Abstract
The aim of this work was to study effects of ketotifen fumarate (KF) on prevention of tissue damage in testes of rats with experimental autoimmune orchitis (EAO) and on the contralateral testis in a model of prolonged testicular cord torsion (TCT). Rats with EAO or TCT were injected intraperitoneally once daily with KF or saline solution (vehicle group). Incidence and severity of testicular damage were evaluated by histopathology using an EAO score or a Johnsen score. Mast cells (MC) were identified by histochemistry and quantified. In EAO model, KF significantly reduced severity of histopathological testicular damage compared to rats in the vehicle group. KF also reduced the number of testicular MC compared to vehicle group. Similarly, in TCT model, multifocal damage of the contralateral testis was observed 30 days after testicular torsion characterized by sloughing of the germinal epithelium, seminiferous tubule atrophy, and interstitial edema. Focal signs of inflammation and fibrosis of seminiferous tubular walls were also observed. In contrast, sections of contralateral testis of rats injected with KF and killed 30 days after surgery showed normal histological features. A significant decrease in the number of MC was observed in rats treated with KF compared to untreated animals. In conclusion, we demonstrated that treatment with KF reduced testicular inflammatory process and MC infiltrates in both EAO and TCT models. The results suggest a promising treatment for infertile male patients with testicular pathologies associated with inflammation and germ cell loss.
Keywords: ketotifen fumarate, male infertility, mast cells, orchitis, testicular cord torsion, testis
INTRODUCTION
Ketotifen fumarate (KF), 4-(methyl-4-piperidylene)-4H-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one hydrogen fumarate, has been widely used as an anti-allergicand anti-anaphylactic agent in adults and children.1 It has been administered to prevent allergic asthma by blocking release of allergic mediators from mast cells (MC) and by antagonizing histamine H1 receptors.2 Significant improvement in semen parameters after KF treatment has been reported in patients with idiopathic oligozoospermia and asthenospermia or as therapy after varicocelectomy.3,4,5 Recently, Baba et al.6 demonstrated that KF dose-dependently inhibits the process of exocytosis of rat MC. In addition to exocytotic release of histamine, leukotrienes, and proteases, MC are also involved in the development of fibrosis in many organs including testis.7
Experimental autoimmune orchitis (EAO) is a useful model to study organ-specific autoimmunity and chronic testicular inflammation. The rodent model mimics pathological changes reported in immunological infertility in men.8,9 EAO is characterized by apoptosis and sloughing of germ cells and the presence of interstitial inflammatory cell infiltrates.10,11 Quantitative and phenotypic analyses of inflammatory infiltrates in testis reveal increased numbers of macrophages,12 dendritic cells,13 MC,14,15 and different T cell subsets including effector Th1 and Th17 cells and Foxp3+ regulatory T cells.11,16 Upregulation of chemokines and pro-inflammatory cytokines as well as nitric oxide (NO) is also involved in the impairment of spermatogenesis.17,18,19 EAO progression is associated with fibrosis, testicular atrophy, and infertility.
Testicular cord torsion (TCT) is an emergency affecting 1:4000 males under 25 years old. Its incidence in general hospitals is between 4 and 20 cases per year.20,21 Surgical detorsion is the only effective treatment to improve testicular function. However, these patients may develop infertility or subfertility in the future, even after surgical detorsion or orchiectomy, and nearly 10% of testicular biopsies for gamete criopreservation are secondary to this pathology.22,23 Other clinical studies have not confirmed these observations and argued that the fertility potential of these patients is normal.24 Our results demonstrated previously that TCT in rats induces tissue damage in the contralateral testis characterized by macrophage infiltrates associated with seminiferous tubules showing increased germ cell apoptosis. Significantly increased MC were also observed in this model.25
Although some publications describe the use of KF in male infertility, to our knowledge, no studies report the effect of MC blockers on EAO and TCT. Therefore, this study aimed to determine whether KF could prevent or ameliorate testicular damage in rats with EAO or prolonged TCT.
MATERIALS AND METHODS
Animals
Adult male Wistar or Sprague–Dawley rats 45–60 days old were purchased from “Bioterio Central,” School of Pharmacy and Biochemistry, University of Buenos Aires (Buenos Aires, Argentina). Animals were kept at 22°C with a 14-h light/10-h dark schedule and fed standard food pellets and water ad libitum. Experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals published by National Institutes of Health. Approval for the study protocol was obtained from the Use and Care of Experimental Animals Committee of School of Medicine, University of Buenos Aires.
