a valid gauge of anxiety in mice. In the current study, we attempted to validate center time as a measure of the anxiolytic activity of mice in the OF. We tested the anti-thigmotactic effects of standard benzodiazepines, CDP and DZ, a serotonin 5-HT1A receptor partial agonist, buspirone (BUSP), and an anxiogenic, meta-chlorophenylpiperazine (mCPP) in the OF. We also assessed the behavioral effects of CDP in two additional inbred strains, BALB/cJ (BALB) and DBA/2J (D2). Moreover, we assessed anxiolytic properties of CDP in other commonly used assays of anxiety-like behavior, the elevated plus maze (EPM), elevated zero maze (EZM) and the light/dark assay (LD). Our results highlight the importance of careful behavioral analysis of animal models of anxiety and the need for cross-validation of such models across species.
2. Materials and methods
2.1. Testing locations
Most experiments were performed at the Genomics Institute of the Novartis Research Foundation (GNF) in San Diego California. Behavioral testing in a brightly lit OF and pharmacokinetic (PK) experiments were performed at the University of North Carolina at Chapel Hill (UNC). Data collected at GNF and UNC were analyzed separately. A complete list of behavioral experiments, mouse strains, drugs and doses tested are presented in Suppl Table 1.
2.2. Drugs
CDP (Sigma-Aldrich, St. Louis, MO and Grace Davison Discovery Sciences, Columbia, MD), mCPP (Sigma-Aldrich), and BUSP (Sigma-Aldrich) were dissolved in 0.9% saline. Mice were injected intraperitoneally (IP) with 0.01 mL solution:g of body weight of testing solution 30 min prior to behavioral and physiologic testing. Mice were returned to their home cages between injection and testing. Control solution and solvent was 0.9% saline for all CDP, BUSP, and mCPP tests. DZ (Sigma-Aldrich) was dissolved in propylene glycol and ethanol for OF testing and suspended in a solution of sterile water and 0.05% Tween-20 for stress-induced hyperthermia (SIH). DZ solvents and controls were 0.8% propylene glycol with 0.2% ethanol vehicle, 1.6% propylene glycol with 0.4% ethanol vehicle, for 1 and 2 mg/kg, respectively (Choleris et al., 2001). For SIH, DZ vehicle control, 3, 6, 9, or 12 mg/kg was administered by oral gavage (Olivier et al., 2002). Subjects were randomized as to dose of drug received.
2.3. Inbred mouse strains
Male mice were used for all studies. For studies conducted at UNC, C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B6, BALB and D2 mice tested at the Genomics Institute of the Novartis Research Foundation (GNF) were bred in an in-house colony and stocks were refreshed regularly from the Jackson Laboratory to avoid genetic drift. At both locations, mice were maintained in AAALAC-accredited specific pathogen-free colonies in ventilated cages (Thoren Caging Systems, Hazelton, PA [GNF] or Tecniplast, Italy [UNC]) on a 12-h light–dark cycle (lights on at 6:00 am [GNF] or 7:00 am [UNC]). Mice were group-housed in cages containing bedding (Bed-o-cob) and a cotton nestlet (GNF) or a cotton nestlet and PVC tube (UNC). Irradiated food (GNF: Purina Pico rodent chow 20, Purina, St. Louis, MO and UNC: Purina RMH 3000, Purina, St. Louis, MO, USA) and water were provided ad libitum. A minimum of eight mice were tested per dose for each behavioral assay (range n = 8 to n = 40).
2.4. General methods
Most behavioral assays were automated and thus non-subjective. Therefore, the investigator was not blinded as to dose or strain during behavioral testing. Mice were 63.7 days of age (±4.7 days) at the start of behavioral testing. All testing was conducted between 8 am and 12 pm during the light part of the light/dark cycle. At GNF, mice were moved to a quiet anteroom adjacent to the testing room 1–2 h prior to testing. At UNC, mice were transported from the colony to the testing room immediately prior to testing. All behavioral testing equipment was cleaned between each animal with a dilute (0.1%) bleach solution. For all tests, mice were naïve to both drug and behavioral testing.
2.5. Behavioral testing procedures and equipment
2.5.1. Open field
The OF (ENV-515–16, Med Associates, St. Albans, VT) was a 43.2 × 43.2 × 30.5 cm Plexiglas arena with a white floor and clear walls surrounded by infrared beams at 2.54 cm intervals on the x-, y- and z-axes that tracked the animals’ position and activity throughout the experiment session. At GNF, the apparatus was isolated within a 73.5 × 59 × 59 cm testing chamber and illuminated by two 28 V lamps (14 lx at testing floor). At UNC, the OF was enclosed in the same chamber but illuminated with two 28 V lamps and overhead fluorescent light (280 lx at testing floor). Mice were placed in a front corner of the OF at the start of testing. Behaviors in the OF were recorded during a single 10-minute testing period and scored in post-session analyses using Activity Monitor 5.83 or 6.02 (Med Associates). Behaviors included distance traveled (in cm), ambulatory episodes (number of times the animal breaks 3 beams before coming to rest), percent time resting, average velocity (in cm per second) and number of rearings. Time spent in a center zone of 3 sizes ranging from 316.1 to 780.6 cm2 and corners of the arena were also assessed. Boli were counted in the brightly lit OF following CDP administration and in the dimly lit OF following BUSP, DZ and mCPP administration.
2.5.2. Elevated plus maze (EPM)
The EPM (7001–0336; San Diego Instruments, San Diego, CA) was beige in color and consisted of two open arms and two closed arms [67.3 × 6.4 cm] that directly opposed each other. The walls of the enclosed arms completely surrounded the end of the runway and were 15.2 cm high. The top of the enclosed arms was open to the testing room and illuminated by room fluorescent lights (441 lx). The entire apparatus was elevated 38.1 cm above the floor. A video camera above the maze captured animals’ location in the maze. The animals were placed in the center of the apparatus and allowed to investigate the maze for 5 min. Regions were defined as closed arms, open arms and the central square at the intersection of all four arms. Experiments were video tracked (Big Brother Recorder Window, Actimetrics, Wilmette, IL, USA) and analyzed (Big Brother Analysis Program) to obtain total distance, time spent in and number of entries into each arm. Experiments were also hand scored in real time to obtain measures of rearing, stretch-attend postures and head dips over the edge of the open arms.
2.5.3. Elevated zero maze (EZM)
The EZM (San Diego Instruments, San Diego, CA) was a white ABS plastic circular runway with an outside diameter of 61 cm and an inside diameter of 51 cm. Two opposing quarters of the circular runway were enclosed by 15.2 cm high walls and the entire apparatus was elevated 51 cm above the floor by 3 metal legs. The tops of the enclosed quadrants were open to the fluorescent room lighting (441 lx). Animals were placed on the EZM in a closed quadrant facing an open quadrant and tested for 5 min. Behaviors were hand scored in real time and included time spent in the open areas of the maze, rearing, head dips over the edge of the open areas, grooming, stretch-attend postures, number of transitions between open and closed areas and number of fecal boli. The experimenter was not blinded to the dose levels of animals undergoing behavioral testing.
2.5.4. Light/dark assay (LD)
The LD enclosure (ENV-511; Med Associates) inserted into the Med Associates OF apparatus and was a light-impermeable box that covered one third of the area of the OF. The dark box had a classic “mouse hole” entry for the animal to enter and exit. Animals were placed in the lighted side of the arena (approximately 212 lx) facing the dark enclosure (approximately 3 lx) and allowed to explore the arena for 10 min. Data were analyzed using Activity Monitor 6.02 (Med Associates) with the OF divided into light and dark quadrants separated by a transition space of 5.1 cm at the junction between zones. Animals were scored for total distance, time spent in the light and dark quadrants and transition space and total transitions between quadrants. Time spent in the transition space is considered a measure of risk-assessment.
