Abstract
Muenke syndrome (MIM #602849), the most common syndromic craniosynostosis, results from the recurrent pathogenic p.P250R variant in FGFR3. Affected patients exhibit wide phenotypic variability. Common features include coronal craniosynostosis, hearing loss, carpal and tarsal anomalies, and developmental/behavioral issues. Our study examined the phenotypic findings, medical management, and surgical outcomes in a cohort of 26 probands with Muenke syndrome identified at the Children’s Hospital of Philadelphia. All probands had craniosynostosis; 69.7% had bicoronal synostosis only, or bicoronal and additional suture synostosis. Three male patients had autism spectrum disorder. Recurrent ear infections were the most common comorbidity, and myringotomy tube placement the most common extracranial surgical procedure. Most patients (76%) required only one fronto-orbital advancement. de novo mutations were confirmed in 33% of the families in which proband and both parents were genetically tested, while in the remaining 66% one of the parents was a mutation carrier. In affected parents, 40% had craniosynostosis, including 71% of mothers and 13% of fathers. We additionally analyzed the medical resource utilization of probands with Muenke syndrome. To our knowledge, these data represent the first comprehensive examination of long-term management in a large cohort of patients with Muenke syndrome. Our study adds valuable information regarding neuropsychiatric and medical comorbidities, and highlights findings in affected relatives.
Keywords: affected family members, FGFR3, FGFR3-related craniosynostosis, Muenke syndrome, reduced penetrance, variable expressivity
1 |. INTRODUCTION
Muenke syndrome (MIM #602849) is caused by a recurrent p.Pro250Arg variant in fibroblast growth factor receptor 3 (FGFR3) (Muenke et al., 1997). It is the most common syndromic craniosynostosis, affecting 1 in 30,000 individuals (Kruszka et al., 1993). Common findings include sensorineural hearing loss, coronal craniosynostosis, and developmental delay (Doherty et al., 2007). FGFR3 is located on chromosome 4, and the pathogenic p.Pro250Arg variant is analogous to the p.Pro253Arg and p.Pro252Arg variants in FGFR2 and FGFR1, etiologically associated with Apert and Pfeiffer syndromes, respectively (Bellus et al., 1996). The p.Pro250Arg variant, like the analogous variants in FGFR2 and FGFR3, is located in the IgII-IgIII linker. These variants act via gain-of-function mechanism to induce cranial and limb abnormalities (González-del Angel et al., 2016; Wilkie, 1997). The syndrome has wide phenotypic variability due to reduced penetrance and variable expressivity. It is estimated that up to 64.7% of cases are inherited (Kruszka et al., 2016).
In 2016, Kruszka et al. reported the clinical characteristics of 106 patients diagnosed with Muenke syndrome. 84.5% had craniosynostosis, underlining the variable penetrance of the condition. Other common findings included hearing loss (70.8%), developmental delay (66.3%), and strabismus (44.9%).
Here we report phenotypic findings in 26 probands, as well as clinical features in a non-biased sample of affected family members who came to attention following the identification of Muenke syndrome in the proband. We report associated medical comorbidities and developmental and behavioral phenotypes in probands. While there have been studies assessing the executive functioning and adaptive behavior in patients with Muenke syndrome, this is the first report of autism spectrum disorder in this population (Yarnell et al., 2015). Additionally, our study aimed to assess data on medical management and surgical outcomes. Previous studies have examined the cranial surgery reoperation rate in this population and outcomes specifically related to cranial surgery, but to our knowledge, this is the first study to report details such as average number of subspecialists, average number of admissions, medical comorbidities, and extracranial surgeries (Honnebier et al., 2008; Ridgway et al., 2011; Thomas et al., 2005).
2 |. MATERIALS AND METHODS
This study was performed under a protocol approved by the Children’s Hospital of Philadelphia Institutional Review Board (IRB) for the Protection of Human Subjects. The study was approved as a retrospective chart review, collecting data in a de-identified manner. Therefore, the IRB approved Waiver of Written Consent.
