Abstract
Inflatable penile prostheses are an important tool in the treatment of medically refractory erectile dysfunction. One of the major complications associated with these prostheses is infections, which ultimately require device explanation and placement of a new device. Over the past several decades, significant work has been done to reduce infection rates and optimize treatment strategies to reduce patient morbidity. This article reviews the current state of knowledge surrounding penile prosthesis infections, with attention to the evidence for methods to prevent infection and best practices for device reimplantation.
Keywords: erectile dysfunction, infection, penile prosthesis
INTRODUCTION
Inflatable penile prostheses (IPPs) were first introduced in the 1970s as a method for treating erectile dysfunction (ED) and are the gold standard for ED refractory to medical therapy. Since their inception, these devices have been complicated by infection. Early pioneers of these devices attempted multiple strategies to reduce infection, including improved skin site preparation and attempting insertion under laminar flow conditions.1 Over the past 50 years, urologists have continued to work to advance our understanding of the pathophysiology of the infectious processes unique to IPP placement and have continued to develop strategies to reduce infections. When infection occurs, the IPP hardware must be removed to achieve source control, and a new device may be implanted. However, care must be taken to prevent penile shortening and reduce the risk of repeat infections. The following article reviews the current state of our understanding of IPP infections, the evidence base for infection prevention strategies, and the current techniques for managing infected IPPs.
PATHOPHYSIOLOGY OF IPP INFECTION
IPP infections vary in timing, symptomatology, and underlying microbiology. Infections can occur weeks, months, or (more rarely) years after implantation. Clinical presentation can range from dramatic local signs of infection accompanied by sepsis to more vague symptoms requiring a higher degree of clinical suspicion. In a review, Carson provided an in-depth discussion of the various organisms involved in IPP infections,2 which typically involve skin flora but may also include enteric organisms.3
Infections presenting within 8 weeks of device placement are classified as immediate infections (Figure 1 and 2) and are believed to originate from direct infection of the device during placement. Skin flora accounts for the largest group of bacteria contributing to IPP infection,4 and those associated with immediate infection are thought to be more virulent strains, such as Staphylococcus aureus.4 Gram-negative enteric bacteria have also been reported to have a faster onset of infection than low virulence skin flora.3 Immediate infections are more recognizable with obvious signs of infection, such as local erythema, induration, warmth, and purulent drainage.
Figure 1.

Immediate penile prosthesis infection. Note the visible edema and erythema
Figure 2.
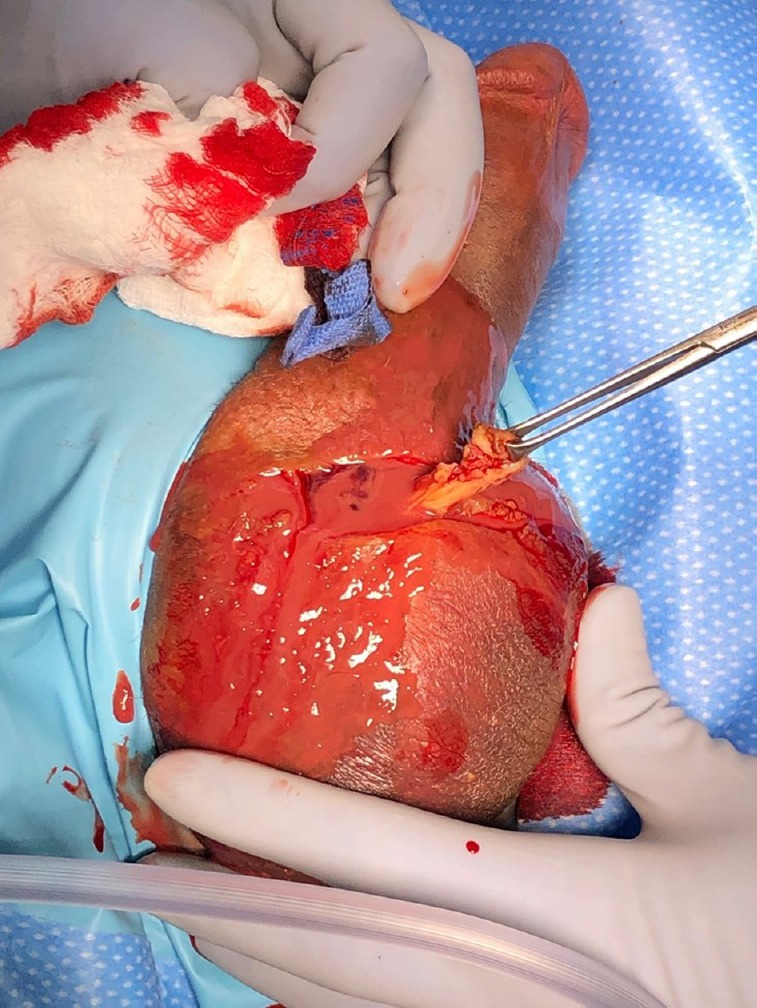
Immediate penile prosthesis infection. Note the purulent fluid. (Image courtesy of Dr. Rafael Carrion).