Induction of EAO and histopathology
Wistar rats were actively immunized with testicular homogenate (TH) prepared as previously described.26 Briefly, the rat testes were decapsulated, diluted in an equal volume of saline, and disrupted in an Omni mixer (17105 OM, Sorvall, Norwalk, CT, USA) for 30 s. The final concentration was 500 mg ml−1 wet weight. The rats were injected three times with 200 mg wet weight of TH per dose per rat at 14-day intervals. TH (0.4 ml) emulsified with 0.4 ml complete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO, USA) was injected into footpads and subcutaneously at multiple sites in flanks and the neck area. The first two immunizations were followed by an intravenous injection of 0.5 ml Bordetella pertussis (Bp) (strain 10536, “Instituto Malbrán,” Buenos Aires, Argentina) containing 1010 microorganisms and the third by intraperitoneal injection of 5 × 109 microorganisms. The rats were killed 56 days after the first immunization. Testes and epididymis were removed, weighed, and studied in paraffin-embedded Bouin's (Sigma-Aldrich)-fixed sections obtained from three different levels and stained with hematoxylin-eosin Y (yellowish; Merck, Darmstadt, Germany). To evaluate the degree of germ cell damage characteristic of EAO, we used a score described previously.12 Briefly, this score includes (a) percentage of damaged seminiferous tubules (ST), (b) degree of germ cell sloughing, and (c) testicular/body weight ratio (T/Bw). Therefore, EAO score = V + T + P, where V is the value assigned to the percentage of damaged ST (presenting germ cell sloughing and degeneration); V = 0 (0%–3% damaged ST); 1 (3.1%–4.9%); 2 (5%–15.9%); 3 (16%–25.9%); 4 (26%–35.9%); 5 (36%–55.9%); 6 (56%–60.9%); 7 (61%–79.9%); 8 (80%–95.9%); or 9 (96%–100%). T is an indicator of degree of germ cell sloughing: T = 0 when mild germ cell sloughing is observed and T = 0.5 when germ sloughing is severe (only spermatogonia and Sertoli cells are still attached to the ST walls). P is a correction factor corresponding to the T/Bw index: P = 0.5 when T/Bw index is <2.5 × 103. The rats with a score over 0 were considered to have orchitis.
Induction of TCT and histopathology
Under anesthesia, a scrototomy on the left side and a 720° unilateral spermatic cord torsion was performed on Sprague–Dawley rats in the experimental (E) group. The testis was fixed into the scrotum using 4-0 silk suture. The rats were killed 30 days after surgery. At the end of each time segment, the contralateral testis was removed, weighed, fixed in Bouin's solution, dehydrated, and embedded in paraffin or quickly frozen to obtain cryostat sections for histopathology or immunohistochemistry, respectively. Paraffin-embedded testis sections (6 μm) were stained with hematoxylin-eosin Y. Tubular damage was quantified by a modified Johnsen score, which analyzes the degree of damage to seminiferous tubule germ cells by a score from 1 to 10.25 Complete spermatogenesis with many spermatozoa is considered 10 and animals with a score under 9 were considered to have testicular damage. The mean tubular score was obtained by evaluating 50 ST per testis per rat. Tubular diameter was calculated in transversal testis sections with the aid of an ocular micrometer (8×, Kpl, Zeiss, Oberkochen, Germany).
Experimental design
In EAO model, a group of immunized rats was injected intraperitoneally with 1 or 4 mg kg−1 of KF (Roma Pharmacy and Laboratory S.A., Buenos Aires, Argentina) or vehicle (saline solution) once daily. Treatment started 48 h before the first immunization and continued daily throughout the experiment (treatment 1). The treatment of another group of rats was started 7 days after the last immunization (treatment 2). In TCT model, the rats were injected intraperitoneally with KF (1 mg kg−1) or vehicle once a day. Treatment was started from the moment of the surgical procedure and continued daily throughout the experiment. The rationale for the doses of KF used in this experimental design was based on prior in vivo study using similar doses of KF and the maximum recommended dose.27
Histochemistry
To identify MC, testis sections were stained with Alcian blue (Sigma-Aldrich). MC were counted using a 25× objective, and the ocular (12.5×; 473014-9901, Zeiss, Oberkochen, Germany) was fitted with a quadratic grid with a total area of 96 100 μm2.
Delayed-type hypersensitivity (DTH)
DTH was measured by a footpad swelling test performed at the end of each experiment. The rats were intradermically challenged in the left footpad with 2.10 mg of TH supernatant in 50 μl saline. This fraction was obtained by TH centrifugation (PR-2 International Equipment Co., Boston, MA, USA) (20 000 g at 4°C). The other footpad was injected with the same volume of saline. Footpad thickness was measured with a micrometer (Teclock Corporation, Naruta-cho Okaya-shi NaganoPref., Japan) 48 h after the challenge. The results of footpad swelling were expressed as mean of at least three measurements per rat (in mm), calculated as the difference between the thickness of the HT-injected footpad and that of the saline injected footpad.
Statistical analyses
Comparisons of groups were assessed by the nonparametric Mann–Whitney U test or one-way ANOVA Kruskal–Wallis test as indicated in each figure's legend. P ≤ 0.05 was considered statistically significant.
RESULTS
Administration of KF reduced severity of EAO and epididymitis
We evaluated the effects of KF administration on rats during EAO. Two experimental designs were used. In treatment 1, KF was administered before the first immunization; in treatment 2, KF was administered after the last immunization when the first signs of disease became apparent28,29 and continued until euthanasia. Histopathology showed that 100% of rats in the vehicle group developed orchitis (Figure 1a and 1b). In contrast, a decrease in EAO incidence and severity was observed in rats injected with KF (4 mg kg−1) in treatment 1 (80%) (Figure 1a). Interestingly, we also found that KF significantly reduced severity of epididymis inflammation compared to the vehicle group (Figure 2). Rats in the vehicle group presented severe orchitis with large areas of aspermatogenic ST, in which only spermatogonia and Sertoli cells attached to the tubular wall and interstitial inflammatory cell infiltrates intermingled with Leydig cells were present (Figure 1c). In contrast, most rats (90%) treated with KF showed ST with scarce germ cell sloughing or normal seminiferous epithelium (Figure 1d). Body weight of KF (4 mg kg−1)-treated rats did not differ from that of untreated normal rats, suggesting that KF treatment induced no apparent adverse effects (P = 0.20) (mean ± standard error of the mean [s.e.m.] of body weight: untreated normal rats [n = 17], 342.90 ± 8.14 g; treatment 1 [n = 10], 336.50 ± 10.90 g; and treatment 2 [n = 6], 341.60 ± 14.90 g). Moreover, all rats survived after KF treatment. A significant reduction of inflammation and damage of ST was observed in KF treatment 1. In KF treatment 2, histopathology was similar in the vehicle and KF groups.
