Abstract
Prostatic carcinoma metastatic to dura is commonly encountered at autopsy, but presenting as a dural or, especially parenchymal brain metastasis during life is far less common. Our group has been interested in two immunohistochemical (IHC) markers previously shown to be downregulated in particularly aggressive primary prostatic carcinomas: CHD1 and MAP3K7. Here we assess protein expression in clinically-relevant CNS metastases. We also assessed how these 2 markers correlated with the most common genetic alteration in prostate cancer: TMPRSS2 fusion to ERG (40–60% of carcinomas at the primary site), which places ERG expression under the control of the androgen-regulated TMPRSS2 gene, increasing expression.
Design:
Database query, 2000–2016, identified 16 metastases to dura, 5 to brain parenchyma.
Results:
Four of 5 intraparenchymal metastases and 15/16 informative dural-based metastases were ERG-negative (90.5% overall). There was reduced expression of CHD1 in 8/21 and reduced MAP3K7 in 17/21 cases; 7/19 (37%) ERG-negative metastases had dual low expression of CHD1/MAP3K7. ERG-positive cases had high expression of one or both markers.
Conclusion:
Metastatic prostatic carcinoma to CNS demonstrates expression patterns consistent with particularly aggressive behavior. Lower ERG expression in dural and intraparenchymal metastases suggests a possibility that ERG-negative tumors with loss of MAP3K7 may become resistant to standard therapies and diffusely metastasize.
Introduction:
Demographics of metastatic disease coming to biopsy or excision in the nervous system have changed dramatically due to the advent of treatment with non-interventional techniques such as stereotactic radiosurgery.[1] Patients with known metastatic nervous system involvement are often treated without histological confirmation.[2] However, single metastases of unknown origin, single presumed metastases in patients with prior malignancy(s) from tumors which seldom spread to the nervous system, and dural/epidural masses requiring emergent decompression are more likely to prompt surgical intervention. In addition, the study of the biology of prostatic disease in clinically-relevant metastases is severely hampered by the fact that most metastases are only rarely biopsied in life. Even when bone metastases do occur, the tissues are suboptimal for immunohistochemical (IHC) studies, given the necessity for decalcification. Similarly, autopsy-obtained tissues often suffer from prolonged fixation issues, making IHC less informative. Thus, prostatic metastatic disease to the nervous system, which more commonly requires surgical intervention than disease to other organ sites, provides an ideal platform to not only study the specifics of the disease as it impacts the central nervous system (CNS), but the biology of metastatic prostatic carcinoma in general.
Considerably more is known about the biology of prostatic carcinoma in its primary site than in metastatic sites. In the primary tumor, the most prevalent reported genetic alteration in prostate cancer is the fusion of the androgen receptor-regulated gene TMPRSS2 (21q22.3) to a transformation-specific transcription factor gene, ERG (21q22.2).[3] This rearrangement results in ERG gene expression at the IHC level being placed under the control of the androgen-regulated TMPRSS2 gene, increasing its expression.[3] Overexpression of ERG has been noted in 40–60% of localized prostate cancer in a number of studies.[3–6] This overexpression decreases in metastatic disease although it has not been specifically investigated in tumors metastatic to the CNS.[3, 4]
In addition to ERG expression, we have previously shown that downregulation of CHD1 and MAP3K7 genes is associated with particularly aggressive primary ERG-negative prostatic carcinomas.[7] MAP3K7 (mitogen-activated kinase kinase kinase7/TGFb-activated kinase-1 or TAK1) is a downstream target of multiple signaling molecules, including TGFb, TNFa, IL1, TLR, Wnt, and TRAIL.[7] The MAP3K7 gene (6q15) is deleted in approximately 40% of primary prostate cancer. [7] CHD1 (chromodomain helicase DNA-binding protein) is a chromatin remodeling factor involved in the regulation of gene transcription through interaction with open chromatin and in the maintenance of embryonic stem cell pluripotency.[7] The CHD1 gene (5q21.1) is deleted in approximately 20% of primary prostate cancer. [7] Approximately 15% of primary prostate cancer has genetic loss of both MAP3K7 and CHD1. [7] However, to our knowledge, no data exists regarding IHC expression for CHD1 and MAP3K7 in metastatic sites. We hypothesized that CHD1 and MAP3K7 expression at the IHC level might be downregulated in metastatic tumors that affected the dura and brain parenchyma. The current study investigated nervous system (CNS) metastases and their specific expression of ERG, CHD1 and MAP3K7. PTEN was also tested as a potential surrogate as an IHC marker of aggressive cancer. Loss of PTEN expression by IHC is reported in ~20% of primary prostate cancer, but up to 61% in metastases. [10] A secondary goal was to see if IHC expression of ERG might prove to be low/absent, thus serving as a caution if using this to identify the origin of metastases from unknown primary sites.