2.1 |. Patient identification and data collection
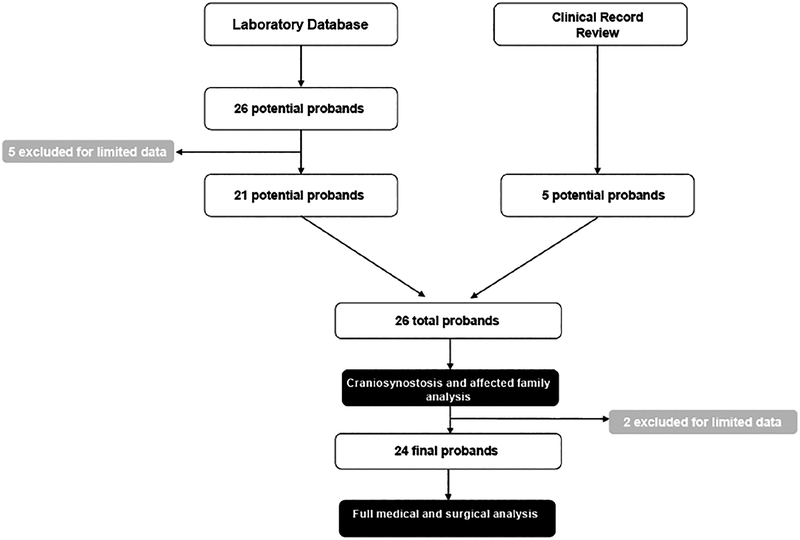
Approach to proband identification is depicted in Figure 1. Patients were identified by querying the Children’s Hospital of Philadelphia (CHOP) laboratory database for all patients who underwent molecular testing for syndromic craniosynostosis between 2004 and 2016, as well as by review of craniofacial genetics specialists’ case logs. Probands whose testing revealed the pathogenic variant for Muenke syndrome, FGFR3 p.P250R, as well as those identified by craniofacial genetics specialists, were selected for further analysis. Querying the laboratory database for all patients with the FGFR3 p.P250R mutation yielded 26 potential probands. Five were excluded because of limited data available in the electronic medical record. This left 21 probands from the laboratory database. An additional five probands were identified by craniofacial genetics specialists from their case logs. These 26 probands underwent analysis for craniosynostosis type and affected family members. Two of the probands were subsequently excluded for limited data available in the medical record, leaving 24 probands who underwent full medical and surgical analysis.
FIGURE 1.
Flowchart depicting selection of probands for analysis from laboratory database and clinical record review
Patients whose medical and surgical care were conducted primarily at CHOP, or at an institution which shared medical records with CHOP’s electronic medical record system, were selected for inclusion. Patients who had very limited or no medical or surgical care at CHOP were excluded from the study. Phenotypic, management, and outcomes data were gathered through accessing each patient’s electronic medical record. In order to ensure the confidentiality of the data, no identifiable information was recorded.
2.2 |. Molecular diagnostic methodology
In the majority of cases, molecular testing for the FGFR3 c.749C > G variant was conducted by Sanger sequencing prior to 2013, and by next-generation sequencing after 2013.
2.3 |. Statistical analysis
Statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, North Carolina) and included the Fisher’s exact test to evaluate the following: (a) the proportion of hearing loss, developmental delay and craniosynostosis type in male versus female subjects; (b) the proportion of subjects with versus without hearing loss that had a history of recurrent ear infections; and (c) the proportion of craniosynostosis in affected mothers versus fathers. Statistical significance was set at p < .05.
2.4 |. Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
3 |. RESULTS
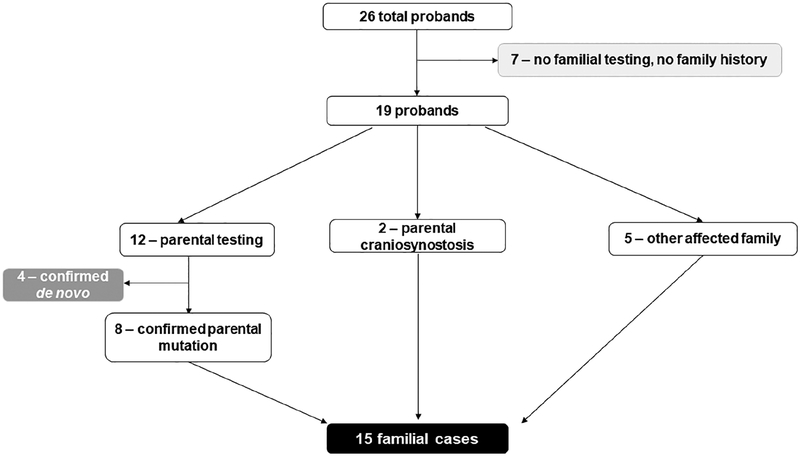
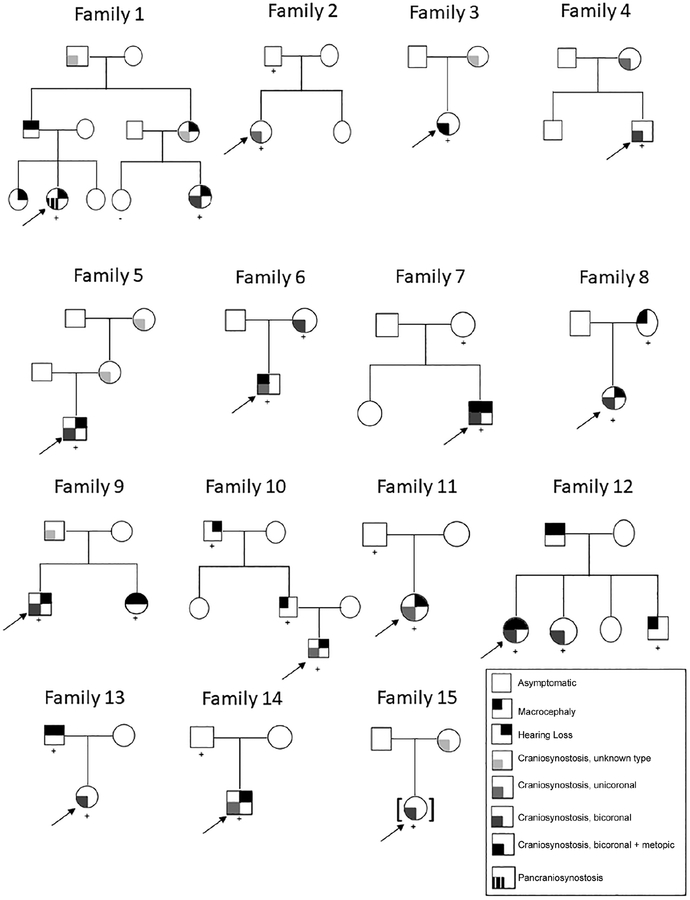