Figure 1.
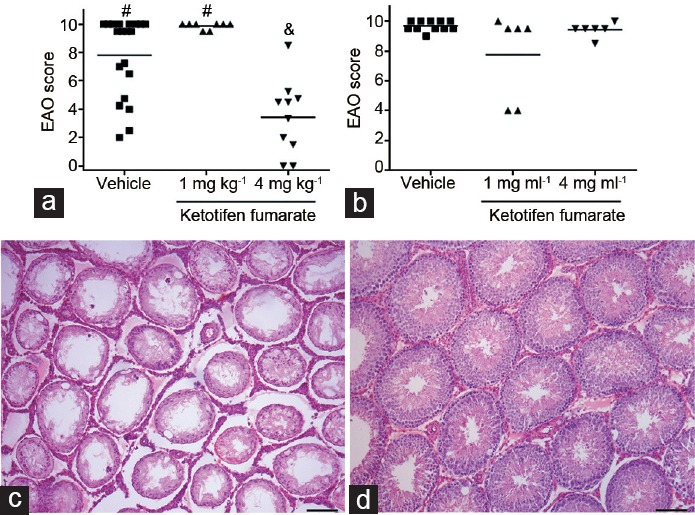
Impact of ketotifen fumarate on incidence and severity of EAO score. All rats were immunized with testicular homogenate and adjuvants. A group of rats was injected with saline solution (vehicle) or ketotifen fumarate. (a) Treatment 1. Administration of ketotifen fumarate (4 mg kg−1) reduced the severity of orchitis. (b) Treatment 2. Similar EAO scores were observed in the two groups studied. Horizontal lines represent the mean. Each symbol represents a single rat. Values with different symbols superscript (#, &) differ significantly P < 0.05 (nonparametric one-way ANOVA Kruskal–Wallis test). (c) Testis section from vehicle group rats killed 56 days after the first immunization showing severe testicular damage characterized by germ cell sloughing of seminiferous tubules and interstitial inflammatory cell infiltrate. Note severe tubular atrophy showing decreased diameter of seminiferous tubules in vehicle rats. (d) In contrast, testis section from rats treated with ketotifen fumarate (4 mg kg−1) shows normal histological features. Scale bars = 100 μm. EAO: experimental autoimmune orchitis.
Figure 2.
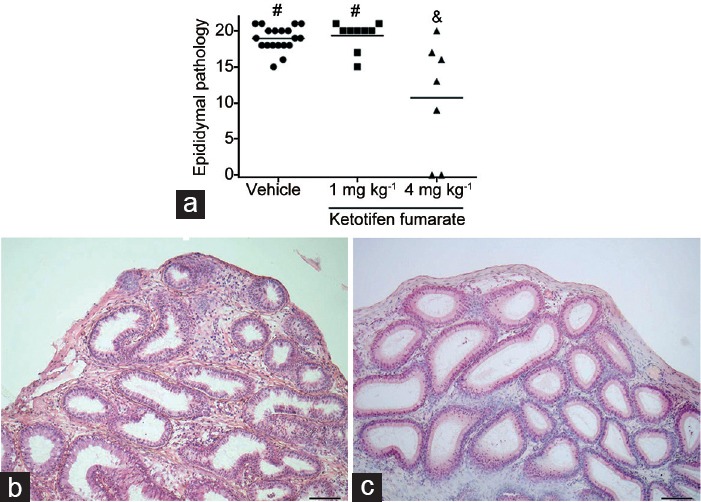
Impact of ketotifen fumarate on epididymis. (a) Epididymal pathology of rats immunized with testicular homogenate and adjuvants and injected with saline solution (vehicle) or ketotifen fumarate. Treatment 1. Administration of ketotifen fumarate (4 mg kg−1) reduces severity of epididimytis. Horizontal lines represent the mean. Each symbol represents a single rat. Values with different symbols superscript (#, &) differ significantly P < 0.05 (nonparametric one-way ANOVA Kruskal–Wallis test). (b) Epididymis section from vehicle group rats killed 56 days after the first immunization showing a severe inflammatory cell infiltrates. (c) In contrast, epididymis section from rats treated with ketotifen fumarate (4 mg kg−1) shows moderate interstitial inflammation. Scale bars = 100 μm.
KF did not alter cell mediated immunity in vivo
We next investigated the effects of KF on T cell mediated immunity by analyzing in vivo DTH response to spermatic antigens. Footpad swelling observed in rats with treatment 1 (Figure 3a) and 2 (Figure 3) was similar to that in rats treated with KF compared to those of vehicle groups.
Figure 3.