Materials and Methods:
Computer-based database queries from January 1, 2000, to June 30, 2016, were conducted for all cases in which metastatic prostatic carcinoma had been diagnosed at biopsy/resection. All intraparenchymal cases were confirmed as being largely or solely intraparenchymal on review of the preoperative neuroimaging studies by the two neurosurgeons on the paper (DRO, DC).
An unexpected finding was that we additionally identified a number of cases by textword search in which metastatic prostatic carcinoma to dura or brain parenchyma had been suspected by the experienced clinical and neuroimaging teams at our tertiary care center, but proved on histological examination after biopsy/resection to be entirely different diseases. Although not central to the current study, we did note these cases when identified and recorded the final diagnosis other than prostatic carcinoma.
All metastases utilized for further study were confirmed at the time of diagnosis as being prostatic in origin using histological and immunohistochemical means. In histologically-challenging examples, IHC for prostate specific antigen (PSA) and/or prostate specific acid phosphatase (PSAP) was utilized per standard practice. All cases were subsequently confirmed as metastatic prostate cancer by both the neuropathologist (BKKD) and prostate carcinoma expert pathologist (MSL) on the study.
Immunohistochemistry for ERG, CHD1 PTEN and MAP3K7: Five micron thick paraffin sections were deparaffinized, antigens unmasked and immunohistochemically stained for CHD1 (Sigma Aldrich; Prestige, St. Louis, MO; rabbit polyclonal; cat# HPA022236; 1:100), ERG (Cell Marque; Rocklin, CA; rabbit monoclonal EP111; cat#434R;) and MAP3K7 (Sigma Aldrich: Prestige, St. Louis, MO; rabbit polyclonal; cat#: HPA007633, 1:50). Antigens for CHD1 were revealed in Cell Conditioner #2 (Ventana Medical Systems (Roche), Tucson, AZ) using the standard protocol. Antigens for MAP3K7 and ERG were revealed in a sodium citrate solution (10 mM, pH 6.0 + 0.1% Tween 20) and BORG Decloaker (Biocare Medical, Pacheco, CA) for 10 minutes at 110°C (NxGen Decloaker, Biocare Medical, Pacheco, CA), respectively, with an ambient cool down for 10 minutes. Immunodetection was performed on the Benchmark XT immunostainer (Ventana) at an operating temperature of 37°C. CHD1 required additional 3% hydrogen peroxide blocker for 8 minutes. Both CHD1 and MAP3K7 antibodies were incubated for 60 minutes and ERG for 30 minutes. Primary antibodies were detected with an UltraView DAB (Ventana) detection kit. All sections were counterstained in Harris hematoxylin for 2 minutes, blued in 1% ammonium hydroxide (v/v), dehydrated in graded alcohols, cleared in xylene and cover-glass mounted using synthetic resin.
Five micron thick paraffin sections were submitted to NeoGenomics (Fort Myers, FL) for PTEN immunohistochemical staining. PTEN staining was scored as positive (>50% of cells) or negative, or not applicable (N/A) if not performed (insufficient tissue).
Immunohistochemical stains were scored independently by two pathologists (MSL, BKKD). Nuclear ERG expression was scored as positive or negative. Since some degree of CHD1 and MAP3K7 staining was present in all tumors, CHD1 and MAP3K7 were assigned H-scores as a combination of stain intensity (0=none, 1=weak, 2=moderate, 3=strong) and percent of tumor cells using the following formula: (% tumor cells intensity 3 X 3) + (% tumor cells intensity 2 X 2) + (% tumor cells intensity X 1). At least 200 cells were counted for each case.
Results:
A total of twenty-one dural or intraparenchymal cases were identified. 16 identified cases were dural-based tumors, and 5 were intraparenchymal brain metastases. Clinical data is presented in Table 1. All patients were diagnosed with CNS metastases an average of 7.5 years following primary diagnosis (four unknown, one identified within the first year of primary diagnosis). 19/21 patients also had bony metastases (2 unknown), all had castration-resistant disease, and most had failed hormonal and cytotoxic therapies, if data was extractable.
Table 1.