Demographics are outlined in Table 1. Fourteen of 26 probands were male. Ages ranged from 8 months to 26 years. Breakdown of known familial, known de novo, and unknown inheritance is displayed in Figure 2. Of the 26 probands, 7 had no familial testing and no family history, for whom inheritance was unknown. Twelve had parental testing, of whom eight parents (67%) had molecular confirmation of mutation. Two probands had parents with known craniosynostosis (both diagnosed prior to their child’s evaluation). Five probands had parents who were considered obligate affected due to family history (i.e., second-degree relatives with the diagnosis). Pedigrees of familial cases are displayed in Figure 3.
TABLE 1.
Proband demographics, inheritance pattern, and craniosynostosis type
| Number | |
|---|---|
| Total | 26 |
| Gender | |
| Male | 14 |
| Female | 12 |
| Age | |
| <2 years | 2 |
| 2–5 years | 7 |
| 6–11 years | 7 |
| 12–18 years | 5 |
| >18 years | 5 |
| Inheritance | |
| de novo | 4 |
| Inherited | 15 |
| Unknown | 7 |
| Craniosynostosis | |
| Unicoronal | 8 |
| Bicoronal | 16 |
| Bicoronal + othersa | 2 |
Bicoronal and metopic—1 patient. Pancraniosynostosis—1 patient.
FIGURE 2.
Flowchart depicting identification of patients with de novo, familial, or unknown inheritance type
FIGURE 3.
Pedigrees of familial probands. Arrow indicates 15 familial probands who were analyzed. + sign indicates presence of p.P250R mutation in FGFR3. − sign indicates negative testing for p. P250R mutation in FGFR3. Craniosynostosis type denoted by color, as noted in legend
All probands presented with craniosynostosis, of which 31% were unicoronal and 62% bicoronal. Two females, representing 7.7% of the total cohort, had bicoronal and other suture synostosis – one with bicoronal and metopic, one with pan-cranial (Table S1). There were similar proportions of unicoronal and multisuture synostosis in males and females (p = .22).
Dysmorphic features and limb anomalies are reviewed in Table S2. Various ear anomalies (uplifted earlobes, squared-off helices, over-folded helices, and others) were the most common dysmorphic feature. High palate, hypertelorism, and other eye anomalies were also fairly common. 20% of our cohort were noted to have downslanting palpebral fissures. One male patient had a cleft lip. Limb anomalies, including brachydactyly, were uncommon. The patients in our cohort did not have imaging to assess for carpal/tarsal fusions and other radiographic abnormalities describe in the syndrome. Two patients had broad fingers.
Most patients had mild or mild to moderate hearing loss (79% total), and 71% of those with hearing loss used amplification (Table 2). Sensorineural hearing loss was the most common type, present in 86%. The vast majority of patients with sensorineural hearing loss utilized hearing aids. There were similar proportions of hearing loss in males and females (p = .40).
TABLE 2.