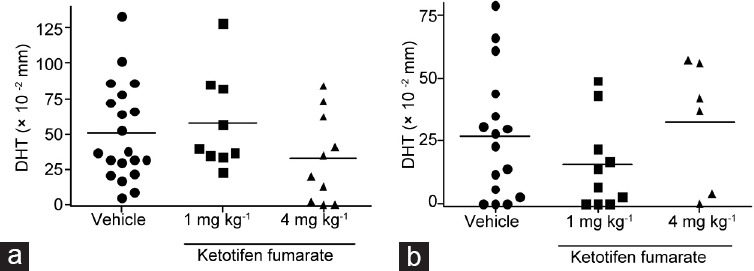
Ketotifen fumarate does not induce changes in cell mediated immunity in vivo. Rats with EAO treated with saline (vehicle) or ketotifen fumarate were tested for DTH to testicular antigens during (a) treatment 1 and (b) treatment 2. Two days before euthanasia (day 54), TH was injected into rats’ left footpad. The right footpad was injected with saline. Footpad swelling was measured after 48 h and results are expressed as the mean difference between the thickness of the HT-injected footpad and that of the saline-injected footpad ± standard error of the mean (s.e.m.) in each group (nonparametric one-way ANOVA Kruskal–Wallis test). EAO: experimental autoimmune orchitis; DTH: delayed-type hypersensitivity.
KF treatment reduced MC numbers
To evaluate the effect of KF on testicular MC, sections from rats treated or untreated with KF were processed by Alcian blue staining. Figure 4a–4d illustrates the presence of MC near tunica albuginea. Quantitative assessment of Alcian blue-positive cells revealed that administration of KF to rats undergoing orchitis reduced the number of MC compared to vehicle-treated rats (P = 0.020; Figure 4e).
Figure 4.
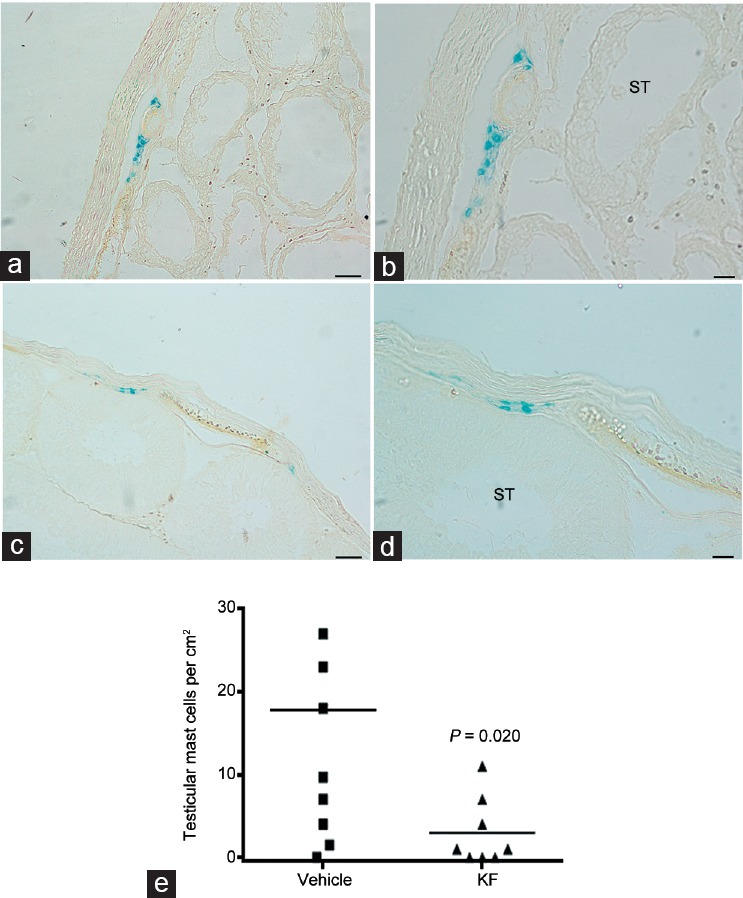
Administration of KF reduced mast cell number in EAO. Representative microphotographs of Alcian blue staining performed on EAO testis sections. Mast cells are observed near the tunica albuginea and vessels of both, (a and b)untreated or (c and d)treated with KF rats. Scale bars = 50 μm in a and c; and 20 μm in b and d. (e) Quantitative assessment of Alcian blue-positive mast cell number in EAO testis sections from rats treated with KF (treatment 1) or saline solution (vehicle). Horizontal lines represent the mean value and each symbol represents a single rat. P = 0.020 (Mann–Whitney U test). EAO: experimental autoimmune orchitis; ST: seminiferous tubule; KF: ketotifen fumarate.
Administration of KF prevented histological damage in the contralateral testis after prolonged TCT
Multifocal damage of the contralateral testis was observed 30 days after testicular torsion in the vehicle group. Testis damage was characterized by sloughing of the germinal epithelium, seminiferous tubule atrophy, and interstitial edema. Focal signs of inflammation and fibrosis of seminiferous tubular walls were also observed (Figure 5a). In contrast, contralateral testis sections of rats treated with KF for 30 days after surgical torsion showed normal histology (Figure 5b). Comparison of tubular diameter and Johnsen score showed significant differences between KF and vehicle group (P = 0.003 and P = 0.008, respectively; Figure 5c and 5d).
Figure 5.
