Abstract
Most of the fetal deformities are caused due to genetic abnormalities. Although magnetic resonance imaging (MRI) may be used to accurately diagnose these deformities, it has been reported that gene analysis is a more accurate diagnostic method. Harlequin ichthyosis (HI) or Ichthyosis fetalis (IF) is a rare and extremely severe hereditary skin disorder with autosomal recessive inheritance. The ultrasound features have been described well and the diagnosis can be made with a fair degree of confidence. However, the final diagnosis needs to be established by prenatal invasive tests. In the present study, we describe the diagnosis of HI in the third trimester on fetal MRI referred to our department with suspicion of anterior encephalocele which was later confirmed through postnatal genetic evaluation.
Keywords: Adenosine triphosphate binding cassette A12, fetal, gene, harlequin, magnetic resonance imaging
Introduction
Harlequin ichthyosis (HI) or Ichthyosis fetalis (IF) is a rare and severe hereditary skin disorder with autosomal recessive inheritance.[1,2] It is a unique condition because of its peculiar clinical appearance and very high perinatal mortality.
The syndrome occurs in about 1 in 300,000 births.[3] Till 2003 (most recent data), 138 cases of HI have been reported. Since 1989, nearly 12 cases of HI have been diagnosed by ultrasound. The first description of congenital ichthyosis was given in 1750 by Hart.[4] The first case of prenatal diagnosis of HI was reported in 1983, which was based on skin biopsy under fetoscopy in a patient who had two infants previously affected by the syndrome.[5] The first case of prenatal sonographic diagnosis of HI was reported by Mihalko et al. in 1989.[6] Finally, the causative gene ABCA12 (Adenosine triphosphate binding cassette A12) was discovered in 2005. Most of the neonates are born prematurely with low Apgar scores.[7] Most of the neonates succumb within days of birth due to mechanical restriction of breathing due to reduced chest movements, pneumonia, sepsis, and electrolyte imbalance.[8]
The aim of the present study is to provide emphasis on MRI combined with genetic analysis which is a more effective diagnostic method as stated by Wang et al.[9]
With the advancement in ultrasound resolution and knowledge of the condition, prenatal diagnosis can be made. However, final diagnosis is done only after prenatal invasive tests are performed or after birth of the fetus.
Case History
A 28-year-old primigravida with 31 weeks of gestation assigned by last menstrual period (LMP) with consanguineous marriage and with no significant medical history was referred for fetal MRI.
There was a suspicion of anterior encephalocele on an ultrasound done at the same gestation age. With ultrasound the diagnosis did not provide any abnormality at 20 weeks. Fetal MRI was performed on Philips-Ingenia 3.0T MR system after taking informed written consent.
T2-weighted fast (turbo) spin-echo (SE) was done in axial, coronal, and sagittal planes with TE of 80 ms and TR of 1500 ms. T1-weighted two-dimensional gradient echo (GRE) was done in axial plane with TE of 1.81 ms and TR of 4.23 ms. An additional diffusion-weighted sequence was done.
There was polyhydramnios assessed subjectively. There was no evidence of any calvarial defect or encephalocele. Eversion of bilateral eyelids (ectropion) was seen [Figure 1]. There were T2 hyperintense and T1 hypointense (not shown) lesions over both orbits in the region of eyelids [Figure 2]. The mouth was wide-open like a fish with slightly protruded tongue [Figure 3] which was persistent on all sequences. These findings lead us to search for other associated features. There was flat nose [Figure 3]. Clinical picture of the neonate few hours after delivery showed ectropion, bilateral supraorbital swellings and wide open mouth [Figure 4]. Limbs were persistently seen in semiflexed position [Figure 5A and B]. Toes were constantly in the plantarflexed (incurved) position [Figure 5C] and fingers were clenched [Figure 5D] in every sequence. Upon very close observation, there were focal discontinuities along the skin surface over the anterior chest wall with a skin flap [Figure 6]. Immediate postpartum clinical picture of the neonate showed semi flexed limbs, incurved toes clenched fingers and skin fissuring [Figure 7].
Figure 1.
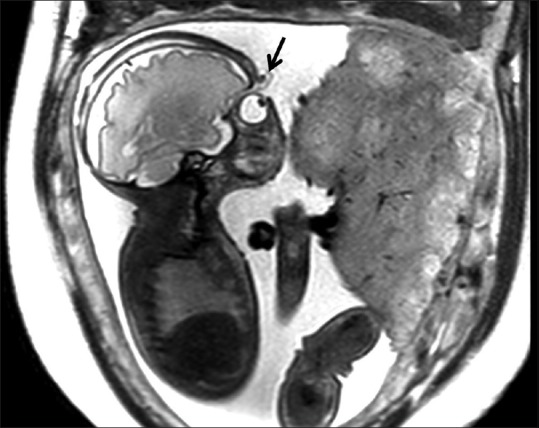
Sagittal T2-weighted image of the fetus showing eversion of the eyelid (arrow)
Figure 2 (A and B).
