Abstract
Background
In Vietnam, lack of animal health information is considered a major challenge for pig production. The main objective of this study was to assess the seroprevalences of five pathogens [porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSV), mycoplasma hyopneumoniae (M. hyo), Japanese encephalitis virus (JEV) and leptospirosis] and to better characterize the farm movements through a survey.
Results
A total of 600 samples were collected from 120 farms from Bac Giang and Nghe An. Among unvaccinated herds, the highest seroprevalence was found for JE with 73.81% (95% CI: 68.39–78.74) in Bac Giang and 53.51% (95% CI 47.68–59.27) in Nghe An. Seroprevalences for PCV2 and M.hyo were 49.43% (95% CI: 45.06–53.80) and 46.06% (95% CI: 41.48–50.69) among unvaccinated animals. Accumulative co-infections for JE (86.25%) showed the highest level followed by M. hyo (66.25%) and PCV2 (62.50%). Three co-infections with JE had the highest positive rate (28.75%) followed by four co-infections (25.0%). Medium farms had relatively higher herd prevalences for all pathogens, except from leptospirosis. Overall, farmers exported/imported their pigs at the most 1–2 times every 6 months. Some respondents (5% for exportation and 20% for importation) had moved pigs more than 6 times over the last 6 months.
Conclusions
Our study provided another pool of evidence that showed that PCV2, PRRS and H. hyo are endemic in pigs in Vietnam. Given the economic impacts of these pathogens elsewhere, the findings confirm the need for studies to evaluate the association between antibody response and clinical relevance as well as to assess the economic impact of co-infections at farm level. We also found that high seroprevalences of JE and leptospirosis were detected in pigs. From a pubic health point of view, it is crucial to raise public awareness especially for high risk occupations (mainly pig farm workers).
Keywords: Vietnam, Pigs, Sero-prevalence, Co-infection, Farm movement
Background
In Vietnam, several constraints to pig production have been identified, most importantly animal diseases, lack of veterinary services, poor nutrition and inadequate animal productivity/genetic make-up [1]. Poor access to animal health information is considered a major challenge for pig production. In addition, more than 70% of producers are smallholders and have low awareness and knowledge on potential disease transmission pathways and biosecurity.
Vietnam’s national animal health surveillance program consists primarily of local authorities who collect data daily/weekly (through email, fax and hard copy paper forms) on major animal production diseases only. These include, classical swine fever (CSF), Foot-and-mouth (FMD) disease and porcine reproductive and respiratory syndrome (PRRS). Most cases are reported from small and medium holder farms via passive surveillance. Among reported cases, only a few are confirmed by laboratories while most reported cases are clinically diagnosed by local animal health workers due to lack of diagnostic facilities in rural areas. It can be assumed therefore that most diseases are underreported under the current national surveillance program.
A few epidemiological and serological surveys have been conducted for major production diseases in pigs in Vietnam [2–5]. Recently, using national animal health data, the first national PRRS study was conducted to identify seasonal patterns and clusters [6]. In the area of zoonotic diseases, while leptospirosis is a notifiable disease in humans, only few cases have been reported to the national surveillance system in Vietnam even though the disease is considered to be endemic [7–9]. A recent study showed that the sero-prevalence was 21.05% in pigs from 10 provinces [8]. Japanese encephalitis (JE) is a vector-borne zoonotic disease and pigs are considered to play an important role in amplifying hosts for transmission to humans given that pigs are often raised near human habitations [10]. A recent study showed that the sero-prevalence of JE in pigs was 73.45% [8].
Previous studies suggest that the major risk factors for animal diseases are the regular movement of animals between farms [11–14]. Moreover, transportation and fomites (e.g. body fluids, soil and droplet) can play a role in disease transmission [15–17]. Therefore, it is very important to understand the movement patterns at farm level.
To our knowledge, few studies in Vietnam have looked at a range of pathogens simultaneously. Therefore, the main objective of this study was to assess the seroprevalences of five pathogens and better characterize the farm movements through a survey.
Results
Farm survey and description of farm movement.
A total of 120 pig farmers [26 (Female): 94 (Male)] were interviewed from two provinces (60 farmers/province) (Additional file 1). The median age of respondents was 49.5 and the range was 25–90 years old. Almost 90% of people reached either primary to high school education levels. A total of 74.17% farms were classified into small farms whereas large farms accounted for 4.17%. Overall, farmers exported/imported their pigs at most 1–2 times every 6 months (Table 1). Some respondents (5% for exportation and 20% for importation) had moved pigs more than 6 times over the last 6 months. More than 60% of pig farms use the continuous flow systems. Veterinarians and animal health workers visited farms more than 2 twice every 6 months while most of farmers (88%) did not allow vehicles onto their premises.
Table 1.
