Abstract
In hospitals, seizures and encephalopathy are one of the common complications observed in critically ill patients. Drug intoxication, metabolic derangements, and anatomical abnormalities can cause altered mental status. We encountered an uncommon case with a diagnostic dilemma due to persistent encephalopathy, where metronidazole toxicity was an etiological factor. A 45-year-old male, who was admitted with the diagnosis of ruptured amoebic liver abscess. During the course of his management, he developed seizures and altered sensorium. After excluding other etiologies for in-hospital de novo seizure, a suspicion of metronidazole toxicity was considered. MRI brain was done which suggested the same. Metronidazole induced encephalopathy (MIE) is an uncommon adverse effect of treatment with metronidazole. Diagnosis is made by identifying specific radiological findings. It characteristically affects the cerebellum and subcortical structures. While the clinical and neuroimaging changes are usually reversible, persistent encephalopathy with poor outcomes may occur as seen in our case.
Keywords: Encephalopathy, metronidazole, seizure
Introduction
Metronidazole is a nitroimidazole antimicrobial drug that is effective against anaerobic bacteria and protozoa. Due to its safety and low cost; it is one of the most commonly used over-the-counter drugs. Generally, well-tolerated, the most common adverse drug reactions observed in patients with metronidazole include nausea, dysgeusia, and abdominal cramping. Neurotoxicity is very rare in the form of peripheral neuropathy, headache, ataxia, seizures, and encephalopathy. While the exact incidence of metronidazole induced encephalopathy (MIE) is not known, most cases in the literature have occurred after prolonged treatment. We report a case of MIE that occurred in a patient after a treatment course for ruptured liver abscess.
Case Report
A 45-year-old male who was a chronic alcohol user presented with high-grade fever, right upper quadrant abdominal pain, and abdominal distension for past 12 days. On abdominal examination, he had tender hepatomegaly and ascites. Ultrasound abdomen revealed ruptured liver abscess and ascites. He was started with intravenous metronidazole 800 mg TDS and piperacillin-tazobactam 2.25 gm QID on the day of admission and the abscess drained subsequently. With the given treatment and supportive care, he was improving clinically.
On the 12th day of admission, he developed one episode of generalized tonic-clonic seizures (GTCS) for 30 s and then he gained consciousness within 10 min. He was loaded with levetiracetam. Metabolic parameters like blood glucose and electrolytes were within the normal range. NCCT head was done to rule out new-onset infarct/intracranial hemorrhage or other gross organic pathology. The next day, he again developed two episodes of seizures following which he had poor responsiveness; hence he was intubated and mechanically ventilated as a result of airway protection.
In view of persistently altered sensorium (GCS–8/15) with multiple liver abscesses and chronic alcohol use, the possibility of hepatic encephalopathy was considered. However, his ultrasound imaging and metabolic parameters were not suggestive for chronic liver disease and hepatic encephalopathy. For further evaluation, we were able to perform MRI on day 29 of admission which showed symmetric areas of FLAIR hyperintensities and restricted diffusion seen in the dentate nuclei, dorsal Pons, and posterior limb of internal capsule and splenium of corpus callosum suggestive of metronidazole toxicity [Figure 1]. Subsequently, metronidazole was stopped on the same day. The total dose of metronidazole received by patient till first episode of seizure was 28.8 g. The total cumulative dose was 69.6 g. His further hospital stay was complicated by cardiac arrest due to hypoxia on next day of stopping metronidazole. He attained return of spontaneous circulation after 20 min of CPR. Following CPR, his sensorium further deteriorated (GCS–3/15) and did not improve further. Since he had cardiac arrest, clinical improvement with reversal of metronidazole toxicity was not seen. Repeat MRI was done after 1 month of previous MRI which showed marked reduction in FLAIR hyperintensities in the dentate nuclei, dorsal pons, and splenium of corpus callosum suggestive of partial resolution in changes due to metronidazole toxicity. However, there were new areas of symmetric FLAIR hyperintensities in basal ganglia and thalamus suggestive of new hypoxic-ischemic changes due to cardiac arrest [Figure 2]. The post-cardiac arrest was managed conservatively, in spite of good supportive care, he succumbed to ventilator-associated pneumonia.