Clinical data on prostatic cancer patients with CNS metastases
| Case No. |
Site | Years after diagnosis presenting with CNS metastases |
Bony metastases |
Castrate- resistant at time of dural/brain metastasis |
Prior treatment |
|---|---|---|---|---|---|
| 1 | C6-T1 (Dural) | 3 | Yes | Yes | Hormonal/unknown |
| 2 | Cerebellum (Dural) | unknown | Unknown | Yes | Unknown |
| 3 | Frontal Lobe (Dural) | 14 | Yes | Yes | Hormonal and cytotoxic |
| 4 | Parieto-occipital (Dural) | 3 | Yes | Yes | Hormonal |
| 5 | L4 (Dural) | 10 | Yes | Yes | Hormonal, cytotoxic and immunotherapy |
| 6 | Cerebellum (Dural) | 4 | Yes | Yes | Hormonal and cytotoxic |
| 7 | T-spine (Dural) | unknown | Yes | Yes | Unknown |
| 8 | C7 (Dural) | 9 | Yes | Yes | Hormonal and cytotoxic |
| 9 | Occipital (Parenchymal) | 3 | Yes | Yes | Hormonal, cytotoxic and immunotherapy |
| 10 | Thalamus (Parenchymal) | 12 | Yes | Yes | Hormonal and cytotoxic |
| 11 | Temporal (Parenchymal) | 2 | Yes | Yes | Hormonal and cytotoxic |
| 12 | Cerebellum (Parenchymal) | 0 | Yes | No | Orchiectomy only (hospice) |
| 13 | Sellar Region (Parenchymal) | unknown | Yes | Yes | Unknown |
| 14 | T10–11 (Dural) | 3 | Yes | Yes | Hormonal and cytotoxic |
| 15 | Frontotemporal (Dural) | 14 | Yes | Yes | Hormonal and cytotoxic |
| 16 | T6–7 (Dural) | 2 | Yes | Yes | Hormonal and cytotoxic |
| 17 | Frontoparietal (Dural) | 26 | Yes | Yes | Hormonal and cytotoxic |
| 18 | T8–10 (Dural) | 13 | Yes | Yes | Hormonal and cytotoxic |
| 19 | T8 (Dural) | 3 | Yes | Yes | Hormonal, cytotoxic and immunotherapy |
| 20 | T1–4 (Dural) | 6 | Yes | Yes | Hormonal and cytotoxic |
| 21 | Not specified (Dural) | unknown | Unknown | Yes | Unknown |
The intraparenchymal metastases occurred in the pituitary region, right frontal and occipital lobes, cerebellum, right temporal lobe and right thalamus (Figure 1A). In the thalamic example, there was extension of metastasis along Virchow-Robin spaces (Figure 1B) with diffuse strong cytoplasmic immunoreactivity for prostate specific antigen (Figure 1C). Most dural and intraparenchymal cases showed prototypical prostatic carcinoma histological features with cribriform gland formation (Figure 1D). One case had neuroendocrine features in a patient with known metastatic neuroendocrine carcinoma of the prostate. That case was negative for PSA but positive for CD56. Four of 5 intraparenchymal metastases and 15/16 dural-based metastases including the neuroendocrine case were ERG-negative (Figure 1E). Only 2 ERG-positive cases were identified, one intraparenchymal and one dural-based metastasis (Figure 1F). Unfortunately, given the retrospective nature of this database, and the referred nature of our patients at a tertiary care center from smaller outside community or regional hospitals, the original slides/tissue blocks from prostate gland were not available for any case for comparison of ERG expression between primary prostate and metastatic sites. Nor was any outside IHC data available regarding ERG expression on any sample.
Figure 1.