2.5.5. Stress induced hyperthermia (SIH)
At 30 min post-dose rectal temperature was measured using the TH-5 Thermalert Monitoring Thermometer with a RET-3 animal rectal probe (Physitemp Instruments Inc., Clifton, NJ) covered with Surgilube surgical lubricant (Fougera, Melville, NY). After the basal temperature (in degrees Celsius) was measured and recorded, mice were returned to the home cage. After 10 min, temperature was measured again following the same procedure as above. The TH-5 Monitoring Thermometer provides fast temperature acquisition and all mice in a cage had their temperature measured in under 1 min. Temperature at time 1 was subtracted from temperature at time 2 to calculate the change in temperature (ΔT).
2.6. Pharmacokinetic analysis
2.6.1. Cardiac puncture
Mice were transported to the procedure area 1 to 2 h prior to blood collection and pseudo randomly assigned to control, 5, 15, 30, or 60-minute time points (n = 3 per group). Mice were anesthetized with isoflurane, USP (Baxter Healthcare Corporation, Deerfield, IL). Blood was collected by cardiac puncture into a tuberculin syringe coated with fresh K2EDTA. Blood was then transferred to a K2EDTA coated Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ). Acetic acid in water (10%) was added at a volume of 35 μL per mL blood. Plasma (supernatant) was extracted after centrifuging whole blood for 15 min at 2500 g in a 4 °C environment.
2.6.2. Whole brain extraction
Immediately after cardiac puncture, mice were sacrificed by cervical dislocation and rapidly decapitated. Whole brain was lifted from the skull and olfactory bulbs removed. The brain was weighed, bisected into hemispheres, and each hemisphere was weighed. Phosphate buffered saline (1% in H2O) was added at a volume of 300 μL per 100 μg brain matter. The right hemisphere was homogenized with a clean Clear Omni Tip™ generator probe equipped to an OmniTip™ Homogenizing Kit (Omni International Inc. Marietta, GA). Homogenate was centrifuged for 15 min at 2500 g in a 4 °C environment.
2.6.3. Plasma preparation for mass spectrometry
All supernatants were kept at 4 °C until manipulation and analysis, both of which were performed within 36 h of obtaining tissues. Supernatant, internal standard (alprazolam 5 μM) and acetonitrile were combined in a glass insert, vortexed for 5 s, then centrifuged for 15 min at 2500 g in a 4 °C environment. After centrifuging, supernatant was extracted. Pilot assays lead to final preparation by diluting 1:1 with 0.1% formic acid to enhance peak resolution.
2.6.4. Mass spectrometry analysis methods
CDP and its metabolites were quantified from the right brain hemisphere using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) with modest modifications from a previously published method (Gunn et al., 2010). A complete description of the LC-MS/MS protocol is available in the supplementary materials (Supplemental Methods). Briefly, sample analysis required 10 μL of unknown sample or prepared known standard and a solvent flow of 0.5 mL/min. Separation was accomplished using a Zorbax SB-C18, 75 × 2.1 mm column, with a 3.5 μm particle size (Agilent, Santa Clara, CA). Optimization of the mobile phase composition (100% HPLC-grade water, 0.1% formic acid and acetonitrile) for each eluting interval was completed prior to experiments. The total run time, including equilibration, was 7 min per injection.
2.6.5. LC-MS/MS quantification
Analytic standards included CDP 1 mg/mL, norchlordiazepoxide 500 μg/mL, demoxepam 1 mg/mL, nordiazepam 1 mg/mL, oxazepam 1 mg/mL and alprazolam 1 mg/mL (Cerilliant Corporation, Round Rock, TX). Each analytic standard was diluted to produce 1 nM to 10,000 nM solutions. Nominal standards were further diluted to create the standard curve used to calculate unknown concentrations. Point calibration curves were constructed from analytic standards by using peak area ratios of analyte alprazolam, the internal standard. All points on the curves were back-calculated to within 15% of the nominal value. Careful analysis ensured that analytes with potentially interfering parent or product ions were separated chromatographically.
2.7. Statistical analysis
Behavioral data were analyzed by IBM© SPSS© Statistics package (Windows version 21 or Macintosh version 16).
The effect of dose was analyzed by one-way ANOVA for all behavioral tests. For several drug studies, mice were tested in multiple batches across several days of testing. For tests where all doses were not tested across all batches, saline control and at least one other dose were repeated and batch effects were tested by ANOVA to determine if batches could be combined.
Differences between groups were evaluated by one-way ANOVA followed by a post-hoc Tukey’s-B. A Dunnett’s T3-adjusted pairwise comparison of treatment and control was computed when equal variance was not assumed. Significance was set at α = 0.05 for all comparisons.
2.8. Pharmacokinetic analysis
PK data were analyzed on Phoenix Build 6.3.0.395 including WinNonlin 6.3, Connect 1.3, NLME 1.2, and IVIVC Toolkit 2.1 (Pharsight a Certara company, St. Louis, MO) for Windows 7. Noncompartmental analysis was performed on all plasma and brain assay data. Parameters extracted from noncompartmental analysis included time to maximum concentration (Tmax), maximum concentration value (Cmax) and area under the curve (AUC). AUC estimation settings included linear up estimation and log down elimination with uniform weighting of concentrations. Brain analyte concentrations were calculated using a density assumption 1 g of brain tissue was equal to 1 mL.
3. Results
3.1. Open field behavior
3.1.1. CDP-induced open field behavior in B6 mice in a dimly lit arena
The effect of batch, dose and size of the center zone were assessed in a single ANOVA. No main effect of batch (p > 0.05) was observed, but significant dose (p < 0.001) and dose by batch interaction (p < 0.001) effects were detected. Time spent in the center at the 10 mg/kg dose was significantly lower than 0 mg/kg, but this was mainly due to a significant decrease in center time in batch 1. There was also a significant effect of center zone size (p < 0.001). As the zone got smaller, time spent in the zone was reduced. There was no dose by center zone size interaction (p > 0.05) indicating that size of the central zone had no effect on the efficacy of CDP (Suppl Fig. 1). A center zone size of 522.6 cm2 was used for all further analyses.
Conducting a separate ANOVA on each testing batch revealed that the 10 mg/kg dose of CDP reduced time spent in the center in batch 1 (p < 0.001; Fig. 1A). Increasing doses of CDP decreased time spent in the center in both batch 2 and 3, but the decrease was not significant (Fig. 1A).
Fig. 1.

Chlordiazepoxide (CDP)-induced behavior in B6 mice in a dimly-lit OF. The effects of CDP (0–15 mg/kg) in male B6 mice are presented by testing batch (A): batch 1 is represented by unfilled diamonds, batch 2 by empty circles and batch 3 by hollow squares. Mean distance traveled in 10 min (B) and number of rearings (C) are presented. Control group mean is indicated by a horizontal dashed line. Significance compared to vehicle is shown as *p < 0.05, **p < 0.01, ***p < 0.001. Error bars are SEM.
Analysis of entries into the center zone produced similar results as those for time spent in the center. Overall, there was a decrease in entries into the center zone at 10 mg/kg but this was only significant in batch 1. Other batches showed a dose-dependent decrease in number of entries into the center, but changes were not significant (data not shown).
No batch effects were observed for total distance or rearing and batches were combined for further analyses. Total distance increased significantly at 10 mg/kg (p < 0.01), but remained unchanged at all other doses (Fig. 1B). A significant decrease in rearing was observed at both 10 and 15 mg/kg doses (Fig. 1C).
To assess temporal effects of CDP in the OF, percent time in the center was analyzed in two-minute bins across the session. Analyses were separated by batch due to significant dose by batch effects described above. For batches 1 and 2, the highest dose of CDP significantly decreased time spent in the center. Time spent in the center increased over time for all batches, however no significant dose by bin effect was observed (p > 0.05; Suppl Fig. 2).
The effect of CDP on time spent in the corners of the arena was also assessed. No batch or dose by batch effects was observed and batches were combined for analyses. Significant effects of both dose and corner location on time spent in the corners were observed. Animals spent more time in the right front and rear corners closest to the location of their placement in the OF. Both 10 and 15 mg/kg doses of CDP significantly increased time spent in the corners (p < 0.05; Suppl Fig. 3).