Unlike immediate infections, indolent infections (Figure 3) may present with vague symptoms and few signs of an overt infectious process; these infections may not become clinically apparent for months after surgery but most commonly present in the first year. Again, skin flora is the most frequently implicated, particularly S. epidermidis, which has been shown to colonize prostheses at high rates in several studies.4,5
Figure 3.

Indolent penile prosthesis infection. Note the pump fixation to the scrotal wall.
Some debate exists over how bacteria gain access to the implanted device. For most infections, bacteria are presumed to enter the wound directly during surgery after which they can either proliferate immediately, causing signs and symptoms of disease, or otherwise lie dormant. In response to the presence of a foreign body, the inflammatory process promotes formation of a fibrous capsule that encases the IPP.6 This capsule restricts blood flow and consequently limits immune system access to area surrounding the device7,8 and may serve to shield bacteria harbored within, thus allowing them to persist and cause infection outside the immediate postoperative period. Bacteria may also form biofilms, which adhere to the surface of the IPP hardware creating a protective layer of extracellular matrix substances that provides nourishment and further limits the exposure to antibiotics and the host immune system.9,10
INFECTION RISK FACTORS
Several factors have previously been shown to increase the risk for IPP infection among certain patients following primary implantation (as recently reviewed by Carrasquillo et al.).11 Diabetes has been proposed as a risk factor for IPP infection, and although some of the largest studies support this, data from the literature have been mixed. In recent analyses, poorly controlled blood sugar at the time of device insertion has been shown to be a risk factor for IPP infection,12,13 and infection rates have also been directly correlated with elevated glycosylated hemoglobin (HgbA1c) levels.14 Older studies have shown that diabetes itself did not contribute to a significantly increased risk for primary IPP infection.15 However, a more recent study of a large statewide database showed that IPP infections occurred in 3% of diabetic patients compared to only 2% of nondiabetic patients (P < 0.01) after primary implantation.16 Despite this, one small case series (n = 6) has demonstrated initial success with immediate salvage in diabetic patients presenting with purulent infections, indicating that diabetes is not contraindication to immediate salvage techniques.17
Patients who are immunocompromised, whether due to HIV or iatrogenic causes, have been shown to be at increased risk for IPP infection in certain cases. In a retrospective cohort study, Wilson and Delk15 showed that 50% (5/10) of patients on chronic steroids for suppression of chronic disease (i.e., chronic lymphocytic, leukemia, lupus, chronic obstructive pulmonary disease, and rheumatoid arthritis) developed IPP infections, but no (0/3) patients on chronic steroids for solid organ transplants. Subsequent data have corroborated the lack of risks associated with immunosuppression for transplant recipients,18,19 though others have indicated that these studies may not be powered to detect small differences in this group. HIV infection has been identified as another risk factor associated with IPP removal secondary to increased infectious complications in a multivariable analysis of a large administrative database.20
An additional medical comorbidity that has been reported to be associated with IPP infection is spinal cord injury. Spinal cord injury patients have been previously reported to be at higher risk of infection based on small retrospective cohorts15 as well as more recent analysis of more population-based administrative data.20 The increased risk has been proposed to be due to several mechanisms, including increased risk for urinary tract infections secondary to intermittent or indwelling catheter placement, decreased tissue perfusion, and delayed diagnosis of early infection/erosion secondary to loss of sensation.11,15
Behavioral risk factors also have been shown to play a role in risk for IPP infection. In a recent study, Balen et al.12 identified a nearly 10-fold increase in the odds of infection among those who had a history of polysubstance abuse and a greater than 12-fold increase for those who are homeless. These risk factors are modifiable and could be mitigated preoperatively to reduce infection risk, especially given the magnitude of the effects reported.
Infection rates among those undergoing revision IPP have previously been reported to be higher than those undergoing primary implantation. Wilson and Delk15 reported infection in 3% (24/823) of patients undergoing primary implantation and 10% (43/428) of patients undergoing revision IPP placement. The fibrous capsule that forms around the IPP hardware after implantation limits the ability of antibiotics and immune system to access bacteria and can lead to increased colonization of the device. Colonizing bacteria organize into biofilms that are particularly difficult to eradicate; one study estimated the rate of biofilm colonization of uninfected IPPs requiring revision to be 70%.5 Complete excision of both the device and the capsule is an essential part of treating the initial infection and preparing the wound for a salvage procedure.