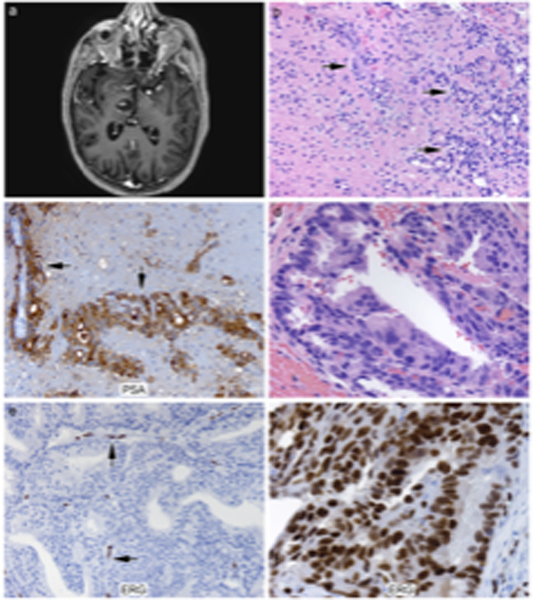
A, a representative MR Image of a right thalamic metastasis. Figure 1B (H&E, 200x), in the thalamic metastasis example, there is extension of metastasis along Virchow-Robin spaces. Figure 1B (H&E, 200x) thalamic metastasis (arrows indicate tumor cells). Figure 1C (200x), the thalamic metastasis showed diffuse strong cytoplasmic immunoreactivity for prostate specific antigen (arrows). Figure 1D (H&E, 400x), most dural and parenchymal cases showed prototypical prostatic carcinoma histological features with cribriform gland formation. Figure 1E (ERG, 200x), a representative case of ERG negative dural-based tumor in contrast to the ERG-positive endothelial cells (arrows). Figure 1F, a representative case of ERG positive tumor with typical nuclear positivity.
In terms of CHD1 and MAP3K7 expression, we defined low expression as an H-score <150 (0–300 possible) and high expression as an H-score ≥ 150 (representative images shown in Figure 2). Table 2 shows the H-scores compared with ERG and PTEN status for the 21 cases. Low expression of MAP3K7 and CHD1 was seen in 17/21 and 8/21 cases, respectively. Of the two ERG-positive cases, both had high expression of CHD1, one also had high expression of MAP3K7, while the other had low MAP3K7. Sixteen of nineteen ERG negative cases had low MAP3K7. Of these, 7 also had low CHD1. Seven of eight cases with low CHD1 also had low MAP3K7. Loss of PTEN by IHC was seen in 9 of 17 cases in which tissue for PTEN staining was available: 7/15 cases that were ERG negative and both ERG positive cases.
Figure 2.
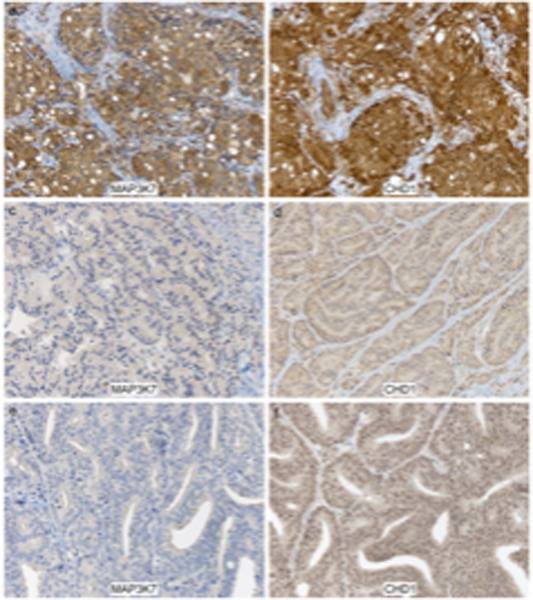
Representative images from a cervical spinal dural metastasis with high immunohistochemical expression for MAP3K7 (A) and CHD1 ( B), in comparison to low dual expression for these markers in 2 different patients with dural metastasis (C-F), the latter supratentorial (left frontal). Immunostaining for MAP3K7 (C, E) and CHD1 (D, F), with light hematoxylin counterstain, all 200X.
Table 2.
IHC expression analysis on patients with prostate cancer CNS metastases
| Case No. |
Site | ERG (+/−) | PTEN (+/−) | CHD1 (H-score) | MAP3K7 (H-score) |
|---|---|---|---|---|---|
| 1 | C6-T1 (Dural) | Neg | N/A | 212 | 90 |
| 2 | Cerebellum (Dural) | Neg | Pos | 106 | 80 |
| 3 | Frontal Lobe (Dural) | Neg | Pos | 110 | 181 |
| 4 | Parieto-occipital (Dural) | Neg | Pos | 224 | 79 |
| 5 | L4 (Dural) | Neg | N/A | 229 | 107 |
| 6 | Cerebellum (Dural) | Neg | Pos | 181 | 97 |
| 7 | T-spine (Dural) | Neg | Pos | 190 | 83 |
| 8 | C7 (Dural) | Neg | Neg | 233 | 194 |
| 9 | Occipital (Parenchymal) | Neg | Neg | 101 | 69 |
| 10 | Thalamus (Parenchymal) | Pos | Neg | 277 | 86 |
| 11 | Temporal (Parenchymal) | Neg | Neg | 215 | 147 |
| 12 | Cerebellum (Parenchymal) | Neg | Neg | 98 | 57 |
| 13 | Sellar Region (Parenchymal) | Neg | Pos | 158 | 90 |
| 14 | T10–11 (Dural) | Pos | Neg | 209 | 157 |
| 15 | Frontotemporal (Dural) | Neg | Neg | 116 | 90 |
| 16 | T6–7 (Dural) | Neg | Pos | 207 | 95 |
| 17 | Frontoparietal (Dural) | Neg | Pos | 92 | 68 |
| 18 | T8–10 (Dural) | Neg | Neg | 98 | 24 |
| 19 | T8 (Dural) | Neg | N/A | 144 | 92 |
| 20 | T1–4 (Dural) | Neg | N/A | 159 | 136 |
| 21 | Not specified (Dural) | Neg | Neg | 190 | 210 |
Although not the main focus of our study, additional malignancies identified in patients with solitary CNS lesions with a known history of prostatic carcinoma included neurocytoma, skull base chordoma, ectopic pituitary adenoma to the clival region, anaplastic meningioma, and undifferentiated malignancy (5 additional lesions, or 19.2% of total suspected cases going to biopsy/excision).