3.1.2. CDP-induced open field behavior in B6 mice in a brightly lit arena
Overall, percent time in the center of the arena decreased under increased lighting conditions. Time spent in the center of the brightly lit arena decreased at higher doses, but there was no significant effect (Fig. 2A). However, entries into the center zone were significantly decreased at 10 mg/kg (p < 0.05; Fig. 2B). No change in distance traveled was observed at any dose under increased lighting conditions (p > 0.05; Fig. 2C). Rearing behavior decreased at 10 mg/kg (p < 0.001; Fig. 2D) under increased lighting, similar to observations in the dimly lit OF.
Fig. 2.

CDP-induced behavior in B6 mice in a brightly-lit OF. Effects of CDP (0–10 mg/kg) in a 280 lx open field assay. Male B6 mice (n = 10) were scored for (A) percent of time spent in the center of the open field, (B) number of entries into the center zone, (C) distance traveled in 10 min and (D) number of rearings. Data are presented as mean ± SEM. Control group mean is indicated by a horizontal dashed line. Significance compared to vehicle is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
3.1.3. CDP-induced open field behavior in BALB and D2
BALB and D2 mice showed no significant change in percent time in the center of the OF at any dose of CDP although a non-significant trend toward increased time in the center was observed in BALB mice. Total distance traveled was increased at all doses in BALB in comparison to vehicle (p < 0.05 for 5 and 15 mg/kg and p < 0.001 for 10 mg/kg; Suppl Table 2) while D2 mice showed an increase in locomotor activity only at 5 mg/kg (p < 0.05). A significant decrease in rearing at 15 mg/kg was observed in D2 mice (p < 0.001). Rearing behavior did not change significantly at any dose in BALB mice, but did show a trend for decreased rearing at doses greater than 5 mg/kg.
3.1.4. DZ-induced open field behavior
The 2 mg/kg dose of DZ reduced percent time in the center (p < 0.05 vs 2 mg/kg vehicle; p < 0.001 vs saline). Both 1 mg/kg and 2 mg/kg vehicles also decreased percent time in the center but the change was not significant (p = 0.060 and p = 0.380, respectively). No significant change in locomotor activity was observed at either 1 or 2 mg/kg doses of DZ. Additionally, rearing behavior in response to DZ was decreased at the 2 mg/kg dose (p < 0.001; Fig. 3A–C).
Fig. 3.
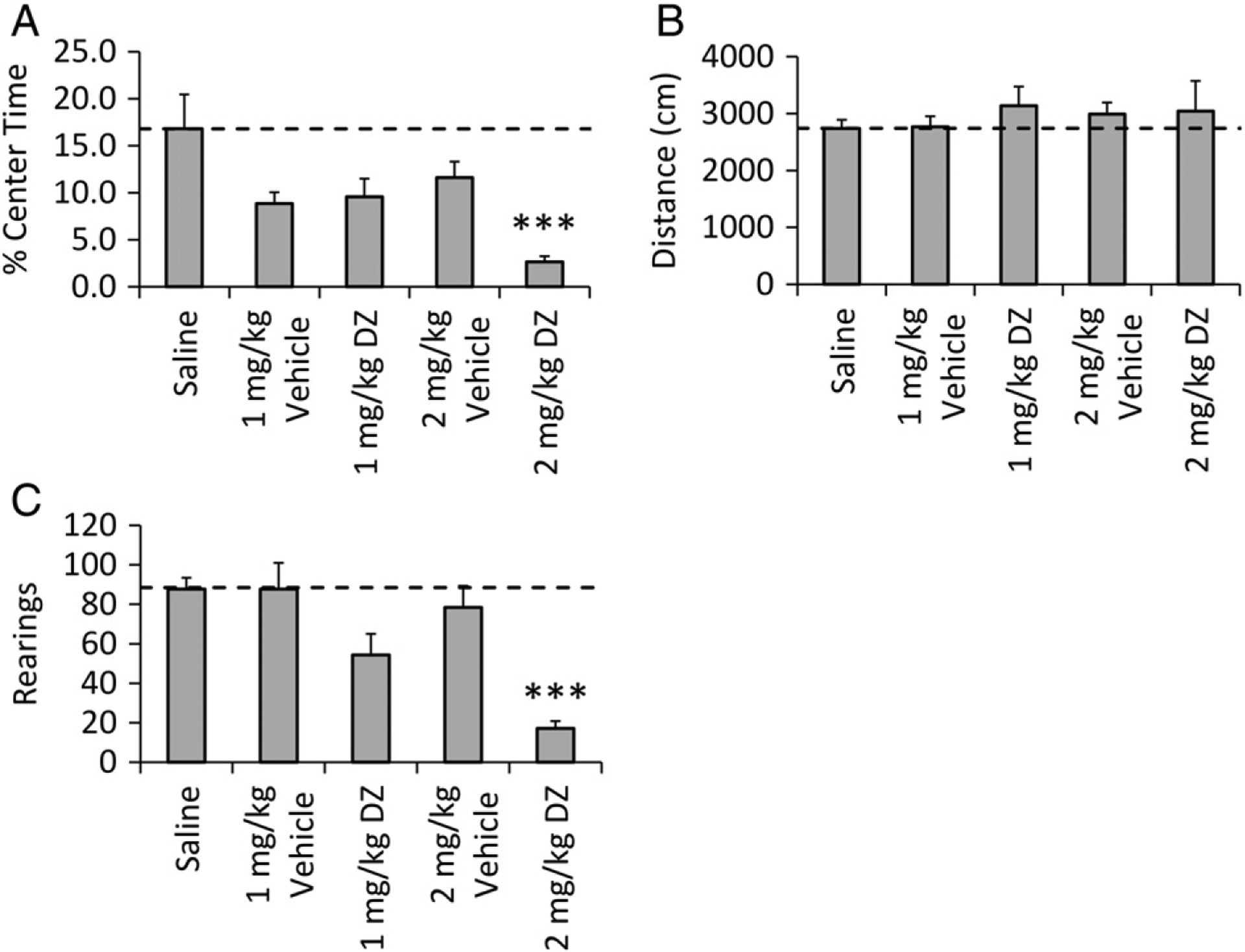
DZ-induced OF behavior in B6 mice. Effects of DZ (0–2 mg/kg), vehicle polyethylene glycol and ethanol and saline in B6 males (n = 8) on (A) percent of time spent in the center of the open field, (B), distance traveled in 10 min and (C) number of rearings. Data are presented as mean ± SEM. Control group mean is indicated by a horizontal dashed line in each graph. Significance compared to saline is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
3.1.5. BUSP-induced open field behavior
BUSP was tested in two batches with 0 and 1 mg/kg doses repeated each day in independent groups of mice. The effect of batch and dose were assessed for percent time in the center, distance and rearing. No significant batch or batch by dose effects were identified for any behavior and batches were combined for further analyses. Percent time spent in the center of the arena was unchanged in response to any dose of BUSP. BUSP significantly decreased both distance and rearing at 3 and 10 mg/kg doses (p < 0.001; Fig. 4A–C).
Fig. 4.

BUSP-induced OF behavior in B6 mice. Effects of BUSP (0–10 mg/kg) in B6 males (n = 20 for saline and 1 mg/kg and n = 10 for all other doses) on (A) percent of time spent in the center of the open field, (B) distance traveled in 10 min, and (C) number of rearings. Control group mean is indicated by a horizontal dashed line. Significance compared to vehicle is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
3.1.6. mCPP-induced open field behavior
The anxiogenic, mCPP, significantly increased percent center time at both 1 and 2 mg/kg doses (p < 0.001). The 2 mg/kg dose significantly increased distance traveled (p < 0.05), but decreased rearing (p < 0.05; Fig. 5A–C).
Fig. 5.