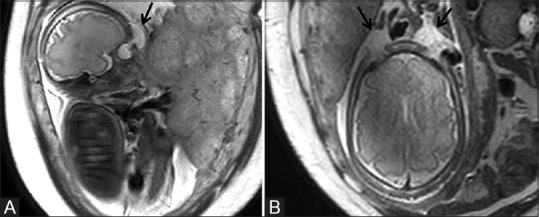
Sagittal T2-weighted image (A) of the fetus showing hyperintense cystic swelling in supraorbital location (arrow). Axial image (B) showing the cystic swelling on both sides (arrows)
Figure 3.
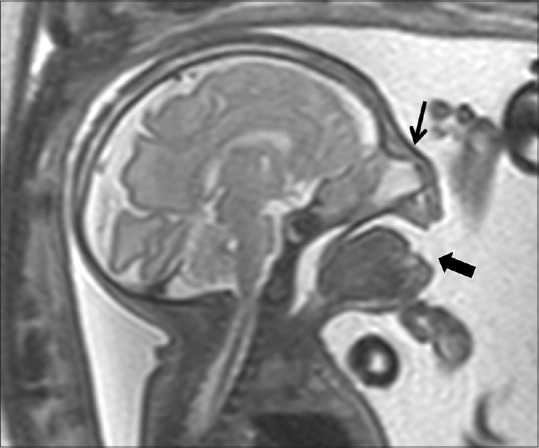
Sagittal T2-weighted image of the fetus showing wide-open mouth (thick arrow) and flat nose (arrow)
Figure 4.
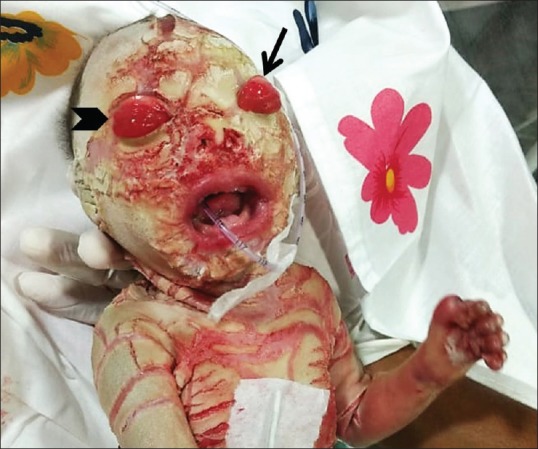
Clinical picture of the neonate few hours after delivery showing ectropion (thin arrow), bilateral supraorbital swellings (chevron), and wide-open mouth (also seen in Figure 7)
Figure 5 (A-D).
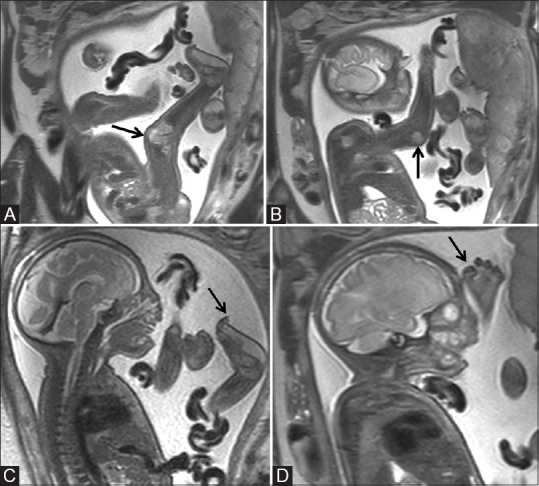
Sagittal T2-weighted image (A) of the fetus showing semiflexed lower limb (arrow), (B) semiflexed upper limb (arrow), (C) showing incurved toes (arrow), and (D) showing clenched fingers (arrow)
Figure 6 (A-C).