Summary of farm movement information through survey
| Questions | Category | Proportion (%) |
|---|---|---|
| How often did you import the pigs on your farm over the last 6 months? | 0 | 41.67 |
| ≤2 times | 41.67 | |
| > 2 times | 16.67 | |
| How often did you export your pigs from your farm over the last 6 months? | 0 | 9.17 |
| ≤2 times | 53.33 | |
| > 2 times | 37.5 | |
| What kind of production system do use on your farm? | All-in-All-out | 35.83 |
| Continuous flow | 64.17 | |
| Are new purchased pigs that are mixed with existing pigs over the last 6 months? | Yes | 15.83 |
| No | 84.17 | |
| How often did other pig farmers visit on your farm over the last 6 months? | 0 | 81.67 |
| ≤2 times | 7.50 | |
| > 2 times | 10.83 | |
| How often did traders visit on your farm over the last 6 months | 0 | 25.0 |
| ≤2 times | 35.83 | |
| > 2 times | 39.17 | |
| How often did butchers visit on your farm over the last 6 months? | 0 | 63.33 |
| ≤2 times | 20.0 | |
| > 2 times | 16.67 | |
| How often did veterinarians/animal health workers visit on your farm over the last 6 months? | 0 | 40.83 |
| ≤2 times | 40.83 | |
| > 2 times | 18.33 | |
| How often were vehicles allowed onto the premises over the last 6 months? | 0 | 88.33 |
| ≤2 times | 7.50 | |
| > 2 times | 4.17 |
Seroprevalences of multi-pathogen
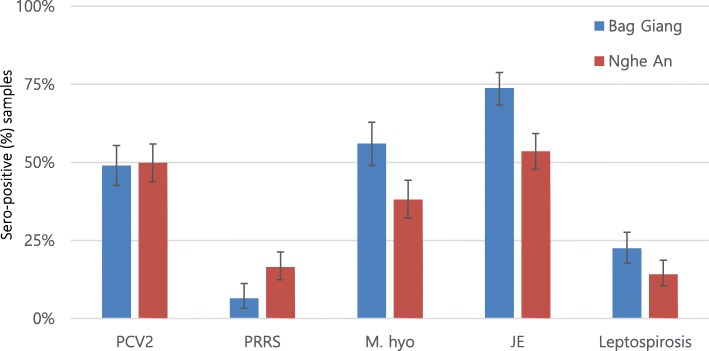
A total of 600 samples were collected from 120 farms across the two provinces. Six samples from Bac Giang (three samples each farm from two farms) and one sample from Nghe An could not be analyzed due to hemolysis, while sufficient sera for 9 samples were not available to perform the microscopic agglutination test (MAT) for leptospirosis. The highest seroprevalence was found for JE with 73.81% [95% confidence interval (CI): 68.39–78.74] in Bac Giang and 53.51% (95% CI 47.68–59.27) in Nghe An (to note: none of the herds were vaccinated against JE). Seroprevalences for porcine circovirus type 2 (PCV2) and mycoplasma hyopneumoniae (M. hyo) were 49.43% (95% CI: 45.06–53.80) and 46.06% (95% CI: 41.48–50.69), respectively whereas leptospirosis and PRRS showed the lowest seroprevalences among unvaccinated animals (Fig. 1).
Fig. 1.
Sero-prevalences with 95% CI of multi-pathogen in unvaccinated pigs in two provinces
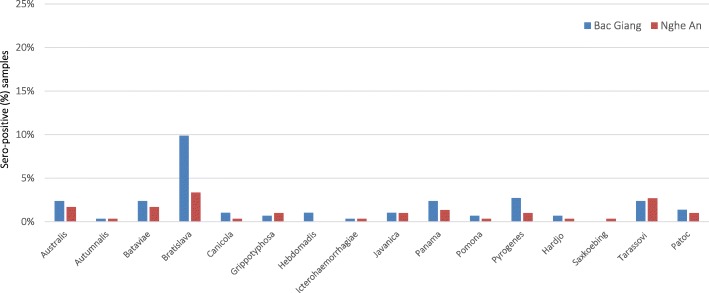
Vaccinated herds with PRRS showed the highest seropositive rate (22.5%) followed by M. hyo (20.83%) and PCV2 (11.67%) whereas none of the herds were vaccinated with JE and leptospirosis (Table 2). Seropositive rates between vaccinated and unvaccinated herds were 55.56% (15 of 27):17.20% (16 of 93) for PRRS and 88.0% (22 of 25): 69.47% (66 of 95) for M. hyo. Overall, medium farms had relatively higher herd prevalences for all pathogens, except from leptospirosis. All medium farms were infected with M. hyo and JE. For leptospirosis, the most frequently detected infective serovar was Bratislava (6.60%), followed by Tarassovi (2.54%), Australis (2.03%) and Bataviae (2.03%) using a cut-off titer of ≥1:100 (Table 3). In Bac Giang, serovar Bratislava had the highest prevalence (9.86%) followed by Pyrogenes (2.72%) while Bratislava (3.37%) had the highest followed by Tarassovi (2.69%) in Nghe An (Fig. 2).
Table 2.
Herd prevalence by farm type [small (< 100), medium (< 500) and large (≥ 500)] in vaccine and unvaccinated farms
| Tested pathogen | No. of vaccinated farm type | Seroprevalence of vaccinated farms with 95% CI | No. of unvaccinated farm type | Seroprevalence of unvaccinated farms with 95% CI |
|---|---|---|---|---|
| PCV2 | Small (6) | 50.0 (11.81–88.19) | Small (83) | 57.83 (46.49–68.60) |
| Medium (6) | 100.0 (54.07–100.0) | Medium (20) | 80.0 (56.34–94.27) | |
| Large (2) | 100.0 (15.81–100.0) | Large (3) | 33.33 (0.84–90.57) | |
| PRRS | Small (12) | 41.67 (15.17–72.33) | Small (77) | 9.09 (3.73–17.84) |
| Medium (13) | 69.23 (38.57–90.91) | Medium (13) | 61.54 (31.58–86.14) | |
| Large (2) | 50.0 (1.26–98.74) | Large (3) | 33.33 (0.84–90.57) | |
| M. hyo | Small (14) | 78.57 (49.20–95.34) | Small (75) | 64.0 (52.09–74.77) |
| Medium (9) | 100.0 (66.37–100.0) | Medium (17) | 100.0 (80.49–100) | |
| Large (2) | 100.0 (15.81–100.0) | Large (3) | 33.33 (0.84–90.57) | |
| JE | Small (0) | 0 | Small (89) | 87.64 (78.96–93.67) |
| Medium (0) | 0 | Medium (26) | 100.0 (86.77–100) | |
| Large (0) | 0 | Large (5) | 100.0 (47.81–100) | |
| Small (0) | 0 | Small (88) | 54.55 (43.58–65.20) | |
| Leptospirosisa | Medium (0) | 0 | Medium (25) | 64 (42.52–82.03) |
| Large (0) | 0 | Large (5) | 80.0 (28.36–99.49) |
asera samples from two farms were not enough volumes for the MAT
Table 3.