Hearing loss findings in probands
| Phenotype (N = 24) | Number total (%)a | Number males (%) | Number females (%) |
|---|---|---|---|
| Hearing loss | |||
| Present | 14/23 (61) | 6/12 (50) | 8/11 (72) |
| Type | |||
| Conductive | 1/14 (7.1) | 1/6 (17)b | 0/8 (0) |
| SNHL | 12/14 (86) | 4/6 (66) | 8/8 (100) |
| Mixed | 1/14 (7.1) | 1/6 (17) | 0/8 (0) |
| Unknown | 1/14 (7.1) | 1/6 (17) | 0/8 (0) |
| Severity | |||
| Mild | 5/14 (36) | 3/6 (50) | 2/8 (25) |
| Mild to moderate | 6/14 (43) | 2/6 (33) | 4/8 (50) |
| Severe | 0/14 (0) | 0/6 (0) | 0/8 (0) |
| Not described | 3/14 (21) | 1/6 (17) | 2/8 (25) |
| Amplification | |||
| Amplification utilized | 10/14 (71) | 5/6 (83) | 5/8 (63) |
| Hearing aid | 9/10 (90) | 5/5 (100) | 4/5 (80) |
| FM system | 4/10 (40) | 0/5 (0) | 4/5 (80) |
Abbreviations: FL, frequency-modulated; HL, hearing loss; SNHL, sensorineural hearing loss.
Variable denominators reflect variability of available information.
One boy had conductive HL which progressed to SNHL.
Ophthalmologic data, available for 10 males and 12 females, is presented in Table S3. Strabismus was the most common finding, present in 50% of the patients.
Table 3 summarizes neuropsychological features. Seizures were noted in three males. Developmental delay was fairly common (68%), with speech delay being the most common type (80%). There were similar proportions of reported developmental delay in males and females (p = .36). Attention deficit-hyperactivity disorder (ADHD) and unspecified hyperactivity were present in four patients (22% overall). Three patients, all males, were diagnosed with autism spectrum disorder by Developmental Pediatrics. Of note, four probands were followed by Developmental Pediatrics and two were followed by Neurology.
TABLE 3.
Neuropsychiatric history and diagnoses in probands
| Diagnosis (N = 24) | Number total (%)a | Number males (%) | Number females (%) |
|---|---|---|---|
| Seizures | 3/24 (13) | 3/12 (25) | 0/12 (0) |
| Developmental delay | 15/22 (68) | 9/11 (81) | 6/11 (54) |
| Speech delay | 12/15 (80) | 8/9 (89) | 4/6 (67) |
| Gross motor delay | 5/15 (33) | 3/9 (33) | 2/6 (33) |
| Fine motor delay | 5/15 (33) | 4/9 (44) | 1/6 (17) |
| Neuropsychiatric diagnoses by patient | |||||||
|---|---|---|---|---|---|---|---|
| Attention deficit-hyperactivity disorder | Hyperactivity | Anxiety | Autism | Obsessive compulsive tendencies | Perfectionism | Aggression | |
| Patient 7 | Present | Present | |||||
| Patient 8 | Present | Present | Present | ||||
| Patient 9 | Present | ||||||
| Patient 12 | Present | Present | |||||
| Patient 14 | Present | Present | |||||
| Patient 23 | Present | ||||||
Notes: Neuropsychiatric diagnoses extrapolated from review of notes in the medical record. Probands not listed did not have neuropsychiatric diagnoses. Four probands were followed by Developmental Pediatrics and two were followed by Neurology.
Variable denominators reflect variability of available information.
Table 4 lists medical comorbidities in this cohort. Recurrent ear infections were the most common, in 21% of patients. There were similar proportions of hearing loss and normal hearing in probands with recurrent ear infections (p = 1.00). Atopic diagnoses, including allergic rhinitis and asthma, were present in 21%. Surgical management data were available for 23 probands, and are presented in Table 5 (one proband had surgery outside of the CHOP medical record system, and was thus not analyzed for surgical management). All but two patients had undergone fronto-orbital advancement (FOA). The two who had not undergone FOA were below 1 year of age, and FOA is not typically performed in this age group. The vast majority underwent only one FOA, but 23.5% underwent multiple FOA (mean number of FOA: 1.3). 48% underwent posterior vault distraction to enlarge the cranial vault prior to undergoing fronto-orbital advancement. Most patients suffered no neurosurgical complications. In terms of extracranial surgical procedures, bilateral myringotomy tube placement was most common, at 38%. Strabismus repair was the second most common extracranial surgery. 46% of patients did not undergo extracranial surgeries.
TABLE 4.
Medical comorbidities in probands
| Medical problem (N = 24) | Number total (%)a | Number males (%) | Number females (%) |
|---|---|---|---|
| Recurrent ear infection | 5/24 (21) | 3/12 (25) | 2/12 (17) |
| Epistaxis | 2/24 (8.3) | 2/12 (17) | 0/12 (0) |
| Reflux | 3/24 (13) | 2/12 (17) | 1/12 (8.3) |
| Atopic featuresb | 5/24 (21) | 3/12 (25) | 2/12 (17) |
| Dysuria/chronic UTI | 1/24 (4.2) | 0/12 (0) | 1/12 (8.3) |
| Nephrotic syndrome | 1/24 (4.2) | 0/12 (0) | 1/12 (8.3) |
| Crohn’s disease | 1/24 (4.2) | 0/12 (0) | 1/12 (8.3) |
| Restless leg syndrome | 1/24 (4.2) | 1/12 (8.3) | 0/12 (0) |
| Headache | 2/24 (8.3) | 1/12 (8.3) | 1/12 (8.3) |
| Structural abnormalitiesc | 1/24 (4.2) | 1/12 (8.3) | 0/12 (0) |
Abbreviation: UTI, urinary tract infection.