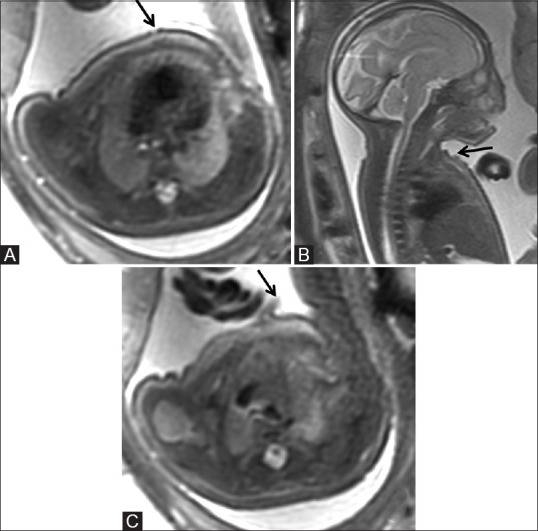
Axial (A) and sagittal (B) T2-weighted images of the fetus showing irregular skin surface with focal areas of discontinuities (arrow) along the anterior chest wall (C) and small focal skin flap, possibly due to exfoliation (arrow)
Figure 7.
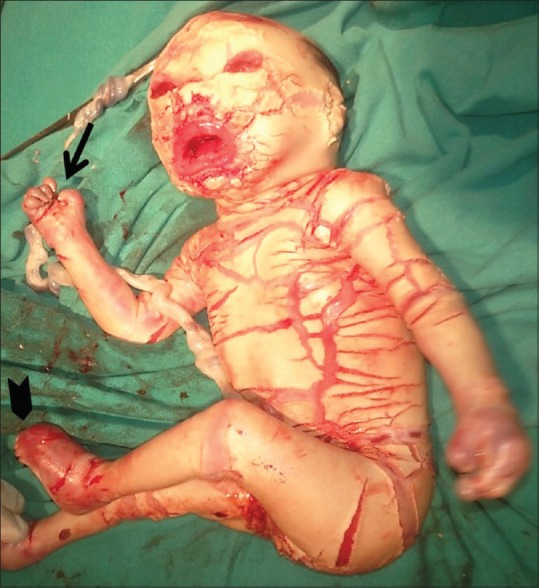
Immediate postpartum clinical picture of the neonate showing semiflexed limbs (thin arrow), incurved toes (arrowhead), clenched fingers (chevron), and skin fissuring which corresponds to the imaging findings
The constellation of findings raised the possibility of HI. The retrospective ultrasound examination showed similar findings. The possible diagnosis of HI was given on MRI. The couple was offered prenatal invasive testing to diagnose the said condition but they refused. The female infant was born prematurely at 35 weeks. All the clinical features of HI were present. The infant died on day 3 of life. Clinical exome sequencing of neonate revealed a homozygous variant in ABCA12 gene with possibility of autosomal recessive congenital ichthyosis type 4B (harlequin).
Discussion
Ichthyoses belong to the group of genodermatoses, characterized by hyperkeratosis and desquamation of the epidermis. Harlequin fetus (OMIM#242500) is the most severe form of congenital ichthyosis.[10] Clinical features of a new born with HI reveals thickened, whitish to yellowish colored, armor-like skin with fissures that divide the skin into polygonal or diamond-shaped sections with reddish oozing cracks all over the body, ectropion, eclabium, and a round, persistently open mouth. The nose is flat with deformed ears. The limbs are usually in semiflexed position. Other features include the absence of scalp hair, eyebrows, and eyelashes.[1,11] Toes are persistently in the plantarflexed (incurved) position and there are persistent clenched fingers.[12]
Abnormal keratinocyte lamellar granules are a typical hallmark of HI skin.[13] In the human fetus, cornification of the skin begins between 14 and 16 weeks of gestation. Under light microscopy, skin from harlequin fetuses shows hyperkeratosis with hypertrophy of the horny layers.[4]
Causative gene ABCA12 (Adenosine triphosphate binding cassette A12) was discovered in 2005. Severe ABCA12 deficiency causes defective lipid transport via lamellar granules (LG) in keratinizing epidermal cells, resulting in the HI phenotype.[13,14,15] ABCA12 is a keratinocyte lipid transporter associated with LG formation and lipid transport via LG on the surface of keratinocytes.[14,15] Previously, the prenatal diagnosis had been established with fetal skin biopsy with fetoscopy using an electron microscope.[14,15] The discovery of this gene enabled prenatal diagnosis of HI by chorionic villus or amniotic fluid sampling in the earlier stages of pregnancy, particularly in cases of previously affected pregnancies.
Diagnostic imaging features include those described above. There may be polyhydramnios debris in amniotic fluid. The characteristic features which lead to suspicion of HI and which are constantly present are, bilateral ectropion with cystic swellings in front of the eyes, persistently wide-open mouth, eclabium, persistently semiflexed limbs, and constantly plantarflexed (incurved) toes.[12,16] The features of HI are typically seen in late gestation so the second-trimester scan can be normal in most of the cases.[12] With the above features diagnosis of HI can be achieved with fair confidence particularly in patients with no previous infant diagnosed with HI. With the history of HI in previous pregnancy, early diagnosis of HI can be achieved with prenatal invasive tests as there is 25% recurrence risk in each pregnancy.