MAT results for Leptospria serovars in pigs using 2 cutoff titers
| Serovar | Total samples | ≥ 1:100 | ≥ 1:200 |
|---|---|---|---|
| N (%, 95 CI) | N (%, 95 CI) | ||
| Australis | 591 | 12 (2.03, 1.05–3.52) | 0 |
| Autumnalis | 591 | 2 (0.3, 0.04–1.21) | 1 (0.17, 0.004–0.94) |
| Bataviae | 591 | 12 (2.03, 1.05–3.52) | 2 (0.3, 0.04–1.21) |
| Bratislava | 591 | 39 (6.60, 4.73–8.91) | 3 (0.5, 0.1–1.48) |
| Canicola | 591 | 4 (0.68, 0.18–1.72) | 0 |
| Grippotyphosa | 591 | 5 (0.8, 0.28–1.96) | 0 |
| Hebdomadis | 591 | 3 (0.5, 0.1–1.48) | 0 |
| Icterohaemorrhagiae | 591 | 2 (0.3, 0.04–1.21) | 0 |
| Javanica | 591 | 6 (1.02, 0.37–2.20) | 1 (0.17, 0.004–0.94) |
| Panama | 591 | 11 (1.86, 0.93–3.31) | 1 (0.17, 0.004–0.94) |
| Pomona | 591 | 3 (0.5, 0.1–1.48) | 0 |
| Pyrogenes | 591 | 11 (1.86, 0.93–3.31) | 0 |
| Hardjo | 591 | 3 (0.5, 0.1–1.48) | 0 |
| Sakoebing | 591 | 1 (0.17, 0.004–0.94) | 0 |
| Tarassovi | 591 | 15 (2.54, 1.42–4.15) | 1 (0.17, 0.004–0.94) |
| Patoc | 591 | 7 (1.18, 0.48–2.43) | 2 (0.3, 0.04–1.21) |
Fig. 2.
Sero-positive samples by Leptospira serovar in two provinces using cut off titer ≥1:100
Co-infections
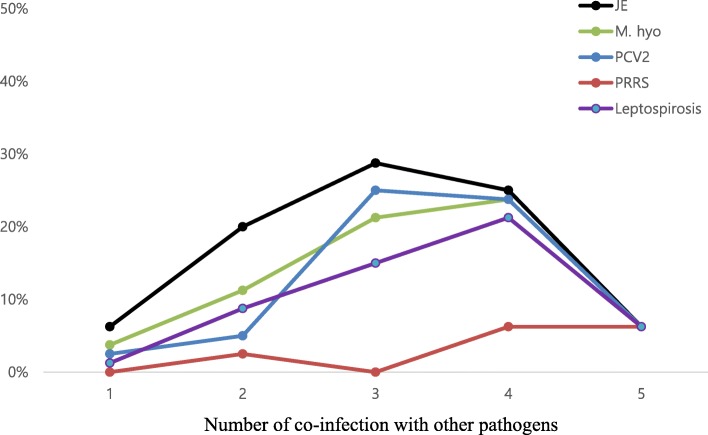
Table 4 demonstrated the proportion of co-infection with different pathogens among unvaccinated farms. The most common co-infections were PRRS-JE (positive rate:16/16, 100%) and JE-leptospirosis (positive rate: 64/68, 94.12%) whereas the least common co-infections were PCV2-PRRS (positive rate: 9/65, 13.84%) and M. hyo-PRRS (positive rate: 9/66, 13.64%). Overall, accumulative co-infections for JE (86.25%) showed the highest level followed by M. hyo (66.25%) and PCV2 (62.50%) (Fig. 3). Three co-infections with JE had the highest positive rate (28.75%), followed by four co-infections (25.0%). A total of five farms (medium farms: 4 and small farms:1) were infected with five pathogens, accounting for 6.25% among unvaccinated farms.
Table 4.
Proportion of co-infection by each positive pathogen among unvaccinated farms in two provinces
| Pathogen (No. of positive samples) | PCV2 | PRRS | M. hyo | JE | Leptospirosis |
|---|---|---|---|---|---|
| PCV2 (65) | N/A | 9 (13.84%) | 41 (63.08%) | 60 (92.31%) | 40 (61.54%) |
| PRRS (16) | 9 (56.25%) | N/A | 9 (56.25%) | 16 (100.0%) | 10 (62.5) |
| M. hyo (66) | 41 (62.12%) | 9 (13.64%) | N/A | 60 (90.91%) | 35 (53.03%) |
| JE (109) | 60 (55.05%) | 16 (14.68%) | 60 (55.05%) | N/A | 64 (58.72%) |
| Leptospirosis (68) | 40 (58.82%) | 16 (23.53%) | 35 (51.47%) | 64 (94.12%) | N/A |
Fig. 3.
Accumulative co-infection trends of five tested pathogens among unvaccinated herds from two provinces
Discussion
Our study found PCV2 (50%) and M. hyo (35%) detection rates among unvaccinated pigs, similar to a previous study, conducted in Hoa Binh and Vinh Phuc whereas our study showed a relatively lower seroprevalence of PRRS (21%) [18]. Another study in southern Vietnam found a prevalence of 25% for PRRS in young pigs, age 4–5 months [19]. PCV2 is characterized by wasting, pale skin, respiratory distress and diarrhoea [20, 21]. Also, PCV2 is associated with the porcine respiratory disease complex (PRDC) [22], underlying its relevance for productivity at farm level. Similarly to Kim et al., we found that co-infection of PCV2 and M. hyo was common. In Vietnam, a study was conducted to evaluate the molecular characterization of PCV2, showing multi circulating clusters of 1A, 1B, 1C and a recombinant cluster [5].