Figure 1 (A-D).
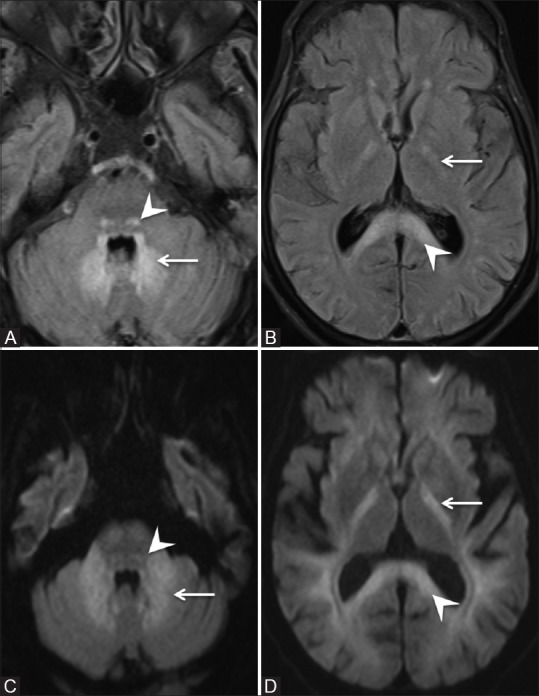
Initial MRI brain scan on day 29 of admission. (A and B) - Axial FLAIR images (C and D) - Axial Diffusion-Weighted Images. Symmetric areas of FLAIR hyperintensity and restricted diffusion are seen involving the dentate nuclei (arrow in A and C), dorsal pons (arrowhead in A and C), posterior limbs of the internal capsule (arrow in B and D) and splenium of corpus callosum (arrowhead in B and D)
Figure 2 (A-D).
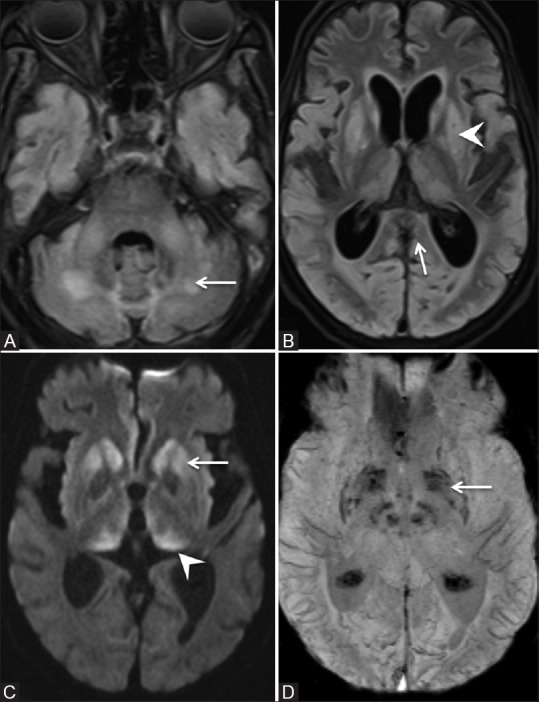
Repeat MRI brain on day 59 of admission. (A and B) Axial FLAIR images. There is marked reduction in the areas of FLAIR hyperintensity in the dentate nuclei (arrow in A), dorsal pons and splenium of corpus callosum (arrow in B). Symmetric FLAIR hyperintensity involving bilateral basal ganglia (arrowhead in B). (C) Axial Diffusion-weighted image. Restriction of diffusion in bilateral basal ganglia (arrow) and thalami (arrowhead). (D) Axial Susceptibility weighted images. Micro-hemorrhages in bilateral basal ganglia (arrow)
Discussion
Metronidazole is a nitroimidazole drug, which has broad-spectrum cidal activity against anaerobic bacterial and protozoal infections, including amoebiasis. The nitro radical of metronidazole acts as an electron sink that competes with biological electron acceptors of the anaerobic organism in the PFOR pathway. Although the drug is relatively safe and well-tolerated in common setting, patient may experience side effects in both long-term and short-term use. It is occasionally associated with an unpleasant metallic taste, nausea, and abdominal pain. These side effects are dose-dependent and often persistent for 24to48 h after consumption. Peripheral neuropathy is the most common nervous system adverse effect.