Variable denominators reflect variability of available information.
Food allergy, asthma, allergic rhinitis, drug allergy, atopic dermatitis.
Hypospadias in one male.
TABLE 5.
Surgical history in probands
| Surgery/complication type (N = 24) | Number total (%)a | Number males (%) | Number females (%) |
|---|---|---|---|
| Cranial surgeries | |||
| Fronto-orbital advancement | 21/23 (91) | 11/11 (100) | 10/12 (83)b |
| x1 | 16/21 (76) | 9/11 (81) | 7/10 (70) |
| x2 | 4/21 (19) | 2/11 (18) | 2/10 (20) |
| x3 | 1/21 (4.8) | 0/11 (0) | 1/10 (10) |
| Posterior vault distraction | 11/23 (48) | 3/11 (27) | 5/12 (42) |
| Smoothing/revision | 3/23 (13) | 1/11 (18) | 2/12 (17) |
| Reason for repeat FOA | |||
| Deformity | 2/5 (40) | 1/2 (50) | 1/3 (33) |
| Headache | 3/5 (60) | 1/2 (50) | 2/3 (67) |
| Neurosurgical complications | |||
| Chiari malformation | 1/23 (4.3) | 0/11 (0) | 1/12 (8.3) |
| Incisional infection | 2/23 (8.7) | 2/11 (18) | 0/12 (0) |
| None | 20/23 (87) | 9/11 (81) | 11/12 (92) |
| Other surgeries | |||
| Myringotomy tubes | 9/24 (38) | 5/12 (42) | 4/12 (33) |
| Once | 4/9 (44) | 2/5 (40) | 2/4 (50) |
| More than once | 5/9 (55) | 3/5 (60) | 2/4 (50) |
| Tonsillectomy and adenoidectomy | 2/24 (8.3) | 0/12 (0) | 2/12 (17) |
| Adenoidectomy only | 1/24 (4.2) | 0/12 (0) | 1/12 (8.3) |
| Nasal cautery | 2/24 (8.3) | 2/12 (17) | 0/12 (0) |
| Dental surgery | 0 24 (0) | 0/12 (0) | 0/12 (0) |
| Eye surgery | 3/24 (13) | 1/12 (8.3) | 2/12 (17) |
| Other surgeryc | 2/24 (8.3) | 2/12 (17) | 0/12 (0) |
| None | 11/24 (46) | 5/12 (42) | 6/12 (50) |
Note: Cranial surgery data were not available for one proband, who had surgery outside of CHOP medical record system.
Variable denominators reflect variability of available information.
Two females have only had posterior vault distraction, and have not yet had fronto-orbital advancement.
Tibial fracture repair, septorhinoplasty.
Table S4 reports data on medical management, including admissions, subspecialty appointments, medications, and day surgeries. On average, each patient had undergone 2.4 admissions and was followed by 5.3 medical subspecialists. 100% of patients had been evaluated by Genetics. 95% of patients followed with Plastic Surgery and Ophthalmology.
In examining family history (Figure 3, Table 6), seven probands inherited the mutation from an affected mother, and eight from an affected father. Of the affected mothers, five had a history of craniosynostosis (unicoronal in one, bicoronal in one, and unknown type in the others) and one had macrocephaly. Of the affected fathers, only one had craniosynostosis, while four had macrocephaly. There was no imaging of the cranial sutures available for review in affected parents. In affected parents, there was a greater proportion of mothers with craniosynostosis than fathers (p = .04). Not all parents had formal audiologic testing, but hearing loss was reported in three affected fathers and no affected mothers. In all, 60% of affected parents had no craniosynostosis, and 27% were completely asymptomatic. Affected parents did not undergo radiologic imaging of the hands and feet to assess for carpal or tarsal fusions, coned epiphyses, or phalangeal abnormalities.
TABLE 6.
Phenotypic findings in affected family members
| Phenotype (N = 15) | Number total (%) | Number mothers (%) | Number fathers (%) |
|---|---|---|---|
| Craniosynostosis | 6/15 (40) | 5/7 (71) | 1/8 (13) |
| Macrocephaly | 6/15 (40) | 1/7 (14) | 4/8 (50) |
| Neither | 4/15 (27) | 1/7 (14) | 3/8 (38) |
| Phenotype (N = 4) | Number total (%) | Number sisters (%) | Number brothers (%) |
| Craniosynostosis | 1/4 (25) | 1/3 (33) | 0/1 (0) |
| Macrocephaly | 3/4 (75) | 2/3 (67) | 1/1 (100) |
| Hearing loss | 1/4 (25) | 1/3 (33) | 0/1 (0) |
In multiplex families, we identified nine siblings of probands with an FGFR3 p.P250R mutation. Adequate phenotypic data was available for four siblings from three families. One female sibling had bicoronal craniosynostosis and was positive for the p.P250R mutation. One brother had macrocephaly and was positive for the p.P250R mutation. One sister had hearing loss, macrocephaly, and was positive for the p. P250R mutation. One sister had hearing loss and had not been tested for the familial mutation.