Our study found a high seroprevalence for M. hyo infection and the likelihood that this pathogen is a cause of respiratory disease in pigs in Vietnam, which has been underestimated and for which awareness is likely to be low. It can be differentiated from PRRS by number of infected pigs affected in herd, clinical signs (e.g. local pneumonia) and spread patterns [23]. The first clinical signs of infected pig herds are coughing, anorexia and shortness of breath. Also, one study reported that the disease spreads slowly within herds, showing 20% of morbidity and 12% of fatality in Vietnam [24].
In 2006, a new strain of PRRS (called “highly pathogenic (HP) PRRS) was detected in China for the first time, characterized by high morbidity and mortality rates [25]. In 2007, HP-PRRS virus was first identified in Hai Dung province, which then spread to other provinces in Vietnam [26]. In 2008, more than 300,000 pig deaths (including controlled pig culling) were recorded in 26 out of 62 provinces which had a huge economic impact on production [27]. Since then, more pig farmers have opted to vaccinate their herds against PRRS vaccination. However, vaccinations are more commonly used in large pig farms, but not in the small and medium farms, that account for more than 95% of total pig production – and are able to sell pigs without PRRS vaccination certification. Our study showed that only 22% of small/medium farms were vaccinating their pig herds against PRRS, compared to 67% in large farms.
From a public health point of view, it has been suggested that infection of PRRS in pigs was increased by co-infection with Streptococcus suis (S. suis) [28, 29], further to one experiment that showed that PRRS infected pigs were more susceptible to S. suis [30]. In Vietnam, some studies showed that significantly more cases of S. suis were observed in a district adjacent to a PRRS district [31, 32]. S. suis infection is a zoonotic disease of growing importance in Asia that causes acute meningitis, septicemia and arthritis in humans [33, 34]. More investigations are needed to evaluate the association between the two diseases in Vietnam.
It is well recognized that JE is endemic in Southeast Asia, which is a major cause of viral encephalitis (VE) in young children [35–37]. In Vietnam, national surveillance for VE in humans is ongoing, with the JE virus considered to be a leading cause of VE, accounting for 12–71% of cases [38–40]. JE is a virus transmitted by mosquitoes and pigs are well known as a major amplifying host for transmission to humans [41, 42].
The seroprevalence (63.58%) of JE was similar or slightly lower compared to other studies in Southeast Asia (Laos, Cambodia and Vietnam [65–75%]) but were higher than other Asian countries (Indonesia, Nepal and Taiwan) (73.45%) [8, 43–47]. Bac Giang province was included in both studies, and interestingly seroprevalence had similar levels (73.81% in our study and 79.0% in Lee et al., 2019). Pigs are the most important amplifying hosts for JE virus transmission because they are raised in close proximity to humans [48, 49]. In order to prevent the spread of the disease, it is important to increase awareness amongst pig farmers.
For leptospirosis, our study showed that the seroprevalence was similar to a previous study conducted by Lee et al. (21.05%) [8]. However, it was significantly higher than in another previous study (8.17%) [7]. A possible explanation is that all samples in the previous study were collected from slaughterhouses where healthy or visually good condition pigs were likely to be sent for butchery. Serovar Bratislava and Tarassovi had the highest seroprevalence which were similar to the previous studies [8, 50]. It is known that pigs are the main host for serovar Bratislava, Muenchen, Pomona and Tarassovi [51–53]. Bratislava and Tarassovi in particular have been commonly detected in wild boars [54, 55]. It has been suggested that wild boars may play a role in transmission to domesticated pigs. However, further study is necessary to evaluate this role in wild boars in Vietnam.
In general, various PRRS and PCV vaccines have been introduced for pigs. However, these vaccinations are commonly used in large farms, but not in small farms that are responsible collectively for 70–75% of the total pig production in Vietnam. Because smallholder farmers are able to trade pigs without certification of vaccination, there is no of incentive for smallholders to use these vaccines.
Medium farms are the major suppliers of piglets to smallholders (accounting for more than 70% of pig production), resulting in the hierarchical structure of animal movement from medium to small farms. One the other hand, large farms have better biosecurity and are managed by large commercial companies, are unlikely to have animal movements toward small and medium farms. Our study found that medium farms had relatively higher seroprevalences of diseases compared to small and large farms. Because biosecurity levels of small and small-to-medium farms are low to non-existent, these provide opportunities for the introduction, spill-over and spread of pathogens. Therefore, medium farms need to be targeted to efficiently reduce and prevent the transmission of disease to small farms in Vietnam.
The main limitation in our study was that our samples may not be representative because these were not proportionally collected depending on farm size. We had to take into account the reality that large and medium farmers were not willingly to cooperate (mainly for biosecurity reasons) with our study.
It is well known that farm movements [i.e. direct contact (animal movement) and indirect contact (e.g., vehicles, equipment and personnel) contact] play an important role in between- farm disease transmission [56]. However, few studies have been conducted to demonstrate these patterns in Vietnam. We found that pig famers still have a poor understanding of biosecurity: more than 60% of pig farmers operate a continuous flow system that increase the access of susceptible pigs to objects contaminated by infectious pigs. In addition, it is necessary to improve the accessibility of veterinary services for 40% of pig farmers.
In Vietnam, after the restructuring of government systems in Oct 2017, veterinary services at the district and commune levels were weakened to an extent that early detection/reporting and rapid control/prevention intervention can not be implemented for transboundary animal diseases, emerging and zoonotic diseases. Our study showed that 40% of farms did not receive any veterinary services in the last 6 months.