Among patients with metronidazole toxicity to the central nervous system, maximum cases were presented with cerebellar dysfunction (75%) followed by altered mental status (33%) and seizures (13%). Among cerebellar dysfunction, dysarthria, ataxia, dysmetria, and nystagmus were the most common findings on examination in descending order of frequency.[1] Altered mental status is generally a part of the encephalopathy; however, it can be due to nonconvulsive status as well. Metronidazole toxicity can also present as extrapyramidal manifestations. There are several reported cases of chorea and myoclonus as presenting symptoms. Sensorineural hearing loss could be a presenting symptom. The duration of treatment with metronidazole before occurrence of cerebellar symptoms is variable, and toxic cumulative doses range from 13.2 g to 228 g.[2] The half-life of metronidazole is 6–8 h; however, the half-life in patients with liver dysfunction or renal dysfunction is approximately 3-folds higher.[3] Accordingly, it is necessary to limit the total cumulative dose to less than 20 g in such patients. Our patient had mild hepatic dysfunction, and the total cumulative dose was 69.6 g.
Kumsi et al. reported an adult patient, who developed encephalopathy and cerebellar dysfunction and sensory neuropathy after receiving oral metronidazole. She recovered fully after the withdrawal of the drug but the features of neuropathy recovered later.[4] On cessation of metronidazole, the symptoms and MRI changes usually resolve. Most cases have a complete resolution of symptoms after the interruption of metronidazole (65%) or at least a significant improvement (29%). However, some cases may suffer a permanent deterioration (3%), even resulting in death. There are two case reports, in which the patients died due to irreversible MIE despite cessation of the drug.[5]
Metronidazole being a lipophilic drug can penetrate cerebrospinal fluid (CSF) and the central nervous system easily. The ratio of serum and CSF concentration is nearly 1.0.[6] The exact mechanisms of metronidazole neurotoxicity remain unclear, several hypotheses have been proposed by various researchers. The signal intensity changes observed on the diffusion-weighted (DW) images most likely represent interstitial edemas. Ahmed et al. first described reversible neuroimaging in MIE and postulated the reversibility by axonal swelling with increased water content rather than a demyelinating process.[7] Most brain lesions induced by metronidazole are reversible. It has been suggested that metabolites of metronidazole inhibit RNA protein synthesis which could lead to neurotoxicity. Once the medication is discontinued, the syndrome may improve within days and can be resolved within several weeks.
The clinical disease concept of MIE was reported over 20 years ago. However, MIE-specific MRI findings have been reported recently. Patients with metronidazole toxicity usually have bilaterally symmetric T2 hyperintense reversible lesions in the cerebellar dentate nucleus and the inferior colliculus. The other regions usually affected are subcortical white matter, anterior commissure of the splenium, basal ganglia, midbrain, cerebellar white matter, and inferior olivary nuclei.[8] The lesions appear as nonenhancing, hyperintense on T2-weighted, and FLAIR images without evidence of a mass effect. The DW image signal is high with variable apparent diffusion coefficient values. The brain MRI findings of MIE were graded as shown in Table 1.[9]
Table 1.