INFECTION PREVENTION
Given the risk for infection at the time of IPP placement, surgeons performing this procedure must have a comprehensive plan for infection prevention in the perioperative period. Interventions can be divided into the following phases with regard to their timing related to surgery: preoperative, intraoperative, and postoperative. Many of the infection mediation strategies discussed below are not unique to urology or IPP implantation but represent established surgical standards from other disciplines. In addition, certain practices that are routinely performed may have little evidence base in the literature but are performed based on anecdotally perceived benefit.
Preoperative
The first step in managing infection for any surgical procedure began with appropriate patient selection and optimization of patient risk factors (Table 1). Patients appropriate for consideration of IPP implantation are those with erectile dysfunction who have failed nonsurgical management options, such as oral medications, intracavernosal injection, and vacuum erection devices. Patients desiring treatment should be evaluated for anatomic suitability for IPP placement. Additional thought should also be given to patient's manual dexterity and ability to operate the device.
Table 1.
Strategies for reducing primary inflatable penile prostheses infections in the preoperative and intraoperative periods
| Preoperative | Intraoperative | |
|---|---|---|
| Strategy | Optimization of comorbid conditions, particularly diabetes control and cardiovascular risk factors | Surgeon specializing in IPP placement |
| Assess for signs of infection or skin integrity issues | Surgical checklist | |
| Nasal swab and treatment for Staphylococcus aureus | Surgical site preparation: hair removal and skin preparation | |
| Preoperative chlorhexidine wash | ||
| Preoperative antibiotics | No-touch technique | |
| Infection retardant prosthetic coatings | ||
| Mummy wrap | ||
| Corporal washout (revisions) |
IPP: inflatable penile prosthesis
Consideration should be given to patient's comorbidities, and risk of infection based on those comorbidities should be discussed before surgery. Preoperative optimization of modifiable factors related to these comorbidities is imperative. Diabetic patients may benefit from preoperative HgbA1c testing as a marker of glucose control. While the data on this intervention are not firmly established, ideally, an appropriate diabetic management plan could be instituted with the patient's primary care provider before surgery. If not achievable, steps should be taken on the day of surgery to manage blood sugars and avoid hyperglycemia.12,14 Patients with cardiovascular risk factors should be assessed and obtain clearance from the cardiologist before surgery. While aspirin may be continued through surgery, it is often advisable to withhold more potent anticoagulation when possible to prevent formation of postoperative hematoma. Preoperative laboratories and an electrocardiogram may be prudent.
Care should also be taken to assess the patient for signs of preoperative infection. A thorough genital examination should be performed both in the office and immediately before surgery to ensure no evidence of local infection exists. Evidence of candidal groin infection can be treated preoperatively with oral antifungals before surgery to decrease risk for fungal IPP infections.6,21 Any open sores or skin compromise identified before surgery would need to be treated and resolved before IPP implantation. Despite inadequate evidence of clear benefit,22 preoperative urine cultures are also typically obtained with positive results treated using culture-directed antibiotics.
Preoperative nasal swab testing for S. aureus is also advisable. A seminal multicenter, randomized controlled trial performed in the Netherlands evaluated the effect of chlorhexidine wash and mupirocin treatment in preventing infections among patients admitted to the hospital. Among those undergoing surgical procedures, 3.4% (17/504) treated with this regimen experienced infections with S. aureus, compared to 7.7% (32/413) in the placebo group.23 Although a recent meta-analysis examining utility of preoperative chlorhexidine washes failed to show benefit,24 these authors recommend that patients bathe with chlorhexidine soap for 3 days before surgery and receive treatment with mupirocin for positive nasal swabs as the risks associated with these interventions are relatively low.
On the day of surgery, preoperative antibiotics should be administered for general prevention of surgical site infection,25,26 though no universal standard exists regarding the choice of antibiotic for IPP implantation. Both the American Urological Association (AUA) and the European Association of Urology (EAU) have developed surgical prophylaxis guidelines, though recent evidence shows that standard were found to be ineffective in 14%–38% of cases with coverage of Candida and methicillin-resistant S. aureus (MRSA) particularly lacking.21 Based on this study, Gross et al.27 outlined a protocol for obtaining cultures and administering antibiotics and antifungals at the time of salvage or explant. Their group recommended the use of vancomycin, piperacillin-tazobactam, and fluconazole perioperatively for empiric treatment of infected IPPs. However, this study did not specifically offer guidance on antibiotics for primary IPP placement or revision surgery. The authors did note that current AUA and EAU guidelines were ineffective in covering for the organisms seen at salvage or explant in their study.
Intraoperative
The literature contains examples of several interventions that have been shown to reduce the rate of IPP infection Table 1. Several of these interventions relate to team efficacy and operating room dynamics and have previously been shown to be effective in other disciplines. Other interventions utilize currently available antimicrobial products to directly decrease bacterial access to the IPP hardware itself.