A typical example is the ongoing outbreak of African swine fever (ASF), which was first detected in February, 2019 at 33-pig farms in Hung Yen, a northern Vietnamese province [57]. By December 2019, more than 5 million pigs have been culled or perished from the disease in all provinces. Poor/slow disease reporting as a result of low compensation rates, uncertainty of time to receive compensation and the complexity of procedures from local authorities was a major reason for the rapid spread of disease across the country. This was further compounded by the generally, low biosecurity levels of small and small-to-medium commercial farms, which provides an opportunity for the introduction of infectious diseases. These are points that would need to be addressed in order to prevent the future spread of infectious diseases in Vietnam.
Conclusions
Our study provided evidence that PCV2, PRRS and H. hyo are endemic in pigs in Vietnam. Given the economic impacts that these pathogens have elsewhere, the findings confirm the need for studies to evaluate the association between antibody response and clinical relevance as well as to assess the economic impact of co-infections at farm level. We also found that high seroprevalences of JE and leptospirosis were detected in pigs. From a pubic health point of view, it is crucial to raise public awareness for high risk occupations (mainly pig farm workers) who have higher chances to come in contact with infected pigs, contaminated materials and vectors.
Methods
Study locations and design
Bac Giang and Nghe An are located in the Red river delta and north central coast regions of Vietnam, with an estimated human population of 1.7 and 3.1 million, respectively (Fig. 4) [58]. As of 2017, there were about 1.08 pigs in Bac Giang and 0.89 million pigs in Nghe An. A total of 120 pig farms (60 farms /province) were randomly selected among registered farms from two provinces. Within each province, two districts were selected. Face-to-face interviews were carried out with adults (> 18 years old) who were mostly involved in pig-rearing. The questionnaires covered demographic information, farm management, vaccination history and farm contact information (on and off farm movements; Additional file 1).
Fig. 4.
Selected pig farm locations (blue dots) in two districts from two provinces (This figure was created by our own team)
For blood sampling, five pigs (16–20 weeks old) were randomly selected in each farm while farmers were being interviewed. A total of 600 blood samples were collected for evaluating the sero-prevalences of five pathogens [PCV2, PRRS, M. hyo, JE and leptospirosis].
Laboratory analysis
The sera were extracted after centrifugation and kept at − 20 °C in a cool box until delivery to the National Institute of Veterinary Research (NIVR) in Hanoi. For four pig pathogens (PCV2, PRRS, M. hyo and JE), enzyme-linked immunosorbent assay (ELISA) was used to measure antibodies in pig samples. We followed the guidelines of manufacturer (VDPro PCV2, PRRS, M. hyo and JE AB ELISA; MEDIAN Diagnostics, Chuncheon-si, Gangwon-do, Korea). For leptospirosis diagnosis, the MAT was used to identify the positive samples. The MAT results were recorded by using 2-fold serial dilutions of serum samples, beginning from 1:100 to 1:1600 (end-point). A total of 16 serovars (Table 5) were tested as the highest dilution point that agglutinated > 50% of live leptospires compared to the control samples were recorded. Positivity was considered as MAT titers ≥1:100 for at least one of the tested serovars.
Table 5.
List of Leptospira antigens used in the MAT
| No. | Genomospecies | Serogroup | Serovar |
|---|---|---|---|
| 1 | L. interrogans | Australis | Australis |
| 2 | L. interrogans | Autumnalis | Autumnaliss |
| 3 | L. interrogans | Bataviae | Bataviae |
| 4 | L. interrogans | Australis | Bratislava |
| 5 | L. interrogans | Canicola | Canicola |
| 6 | L. kirschneri | Grippotyphosa | Grippotyphosa |
| 7 | L. interrogans | Hebdomadis | Hebdomadis |
| 8 | L. interrogans | Icterohaemorrhagiae | Icterohaemorrhagiae |
| 9 | L. borgpetersenii | Javanica | Javanica |
| 10 | L. noguchii | Panama | Panama |
| 11 | L. interrogans | Pomona | Pomona |
| 12 | L. interrogans | Pyrogenes | Pyrogenes |
| 13 | L. borgpetersenii | Sejroe | Hardjo |
| 14 | L. borgpetersenii | Sejroe | Saxkoebing |
| 15 | L. biflexa | Semaranga | Patoc |
| 16 | L. borgpetersenii | Tarassovi | Tarassovi |
Data analysis
The selected farms were classified into types based on number of pigs held by farms: small< 100; medium between 100 and 500; large farms≥500 pigs. The seroprevalence was calculated based on the proportion of positive samples with a 95% Clopper-Pearson/Exact CI. It was calculated by vaccinated and unvaccinated at animal/herd level, respectively as vaccination history was collected. Herd prevalence was estimated by the farm type (small, medium and large), which was defined as positive when at least one sampled pig showed positivity.
For positive pathogens, we assessed the proportion of co-infection with other pathogens. In addition, we evaluated the proportion of accumulative co-infection trends among five pathogens. All data were entered into Microsoft Excel 2016 and analyzed using STATA (version 15.1 StataCorp, College Station, TX, USA). 3.5.2). QGIS (Quantum GIS development Team 2018. QGIS version number 3.6.0) was used to create the map.
Supplementary information
Additional file 1. Summary of survey data for pig farms in Bac Giang and Nghe An province of Vietnam.
Additional file 2. Questionnaire for pig farmers.
Acknowledgments
The authors thank the National Institute of Veterinary Research (NIVR), Ministry of Agriculture and Rural Development (MARD). The authors also thank the staff of the sub-department of animal health from Bac Giang and Nghe An provinces for the collaboration with research team in conducting this study.