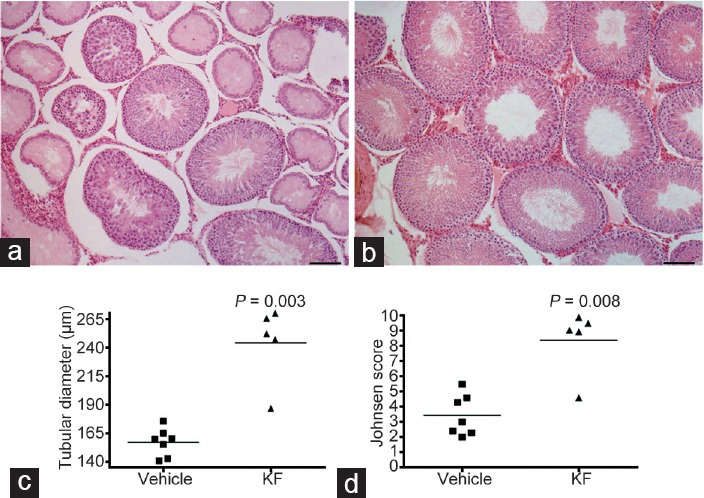
Impact of KF in testicular damage after prolonged testicular cord torsion. Testicular histopathology. Representative microphotographs of sections of contralateral testis from rats (a) untreated or (b) treated with KF. Germ cell sloughing and seminiferous tubule atrophy in an untreated rat. Normal histology in a rat treated with KF. Scale bars = 100 μm. Quantitative assessment of tubular damage shows that (c) the mean tubular diameter and (d) Johnsen score are higher in KF versus vehicle group (P = 0.003 and P = 0.008, respectively). Horizontal lines represent the mean. Each symbol represents a single rat. (Mann–Whitney U test). KF: ketotifen fumarate.
KF reduced the number of MC in the contralateral testis after prolonged TCT
MC were observed near the tunica albuginea and vessels (Figure 6a–6d). The number of MC decreased significantly in the contralateral testis of rats treated with KF compared to vehicle group (P = 0.019; Figure 6e).
Figure 6.
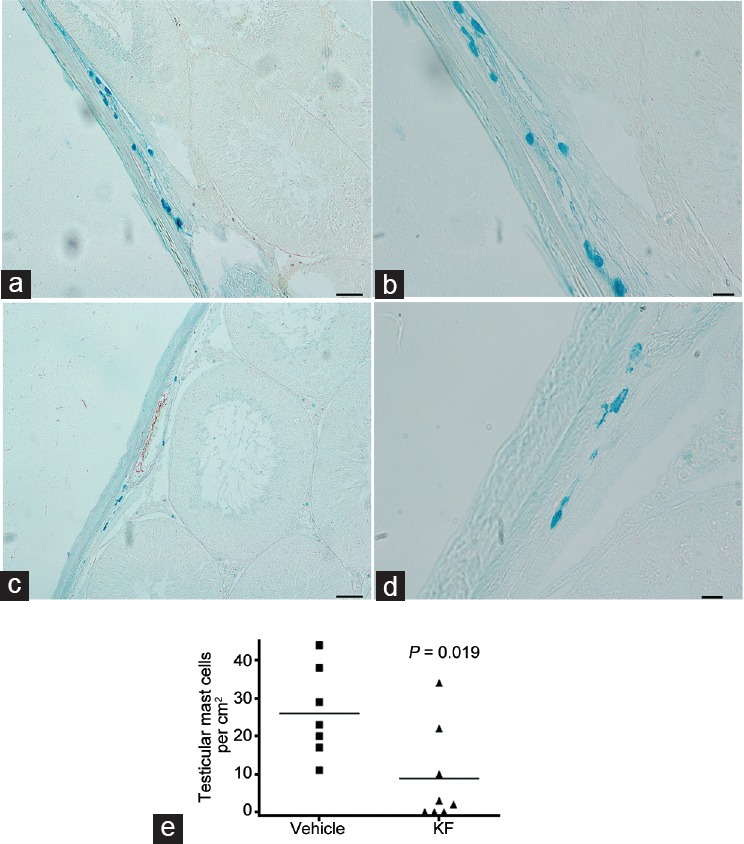
Administration of KF reduced mast cell number in testicular cord torsion model. Representative microphotographs of contralateral testis sections showing mast cells stained with Alcian blue. Mast cells are observed near the tunica albuginea and vessels of both, (a and b) untreated or (c and d) treated with KF rats. Scale bars = 50 μm in a and c; and 20 μm in b and d. (e) Quantitative assessment of Alcian blue-positive mast cell number in contralateral testis sections from rats treated with KF or saline solution (vehicle). P = 0.019. Horizontal lines represent the mean. Each symbol represents a single rat. (Mann–Whitney U test). KF: ketotifen fumarate.
DISCUSSION
Inflammatory processes in the testis are frequently associated with infertility.9 In fact, a high percentage of patients with subfertility or infertility present different degrees of testicular immune cell infiltrates.30
TCT and EAO are interesting models to study testicular inflammation and infertility. Testicular inflammatory infiltrates are composed mainly of dendritic cells, macrophages, lymphocytes, and MC.12,16,25 The inflammatory process and oxidative stress generate germ cell sloughing and apoptosis, resulting in aspermatogenesis and atrophy of ST.19,31
MC have a key role in inflammation and tissue fibrosis in several models of autoimmune diseases.32,33 In vitro, MC can promote fibroblast proliferation and collagen synthesis.34 Presynthesized substances such as histamine, chymase, tryptase, carboxipeptidase A, and tumor necrosis factor-alpha (TNFα) are stored in granules and released immediately after MC activation. TNFα, interleukin (IL) 6, and IL1b are de novo synthesized after MC activation.35 Because the present work focuses on MC, we evaluated the effect of KF, a high affinity blocker of histamine H1 receptor, on testicular inflammation and spermatogenesis in TCT and EAO experimental models.