With regard to care team composition, evidence suggests that surgeons performing more IPP placements may have better infection outcomes. A study by Henry28 demonstrated lower infection rates among IPPs placed by different surgeons in a large multi-surgeon group (14%) compared to the rate of infection for a single surgeon specializing in this procedure (0), which may be the result of a decrease in operative time (94 min vs 34 min). However, this study was limited to a single surgical center and may also be attributable to individual skill that is not generalizable to other providers. Although studies have suggested that increased time is associated with increased infection29 potentially due to increased exposure time of the IPP, other studies looking at infection rates in patients with placements of IPPs alone compared to simultaneous IPP and artificial urethral sphincter placement (thus increasing operative time) have not shown a significant difference.30,31
Another tool shown to decrease IPP infection is a surgical checklist. One group reported initial infection rates of 2.9% followed by an outbreak period during which IPP infection rates climbed to 54.5%. After implementing a checklist to ensure completion of a preoperative protocol to reduce risk of IPP device contamination, a single academic teaching center was able to reduce their IPP infection rate to 0 over the subsequent 2 years.32 Criticism of this method included the mix of data-driven and nonempiric items on the checklist, but it remains a simple tool with demonstrable results in reducing IPP infection.
During the procedure, contact of the IPP hardware can be minimized in several ways to reduce bacterial contamination. The “no-touch technique” was introduced by Eid as a protocol to maximize these efforts.33 In this system, contact barriers are used and a small opening is created to access the minimum amount of tissue necessary for device implantation. Results indicated that the adoption of this technique reduced IPP infection rates from 2% using the standard implantation technique to 0.46% using the no-touch technique.34 Contact minimization methods can be modified to surgeon preference but certainly have been shown to be useful.
Multiple studies have been performed looking at surgical site preparation with regard to hair removal and choice of skin preparation solution. Data regarding hair removal have not shown a definite benefit. While a meta-analysis suggests that clipping is associated with fewer surgical site infections than shaving,35 one clinical trial comparing clipping and shaving of the male genitals showed that shaving caused less skin trauma and more complete hair removal.36 With regard to skin preparation, recent data support the use of chlorhexidine-alcohol compared to povidone-iodine with surgical site infection rates of 9.5% and 16.1%, respectively.37 Skin preparation solution selection has also been examined in patients undergoing genitourinary prosthetic implantation; rates of positive skin cultures were 8% in patients prepped with chlorhexidine-alcohol and 32% of patients prepped with povidone-iodine.38
A major technological advancement in the prevention of IPP infections has been the creation of devices with infection retardant coatings. These coatings kill contaminant bacteria and prevent the formation of biofilms on implanted devices. A large meta-analysis showed infection rates for coated prostheses of 0.89% compared to 2.32% for uncoated devices.39 Currently, both IPP manufacturers offer two different forms of infection retardant IPP coatings. Boston Scientific (Marlborough, MA, USA) offers a coating called InhibiZone, which is composed of rifampin and minocycline, coated onto the device during manufacturing. Coloplast (Humlebæk, Denmark) offers a hydrophilic coating that absorbs antibiotics when submerged in solutions before implantation.40 A recent systematic review examined the various antibiotic dip combinations studied to date and recommends that Coloplast devices be used with a combination of rifampin/gentamicin.41
After the operation, a Mummy wrap28 is used which may aid in hemostasis but has also been shown to be associated with decreased odds of infection.12 A potential mechanism for reduced infection is the prevention of hematoma, which can be a nutrient source for bacteria. Postoperative hematoma prevention can also include tight corporotomy closure, device postoperative inflation, and surgical drain placement. However, none of these methods have been directly shown to decrease the rates of infection.
With regard to revision IPPs, a thorough washout of the corpora has been shown to be crucial in preventing subsequent infections of reimplanted devices. A large multicenter study compared patients undergoing revision implants either with or without an antiseptic washout; the antiseptic washout reduced the infection rate during revision surgery from 11.6% to 2.9%.42 However, choice of washout solution does not appear to have a significant effect, rather the act of vigorous irrigation is associated with lower infection rates,43 potentially due to disruption and eradication of residual biofilm components.
Postoperative
Ambiguity exists regarding the need and duration for postoperative antibiotics. A recent survey of urologists performing IPP placements showed that 89% prescribed postoperative antibiotics,44 but there is no evidence to guide this practice. Recommendations for overall surgical care indicate that antibiotics should be discontinued within 24 h of the procedure,45 but the risk factors associated with general surgical procedures do not necessarily coincide with those surrounding IPP placement. The optimal protocol for postoperative antibiotics remains unclear.