Discussion:
Of our 21 confirmed prostate cancer metastases to the nervous system, 16 were dural-based tumors and 5 were intraparenchymal brain metastases, a feature of metastatic prostatic carcinoma that has been reported only rarely. Neuroimaging was typical in most cases for metastatic disease, although patients with known histories of prostatic carcinoma and suspected metastases were identified in our database search who eventually proved to have neurocytoma, skull base chordoma, ectopic pituitary adenoma to the clival region,[8] anaplastic meningioma, and undifferentiated malignancy, underscoring the clinical confusion that can sometimes result. Additional clinical data in the 21 patients revealed what would be suspected in a CNS metastatic cohort of prostate cancer patients: these patients were all late-stage patients who had developed castrate-resistant disease prior to CNS metastatic diagnosis.
Interestingly, 19 of the 21 prostate metastasis cases were ERG-negative (90.5%) which is significantly skewed from the reported rate of ~40% of ERG translocation in prostatic carcinoma in the primary site. We identified low expression of CHD1 in 8/21 cases and reduced MAP3K7 in 17/21 cases. Sixteen of the 19 ERG-negative tumors (84%) had low expression of MAP3K7. Seven of the 19 ERG-negative cases (37%) also had low expression of CHD1. Nine of 17 cases (53%) stained for PTEN showed loss of PTEN in line with previous literature.
Significant limitations of our study include relatively low case numbers and the absence of the primary tumor in our system with which to compare our results in the metastases. However, as noted above, metastases of prostatic carcinoma to other systemic sites seldom come to biopsy, and even if bone metastases are biopsied, the decalcification treatment necessary on the tissue specimen often renders it unfit for IHC expression analysis. Thus, we utilized what was available. In terms of our interest specifically in those patients where the metastatic disease impacted dura or brain parenchyma, the latter is very rare, even in large institutions, as illustrated by our ability to only identify 5 cases over a >15 year time period, despite our tertiary status. The absence of the primary tumor in our databases with which to compare the results in our metastatic cases was to be expected, given how common prostatic carcinoma is in general, and the limited capability of smaller-sized non-tertiary institutions to deal with pathology beyond basic histological diagnosis.
With these limitations, our findings are far from conclusive, and we fully acknowledge the need for larger studies which would likely require multi-institutional involvement, especially for parenchymal brain metastatic disease. However, our results imply that more aggressive prostate cancer is more likely to be ERG negative.
Our results have implications for using ERG IHC in making diagnoses in the setting of metastases of unknown primary. While previous studies have shown ERG-negative tumors, along with downregulation of MAP3K7 and CHD1, were associated with more aggressive phenotypes (7), this is the first study, to our knowledge, to specifically assess ERG expression in relation to CHD1 and MAP3K7 expression in dural/brain parenchymal metastases.
The high prevalence of loss of MAP3K7 expression in brain metastases is suggestive of a mechanistic underpinning that will need testing in future studies. Interestingly, we observed cribriform patterning in many of our metastases. Recent data has demonstrated a significant association between cribriform primary prostate cancer and genetic deletion of MAP3K7 (9). A causal link between loss of MAP3K7 and cribriform patterning is not established, but our observations here are supportive of such a link. Future studies will also need to be conducted to better understand under what circumstances the rare event of intraparenchymal brain metastasis from prostate cancer occurs.