4 |. DISCUSSION
Our study offers heretofore unreported phenotypic data in a cohort of patients with Muenke syndrome, including occurrence of autism and medical comorbidities, as well as information on medical and surgical management. Additionally, we examined phenotypic features in affected parents and siblings to differentiate the clinical findings of probands from affected family members.
The prevalence of bicoronal synostosis in our cohort, 62% for bicoronal only and 69.7% for bicoronal only or in combination with other sutures, was higher than previously reported (Kruszka et al., 2016). Notably, only females in our cohort had multisuture synostosis, though there were overall similar proportions of unicoronal and multisuture synostosis among males and females (p = .22). This is in contrast to previous reports of females being more severely affected than males (Lajeunie et al., 1999). Compared to previous reports, our cohort generally had fewer reported dysmorphic craniofacial features, but ear differences were the most common.
Examination of the medical comorbidities in this cohort was revealing. Recurrent ear infections were fairly common, and bilateral myringotomy tube placement was a common extracranial surgical procedure, though there was not a statistically significant difference in proportions of hearing loss and normal hearing among patients with recurrent ear infections (p = 1.00). The prevalence of hearing loss in our cohort, 61%, is similar to that seen in a study of pediatric patients in the Netherlands with various syndromic craniosynostoses, which found mild or moderate hearing loss in 62.1% of patients with Muenke syndrome. This study identified predominantly sensorineural hearing loss in children with Muenke syndrome, similar to our cohort, in which 86% had sensorineural hearing loss and 7.1% had conductive hearing loss. In this study, 48% of patients had recurrent otitis media with effusions, compared to 21% of our cohort. de Jong et al. note that most patients with syndromic craniosynostosis experience recurrent otitis media with effusion (de Jong et al., 2011).
Neuropsychiatric comorbidities in our population exhibited similarities to the published literature, as well as previously unreported findings. Three male patients in our cohort presented with seizures, which have been reported in other patients with Muenke syndrome (Abdel-Salam et al., 2011; Agochukwu et al., 2012; Okubo et al., 2017). Notably, three male patients were diagnosed with autism spectrum disorder. Only four patients were regularly followed by Developmental Pediatrics and two by Neurology. The prevalence of autism spectrum disorder in our cohort, along with the prevalence of developmental delay (68%), underscores the need for neurocognitive follow-up and periodic neuropsychological evaluation in children with Muenke syndrome, as suggested by other reports of developmental delay in this population (Doherty et al., 2007; Escobar et al., 2009; González-del Angel et al., 2016; Kruszka et al., 2016; Lajeunie et al., 1999; Lowry et al., 2001; Okubo et al., 2017; Yarnell et al., 2015).
Examination of craniosurgical data revealed that most patients in our cohort underwent only one fronto-orbital advancement (FOA). Previous studies have associated the presence of the FGFR3 p.P250R mutation with a higher risk of reoperation, often due to increased intracranial pressure (Thomas et al., 2005; Wilkie et al., 2010). In our cohort, postoperative increased intracranial pressure was not noted. One study from 2011 found that in a cohort of 21 patients with Muenke syndrome, 40% required secondary FOA. Increased age at initial FOA was inversely correlated with need for repeat FOA (Ridgway et al., 2011). The relatively new practice of performing posterior vault distraction as the initial surgery, thus delaying the timing of the first FOA, may account for the decreased reoperation rate in our cohort compared to the literature (Taylor and Bartlett, 2017). One patient in our cohort was diagnosed with Chiari malformation, which has been reported previously (Abdel-Salam et al., 2011).