Abbreviations
- ASF
African swine fever
- CI
Confidence interval
- CSF
Classical swine fever
- ELISA
Enzyme-linked immunosorbent assay
- FMD
Foot-and-mouth
- HP
highly pathogenic
- JE
Japanese encephalitis
- M. hyo
Mycoplasma hyopneumoniae
- MAT
microscopic agglutination test
- NIVR
National Institute of Veterinary Research
- PCV2
Porcine circovirus type 2
- PRDC
Porcine respiratory disease complex
- PRRS
Porcine reproductive and respiratory syndrome
- VE
Viral encephalitis
Authors’ contributions
Conceived and designed the experiments: HSL Performed the experiments: HSL, VNB, HNX, ANB and TDH Analyzed the data: HSL. Wrote the paper: HSL, HNV, DGR and BW. All authors read and approved the final manuscript.
Funding
This study was funded by the CGIAR Research Program on Livestock and is supported by contributors to the CGIAR Trust Fund. CGIAR is a global research partnership for a food-secure future. This study was also supported by the CGIAR Research Program on Agriculture for Nutrition and Health, led by the International Food Policy Research Institute. The funders did not involve in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
Our questionnaires are available (Additional file 2). All datasets supporting our findings are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Hanoi University of Public Health (HUPH), Vietnam (Reference Number IRB/018–186/DD-YTCC. Verbal informed consent was obtained from all owners to collect samples from the pigs. IRB approved to receive the verbal informed consent as most of the farmers were either illiterate or semi-literate. This consent form was dated and signed by the researchers indicating that “I have read and explained this consent form to the participant before receiving the participant’s consent, and the participant had knowledge of its contents and appeared to understand it”.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. Barbara Wieland is a member of the editorial board.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hu Suk Lee, Email: H.S.Lee@cgiar.org.
Vuong Nghia Bui, Email: buinghiavuong@gmail.com.
Huyen Xuan Nguyen, Email: nxhuyen7@gmail.com.
Anh Ngoc Bui, Email: buingocanh_1980@yahoo.com.
Trung Duc Hoang, Email: hdtrung.hau@gmail.com.
Hung Nguyen-Viet, Email: H.Nguyen@cgiar.org.
Delia Grace Randolph, Email: d.randolph@cgiar.org.
Barbara Wieland, Email: b.wieland@cgiar.org.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12917-020-2236-7.
References
- 1.Huynh TTT, Aarnink AJA, Drucker A, Verstegen MWA. Pig production in Cambodia, Laos, Philippines, and Vietnam: a review. Asian J Agric Dev. 2006;3:69–90. [Google Scholar]
- 2.Le H, Poljak Z, Deardon R, Dewey CE. Clustering of and risk factors for the porcine high fever disease in a region of Vietnam. Transbound Emerg Dis. 2012;59:49–61. doi: 10.1111/j.1865-1682.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 3.Vu LT, Long NT, Brito B, Stenfeldt C, Phuong NT, Hoang BH, et al. First detection of foot-and-mouth disease virus O/Ind-2001d in Vietnam. PLoS One. 2017;12:e0177361. doi: 10.1371/journal.pone.0177361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamakawa A, Thu HTV, Yamada S. Epidemiological survey of viral diseases of pigs in the Mekong delta of Vietnam between 1999 and 2003. Vet Microbiol. 2006;118:47–56. doi: 10.1016/j.vetmic.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Huynh TML, Nguyen BH, Nguyen VG, Dang HA, Mai TN, Tran THG, et al. Phylogenetic and Phylogeographic analyses of P orcine Circovirus type 2 among pig farms in V ietnam. Transbound Emerg Dis. 2014;61:e25–e34. doi: 10.1111/tbed.12066. [DOI] [PubMed] [Google Scholar]
- 6.Lee HS, Pham TL, Nguyen TN, Lee M, Wieland B. Seasonal patterns and space-time clustering of porcine reproductive and respiratory syndrome (PRRS) cases from 2008 to 2016 in Vietnam. Transbound Emerg Dis. 2019;66:986–994. doi: 10.1111/tbed.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HS, Khong NV, Xuan HN, Nghia VB, Nguyen-Viet H, Grace D. Sero-prevalence of specific Leptospira serovars in fattening pigs from 5 provinces in Vietnam. BMC Vet Res. 2017;13:125. doi: 10.1186/s12917-017-1044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HS, Thanh TL, Ly NK, Nguyen-Viet H, Thakur KK, Grace D. Seroprevalence of leptospirosis and Japanese encephalitis in swine in ten provinces of Vietnam. PLoS One. 2019;14:e0214701. doi: 10.1371/journal.pone.0214701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laras K, Van Cao B, Bounlu K, Nguyen TKT, Olson JG, Thongchanh S, et al. The importance of leptospirosis in Southeast Asia. Am J Trop Med Hyg. 2002;67:278–286. doi: 10.4269/ajtmh.2002.67.278. [DOI] [PubMed] [Google Scholar]