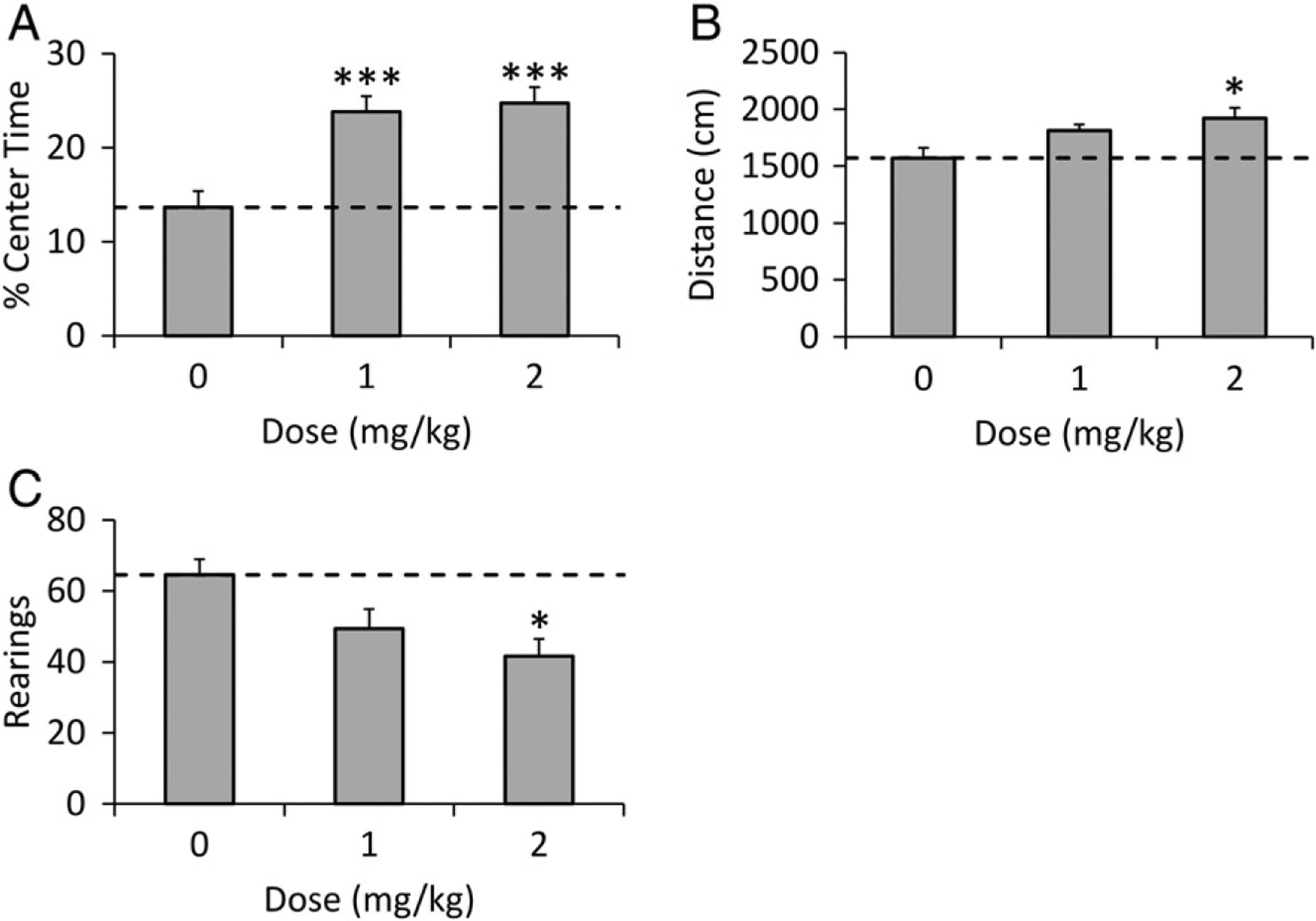
mCPP-induced OF behavior in B6 mice. Effects of mCPP (0–2 mg/kg) in B6 males (n = 10 and n = 15 for saline and mCPP doses, respectively) on (A) percent of time spent in the center of the open field, (B) distance traveled in 10 min, and (C) number of rearings. Data are presented as mean ± SEM. Control group mean is indicated by a horizontal dashed line. Significance compared to vehicle is shown as *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Elevated plus and zero maze behavior
EPM testing of B6 mice was conducted in two batches with 0, 1, 5 and 10 mg/kg doses represented in both groups. A significant batch effect was observed for total transitions (p < 0.01) and percent time in the open arms (p < 0.001). Therefore, batches were analyzed separately for all behaviors. No significant effect of dose was observed for time spent in the open arms of the EPM for either batch (p > 0.05). Animals in batch 1 that received a 10 mg/kg dose of CDP made more total transitions compared to control (p < 0.05) but this finding was not replicated in batch 2. However, transitions into the open arms of the EPM were significantly increased for the 15 mg/kg dose (p < 0.01) in batch 2. There was also an increase in transitions into the open arms at the highest dose in batch 1, but it was not statistically significant (p = 0.07; Fig. 6A–B). Rearing, head dips, grooming and stretch-attend behaviors were not altered at any dose in comparison to control (data not shown).
Fig. 6.

CDP-induced elevated plus maze (EPM) behavior in B6 mice. Effects of CDP (0–15 mg/kg) in B6 males on (A) total number of transitions between open and closed arms (B) transitions from the center square to the open arms. Batch 1 means are shown as unfilled diamonds and batch 2 are shown as empty circles. Error bars are SEM.
Percent time in the open quadrants, total transitions between quadrants, transitions into the open quadrants, grooming behavior, stretch-attend postures, head dips and fecal boli in the EZM were not significantly changed at any dose of CDP. Rearing behavior in both open and closed quadrants increased at the 1 mg/kg dose (p < 0.05), but was unaffected at all other doses (data not shown).
mCPP did not alter any behavior in the EZM (data not shown).
3.3. Light/dark behavior
The LD assay yielded no significant differences in percent time or distance traveled in the lighted compartment or transitions into either the lighted or dark compartment at any dose of CDP. CDP had no effect on time spent in the transition area between the compartments (p > 0.05; Fig. 7A). CDP did not affect percent of time in the dark or change the entries into the dark compartment. However, there was an increase in distance traveled in the dark compartment at all doses of CDP (p < 0.001; Fig. 7A–C).
Fig. 7.

CDP-induced light/dark (LD) behavior in B6 mice. Effects of CDP (0–15 mg/kg) in B6 males (n = 10) on (A) percent of time in light and dark compartments and the transition area between compartments, (B) number of transitions between the light and dark compartments, and (C) distance traveled in each compartment. Data are presented as mean ± SEM. Mean entries of the control group are indicated by a horizontal dashed line. Significance for distance is compared to saline control. ***p < 0.001.
3.4. Stress-induced hyperthermia
DZ significantly decreased basal temperature in a dose-dependent manner (p < 0.001). However, ΔT decreased significantly at both the 6 mg/kg (p < 0.05) and 12 mg/kg (p < 0.01) doses compared to vehicle (Fig. 8).
Fig. 8.