TREATMENT OF IPP INFECTION
A tenet of modern treatment strategies for IPP infection is the need to maintain corporal volume, and thus penile length, while still eradicating the infection. Historically, IPP infection was treated by device removal followed by systemic antibiotics and local irrigation/drainage. Only after the infection had been cleared would consideration be given to implanting a new device. In the interim, inflammation secondary to the infectious process would lead to scarring and fibrosis of the corpora, limiting their ability to accept the cylinders of a new device without additional procedures.46 A recent observational study showed that those men undergoing delayed reimplant of a prosthetic device experienced a mean loss of 3.7 cm in total corporal length, compared to 0.6 cm loss in length in patients undergoing salvage therapy.47
In 1996, Brant et al.48 described initial success with a salvage operation and immediate replacement of a new device. The infected device and its fibrotic capsule are removed completely, and the wound cavities, including the corpora, scrotal pump pocket, and reservoir pump pocket, are irrigated with a series of antimicrobial solutions. After a re-prep, re-drape, and instrument change, a new device is implanted. Systemic antibiotics are used intraoperatively, and culture-directed antibiotics are continued for 1 month postoperatively. In the initial report, 91% (10/11) patients remained free of infection after a mean follow-up period of 21 months. The high success rate for this technique was again demonstrated in a subsequent study by the same group, which showed that 82% (45/55) of the patients with a mean follow-up of 35 months remained free of infection.49 Thus, salvage in appropriate patients became considered the gold standard in care following IPP infection.
In 2016, a modification of the original Mulcahy technique was introduced using a malleable prosthesis for reimplantation to reduce infection rates after salvage.27 The purpose of this modification is to eliminate the scrotal hardware and allow for closure of the scrotal wound to minimize the risk of infection. The placement of a malleable prosthesis still allows for the preservation of corporal space and penile length, and the malleable implant can be converted over to an IPP with a subsequent surgery per patient preference. In a multicenter study of 58 patients undergoing this technique, 93% (54/58) of patients remained infection-free following salvage treatment. Of those, 31% (17/54) patients eventually chose to exchange the malleable implant for an IPP.27
An alternative method for preserving corporal volume without implantation of new prosthesis is placement of a Carrion cast, which uses an injectable compound to act as a temporary spacer within corporal bodies. A mixture of calcium sulfate and antibiotics (vancomycin and tobramycin) is prepared and injected via bilateral coporotomies to fill the intracorporal space after device removal. The calcium sulfate mixture hardens to form a cast which hardens and then is slowly reabsorbed by the body with complete reabsorption occurring at approximately 6 weeks.50 Long-term studies examining the infection rates with this technique have yet to be reported.
Despite the evidence to suggest improved patient outcomes with salvage procedures, a recent analysis of a national inpatient sample shows that salvage procedures are performed in a minority of cases. Of 1557 patients examined, only 17% received a salvage prosthesis.51 Those receiving salvage were statistically more likely to be younger with less severe presentation and to have received care in an urban teaching hospital. This may in part be due to the availability of trained urologists and/or IPP devices and support staff at nonteaching hospitals.
FUTURE DIRECTIONS AND EMERGING WORK
One area of active research with regard to IPP infection is understanding the underlying bacterial physiology, particularly their ability to form and persist as biofilms. Bacteria within biofilms exist as a protected sessile community with a distinct phenotype that allows them to adhere, persist, and even inhibit innate immune responses.52 Furthermore, the bacteria within these multicellular biofilm communities have been shown to have increased ability to resist eradication with antibiotics due to several mechanisms, including limited diffusion of antibiotics through the biofilm matrix, alteration in the microstate of the chemical environment, disabling antibiotics, and entry of certain bacteria within the biofilm into a protected spore-like state.53
Current strategies for infection control include antimicrobial skin barriers and infection retardant device coatings. Alternate strategies to limit the ability of bacteria to adhere to IPP devices are plausible and may further enhance infection prevention, such as altering the hydrophobicity or smoothness of device surfaces.54 Other approaches for preventing biofilm formation have been proposed, including agents to disrupt the biofilm extracellular matrix material, which disrupt the protected state of embedded bacteria, or interference with cell signaling to promote dispersion of bacteria and inhibit community formation.54
Further work is needed to understand IPP infection patterns with respect to microbial species and patient susceptibilities and to understand the effect of current infection prevention strategies. A systematic review of studies looking at the isolates from infected IPPs noted that these interventions may be changing the bacterial composition of biofilms with a shift away from low virulence skin flora and toward enteric bacteria, leading to more aggressive infections.55 A more recent multicenter study analyzed culture data from explanted IPPs and also showed that patients with coated IPPs were more likely to have atypical bacteria and had a lower rate of infection with Staphylococcus species.56 This suggests that while infection retardant IPP coatings decrease biofilm formation from skin flora, they may be shifting the pattern of infectious organisms toward more virulent pathogens. More generally, further creation and dissemination of clinical and bench research are needed to minimize IPP infection risk while maximizing ideal outcomes for all patients.