- 10.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortensen S, Stryhn H, Søgaard R, Boklund A, Stärk KDC, Christensen J, et al. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Prev Vet Med. 2002;53:83–101. doi: 10.1016/S0167-5877(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R, Wint W. Cattle movements and bovine tuberculosis in Great Britain. Nature. 2005;435:491. doi: 10.1038/nature03548. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Pelaez A, Pfeiffer DU, Soares-Magalhaes RJ, Guitian FJ. Use of social network analysis to characterize the pattern of animal movements in the initial phases of the 2001 foot and mouth disease (FMD) epidemic in the UK. Prev Vet Med. 2006;76:40–55. doi: 10.1016/j.prevetmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Rossi G, Smith RL, Pongolini S, Bolzoni L. Modelling farm-to-farm disease transmission through personnel movements: from visits to contacts, and back. Sci Rep. 2017;7:2375. doi: 10.1038/s41598-017-02567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otake S, Dee SA, Jacobson L, Pijoan C, Torremorell M. Evaluation of aerosol transmission of porcine reproductive and respiratory syndrome virus under controlled field conditions. Vet Rec. 2002;150:804. doi: 10.1136/vr.150.26.804. [DOI] [PubMed] [Google Scholar]
- 16.Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of transmission of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128. [PMC free article] [PubMed] [Google Scholar]
- 17.Gloster J, Champion HJ, Sørensen JH, Mikkelsen T, Ryall DB, Astrup P, et al. Airborne transmission of foot-and-mouth disease virus from Burnside farm, Heddonon-The-Wall, Northumberland, during the 2001 epidemic in the United Kingdom. Vet Rec. 2003;152:525–533. doi: 10.1136/vr.152.17.525. [DOI] [PubMed] [Google Scholar]
- 18.Unger F, Nghia VB, Wieland B. Prevalence of major pig production diseases in 2 provinces of northern Vietnam (Hoa Binh and Vinh Phuc) 2016. [Google Scholar]
- 19.Van Cuong N, Carrique-Mas J, Thu HTV, Hien ND, Hoa NT, Nguyet LA, et al. Serological and virological surveillance for porcine reproductive and respiratory syndrome virus, porcine circovirus type 2, and influenza a viruses among smallholder swine farms of the Mekong Delta, Vietnam. J Swine Heal Prod. 2014;22:224–231. [Google Scholar]
- 20.Segalés J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6:119–142. doi: 10.1079/AHR2005106. [DOI] [PubMed] [Google Scholar]
- 21.Segalés J. Porcine circovirus type 2 (PCV2) infections: clinical signs, pathology and laboratory diagnosis. Virus Res. 2012;164:10–19. doi: 10.1016/j.virusres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Chung H-K, Chae C. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet J. 2003;166:251–256. doi: 10.1016/S1090-0233(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 23.Pham HTT, Antoine-Moussiaux N, Grosbois V, Moula N, Truong BD, Phan TD, et al. Financial impacts of priority swine diseases to pig farmers in Red River and Mekong River Delta, Vietnam. Transbound Emerg Dis. 2017;64:1168–1177. doi: 10.1111/tbed.12482. [DOI] [PubMed] [Google Scholar]
- 24.Le V, Huynh T, Trinh D, Dang H, Do N, Nguyen B. Epidemiology of enzootic pneumonia and using semi-nested PCR to detect. Mycoplasma hyopneumoniae. Vietnam Vet Sci Tech. 2012;19:9–15. [Google Scholar]
- 25.Feng Y, Zhao T, Nguyen T, Inui K, Ma Y, Nguyen TH, et al. Porcine respiratory and reproductive syndrome virus variants, Vietnam and China, 2007. Emerg Infect Dis. 2008;14:1774. doi: 10.3201/eid1411.071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tung N. Molecular epidemiology of highly pathogenic PRRS in Vietnam in 2010. Conference Proceeding on 5th Asian Pig Veterinary Society Congress. 2010. [Google Scholar]
- 27.N.N. T. Status of PRRS and disease control in Vietnam . Department of Animal Health. Annual report. 2009. [Google Scholar]
- 28.Rossow KD. Porcine reproductive and respiratory syndrome. Vet Pathol. 1998;35:1–20. doi: 10.1177/030098589803500101. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Wang S, Li L, Lei L, Liu Y, Shi W, et al. Secondary infection with Streptococcus suis serotype 7 increases the virulence of highly pathogenic porcine reproductive and respiratory syndrome virus in pigs. Virol J. 2010;7:184. doi: 10.1186/1743-422X-7-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W, Laster SM, Tompkins M, Brown T, Xu J-S, Altier C, et al. In utero infection by porcine reproductive and respiratory syndrome virus is sufficient to increase susceptibility of piglets to challenge by Streptococcus suis type II. J Virol. 2001;75:4889–4895. doi: 10.1128/JVI.75.10.4889-4895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huong VTL, Thanh LV, Phu VD, Trinh DT, Inui K, Tung N, et al. Temporal and spatial association of Streptococcus suis infection in humans and porcine reproductive and respiratory syndrome outbreaks in pigs in northern Vietnam. Epidemiol Infect. 2016;144:35–44. doi: 10.1017/S0950268815000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wertheim Heiman F. L., Nguyen Huyen Nguyen, Taylor Walter, Lien Trinh Thi Minh, Ngo Hoa Thi, Nguyen Thai Quoc, Nguyen Bich Ngoc Thi, Nguyen Ha Hong, Nguyen Ha Minh, Nguyen Cap Trung, Dao Trinh Tuyet, Nguyen Trung Vu, Fox Annette, Farrar Jeremy, Schultsz Constance, Nguyen Hien Duc, Nguyen Kinh Van, Horby Peter. Streptococcus suis, an Important Cause of Adult Bacterial Meningitis in Northern Vietnam. PLoS ONE. 2009;4(6):e5973. doi: 10.1371/journal.pone.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thi Hoang Mai N, Thi Hoa N, Vu Thieu Nga T, Dieu Linh L, Thi Hong Chau T, Xuan Sinh D, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46:659–667. doi: 10.1086/527385. [DOI] [PubMed] [Google Scholar]
- 34.Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM An Int J Med. 1995;88:39–47. [PubMed] [Google Scholar]
- 35.WHO. Japanese encephalitis. http://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis. Accessed 8 Aug 2019.