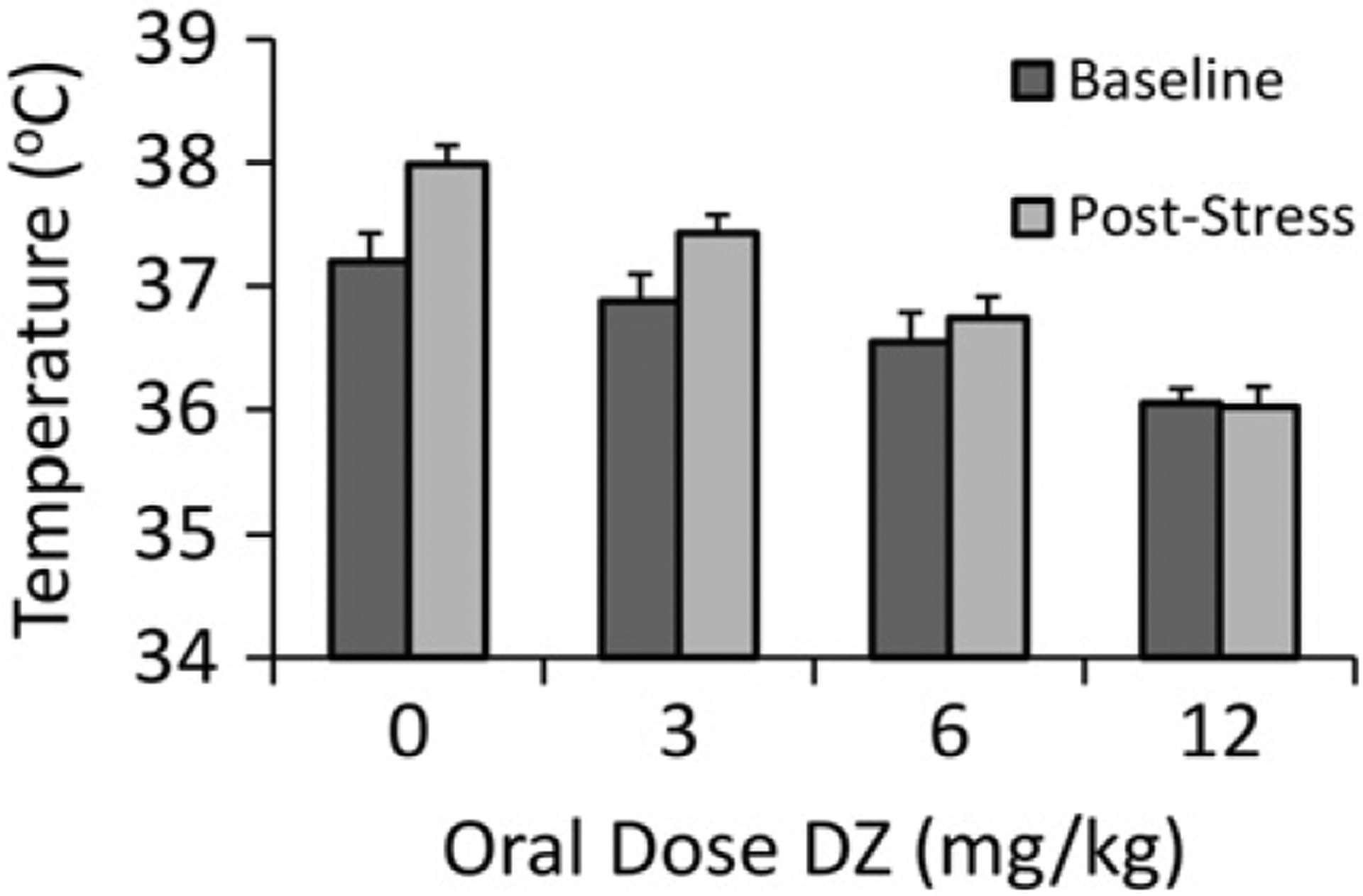
DZ-induced physiologic effects in B6 mice in the SIH assay. Effects of DZ (0–12 mg/kg) in B6 males (n = 9 for vehicle and n = 10 for all other doses) on temperature at baseline and 10 min after stressful stimulus. Sample means ± SEM for baseline and post-stress are shown for each dosing level as a dark gray and light gray bars, respectively.
3.5. Fecal boli
DZ produced a significant increase in boli in the OF at 1 mg/kg compared to vehicle (p < 0.05). No other significant change in the number of fecal boli was observed (Suppl Table 3).
3.6. Pharmacokinetic quantification
The plasma concentration peak for the parent drug, CDP, was recorded at 5 min (Suppl Table 4). Peak concentrations of each metabolite occurred after the parent peak. There was a lower peak concentration of each metabolite sequentially in the anticipated metabolic pathway (Fig. 9). PK characterization of plasma and brain tissue showed a constant elimination of CDP after 15 min. The plasma AUCs were 6.8 × 104, 2.4 × 105, 3.5 × 104, 882 and 204 nM*min for CDP, NorCDP, demoxepam, NorDZ and oxazepam, respectively.
Fig. 9.

CDP and CDP metabolites’ concentration versus time in B6 mice. Concentration of CDP and metabolites in (A) plasma, and (B) brain homogenate after administration of 10 mg/kg CDP. Data are presented as mean ± SEM (n = 3 per time point except n = 2 for NorCDP in plasma at 60 min).
Brain CDP concentration peaked at 5 min. CDP metabolites increased in the brain at each time point and peak concentrations were lower corresponding to their position in the biotransformation pathway. NorDZ was not detected in the brain and brain concentrations of oxazepam were below the lower limit of quantification. The brain AUCs for CDP, NorCDP and demoxepam were calculated as 35.4, 81.5 and 4.61 μg analyte*min/g brain tissue, respectively.
4. Discussion
The OF was introduced by Calvin Hall as a rodent test of emotionality. Hall’s original studies examined urination, defecation and locomotor activity in a large circular arena and concluded that rats that were more emotional defecated more and were less active (Hall, 1934, 1936). In the 80 years since its inception, the OF has become one of the most utilized behavioral tests for unconditioned anxiety. However, in the ensuing years many aspects of the test have changed. Arena size, shape and color along with other environmental variables such as illumination level, floor texture, inclusion of objects and background noise levels all vary widely across laboratories (Broadhurst, 1957; Walsh and Cummins, 1976). Early studies also employed a protocol of multiple test sessions over many days while more recent studies often conduct only one test session. In addition, the parameters that are examined in the OF have evolved considerably from defecation and locomotor activity to include numerous dependent variables including time spent in or number of crossings into the center of the arena which are now most often reported as the primary anxiety-related behaviors. After years of studying OF behavior, only one thing is clear — we still do not completely understand the complex mix of behaviors it is assessing (Ramos et al., 2008; Stanford, 2007).
The predictive validity of the OF is based primarily on studies in rats using benzodiazepines. Acute administration of benzodiazepines in rats has been shown to increase entries into the central part of the OF (Prut and Belzung, 2003). However, the OF that was designed for and validated in rats has been transferred to mice to take advantage of advances in genetic and genomic tools. Our observation that CDP failed to increase center time in B6 mice in the OF was surprising, if not completely unexpected based on studies in which no effect or the opposite effect has been observed (Heredia et al., 2013a; Seredenin et al., 1990). However, a confirmation of the CDP-induced behavioral results using a different benzodiazepine, DZ, led us to start considering potential factors that might explain the lack of validation in B6 mice.
It is widely acknowledged that inbred mouse strains differ in anxiety-like behavior (Crawley et al., 1997; Williams et al., 2009). Therefore, floor or ceiling effects may interfere with the ability to detect the effects of anxiolytic drugs. In fact, B6 mice have been reported as having a low baseline level of anxiety-like behavior (Carola et al., 2002; Crawley, 2007; Crawley and Davis, 1982; Griebel et al., 2000; Lepicard et al., 2000; Ohl et al., 2001; Rogers et al., 1999; Tarantino et al., 2000) that might result in an inability to detect anxiolytic effects. Further validation attempts in the OF incorporating two strains with higher baseline anxiety behavior, BALB and D2, also resulted in no change in center time. These results concur with previously published studies of the effects of benzodiazepines on OF behavior in BALB and D2 mice (Birkett et al., 2011; Salomons et al., 2012; Seredenin et al., 1990).