Although a small number of MC are present in the normal mammal testis, in inflammatory conditions, the number of MC increases.35,36,37 Testicular MC are also associated with sclerosis and fibrosis of ST in patients with varicocele and defective spermatogenesis.38,39
In the TCT model, multifocal damage of the contralateral testis characterized by focal signs of inflammation and fibrosis of seminiferous tubular walls was observed 30 days after testicular torsion in the vehicle group. In contrast, the present results showed normal testicular histology in rats treated with 1 mg kg−1 day−1 of KF and a significantly decreased number of MC in the contralateral testis of rats treated with KF compared to the vehicle group. Similar to our findings, Acikgoz et al.27 observed that the number of MC decreased in the contralateral descended testis in rats treated with MC blocker in experimental unilateral undescended testis, supporting its therapeutic use for autoimmune testicular inflammation.
In the EAO model, we also observed decreased incidence and severity of orchitis and epididymal inflammation in rats injected with 4 mg kg−1 day−1 of KF compared to the vehicle. We observed no adverse effects on weight or behavior in rats treated with KF in spite of the high doses used. By analyzing in vivo DTH response to spermatic antigens, we showed that KF treatment does not modify cell mediated immunity. In effect, this drug is mainly an immunomodulator with anti-histaminic action but does not block molecular steps that activate cell-mediated immunity.
Reduction of the number of MC is associated with reduction of testicular damage and improved spermatogenesis. The results suggest that KF inhibits testicular MC degranulation preventing the release of inflammatory and fibrotic mediators, consequently improving histopathological parameters. MC are a source of numerous cytokines, chemokines, and growth factors with potential autocrine, paracrine, local, and systemic effects.40 In fact, some of these, such as TNFα, IL6, C-C chemokine ligand (CCL) 2, and CCL4, play a relevant role in the testis during inflammation.17 KF, like other MC stabilizers, was reported to restrain serum TNFα levels and improve survival in a murine experimental model of sepsis.41
KF also has a very important role in regulating macrophage metabolic activity, modulating nitric oxide (NO) production by inhibition of inducible oxide nitric synthase (iNOS) expression in lipopolysaccharide-stimulated cells. This inhibition is followed by a significant reduction in nitrite levels. This drug had no scavenger activity against NO in the chemical system. Therefore, it is possible that the inhibitory effect on macrophage iNOS expression could be due to its ability to affect intracellular signaling pathways leading NO production.42
MC act in concert with macrophages and Leydig cells, germ cells, and peritubular cells which also express histamine receptor H1.43,44 Histamine has also been reported to have an important role in controlling Leydig cell steroidogenesis.44 Hence, we cannot exclude the possibility that KF as an antagonist of H1 may modulate other parameters beyond those we describe in this report.
MC blockers have been used empirically for the treatment of male infertility with controversial results. Yamamoto et al.45 reported a significant improvement of semen parameters and pregnancies in a group of oligozoospermic patients treated with tranilast, and Matsuki et al.46 reported similar results in infertile male patients treated with the MC blocker ebastine. However, Cayan et al.47 reported that treatment with an antihistamine drug, fexofenadine, in infertile patients with a significant increase of testicular MC appears to have no benefit; no data concerning semen quality and pregnancies were reported. Use of this drug was discontinued due to lack of homogeneous results from different authors. Recently, Mondillo et al.48 summarized the negative impact of anti-histamines loratadine, desloratadine, cimetidine, and ranitidine on male reproduction.
In contrast, several authors reported that KF treatment of infertile patients with idiopathic oligozoospermia and asthenozoospermia resulted in moderate, but statistically significant improvement in sperm count and motility and reduction of leukocytospermia and necrospermia.3,5,49 Finally, in a preliminary study, we observed a significant improvement in seminal parameters and pregnancy rates after intracytoplasmic sperm injection (ICSI) in male patients with severe oligo-asthenozoospermia treated with KF.50
CONCLUSION
Our results indicate that MC are involved in the pathogenesis of testicular inflammatory process. We demonstrated that treatment with KF can significantly reduce testis damage in experimental models of orchitis and prevent impairment of contralateral testis after testicular torsion. The present work enlarges the use of KF as treatment in testicular pathologies associated with inflammation and germ cell loss.
AUTHOR CONTRIBUTIONS
DM performed EAO experiments and was involved in interpretation of results. CMS contributed with histochemistry experiments. LL was involved in the study's conception, interpretation and discussion of data, and contributed to drafting of the article. MGRP performed TCT experiments and contributed to drafting of the article. VAG performed experiments and was involved in the study's conception and design, interpretation and discussion of data, and drafting of the article. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We thank Miss Cecilia Garcia and Miss Mercedes Imsem for their excellent technical assistance and the National Institute of Microbiology “A. Malbrán,” Bacterial Vaccines Department, for their generous donation of B. pertussis. This work was supported by grants from Foundation “Florencio Fiorini” and the University of Buenos Aires (UBACYT 2014-2017 22320160100058BA).