CONCLUSION
IPPs are a safe and effective treatment option for medically refractory erectile dysfunction. Many gains have been made in our understanding of infection prevention over the past four decades, particularly in preventing device colonization by organisms that increase infection risk. If infection is encountered, vigorous washout followed by salvage procedures, in appropriate patients, with immediate reimplantation to maintain corporal volumes is recommended. Future work is needed to further understand the infectious process and to examine new technologies and techniques for prevention and intervention.
AUTHOR CONTRIBUTIONS
All authors contributed to the literature review and writing of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Mobley DF. Early history of inflatable penile prosthesis surgery: a view from someone who was there. Asian J Androl. 2015;17:225–9. doi: 10.4103/1008-682X.140962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson CC. Diagnosis, treatment and prevention of penile prosthesis infection. Int J Impot Res. 2003;15(Suppl 5):S139–46. doi: 10.1038/sj.ijir.3901091. [DOI] [PubMed] [Google Scholar]
- 3.Montague DK, Angermeier KW, Lakin MM. Penile prosthesis infections. Int J Impot Res. 2001;13:326–8. doi: 10.1038/sj.ijir.3900768. [DOI] [PubMed] [Google Scholar]
- 4.Licht MR, Montague DK, Angermeier KW, Lakin MM. Cultures from genitourinary prostheses at reoperation: questioning the role of Staphylococcusepidermidis in periprosthetic infection. J Urol. 1995;154:387–90. [PubMed] [Google Scholar]
- 5.Henry GD, Wilson SK, Delk JR, 2nd, Carson CC, Silverstein A, et al. Penile prosthesis cultures during revision surgery: a multicenter study. J Urol. 2004;172:153–6. doi: 10.1097/01.ju.0000132141.48587.f1. [DOI] [PubMed] [Google Scholar]
- 6.Mulcahy JJ. Current approach to the treatment of penile implant infections. Ther Adv Urol. 2010;2:69–75. doi: 10.1177/1756287210370330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson SK, Costerton JW. Biofilm and penile prosthesis infections in the era of coated implants: a review. J Sex Med. 2012;9:44–53. doi: 10.1111/j.1743-6109.2011.02428.x. [DOI] [PubMed] [Google Scholar]
- 8.Elmussareh M, Goddard JC, Summerton JD, Terry TR. Minimising the risk of device infection in penile prosthetic surgery: a UK perspective. J Clin Urol. 2013;6:280–8. [Google Scholar]
- 9.Welliver RC, Jr, Hanerhoff BL, Henry GD, Kohler TS. Significance of biofilm for the prosthetic surgeon. Curr Urol Rep. 2014;15:411. doi: 10.1007/s11934-014-0411-8. [DOI] [PubMed] [Google Scholar]
- 10.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18:843–6. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 11.Carrasquillo RJ, Munarriz RM, Gross MS. Infection prevention considerations for complex penile prosthesis recipients. Curr Urol Rep. 2019;20:12. doi: 10.1007/s11934-019-0875-7. [DOI] [PubMed] [Google Scholar]
- 12.Balen A, Gross MS, Phillips EA, Henry GD, Munarriz R. Active polysubstance abuse concurrent with surgery as a possible newly identified infection risk factor in inflatable penile prosthesis placement based on a retrospective analysis of health and socioeconomic factors. J Sex Med. 2016;13:697–701. doi: 10.1016/j.jsxm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SK, Carson CC, Cleves MA, Delk JR., 2nd Quantifying risk of penile prosthesis infection with elevated glycosylated hemoglobin. J Urol. 1998;159:1537–9. doi: 10.1097/00005392-199805000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Habous M, Tal R, Tealab A, Soliman T, Nassar M, et al. Defining a glycated haemoglobin (HbA1c) level that predicts increased risk of penile implant infection. BJU Int. 2018;121:293–300. doi: 10.1111/bju.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SK, Delk JR., 2nd Inflatable penile implant infection: predisposing factors and treatment suggestions. J Urol. 1995;153:659–61. [PubMed] [Google Scholar]
- 16.Lipsky MJ, Onyeji I, Golan R, Munarriz R, Kashanian JA, et al. Diabetes is a risk factor for inflatable penile prosthesis infection: analysis of a large statewide database. Sex Med. 2019;7:35–40. doi: 10.1016/j.esxm.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters CE, Carlos EC, Lentz AC. Purulent inflatable penile prostheses can be safely immediately salvaged in insulin-dependent diabetics. J Sex Med. 2018;15:1673–7. doi: 10.1016/j.jsxm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Cuellar DC, Sklar GN. Penile prosthesis in the organ transplant recipient. Urology. 2001;57:138–41. doi: 10.1016/s0090-4295(00)00876-1. [DOI] [PubMed] [Google Scholar]