Although the OF is among the most commonly used tests for rodent anxiety, there are additional assays that model approach-avoidance behavior such as the EPM, EZM and LD. Our attempts to validate the activity of CDP in B6 mice using these assays resulted in significant increases in locomotor behavior in the EPM and LD, but only a trend toward increased transitions into the open arms of the EPM. CDP had no effect on any behavior in the EZM. These results replicate previous studies that report insensitivity of B6 mice to the anxiolytic-like effects of CDP using the same behavioral assays (Heredia et al., 2013a; Heredia et al., 2013b; Mathiasen et al., 2008) although others have reported anxiolytic effects in both the EPM and LD assays (Belzung and Dubreuil, 1998; Crawley and Goodwin, 1980; Smith et al., 2012). It should be noted that there might be strain-specific effects that were not assessed since we focused our studies in the EPM, EZM and LD on B6 mice to provide a comprehensive assessment of this commonly used inbred strain.
We next turned to different classes of drugs. The 5-HT1A receptor agonist, BUSP has been shown to increase center time in numerous studies in rats (Prut and Belzung, 2003) although contradictory findings have also been published (Angrini et al., 1998). In the current study, BUSP reduced locomotor activity at higher doses but had no significant effect on center time in B6 mice. The anxiogenic, mCPP, has been shown to reduce locomotor activity and decrease center time in rats (Lucki et al., 1989; Meert et al., 1997), but actually increased center time and moderately altered locomotor activity at the highest dose tested in our B6 mice.
Behavior in the OF and other behavioral tests can be influenced by test environment. It has generally been reported that locomotor activity is diminished and center time decreases under high levels of illumination (Dixon and Defries, 1968; Livesey and Egger, 1970; McClearn, 1960; Valle, 1970). Our initial OF study in dim lighting may have resulted in an increased basal level of center time and a ceiling effect. However, our follow-up study under higher lighting conditions showed very similar results, a lack of change in percent center time. These results are particularly striking because the two experiments were performed in two separate locations, under different environmental conditions and by a different experimenter. Although most studies have found that levels of illumination are more important (Blizard, 1971), circadian changes in behavioral models of anxiety have also been reported (Jones and King, 2001; Post et al., 2011; Valentinuzzi et al., 2000). Furthermore, there are reports in the literature of changes in benzodiazepine receptor expression across the light/dark cycle (Acuna-Castroviejo et al., 1986; Brennan et al., 1985; Kafka et al., 1986). Our study was conducted during the light part of the light/dark cycle and further studies investigating the efficacy of anxiolytics during the dark phase, when the mouse is more active, are warranted.
Lighting levels during behavioral testing in the OF and LD were consistent with ranges reported in the literature (Blizard, 1971; Milner and Crabbe, 2008; Rodgers et al., 2002a). However, lower lighting levels of ~20–50 lx are often suggested for tests like the EPM and EZM (Milner and Crabbe, 2008; Rodgers et al., 2002a,b). Higher lighting levels have been advocated for testing anxiolytic effects in the EPM (Leo et al., 2014), but it is possible that the anxiogenic effects of harsh lighting did not allow for anxiolytic effects to be observed.
Another potential consideration is the effective concentration of drug in the brain during behavioral testing. We used a 30-minute interval between drug administration and behavioral testing based on published protocols (Angrini et al., 1998; Birkett et al., 2011; Choleris et al., 2001; Heredia et al., 2013a; Lalonde and Strazielle, 2010; Salomons et al., 2012; Seredenin et al., 1990, 2013). Our PK studies determined that peak CDP concentration occurred prior to testing. Therefore, although appreciable levels of both the drug and active metabolites were present in the brain during behavioral studies (Serfozo and Cash, 1992) we cannot exclude the possibility that we may have missed the appropriate therapeutic window. Our 10-minute test session in the OF is consistent with protocols reported in the literature (Blizard, 1971; Clement et al., 1995; Crusio and Gerlai, 1999; Rogers et al., 1999). It is possible that extending our observation to a 30-minute session (Choleris et al., 2001; Paylor et al., 2006) may have allowed us to observe anxiolytic effects, particularly if CDP metabolites have a significant effect on efficacy. However, one must be cognizant of habituation that occurs over a 30-minute test session to avoid misinterpreting habituation effects and anxiolytic effects (Bolivar, 2009).
There exists very little literature describing the PK of benzodiazepines in mice and, in particular, inbred mouse strains including B6, BALB and D2. Understanding the role of benzodiazepine PK on anxiety-related behaviors in different genetic backgrounds is an important undertaking if we are to develop a more thorough appreciation of the validity of these behaviors in mice. The PK data reported here represent first steps in this area.
The results of these studies indicate that the amount of time B6 mice spend in the center of the OF is not increased by the benzodiazepines CDP and DZ or the 5-HT1A partial agonist BUSP. CDP does not alter center time in BALB or D2 mouse strains that are reported to have higher baseline anxiety. These data along with other published reports call into question the predictive validity of center time as a measure of anxiety-related behavior in mice.
Early studies of OF behavior determined that increased locomotor activity is indicative of lower emotionality (Denenberg, 1969; Hall, 1936). Benzodiazepines have been shown to increase locomotor activity at low doses, although this pattern appears to be strain-dependent in mice (Crabbe et al., 1998). We observed increased locomotor behavior at a higher dose of CDP in the dimly-lit OF but this observation was not consistent at higher illumination, in response to DZ or across other behavioral tests. The finding that lower doses of CDP do not increase locomotor behavior under higher illumination is not surprising based on suppression of activity often observed under these conditions (Walsh and Cummins, 1976). We observed a suppression of locomotor activity in B6 mice in the brightly-lit OF in comparison with the dimly-lit arena. Interestingly, we also noted that all doses of CDP increased locomotor activity in the dark compartment of the LD apparatus, but had no effect on activity in the lighted compartment. These observations lead us to consider the possibility that locomotor activity may be a more valid measure of anxiety-like behavior in mice in response to anxiolytics. However, the observation that BUSP decreases and mCPP increases activity in the OF contradicts that hypothesis. Further experiments employing additional drugs, expanded dose ranges and varied behavioral tasks must be conducted in order to make any firm conclusions.
One consistent finding from this study and others was the significant dose-dependent reduction in rearing behavior after exposure to benzodiazepines (Crabbe et al., 1998; Quock et al., 1992). Rearing behavior is thought to be a novelty-evoked exploratory behavior that may contain components of both activity and anxiety (Bailey et al., 2008; Eisener-Dorman et al., 2010; Henderson et al., 2004). However, in our study, rearing behavior appeared to be independent of locomotor activity as evidenced by the observation of significantly decreased rearing along with no change in locomotor activity in multiple tests. It is interesting to speculate that decreased rearing may reflect anxiolytic effects of CDP and DZ, but others have noted that suppression of rearing may actually be indicative of increased anxiety (Henderson et al., 2004). Moreover, rearing was decreased by anxiolytics and the anxiogenic, mCPP.
Finally, early OF studies examined defecations or number of fecal boli as a measure of emotionality. Higher numbers of fecal boli are indicative of increased emotionality (Hall, 1934). However, we observed no decrease in number of fecal boli after administration of CDP, DZ or BUSP nor an increase with mCPP.
Several more recent papers (Carola et al., 2002; Choleris et al., 2001; Henderson et al., 2004) describe detailed ethological analysis of OF behavior focusing on a complete set of behaviors, many of which (e.g. stretch attends) are often not collected using high-throughput or automated systems (Blizard et al., 2007). The effects of benzodiazepines in these studies are complex and depend on how the central portion of the arena is defined and what dependent variables are examined (Choleris et al., 2001). Conducting multiple and diverse tests of anxiety (conditioned, unconditioned and other) is always advised to develop a more complete behavioral profile (Chadman et al., 2009; Ramos et al., 2008).
We examined the EPM, EZM and LD and observed very few behavioral effects of benzodiazepines in these assays. However, our pharmacological assessment of these behavioral tests was limited to CDP and B6 mice. A systematic analysis of additional anxiolytics in different strain backgrounds and under varied environmental conditions is warranted before a conclusion can be made about the utility of these assays as models of anxiety-like behavior. Notably, the EZM was not scored in an automated fashion and the experimenter was not blinded to dosing of the animals. However, even knowing the dose assignment for each animal, the experimenter did not report findings biased toward identifying the expected effects of CDP. However, we cannot exclude the potential for bias in scoring of the EZM.