Conclusion
HI is a rare autosomal recessive genetic disorder presenting in utero which is extremely difficult to diagnose with imaging. It is important for the radiologist to be aware of this disease entity and should specifically look for the characteristic imaging findings of HI. These findings may appear during the late second or early third trimester and may be suspected on ultrasound and confirmed on MRI. Prenatal genetic diagnosis should be advice to couples with previously affected infants.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Akiyama M. The pathogenesis of severe congenital Ichthyosis of the neonate. J Dermatol Sci. 1999;21:96–104. doi: 10.1016/s0923-1811(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed H, O'Toole EA. Recent advances in the genetics and management of harlequin ichthyosis. Pediatric Dermatology. 2014;31:539–46. doi: 10.1111/pde.12383. [DOI] [PubMed] [Google Scholar]
- 3.Multani AS, Sheth FJ, Shah VC, Chinoy NJ, Pathak S. Three siblings with harlequin Ichthyosis in an Indian family. Early Hum Dev. 1996;45:229–33. doi: 10.1016/0378-3782(96)01733-1. [DOI] [PubMed] [Google Scholar]
- 4.Bongain A, Benoit B, Ejnes L, Lambert JC, Gillet JY. Harlequin fetus: Three-dimensional sonographic findings and new diagnostic approach. Ultrasound Obstet Gynecol. 2002;20:82–5. doi: 10.1046/j.1469-0705.2002.00708.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanchet-Bardon C, Dumez Y, Labbé F, Lutzner M, Puissant A, Henrion R, et al. Prenatal diagnosis of harlequin fetus. The Lancet. 1983;321:132. doi: 10.1016/s0140-6736(83)91780-4. [DOI] [PubMed] [Google Scholar]
- 6.Mihalko M, Lindfors KK, Grix AW, Brant WE, McGahan JP. Prenatal sonographic diagnosis of harlequin ichthyosis. Am J Roentgenol. 1989;153:827–8. doi: 10.2214/ajr.153.4.827. [DOI] [PubMed] [Google Scholar]
- 7.Zapałowicz K, Wyględowska G, Roszkowski T, Bednarowska A. Harlequin ichthyosis—difficulties in prenatal diagnosis. J Appl Genet. 2006;47:195–7. doi: 10.1007/BF03194622. [DOI] [PubMed] [Google Scholar]
- 8.Elias S, Mazur M, Sabbagha R, Esterly NB, Simpson JL. Prenatal diagnosis of harlequin ichthyosis. Clin Genet. 1980;17:275–80. doi: 10.1111/j.1399-0004.1980.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Nie H, Wang Q, Zhang G, Li G, Bai L, et al. Use of magnetic resonance imaging combined with gene analysis for the diagnosis of fetal congenital heart disease. BMC Med Imaging. 2019;19:12. doi: 10.1186/s12880-019-0314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy-Brown L, Vella J, Lawlor-Klean P. Harlequin ichthyosis: A case study. Neonatal Netw. 2004;23:7–12. doi: 10.1891/0730-0832.23.3.7. [DOI] [PubMed] [Google Scholar]
- 11.Plocoste V, Bonneau D, Deshayes M. Le syndrome dube´be´ arlequin. J Gynecol Obstet Biol Reprod. 1992;21:247–50. [PubMed] [Google Scholar]
- 12.Rathore S, David LS, Beck MM, Bindra MS, Arunachal G. Harlequin ichthyosis: Prenatal diagnosis of a rare yet severe genetic dermatosis. J Clin Diagn Res. 2015;9:QD04. doi: 10.7860/JCDR/2015/15250.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, et al. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–84. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelsell PD, Norgett EE, Unsworth H, Teh MT, Cullup T, Mein CA, et al. Mutations in ABCA12 underlie the severe congenital skin disease harlequin ichthyosis. Am J Hum Genet. 2005;76:794–803. doi: 10.1086/429844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu A, Akiyama M, Ishiko A, Yoshiike T, Suzumori K, Shimizu H. Prenatal exclusion of harlequin ichthyosis; potential pitfalls in the timing of the fetal skin biopsy. Br J Dermatol. 2005;153:811–4. doi: 10.1111/j.1365-2133.2005.06778.x. [DOI] [PubMed] [Google Scholar]
- 16.Wen Y, Zhang SL, He J, Zhang XX, Yu XW. Prenatal diagnosis of congenital harlequin ichthyosis with two-and three-dimensional ultrasound in the third trimester. J Med Ultrasound. 2013;21:221–5. [Google Scholar]