In analyzing probands and affected relatives separately, we were able to glean useful data on familial cases. Craniosynostosis was more prevalent among affected mothers than fathers, which has been previously reported in affected parents of probands (Lajeunie et al., 1999). It is notable that 27% of affected parents had neither macrocephaly nor craniosynostosis. A similar review of familial cases by Gonzalezdel Angel et al. found that some family members carrying the pathogenic variant were considered unaffected prior to molecular testing. However, these family members exhibited hearing loss, dysmorphic features, and hand and foot abnormalities (González-del Angel et al., 2016). In our cohort, parents did not undergo radiography of the hands and feet, therefore carpal or tarsal fusions, coned epiphyses, and abnormalities of the phalanges, which have been reported in carrier family members previously considered unaffected, were not identified (González-del Angel et al., 2016; Graham et al., 1998; Lajeunie et al., 1999; Lowry et al., 2001). Of note, Singh et al. reported one Indian family in which a clinically asymptomatic mother transmitted the p.P250R variant to her daughter, who presented with classic signs of the syndrome (Singh et al., 2014). In our cohort, there were four families in which an affected parent did not exhibit any signs or symptoms of Muenke syndrome (Families 2, 7, 11, 14, Figure 3), underscoring the variable and reduced penetrance of the syndrome. Our findings further underscore the importance of testing all family members, including those who are asymptomatic, when a proband is diagnosed with Muenke syndrome.
This study was limited by its retrospective nature. The nature of patient selection, including review of case logs by clinical geneticists, may have resulted in sampling bias, affecting parameters such as types of subspecialists who followed the patients, as well as sampling patients with relatively severe phenotypes. In some areas, data was incomplete or unclear for certain patients. Additionally, our sample size, while sizable from one institution, is underpowered to perform robust statistical analyses. We hope to enlarge our sample size in the future by collaborating with other large craniofacial centers.
5 |. CONCLUSION
Our study gathered phenotypic data, including heretofore unreported information such as certain medical comorbidities and the occurrence of autism spectrum disorder, as well as surgical and medical management data, in a cohort of patients with Muenke syndrome. These data suggest that patients with syndromic craniosynostosis are not necessarily more likely to require multiple corrective surgeries, possibly due to utilization of posterior vault distraction. Neuropsychiatric diagnoses such as autism spectrum disorder and ADHD are present among those with Muenke syndrome, and these children should have timely regular follow-up with Developmental Pediatric specialists. Recurrent ear infections and bilateral myringotomy tube placement appear fairly common in this population. Taken together, these data begin to suggest a way forward to create a management guideline for clinicians caring for this population, as well as a family-friendly prognostic guideline to help families understand the implications of the diagnosis.
Additionally, examination of affected relatives revealed that some were completely asymptomatic, though our cohort did not have limb imaging to assess for radiographic findings. The lack of clinical findings in affected relatives underscores the importance of mutational analysis in all family members, regardless of phenotypic features, when a proband is diagnosed with Muenke syndrome.
Supplementary Material
ACKNOWLEDGMENTS
Chaya Nautiyal Murali was supported by the Ruth L. Kirschstein National Research Service Award, Grant number 2T32GM008638-21. She is currently supported by the T32GM07526-41 Medical Genetics Research Fellowship Program. Bridget M. Stroup was supported by NIH T32DK007664-28 and the US Public Health Service grant P30DK56338, which funds the Texas Medical Center Digestive Disease Center.
Funding information
National Institutes of Health, Grant/Award Numbers: 2T32GM008638-21, GM07526-41, T32DK007664-28; U.S. Public Health Service, Grant/Award Number: P30DK56338; Medical Genetics Research Fellowship Program, Grant/Award Number: T32GM07526-41; Ruth L. Kirschstein National Research Service Award, Grant/Award Number: 2T32GM008638-21
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Abdel-Salam GMH, Flores-Sarnat L, El-Ruby MO, Parboosingh J, Bridge P, Eid MM, … Temtamy SA (2011). Muenke syndrome with pigmentary disorder and probable hemimegalencephaly: An expansion of the phenotype. American Journal of Medical Genetics, Part A, 155, 207–214. [DOI] [PubMed] [Google Scholar]