It is interesting to note that the non-behavioral or physiological model of anxiety that we studied, SIH, does appear to be sensitive to DZ in B6 mice (Groenink et al., 1995; Olivier et al., 2003).
A major limitation of these studies is the use of only males. The experiments described here were aimed at validating the use of open field behavior as a model of anxiety for a high-throughput ENU mutagenesis program in which only males were being tested. Sex differences have been reported for anxiety behavior in mice (Archer, 1977; Palanza, 2001) and we cannot make any conclusions from this study about the validity of these tests in females.
We do not believe that our results negate the use of the OF as a tool for behavioral research. The OF has a long history and rich literature; its utility as a task for measuring locomotor activity and response to novelty (“emotionality”) has been proven. Instead, we are cautioning that simplifying complex behavioral tests into a single variable as a primary measure of anxiety can be misleading. In particular, the results of this study call into question the predictive validity of OF center time as a measure of anxiety in mice. A recent set of papers discussed the utility of B6 mice as a model of anxiety and concluded that caution must be used when selecting drug class and dose range, appropriate behavioral tests and inbred strain background (Heredia et al., 2014; Kalueff and Nguyen, 2014). Based on our results, we reach the same conclusions and suggest that for any behavioral test, the entire set of variables collected should be analyzed and described so that changes in one behavior can be interpreted in relation to changes in other parameters.
Supplementary Material
Acknowledgments
We thank Joseph Farrington for the advice, coordination of animal studies and procedural help and Pamela Metten for the useful comments on the manuscript. The authors would like to acknowledge Arlene Bridges for her extensive help in facilitating and performing the LC/MS/MS studies.
Abbreviations:
- CDP
chlordiazepoxide
- norCDP
norchlordiazepoxide
- DZ
diazepam
- norDZ
nordiazepam
- mCPP
meta-chlorophenylpiperazine
- BUSP
buspirone
- LC
liquid chromatography
- MS
mass spectrometry
- LD
light–dark
- B6
C57BL/6J
- OF
open field
- EZM
elevated zero maze
- EPM
elevated plus maze
- SIH
stress-induced hyperthermia
- BALB
BALB/cJ
- D2
DBA/2J
- IP
intraperitoneal
- AUC
area under the curve
- Cmax
maximum concentration
- Tmax
time to maximum concentration
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.pbb.2015.03.011.
References
- Acuna-Castroviejo D, Lowenstein PR, Rosenstein R, Cardinali DP. Diurnal variations of benzodiazepine binding in rat cerebral cortex: disruption by pinealectomy. J Pineal Res 1986;3:101–9. [DOI] [PubMed] [Google Scholar]
- Altamura AC, Moliterno D, Paletta S, Maffini M, Mauri MC, Bareggi S. Understanding the pharmacokinetics of anxiolytic drugs. Expert Opin Drug Metab Toxicol 2013;9: 423–40. [DOI] [PubMed] [Google Scholar]
- Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav 1998;59: 387–97. [DOI] [PubMed] [Google Scholar]
- Archer J. Sex differences in the emotional behaviour of laboratory mice. Br J Psychol 1977; 68:125–31. [DOI] [PubMed] [Google Scholar]
- Bailey JS, Grabowski-Boase L, Steffy BM, Wiltshire T, Churchill GA, Tarantino LM. Identification of QTL for locomotor activation and anxiety using closely-related inbred strains. Genes Brain Behav 2008;7:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. The rat; a study in behaviour. Chicago: Aldine Pub. Co; 1963. [Google Scholar]
- Belzung C, Dubreuil D. Naloxone potentiates the anxiolytic but not the amnestic action of chlordiazepoxide in C57BL/6 mice. Behav Pharmacol 1998;9:691–8. [DOI] [PubMed] [Google Scholar]
- Birkett MA, Shinday NM, Kessler EJ, Meyer JS, Ritchie S, Rowlett JK. Acute anxiogenic-like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol Biochem Behav 2011;98: 544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Situational determinants of open-field behaviour in Mus-musculus. Br J Psychol 1971;62:245–52. [Google Scholar]
- Blizard DA, Takahashi A, Galsworthy MJ, Martin B, Koide T. Test standardization in behavioural neuroscience: a response to Stanford. J Psychopharmacol 2007;21:136–9. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol Learn Mem 2009;92:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MJ, Volicer L, Moore-Ede MC, Borsook D. Daily rhythms of benzodiazepine receptor numbers in frontal lobe and cerebellum of the rat. Life Sci 1985;36:2333–7. [DOI] [PubMed] [Google Scholar]
- Broadhurst PL. Determinants of emotionality in the rat. I. Situational factors. Br J Psychol 1957;48:1–12. [DOI] [PubMed] [Google Scholar]
- Bruhwyler J. Anxiolytic potential of a microgram dose of chlordiazepoxide in the open-field test. Eur J Pharmacol 1990;187:547–9. [DOI] [PubMed] [Google Scholar]
- Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 2002;134:49–57. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Yang M, Crawley JN. Criteria for validating mouse models of psychiatric diseases. Am J Med Genet B Neuropsychiatr Genet 2009;150B:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 2001;25:235–60. [DOI] [PubMed] [Google Scholar]
- Clement Y, Martin B, Venault P, Chapouthier G. Involvement of regions of the 4th and 7th chromosomes in the open-field activity of mice. Behav Brain Res 1995;70:51–7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Gallaher EJ, Cross SJ, Belknap JK. Genetic determinants of sensitivity to diazepam in inbred mice. Behav Neurosci 1998;112:668–77. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav 1981;15:695–9. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse?: behavioral phenotyping of transgenic and knockout mice. 2nd ed Hoboken, N.J.: Wiley-Interscience; 2007 [Google Scholar]
- Crawley JN, Davis LG. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull 1982;8:609–12. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 1980;13:167–70. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. [DOI] [PubMed] [Google Scholar]
- Crusio WE, Gerlai RT. Handbook of molecular-genetic techniques for brain and behavior research. 1st ed Amsterdam; New York: Elsevier; 1999. [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA 2004;291:2581–90. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Open-field behavior in the rat: what does it mean? Ann N Y Acad Sci 1969;159:852–9. [DOI] [PubMed] [Google Scholar]
- Dixon LK, Defries JC. Effects of illumination on open field behavior in mice. J Comp Physiol Psychol 1968;66:803–5. [Google Scholar]
- Eisener-Dorman AF, Grabowski-Boase L, Steffy BM, Wiltshire T, Tarantino LM. Quantitative trait locus and haplotype mapping in closely related inbred strains identifies a locus for open field behavior. Mamm Genome 2010;21:231–46. [DOI] [PubMed] [Google Scholar]
- Fahey JM, Pritchard GA, Pratt JS, Shader RI, Greenblatt DJ. Lorazepam attenuates the behavioral effects of dizocilpine. Pharmacol Biochem Behav 1999;62:103–10. [DOI] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav Brain Res 1987;25:101–7. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–70. [DOI] [PubMed] [Google Scholar]
- Groenink L, Compaan J, van der Gugten J, Zethof T, van der Heyden J, Olivier B. Stress-induced hyperthermia in mice. Pharmacological and endocrinological aspects. Ann N Y Acad Sci 1995;771:252–6. [DOI] [PubMed] [Google Scholar]
- Grossen NE, Kelley MJ. Species-specific behavior and acquisition of avoidance behavior in rats. J Comp Physiol Psychol 1972;81:307–10. [DOI] [PubMed] [Google Scholar]
- Gunn J, Kriger S, Terrell AR. Simultaneous determination and quantification of 12 benzodiazepines in serum or whole blood using UPLC/MS/MS. Methods Mol Biol 2010;603: 107–19. [DOI] [PubMed] [Google Scholar]
- Hall CS. Emotional behavior in the rat: defecation and urination as measures of individual differences in emotionality. J Comp Psychol 1934;18:385–403. [Google Scholar]