REFERENCES
- 1.Slater JW, Zechnich AD, Haxby DG. Second-generation antihistamines: a comparative review. Drugs. 1999;57:31–47. doi: 10.2165/00003495-199957010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. 1990;40:412–48. doi: 10.2165/00003495-199040030-00006. [DOI] [PubMed] [Google Scholar]
- 3.Schill WB, Schneider J, Ring J. The use of ketotifen, a mast cell blocker, for treatment of oligo- and asthenozoospermia. Andrologia. 1986;18:570–3. doi: 10.1111/j.1439-0272.1986.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 4.Azadi L, Abbasi H, Deemeh MR, Tavalaee M, Arbabian M, et al. Zaditen (Ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int J Androl. 2011;34:446–52. doi: 10.1111/j.1365-2605.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 5.Saharkhiz N, Nikbakht R, Hemadi M. Ketotifen, a mast cell blocker improves sperm motility in asthenospermic infertile men. J Hum Reprod Sci. 2013;6:19–22. doi: 10.4103/0974-1208.112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, et al. Anti-allergic drugs tranilast and ketotifen dose-dependently exert mast cell-stabilizing properties. Cell Physiol Biochem. 2016;38:15–27. doi: 10.1159/000438605. [DOI] [PubMed] [Google Scholar]
- 7.Frungieri MB, Weidinger S, Meineke V, Köhn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPAR gamma: possible relevance to human fibrotic disorders. Proc Natl Acad U S A. 2002;99:15072–7. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuppe HC, Bergmann M. Inflammatory conditions of the testis. In: Davor J, editor. Atlas on the Human Testis. London: Springer; 2013. p. 113. [Google Scholar]
- 9.Lustig L, Rival C, Tung KS. Autoimmune orchitis and autoimmune oophoritis. In: Rose NR, Mackay IR, editors. The Autoimmune Disease. Cambridge: Elsevier Inc; 2014. p. 1007. [Google Scholar]
- 10.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev. 2011;10:201–4. doi: 10.1016/j.autrev.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–17. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- 13.Rival C, Guazzone VA, von Wulffen W, Hackstein H, Schneider E, et al. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod. 2007;13:853–61. doi: 10.1093/molehr/gam067. [DOI] [PubMed] [Google Scholar]
- 14.Iosub R, Klug J, Fijak M, Schneider E, Fröhlich S, et al. Development of testicular inflammation in the rat involves activation of proteinase-activated receptor-2. J Pathol. 2006;208:686–98. doi: 10.1002/path.1938. [DOI] [PubMed] [Google Scholar]
- 15.Lustig L, Rodriguez M, Denduchis B, Suescun O. Testicular mast cells in autoimmune orchitis. Am J Reprod Immunol. 1995;33:459. (Abstract) [Google Scholar]
- 16.Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol. 2009;81:44–54. doi: 10.1016/j.jri.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: a brief review. Microsc Res Tech. 2009;72:620–8. doi: 10.1002/jemt.20704. [DOI] [PubMed] [Google Scholar]
- 18.Pérez CV, Theas MS, Jacobo PV, Jarazo-Dietrich S, Guazzone VA, et al. Dual role of immune cells in the testis: protective or pathogenic for germ cells? Spermatogenesis. 2013;3:e23870. doi: 10.4161/spmg.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarazo-Dietrich S, Fass MI, Jacobo PV, Sobarzo CM, Lustig L, et al. Inhibition of NOS-NO system prevents autoimmune orchitis development in rats: relevance of NO released by testicular macrophages in germ cell apoptosis and testosterone secretion. PLoS One. 2015;10:e0128709. doi: 10.1371/journal.pone.0128709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age related delay in the presentation of testicular torsion. J Urol. 1989;142:746–8. doi: 10.1016/s0022-5347(17)38875-4. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JB, Williamson RC. Testicular torsion in Bristol: a 25-year review. Br J Surg. 1988;75:988–92. doi: 10.1002/bjs.1800751015. [DOI] [PubMed] [Google Scholar]
- 22.Krarup T. The testes after torsion. Br J Urol. 1978;50:43–6. doi: 10.1111/j.1464-410x.1978.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 23.Klami R, Mankonen H, Perhentupa A. Microdissection testicular sperm extraction in Finland – results of the first 100 patients. Acta Obstet Gynecol Scand. 2018;97:53–8. doi: 10.1111/aogs.13243. [DOI] [PubMed] [Google Scholar]
- 24.Gielchinsky I, Suraqui E, Hidar G, Zuaiter M, Londau EH, et al. Pregnancy rates after testicular torsion. J Urol. 2016;196:852–5. doi: 10.1016/j.juro.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez MG, Rival C, Theas MS, Lustig L. Immunohistopathology of the contralateral testis of rats undergoing experimental torsion of the spermatic cord. Asian J Androl. 2006;8:576–83. doi: 10.1111/j.1745-7262.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 26.Doncel GF, Di Paola JA, Lustig L. Sequential study of the histopathology and cellular and humoral immune response during the development of an autoimmune orchitis in Wistar rats. Am J Reprod Immunol. 1989;20:44–51. doi: 10.1111/j.1600-0897.1989.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 27.Acikgoz A, Asci R, Aydin O, Çavuş H, Donmez G, et al. The role of ketotifen in the prevention of testicular damage in rats with experimental unilateral undescended testes. Drug Des Devel Ther. 2014;23:2089–97. doi: 10.2147/DDDT.S67941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guazzone VA, Rival C, Denduchis B, Lustig L. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in experimental autoimmune orchitis. J Reprod Immunol. 2003;60:143–57. doi: 10.1016/j.jri.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Guazzone VA, Jacobo P, Denduchis B, Lustig L. Expression of cell adhesion molecules, chemokines and chemokine receptors involved in leukocyte traffic in rats undergoing autoimmune orchitis. Reproduction. 2012;143:651–62. doi: 10.1530/REP-11-0079. [DOI] [PubMed] [Google Scholar]