- 19.Sun AY, Babbar P, Gill BC, Angermeier KW, Montague DK. Penile prosthesis in solid organ transplant recipients-a matched cohort study. Urology. 2018;117:86–8. doi: 10.1016/j.urology.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Brandes ER, Chang SL, Leow JJ, Chung BI, et al. Trends in penile prosthesis implantation and analysis of predictive factors for removal. World J Urol. 2019;37:639–46. doi: 10.1007/s00345-018-2491-4. [DOI] [PubMed] [Google Scholar]
- 21.Gross MS, Phillips EA, Carrasquillo RJ, Thornton A, Greenfield JM, et al. Multicenter investigation of the micro-organisms involved in penile prosthesis infection: an analysis of the efficacy of the AUA and EAU guidelines for penile prosthesis prophylaxis. J Sex Med. 2017;14:455–63. doi: 10.1016/j.jsxm.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Kavoussi NL, Siegel JA, Viers BR, Pagliara TJ, Hofer MD, et al. Preoperative urine culture results correlate poorly with bacteriology of urologic prosthetic device infections. J Sex Med. 2017;14:163–8. doi: 10.1016/j.jsxm.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 23.van der Sluis WB, Bouman MB, de Boer NK, Buncamper ME, van Bodegraven AA, et al. Long-term follow-up of transgender women after secondary intestinal vaginoplasty. J Sex Med. 2016;13:702–10. doi: 10.1016/j.jsxm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Chlebicki MP, Safdar N, O’Horo JC, Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis. Am J Infect Control. 2013;41:167–73. doi: 10.1016/j.ajic.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–6. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 26.Hawn MT, Richman JS, Vick CC, Deierhoi RJ, Graham LA, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013;148:649–57. doi: 10.1001/jamasurg.2013.134. [DOI] [PubMed] [Google Scholar]
- 27.Gross MS, Phillips EA, Balen A, Eid JF, Yang C, et al. The malleable implant salvage technique: infection outcomes after Mulcahy salvage procedure and replacement of infected inflatable penile prosthesis with malleable prosthesis. J Urol. 2016;195:694–7. doi: 10.1016/j.juro.2015.08.091. [DOI] [PubMed] [Google Scholar]
- 28.Henry GD. The Henry Mummy Wrap™ and the Henry Finger Sweep™ surgical techniques. J Sex Med. 2009;6:619–22. doi: 10.1111/j.1743-6109.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 29.Jarow JP. Risk factors for penile prosthetic infection. J Urol. 1996;156:402–4. doi: 10.1097/00005392-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Kumar R, Nehra A. Dual implantation of penile and sphincter implants in the post-prostatectomy patient. Curr Urol Rep. 2007;8:477–81. doi: 10.1007/s11934-007-0052-2. [DOI] [PubMed] [Google Scholar]
- 31.Kendirci M, Gupta S, Shaw K, Morey A, Jones L, et al. Synchronous prosthetic implantation through a transscrotal incision: an outcome analysis. J Urol. 2006;175:2218–22. doi: 10.1016/S0022-5347(06)00345-4. [DOI] [PubMed] [Google Scholar]
- 32.Katz BF, Gaunay GS, Barazani Y, Nelson CJ, Moreira DM, et al. Use of a preoperative checklist reduces risk of penile prosthesis infection. J Urol. 2014;192:130–5. doi: 10.1016/j.juro.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 33.Eid JF. No-touch technique. J Sex Med. 2011;8:5–8. doi: 10.1111/j.1743-6109.2010.02137.x. [DOI] [PubMed] [Google Scholar]
- 34.Eid JF, Wilson SK, Cleves M, Salem EA. Coated implants and “no touch” surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46% Urology. 2012;79:1310–5. doi: 10.1016/j.urology.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 35.Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD004122.pub4. doi: CD004122. [DOI] [PubMed] [Google Scholar]
- 36.Grober ED, Domes T, Fanipour M, Copp JE. Preoperative hair removal on the male genitalia: clippers vs razors. J Sex Med. 2013;10:589–94. doi: 10.1111/j.1743-6109.2012.02904.x. [DOI] [PubMed] [Google Scholar]