- Hall C. Emotional behaviour in the rat: III. The relationship between emotionality and ambulatory activity. J Comp Psychol 1936;22:345–52. [Google Scholar]
- Hall CS, Ballechey EL. A study of the rat’s behavior in a field: a contribution to method in comparative psychology. University of California Publications in Psychology, 6; 1932. p. 1–12. [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet 2004;34:267–93. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Colomina MT, Domingo JL. Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the open-field and light/dark tests. J Pharmacol Toxicol Methods 2013a;69:108–14. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Colomina MT, Domingo JL. Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the zero maze test. J Pharmacol Toxicol Methods 2013b;68:275–83. [DOI] [PubMed] [Google Scholar]
- Heredia L, Torrente M, Domingo JL. Reply to Kalueff and Nguyen. J Pharmacol Toxicol Methods 2014;69:208–9. [DOI] [PubMed] [Google Scholar]
- Jones N, King SM. Influence of circadian phase and test illumination on pre-clinical models of anxiety. Physiol Behav 2001;72:99–106. [DOI] [PubMed] [Google Scholar]
- Kafka MS, Benedito MA, Blendy JA, Tokola NS. Circadian rhythms in neurotransmitter receptors in discrete rat brain regions. Chronobiol Int 1986;3:91–100. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nguyen M. Testing anxiolytic drugs in the C57BL/6J mouse strain. J Pharmacol Toxicol Methods 2014;69:205–7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc 2009;18:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lader M. Long-term anxiolytic therapy: the issue of drug withdrawal. J Clin Psychiatry 1987;48:12–6. (Suppl.). [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests in C57BL/6J and BALB/c mice injected with GABA- and 5HT-anxiolytic agents. Fundam Clin Pharmacol 2010;24:365–76. [DOI] [PubMed] [Google Scholar]
- Leo LM, Almeida-Correa S, Canetti CA, Amaral OB, Bozza FA, Pamplona FA. Age-dependent relevance of endogenous 5-lipoxygenase derivatives in anxiety-like behavior in mice. PLoS One 2014;9:e85009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav 2000;67:739–48. [DOI] [PubMed] [Google Scholar]
- Livesey PJ, Egger GJ. Age as a factor in open-field responsiveness in the white rat. J Comp Physiol Psychol 1970;73:93–9. [DOI] [PubMed] [Google Scholar]
- Lopez F, Miller LG, Greenblatt DJ, Paul SM, Shader RI. Low-dose alprazolam augments motor activity in mice. Pharmacol Biochem Behav 1988;30:511–3. [DOI] [PubMed] [Google Scholar]
- Lucki I, Ward HR, Frazer A. Effect of 1-(m-chlorophenyl)piperazine and 1-(m-trifluoro-methylphenyl)piperazine on locomotor activity. J Pharmacol Exp Ther 1989;249: 155–64. [PubMed] [Google Scholar]
- Mathiasen LS, Mirza NR, Rodgers RJ. Strain- and model-dependent effects of chlordiazepoxide, L-838,417 and zolpidem on anxiety-like behaviours in laboratory mice. Pharmacol Biochem Behav 2008;90:19–36. [DOI] [PubMed] [Google Scholar]
- McClearn GE. Strain differences in activity in mice: influence of illumination. J Comp Physiol Psychol 1960;53:142–3. [Google Scholar]
- Meert TF, Melis W, Aerts N, Clincke G. Antagonism of meta-chlorophenylpiperazine-induced inhibition of exploratory activity in an emergence procedure, the open field test, in rats. Behav Pharmacol 1997;8:353–63. [DOI] [PubMed] [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav 2008;7:496–505. [DOI] [PubMed] [Google Scholar]
- Nichols NS, Schreur PJKD. Centre time: a test for anxiolytic activity in rats. Society for Neurosciences Abstract: Society for Neurosciences Abstract; 1987. p. 274. [Google Scholar]
- Novas ML, Wolfman C, Medina JH, de Robertis E. Proconvulsant and ‘anxiogenic’ effects of n-butyl beta carboline-3-carboxylate, an endogenous benzodiazepine binding inhibitor from brain. Pharmacol Biochem Behav 1988;30:331–6. [DOI] [PubMed] [Google Scholar]
- Ohl F, Sillaber I, Binder E, Keck ME, Holsboer F. Differential analysis of behavior and diazepam-induced alterations in C57BL/6 N and BALB/c mice using the modified hole board test. J Psychiatr Res 2001;35:147–54. [DOI] [PubMed] [Google Scholar]
- Olivier B, Bouwknecht JA, Pattij T, Leahy C, van Oorschot R, Zethof TJ. GABAA-benzodiazepine receptor complex ligands and stress-induced hyperthermia in singly housed mice. Pharmacol Biochem Behav 2002;72:179–88. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol 2003;463: 117–32. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev 2001;25:219–33. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav 2006;87:95–102. [DOI] [PubMed] [Google Scholar]
- Post AM, Weyers P, Holzer P, Painsipp E, Pauli P, Wultsch T, et al. Gene-environment interaction influences anxiety-like behavior in ethologically based mouse models. Behav Brain Res 2011;218:99–105. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 2003;463:3–33. [DOI] [PubMed] [Google Scholar]
- Quock RM, Emmanouil DE, Vaughn LK, Pruhs RJ. Benzodiazepine receptor mediation of behavioral effects of nitrous oxide in mice. Psychopharmacology (Berl) 1992;107: 310–4. [DOI] [PubMed] [Google Scholar]
- Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izidio GS. Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res 2008;193:277–88. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Boullier E, Chatzimichalaki P, Cooper GD, Shorten A. Contrasting phenotypes of C57BL/6JOlaHsd, 129S2/SvHsd and 129/SvEv mice in two exploration-based tests of anxiety-related behaviour. Physiol Behav 2002a;77:301–10. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Davies B, Shore R. Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav 2002b;1:242–51. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Jones DN, Nelson PR, Jones CM, Quilter CA, Robinson TL, et al. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res 1999;105:207–17. [DOI] [PubMed] [Google Scholar]
- Salomons AR, Pinzon NE, Boleij H, Kirchhoff S, Arndt SS, Nordquist RE, et al. Differential effects of diazepam and MPEP on habituation and neuro-behavioural processes in inbred mice. Behav Brain Funct 2012;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenin SB, Blednov Yu A, Badyshtov BA, Gordey ML, Nagovitsina YA. Pharmacogenetic analysis of mechanisms of emotional stress: effects of benzodiazepines. Ann Ist Super Sanita 1990;26:81–7. [PubMed] [Google Scholar]
- Seredenin SB, Nadorova AV, Kolik LG, Yarkova MA. Effects of phenazepam on the behavior of C57BL/6 and BALB/c mice in the open field test after naloxone pretreatment. Bull Exp Biol Med 2013;155:346–9. [DOI] [PubMed] [Google Scholar]
- Serfozo P, Cash DJ. Effect of a benzodiazepine (chlordiazepoxide) on a GABAA receptor from rat brain. Requirement of only one bound GABA molecule for channel opening. FEBS Lett 1992;310:55–9. [DOI] [PubMed] [Google Scholar]
- Smith KS, Engin E, Meloni EG, Rudolph U. Benzodiazepine-induced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice. Neuropharmacology 2012;63:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SC. The Open Field Test: reinventing the wheel. J Psychopharmacol 2007;21: 134–5. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome 2000;11:555–64. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EA, Takahashi JS, et al. Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol Behav 2000;69:269–75. [DOI] [PubMed] [Google Scholar]
- Valle FP. Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol 1970;83:103–11. [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open-field test: a critical review. Psychol Bull 1976;83: 482–504. [PubMed] [Google Scholar]
- Williams Rt, Lim JE, Harr B, Wing C, Walters R, Distler MG, et al. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One 2009;4:e4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev 1992;44:151–347. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