- 36.Trung NHD, Phuong TLT, Wolbers M, Van Minh HN, Thanh VN, Van MP, et al. Aetiologies of central nervous system infection in Viet Nam: a prospective provincial hospital-based descriptive surveillance study. PLoS One. 2012;7:e37825. doi: 10.1371/journal.pone.0037825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen NT, Duffy MR, Hong NM, Hien NT, Fischer M, Hills SL. Surveillance for Japanese encephalitis in Vietnam, 1998–2007. Am J Trop Med Hyg. 2010;83:816–819. doi: 10.4269/ajtmh.2010.10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon T, Dung NM, Kneen R, Thao LTT, Gainsborough M, Nisalak A, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–1093. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- 39.Phu NH, Nghia HDT, Van Chuong L, Sinh DX, Phong ND, Mai NTH, et al. Viral aetiology of central nervous system infections in adults admitted to a tertiary referral hospital in southern Vietnam over 12 years. PLoS Negl Trop Dis. 2014;8:e3127. doi: 10.1371/journal.pntd.0003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HS, Nguyen-Viet H, Lee M, Duc PP, Grace D. Seasonality of viral encephalitis and associated environmental risk factors in son La and Thai Binh provinces in Vietnam from 2004 to 2013. Am J Trop Med Hyg. 2017;96:110–117. doi: 10.4269/ajtmh.16-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monath TP. The arboviruses: epidemiology and ecology. Boca Raton: CRC Press; 2019.
- 42.Hanna JN, Ritchie SA, Hills SL, van den Hurk AF, Phillips DA, Pyke AT, et al. Japanese encephalitis in North Queensland, Australia, 1998. Med J Aust. 1999;170:533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 43.Conlan JV, Vongxay K, Jarman RG, Gibbons RV, Lunt RA, Fenwick S, et al. Serologic study of pig-associated viral zoonoses in Laos. Am J Trop Med Hyg. 2012;86:1077–1084. doi: 10.4269/ajtmh.2012.11-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duong V, Sorn S, Holl D, Rani M, Deubel V, Buchy P. Evidence of Japanese encephalitis virus infections in swine populations in 8 provinces of Cambodia: implications for national Japanese encephalitis vaccination policy. Acta Trop. 2011;120:146–150. doi: 10.1016/j.actatropica.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Pant GR. A serological survey of pigs, horses, and ducks in Nepal for evidence of infection with Japanese encephalitis virus. Ann N Y Acad Sci. 2006;1081:124–129. doi: 10.1196/annals.1373.013. [DOI] [PubMed] [Google Scholar]
- 46.Chang KJ. Seasonal prevalence of anti-Japanese encephalitis virus antibody in pigs in different regions of Taiwan. J Microbiol Immunol Infect Wei mian yu gan ran za zhi. 2002;35:12–16. [PubMed] [Google Scholar]
- 47.Yamanaka A, Mulyatno KC, Susilowati H, Hendrianto E, Utsumi T, Amin M, et al. Prevalence of antibodies to Japanese encephalitis virus among pigs in Bali and East Java, Indonesia, 2008. Jpn J Infect Dis. 2010;63:58–60. [PubMed] [Google Scholar]
- 48.Hills SL, Phillips DC. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1333. doi: 10.3201/eid1508.090149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanna JN, Ritchie SA, Phillips DA, Lee JM, Hills SL, Van Den Hurk AF, et al. Japanese encephalitis in North Queensland, Australia, 1998. Med J Aust. 1999;170:533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 50.Boqvist S, Chau BL, Gunnarsson A, Engvall EO, Vågsholm I, Magnusson U. Animal-and herd-level risk factors for leptospiral seropositivity among sows in the Mekong deltan, Vietnam. Prev Vet Med. 2002;53:233–245. doi: 10.1016/S0167-5877(01)00263-X. [DOI] [PubMed] [Google Scholar]
- 51.Ryan TJ, Marshall RB. Isolation of a leptospire belonging to serogroup tarassovi. 1976. [DOI] [PubMed] [Google Scholar]
- 52.Rocha T. Isolation of Leptospira interrogans serovar Mozdok from aborted swine fetuses in Portugal. Vet Rec. 1990;126(24). [PubMed]
- 53.Ellis WA, McParland PJ, Bryson DG, Cassells JA. Prevalence of Leptospira infection in aborted pigs in Northern Ireland. Vet Rec. 1986;118:63–65. doi: 10.1136/vr.118.3.63. [DOI] [PubMed] [Google Scholar]
- 54.Jansen A, Luge E, Guerra B, Wittschen P, Gruber AD, Loddenkemper C, et al. Leptospirosis in urban wild boars, Berlin, Germany. Emerg Infect Dis. 2007;13:739. doi: 10.3201/eid1305.061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vale-Gonçalves HM, Cabral JA, Faria MC, Nunes-Pereira M, Faria AS, Veloso O, et al. Prevalence of Leptospira antibodies in wild boars (Sus scrofa) from northern Portugal: risk factor analysis. Epidemiol Infect. 2015;143:2126–2130. doi: 10.1017/S0950268814003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan ML, Kemp R, Christley RM. Direct and indirect contacts between cattle farms in north-West England. Prev Vet Med. 2008;84:242–260. doi: 10.1016/j.prevetmed.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 57.FAO. ASF updated http://www.fao.org/ag/againfo/programmes/en/empres/ASF/situation_update.html. Accessed 9 Sept 2019.
- 58.GSO. https://www.gso.gov.vn/Default_en.aspx?tabid=766. Accessed 12 Aug 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Summary of survey data for pig farms in Bac Giang and Nghe An province of Vietnam.
Additional file 2. Questionnaire for pig farmers.
Data Availability Statement
Our questionnaires are available (Additional file 2). All datasets supporting our findings are available from the corresponding author on reasonable request.