- 37.Darouiche RO, Wall MJ, Jr, Itani KM, Otterson MF, Webb AL, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. doi: 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 38.Yeung LL, Grewal S, Bullock A, Lai HH, Brandes SB. A comparison of chlorhexidine-alcohol versus povidone-iodine for eliminating skin flora before genitourinary prosthetic surgery: a randomized controlled trial. J Urol. 2013;189:136–40. doi: 10.1016/j.juro.2012.08.086. [DOI] [PubMed] [Google Scholar]
- 39.Mandava SH, Serefoglu EC, Freier MT, Wilson SK, Hellstrom WJ. Infection retardant coated inflatable penile prostheses decrease the incidence of infection: a systematic review and meta-analysis. J Urol. 2012;188:1855–60. doi: 10.1016/j.juro.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Pastuszak AW, Lentz AC, Farooq A, Jones L, Bella AJ. Technological improvements in three-piece inflatable penile prosthesis design over the past 40 years. J Sex Med. 2015;12(Suppl 7):415–21. doi: 10.1111/jsm.13004. [DOI] [PubMed] [Google Scholar]
- 41.Lokeshwar SD, Bitran J, Madhusoodanan V, Kava B, Ramasamy R. A surgeon's guide to the various antibiotic dips available during penile prosthesis implantation. Curr Urol Rep. 2019;20:11. doi: 10.1007/s11934-019-0874-8. [DOI] [PubMed] [Google Scholar]
- 42.Henry GD, Wilson SK, Delk JR, 2nd, Carson CC, Wiygul J, et al. Revision washout decreases penile prosthesis infection in revision surgery: a multicenter study. J Urol. 2005;173:89–92. doi: 10.1097/01.ju.0000146717.62215.6f. [DOI] [PubMed] [Google Scholar]
- 43.Hinds PR, Wilson SK, Sadeghi-Nejad H. Dilemmas of inflatable penile prosthesis revision surgery: what practices achieve the best outcomes and the lowest infection rates.(CME)? J Sex Med. 2012;9:2483–92. doi: 10.1111/j.1743-6109.2012.02932.x. [DOI] [PubMed] [Google Scholar]
- 44.Wosnitzer MS, Greenfield JM. Antibiotic patterns with inflatable penile prosthesis insertion. J Sex Med. 2011;8:1521–8. doi: 10.1111/j.1743-6109.2011.02207.x. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberger LH, Politano AD, Sawyer RG. The surgical care improvement project and prevention of post-operative infection, including surgical site infection. Surg Infect (Larchmt) 2011;12:163–8. doi: 10.1089/sur.2010.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finney RP. Coring fibrotic corpora for penile implants. Urology. 1984;24:73–4. doi: 10.1016/0090-4295(84)90393-5. [DOI] [PubMed] [Google Scholar]
- 47.Lopategui DM, Balise RR, Bouzoubaa LA, Wilson SK, Kava BR. The impact of immediate salvage surgery on corporeal length preservation in patients presenting with penile implant infections. J Urol. 2018;200:171–7. doi: 10.1016/j.juro.2018.01.082. [DOI] [PubMed] [Google Scholar]
- 48.Brant MD, Ludlow JK, Mulcahy JJ. The prosthesis salvage operation: immediate replacement of the infected penile prosthesis. J Urol. 1996;155:155–7. doi: 10.1016/s0022-5347(01)66580-7. [DOI] [PubMed] [Google Scholar]
- 49.Mulcahy JJ. Long-term experience with salvage of infected penile implants. J Urol. 2000;163:481–2. [PubMed] [Google Scholar]
- 50.Martinez DR, Alhammali E, Hakky TS, Carrion R. The “carrion cast”: an intracavernosal antimicrobial cast for the treatment of infected penile implant. J Sex Med. 2014;11:1355–8. doi: 10.1111/jsm.12578. [DOI] [PubMed] [Google Scholar]
- 51.Zargaroff S, Sharma V, Berhanu D, Pearl JA, Meeks JJ, et al. National trends in the treatment of penile prosthesis infections by explantation alone vs immediate salvage and reimplantation. J Sex Med. 2014;11:1078–85. doi: 10.1111/jsm.12446. [DOI] [PubMed] [Google Scholar]
- 52.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, et al. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003;112:1466–77. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 54.Herati AS, Lo EM. Penile prosthesis biofilm formation and emerging therapies against them. Transl Androl Urol. 2018;7:960–7. doi: 10.21037/tau.2018.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawn LE, Henry GD, Tan GK, Wilson SK. Biofilm and infectious agents present at the time of penile prosthesis revision surgery: times are a changing. Sex Med Rev. 2017;5:236–43. doi: 10.1016/j.sxmr.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Jani K, Smith C, Delk JR, 2nd, Carson CC, Donatucci CF, et al. Infection retardant coatings impact on bacterial presence in penile prosthesis surgery: a multicenter study. Urology. 2018;119:104–8. doi: 10.1016/j.urology.2018.05.028. [DOI] [PubMed] [Google Scholar]