- Agochukwu NB, Solomon BD, Gropman AL, & Muenke M (2012). Epilepsy in Muenke syndrome: FGFR3-related craniosynostosis. Pediatric Neurology, 47, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellus GA, Gaudenz K, Zackai EH, Clarke LA, Szabo J, Francomano CA, & Muenke M (1996). Identical mutations in three different fibroblast growth factor receptor genes in autosomal domi-nant craniosynostosis syndromes. Nature Genetics, 14, 174–176. [DOI] [PubMed] [Google Scholar]
- Doherty ES, Lacbawan F, Hadley DW, Brewer C, Zalewski C, Kim HJ, … Muenke M (2007). Muenke syndrome (FGFR3-related craniosynostosis): Expansion of the phenotype and review of the literature. American Journal of Medical Genetics, Part A, 143A, 3204–3215. [DOI] [PubMed] [Google Scholar]
- Escobar LF, Hiett AK, & Marnocha A (2009). Significant phenotypic variability of Muenke syndrome in identical twins. American Journal of Medical Genetics, Part A, 149A, 1273–1276. [DOI] [PubMed] [Google Scholar]
- González-del Angel A, Estandía-Ortega B, Alcántara-Ortigoza MA, Martínez-Cruz V, Gutiérrez-Tinajero DJ, Rasmussen A, & Gómez-González CS (2016). Expansion of the variable expression of Muenke syndrome: Hydrocephalus without craniosynostosis. American Journal of Medical Genetics, Part A, 170, 3189–3196. [DOI] [PubMed] [Google Scholar]
- Graham JM, Braddock SR, Mortier GR, Lachman R, Van Dop C, & Jabs EW (1998). Syndrome of coronal craniosynostosis with brachydactyly and carpal/tarsal coalition due to Pro250Arg mutation in FGFR3 gene. American Journal of Medical Genetics, 77, 322–329. [DOI] [PubMed] [Google Scholar]
- Honnebier MB, Cabiling DS, Hetlinger M, McDonald-Mcginn DM, Zackai EH, & Bartlett SP (2008). The natural history of patients treated for FGFR3-associated (Muenke-type) craniosynostosis. Plastic and Reconstructive Surgery, 121, 919–931. [DOI] [PubMed] [Google Scholar]
- de Jong T, Toll MS, de Gier HHW, & Mathijssen IMJ (2011). Audiological profile of children and young adults with syndromic and complex craniosynostosis. Archives of Otolaryngology, Head & Neck Surgery, 137, 775–778. [DOI] [PubMed] [Google Scholar]
- Kruszka P, Addissie YA, Agochukwu NB, Doherty ES, & Muenke M (1993). Muenke Syndrome. Seattle: University of Washington. [Google Scholar]
- Kruszka P, Addissie YA, Yarnell CMP, Hadley DW, Guillen Sacoto MJ, Platte P, … Muenke M (2016). Muenke syndrome: An international multicenter natural history study. American Journal of Medical Genetics, Part A, 170, 918–929. [DOI] [PubMed] [Google Scholar]
- Lajeunie E, El Ghouzzi V, Le Merrer M, Munnich A, Bonaventure J, & Renier D (1999). Sex related expressivity of the phenotype in coronal craniosynostosis caused by the recurrent P250R FGFR3 mutation. Journal of Medical Genetics, 36, 9–13. [PMC free article] [PubMed] [Google Scholar]
- Lowry RB, Wang Jabs E, Graham GE, Gerritsen J, & Fleming J (2001). Syndrome of coronal craniosynostosis, Klippel-Feil anomaly, and sprengel shoulder with and without Pro250Arg mutation in the FGFR3 gene. American Journal of Medical Genetics, 104, 112–119. [DOI] [PubMed] [Google Scholar]
- Muenke M, Gripp KW, McDonald-McGinn DM, Gaudenz K, Whitaker LA, Bartlett SP, … Zackai EH (1997). A unique point mutation in the fibroblast growth factor receptor 3 gene (FGFR3) defines a new craniosynostosis syndrome. American Journal of Human Genetics, 60, 555–564. [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Kitamura T, Anzai M, Endo W, Inui T, Takezawa Y, …Haginoya K (2017). A patient with Muenke syndrome manifesting migrating neonatal seizures. Brain & Development, 39, 873–876. [DOI] [PubMed] [Google Scholar]
- Ridgway EB, Wu JK, Sullivan SR, Vasudavan S, Padwa BL, Rogers GF, & Mulliken JB (2011). Craniofacial growth in patients with FGFR3Pro250Arg mutation after fronto-orbital advancement in infancy. The Journal of Craniofacial Surgery, 22, 455–461. [DOI] [PubMed] [Google Scholar]
- Singh A, Goyal M, Kumar S, Kress W, & Kapoor S (2014). Phenotypic variability in two families of Muenke syndrome with FGFR3 mutation. Indian Journal of Pediatrics, 81, 1230–1232. [DOI] [PubMed] [Google Scholar]
- Taylor JA, & Bartlett SP (2017). What’s new in syndromic craniosynostosis surgery? Plastic and Reconstructive Surgery, 140, 82e–93e. [DOI] [PubMed] [Google Scholar]
- Thomas GPL, Wilkie AOM, Richards PG, & Wall SA (2005). FGFR3 P250R mutation increases the risk of reoperation in apparent “nonsyndromic” coronal craniosynostosis. The Journal of Craniofacial Surgery, 16, 347–352 discussion 353–4. [DOI] [PubMed] [Google Scholar]
- Wilkie AO (1997). Craniosynostosis: Genes and mechanisms. Human Molecular Genetics, 6, 1647–1656. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM, Byren JC, Hurst JA, Jayamohan J, Johnson D, Knight SJL, … Wall SA (2010). Prevalence and complications of single-gene and chromosomal disorders in craniosynostosis. Pediatrics, 126, e391–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell CMP, Addissie YA, Hadley DW, Guillen Sacoto MJ, Agochukwu NB, Hart RA, … Muenke M (2015). Executive function and adaptive behavior in Muenke syndrome. The Journal of Pediatrics, 167, 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.