- 30.Schuppe HC, Meinhardt A. Immune privilege and inflammation of the testis. Chem Immunol Allergy. 2005;88:1–14. doi: 10.1159/000087816. [DOI] [PubMed] [Google Scholar]
- 31.Theas S, Rival C, Lustig L. Germ cell apoptosis in autoimmune orchitis: involvement of the Fas-FasL system. Am J Reprod Immunol. 2003;50:166–76. doi: 10.1034/j.1600-0897.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 32.Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril. 2000;74:239–44. doi: 10.1016/s0015-0282(00)00626-9. [DOI] [PubMed] [Google Scholar]
- 33.Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–90. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 34.Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, et al. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy. 2002;32:237–46. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 35.Haidl G, Duan YG, Chen SJ, Kohn FM, Schuppe HC, et al. The role of mast cells in male infertility. Expert Rev Clin Immunol. 2011;7:627–34. doi: 10.1586/eci.11.57. [DOI] [PubMed] [Google Scholar]
- 36.Roaiah MM, Khatab H, Mostafa T. Mast cells in testicular biopsies of azoospermic men. Andrologia. 2007;39:185–9. doi: 10.1111/j.1439-0272.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 37.Welter H, Köhn FM, Mayerhofer A. Mast cells in human testicular biopsies from patients with mixed atrophy: increased numbers, heterogeneity, and expression of cyclooxygenase 2 and prostaglandin D2 synthase. Fertil Steril. 2011;96:309–13. doi: 10.1016/j.fertnstert.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka K, Fujisawa M, Tanaka H, Okada H, Arakawa S, et al. Significance of human testicular mast cells and their subtypes in male infertility. Hum Reprod. 2000;15:1543–7. doi: 10.1093/humrep/15.7.1543. [DOI] [PubMed] [Google Scholar]
- 39.Apa DD, Cayan S, Polat A, Akbay E. Mast cells and fibrosis on testicular biopsies in male infertility. Arch Androl. 2002;48:337–44. doi: 10.1080/01485010290099183. [DOI] [PubMed] [Google Scholar]
- 40.Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282:121–50. doi: 10.1111/imr.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos L, Peña G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J Immunol. 2010;185:709–16. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lojek A, Číž M, Pekarová M, Ambrožová M, Vašíček O, et al. Modulation of metabolic activity of phagocytes by antihistamines. Interdiscip Toxicol. 2011;4:15–9. doi: 10.2478/v10102-011-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrecht M, Frungieri MB, Gonzalez-Calvar S, Meineke V, Köhn FM, et al. Evidence for a histaminergic system in the human testis. Fertil Steril. 2005;83:1060–3. doi: 10.1016/j.fertnstert.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Mondillo C, Patrignani Z, Reche C, Rivera E, Pignataro O. Dual role of histamine in modulation of Leydig cell steroidogenesis via HRH1 and HRH2 receptor subtypes. Biol Reprod. 2005;73:899–907. doi: 10.1095/biolreprod.105.041285. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto M, Hibi H, Miyake K. New treatment of idiopathic severe oligozoospermia with mast cell blocker: results of a single-blind study. Fertil Steril. 1995;64:1221–3. doi: 10.1016/s0015-0282(16)57992-8. [DOI] [PubMed] [Google Scholar]
- 46.Matsuki S, Sasagawa I, Suzuki Y, Yazawa H, Tateno T, et al. The use of ebastine, a mast cell blocker, for treatment of oligozoospermia. Arch Androl. 2000;44:129–32. doi: 10.1080/014850100262290. [DOI] [PubMed] [Google Scholar]
- 47.Cayan S, Apa DD, Akbay E. Effect of fexofenadine, a mast cell blocker, in infertile men with significantly increased testicular mast cells. Asian J Androl. 2002;4:291–4. [PubMed] [Google Scholar]
- 48.Mondillo C, Varela ML, Abiuso AM, Vázquez R. Potential negative effects of anti-histamines on male reproductive function. Reproduction. 2018;155:R221–7. doi: 10.1530/REP-17-0685. [DOI] [PubMed] [Google Scholar]
- 49.Oliva A, Multigner L. Ketotifen improves sperm motility and sperm morphology in male patients with leukocytospermia and unexplained infertility. Fertil Steril. 2006;85:240–3. doi: 10.1016/j.fertnstert.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez Peña M, Ovando E, Calamera P, Filardi P, De Vincentis S, et al. Benefits of using ketotifen in severe oligoasthenoteratozoospermic (OAT) male patients: significant improvement in both seminal parameters and pregnancy rates using ICSI. Proceedings of 11th International Congress of Andrology; Copenhagen, Denmark. 2017 (Abstract) [Google Scholar]