In conclusion, metastatic prostatic carcinoma to the nervous system appears to have a significantly lower rate of dural-based, and especially parenchymal, brain metastases with ERG nuclear protein expression, compared to primary prostate carcinoma.[3] Negative ERG immunohistochemistry can add to the diagnostic challenge of metastatic prostate carcinoma, but almost no prior data has been published regarding ERG protein status in brain metastases, or its biological implications. Our results are, however, concordant with the limited literature suggesting lower ERG protein expression in lymph node metastases from prostatic carcinoma. The interesting result of lower ERG expression with dural metastases and in intraparenchymal metastases suggests a possibility that ERG-negative tumors (i.e., androgen-deprivation-resistant tumors) may become resistant to standard therapies and diffusely metastasize. Additional work will need to be done to confirm these preliminary findings.
Acknowledgements:
We are grateful to Ms. Lisa Litzenberger for her assistance with photography and figure construction.
Sources of support: R01-CA199741 to SDC
Funding: This study was unfunded.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Dr. Ormond does, however, have unrelated research funded by Synaptive Medical.
Footnotes
Portions of this manuscript were presented in abstract format at the Annual Meeting, Society of Neuro-Oncology, Scottsdale, AZ, USA, November, 2016. BMET-38. Prostatic carcinoma metastatic to the nervous system: parenchymal and problematic cases. Neuro-Oncology 2016;18(suppl 6):vi34-vi35.
Conflict of interest: The authors have no conflicts or potential conflicts of interest related to this manuscript.
References:
- 1.Hatzoglou V, Patel G, Morris M, Curtis K, Zhang Z, Shi W, Huse J, Rosenblum M, Holodny A, Young R (2014) Brain metastases from prostate cancer: an 11-year analysis in the MRI era with emphasis on imaging characteristics, incidence, and prognosis. J Neuroimaging 24: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tremont-Lukats I, Bobustuc G, Lagos G, Lolas K, Kyritsis A, Pudavalli V (2003) Brain metastasis from prostate carcinoma: The M. D. Anderson Cancer Center experience. Cancer 98: 363–368 [DOI] [PubMed] [Google Scholar]
- 3.Teng L-H, Wang C, Begin L, Dolph M, Yilmaz A, Trpkov K, Donnelly B, Bismar T (2013) ERG Protein Expression and Gene Rearrangements Are Present at Lower Rates in Metastatic and Locally Advanced Castration-resistant Prostate Cancer Compared to Localized Disease. Urology 82: 394–399 [DOI] [PubMed] [Google Scholar]
- 4.Guo C, Wang Y, Xiao L, Troncoso P, Czerniak B (2012) The relationship of TMPRSS2-ERG gene fusion between primary and metastatic prostate cancers. Human Pathol 43: 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Ouf H, Zhao L, Bismar T (2016) ERG expression in prostate cancer: biological relevance and clinical implication. J Cancer Res Clin Oncol 142: 1781–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gsponer J, Braun M, Scheble V, Zellweger T, Bachmann A, Perner S, Vlajnic T, Srivastava M, Tan S, Dobi A, Sesterhenn I, Srivastava S, Bubendorf L, Ruiz C (2014) ERG rearrangement and protein expression in the progression to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 17: 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues L, Rider L, Nieto C, Romero L, Karimpour-Fard A, Loda M, Lucia M, Wu M, Shi L, Cimic A, Sirintrapun S, Nolley R, Pac C, Chen H, Peehl D, Xu J, Liu W, Costello J, Cramer S (2015) Coordinate loss of MAP3K7 and CHD1 promotes aggressive prostate cancer. Cancer Res 75: 1021–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mudd P, Hohensee S, Lillehei K, Kingdom T, Kleinschmidt-DeMasters B (2012) Ectopic pituitary adenoma of the clivus presenting with apoplexy: case report and review of the literature. Clin Neuropathol 31: 24–30 [DOI] [PubMed] [Google Scholar]
- 9.Elfandy H, Armenia J, Pederzoli F, Pullman E, Pertega-Gomes N, Schultz N, Viswanathan K, Vosoughi A, Blattner M, Stopsack KH, Zadra G, Penney KL, Mosquera JM, Tyekucheva S, Mucci LA, Barbieri C, Loda M. Genetic and Epigenetic Determinants of Aggressiveness in Cribriform Carcinoma of the Prostate. Molecular Cancer Research: 2018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, and Lotan TL (2018) Clinical implications of PTEN loss in prostate cancer. Nat Rev Urology 15: 222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