Grading of CNS findings in MIE[9]
| Grade | Severity | Extent of involvement of brain |
|---|---|---|
| Grade 1 | Minimal | Symmetric involvement of one lobe (frontal, temporal, parietal, or occipital) without the involvement of the corpus callosum, basal ganglia, thalami, or internal capsules |
| Grade 2 | Mild | Symmetric involvement of two lobes, or of one lobe plus symmetric involvement of one of the corpus callosum, basal ganglia, thalami, or internal capsules |
| Grade 3 | Moderate | Symmetric involvement of two lobes plus symmetric involvement of one of the corpus callosum, basal ganglia, thalami, or internal capsules |
| Grade 4 | Severe | symmetric extensive, and confluent involvement of three or all lobes from the ventricular margin to the subcortical white matter, or of two lobes plus symmetric involvement of two of the following: corpus callosum, basal ganglia, thalami, or internal capsules |
In view of a lack of accurate diagnostic tools or clinical criteria, diagnosis always depends upon high clinical suspicion, specific imaging abnormalities, ruling out other clinical and radiological differentials and significant improvement after the prompt withdrawal of the metronidazole. It is important to rule out another differential diagnosis such as demyelinating diseases, metabolic and toxic diseases like Wernicke's encephalopathy and osmotic myelinolysis with pontine or extrapontine damage.
Withdrawing metronidazole as early as possible along with supportive care is the only proven measure. Nevertheless, where a 5-nitroimidazole is indispensable, replacement with other 5-nitroimidazole like tinidazole or ornidazole may be tried; however, similar side effects have been observed with these drugs also.[10] Though there has been a positive report of diazepam as a measure to shorten the time of recovery in dogs, no such reports are published in the case of human beings.
Learning points:
In the hospital setting, new-onset seizures have various causes, of which drugs are common culprits. Hence, reviewing drug history is important
The side effects of metronidazole may vary from nausea, metallic taste to MIE, or cerebellar dysfunction
Metronidazole-specific MRI findings are bilaterally symmetric T2 hyperintense cerebellar dentate nuclei and the inferior colliculi. The other regions usually affected are subcortical white matter, anterior commissure of the splenium, basal ganglia, midbrain, cerebellar white matter, and inferior olivary nuclei
Patients with hepatic dysfunction or renal dysfunction are at an increased risk of MIE.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: A systematic review. Clin Neuropharmacol. 2011;34:241–7. doi: 10.1097/WNF.0b013e3182334b35. [DOI] [PubMed] [Google Scholar]
- 2.Ish P, Sethi P, Anuradha S, Dewan R, Nischal N. Metronidazole toxicity presenting as acute cerebellar syndrome. Astrocyte. 2015;1:320–1. [Google Scholar]
- 3.Cheong HC, Jeong TG, Cho YB, Yang BJ, Kim TH, Kim HC, et al. Metronidazole-induced encephalopathy in a patient with liver cirrhosis. Korean J Hepatol. 2011;17:157–60. doi: 10.3350/kjhep.2011.17.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusumi RK, Plouffe JF, Wyatt RH, Fass RJ. Central nervous system toxicity associated with metronidazole therapy. Ann Intern Med. 1980;93:59–60. doi: 10.7326/0003-4819-93-1-59. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs K, Stern-Nezer S, Buckwalter MS, Fischbein N, Finley Caulfield A. Metronidazole-induced encephalopathy: Not always a reversible situation. Neurocrit Care. 2015;22:429–36. doi: 10.1007/s12028-014-0102-9. [DOI] [PubMed] [Google Scholar]
- 6.Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–83. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed A, Loes DJ, Bressler EL. Reversible magnetic resonance imaging findings in metronidazole-induced encephalopathy. Neurology. 1995;45:588–9. doi: 10.1212/wnl.45.3.588. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR Imaging of Metronidazole-Induced Encephalopathy: Lesion distribution and diffusion-weighted imaging findings. Am J Neuroradiol. 2007;28:1652–8. doi: 10.3174/ajnr.A0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinney AM, Kieffer SA, Paylor RT, SantaCruz KS, Kendi A, Lucato L. Acute toxic leukoencephalopathy: Potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. Am J Roentgenol. 2009;193:192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 10.Sinha S, Mahadevan A, Bindu P, Taly A, Chacko J, Pramod K, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: Insights into possible pathogenesis. Neurol India. 2011;59:743–7. doi: 10.4103/0028-3886.86552. [DOI] [PubMed] [Google Scholar]


