Abstract
Rationale:
Vascular endothelial growth factor (VEGF) is the main driver of angiogenesis and vascular permeability via VEGF receptor 2 (VEGFR2), whereas lymphangiogenesis signals are transduced by VEGFC/D via VEGFR3. VEGFR3 also regulates sprouting angiogenesis and blood vessel growth, but to what extent VEGFR3 signaling controls blood vessel permeability remains unknown.
Objective:
To investigate the role of VEGFR3 in the regulation of VEGF-induced vascular permeability.
Methods and Results:
Long-term global Vegfr3 gene deletion in adult mice resulted in increased fibrinogen deposition in lungs and kidneys, indicating enhanced vascular leakage at the steady state. Short-term deletion of Vegfr3 in blood vascular endothelial cells increased baseline leakage in various tissues, as well as in tumors, and exacerbated vascular permeability in response to VEGF, administered via intradermal adenoviral delivery or through systemic injection of recombinant protein. VEGFR3 gene silencing upregulated VEGFR2 protein levels and phosphorylation in cultured endothelial cells. Consistent with elevated VEGFR2 activity, vascular endothelial cadherin showed reduced localization at endothelial cell–cell junctions in postnatal retinas after Vegfr3 deletion, or after VEGFR3 silencing in cultured endothelial cells. Furthermore, concurrent deletion of Vegfr2 prevented VEGF-induced excessive vascular leakage in mice lacking Vegfr3.
Conclusions:
VEGFR3 limits VEGFR2 expression and VEGF/VEGFR2 pathway activity in quiescent and angiogenic blood vascular endothelial cells, thereby preventing excessive vascular permeability.
Keywords: blood vessels, vascular biology, vascular leakage, VE-Cadherin, VEGF receptor regulation
Endothelial cells (ECs) are essential regulators of blood vessel function. ECs are connected via adherens and tight junctions, which control the integrity of the EC barrier.1 Vascular endothelial growth factor (VEGF) receptor-2 (VEGFR2) is the main transducer of VEGF signals in ECs.2 VEGFR2 is highly expressed during physiological and pathological angiogenesis, and VEGFR2 levels are downregulated during vascular maturation.3 Yet, low expression of VEGF is required for EC homeostasis in adults.4 Vascular endothelial cadherin (VE-Cadherin), the most prominent cadherin at the adherens junctions of ECs, forms a complex with VEGFR2. Activation of VEGFR2 by VEGF dissociates this complex, leading to receptor internalization and increased vascular permeability and ECs motility, an effect that is mediated by VEGFR2 tyrosine residue Y949 and c-src.5,6
The VEGFR3 tyrosine kinase is expressed mainly in lymphatic vessels. VEGFR3 transmits signals for lymphatic endothelial migration, survival, and proliferation and is involved in the biology and pathology of the lymphatic vasculature.7–9 VEGFR3 is additionally expressed in blood vessel endothelia during developmental, physiological, and pathological angiogenic processes, where it is important for activation of Notch signaling.10–13 Lowlevel VEGFR3 expression is detected in the steady state in high endothelial venules and fenestrated capillaries of several organs.14,15
Here, we analyzed adult mice with either global or endothelial-specific deletion of Vegfr3, using the conditional Cre/loxP recombination approach. We show that loss of Vegfr3 results in increased VEGFR2 levels, which in turn lead to increased vascular permeability, without affecting EC integrity, vascular density, or pericyte coverage. Our results demonstrate a novel mechanism controlling vascular permeability by VEGFR3/VEGFR2 cross talk in blood vascular ECs.
Methods
Analysis of Vascular Leakage
The permeability tracers used in this study are summarized in Table 1. For the Miles assay after adenoviral transduction of the ear skin, the mice were anesthetized with 70 mg/kg ketamine (Ketaminol vet; Intervet) and 9 mg/kg xylazine (Rompun; Orion Pharma). Thereafter, 100 μL of Evans blue 3% in phosphate buffered saline (PBS) was injected intravenously via the tail vein. The dye was allowed to circulate for 12 minutes, and the mice were euthanized and perfused with PBS through the left ventricle. The ears were dissected, weighed, and Evans blue was extracted by incubating the tissues in deionized formamide overnight, at 55°C. Evans blue absorbance was measured at 620 nm. For the basal permeability study, the mice were anesthetized and 100 μL of 3% Evans blue and 0.5% 50-nm polystyrene dragon green nanoparticles in PBS or 100 μL of a mix of 50-μL 1% Texas red dextran (70 kDa) and 50-μL dragon green beads (1%) was injected into the lateral tail vein. After 12 or 30 minutes, mice were euthanized and perfused transcardially with 20 mL PBS. The retinas were then collected and processed for whole mount staining. Sites of vascular leakage in the trachea after administration of VEGF or bradykinin were identified by extravasation of fluorescent nanoparticles. Recombinant human VEGF165 (0.25 μg/g) or bradykinin (1 mg/kg; SIGMA, B3259) was injected intravenously together with 40 μL of 2% nanoparticles (FluoSpheres Carboxylate-Modified Microspheres, 0.1 μm, red fluorescent 580/605), diluted to 100 μL with saline. The intravascular nanoparticles were removed from the circulation by transcardiac perfusion with 10 mL of 1% paraformaldehyde in PBS, 15 (VEGF165) or 4 (bradykinin) minutes after injection, and the tissues were collected for analysis. To quantify leakage based on microscopic images, the amount of tracer extravasation was normalized to blood vessel density.
Table 1.
List of Tracers Used in this Study
| Tracer | Label | MW | Dosage | Comments | References |
|---|---|---|---|---|---|
| Evans blue | Visible blue (absorbance 620 nm) | 961 Da (binds to albumin; MW=66 kDa) | 3% in PBS (100 μL IV) | Evans blue extravasation represents plasma extravasation and can been quantified in the Miles assay. | 16 |
| 70-kDa Dextran | Texas red (589/615 nm) | 70 kDa | 1% in PBS (50 μL IV) | Shows a slow leakage profile similar to albumin and can be used to access plasma extravasation by molecular imaging. | 17 |
| Nanoparticles | Polystyrene nanoparticles, dragon green (480/520 nm), 50 nm FluoSpheres Carboxylate-Modified Microspheres, red fluorescent (580/605 nm), 100 nm |
Corresponds approximately to 50 MDa for 50-nm and 375 MDa for 100-nm diameter nanoparticles (http://www.calctool.org/CALC/prof/bio/protein_length) | 0.5 or 1% for 50 nm and 100 nm, respectively, in PBS (40–50 μL IV) | Allow the titration of the cutoff leakage size of endothelial cell gaps; 50-nm microspheres were used for basal permeability and 100-nm microspheres were used for VEGF/bradykinin-induced permeability studies. | 6, 18–21 |
IV indicates intravenously; MW, molecular weight; and VEGF, vascular endothelial growth factor.
Cell Culture
Murine Lewis lung carcinoma cells were maintained in DMEM, supplemented with 2 mmol/L L-glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% fetal calf serum (Promo Cell). The syngeneic tumor grafts were implanted by injecting 5×105 cells into the subcutaneous space of the abdominal flank. Human umbilical vein endothelial cells (HUVECs) were grown on 0.1% gelatin-coated dishes in endothelial cell basal medium (PromoCell) with supplements provided by the manufacturer. For the growth factor stimulation experiments, transfected HUVECs were starved for 6 to 8 hours and stimulated with recombinant human VEGF165 or BSA (100 ng/mL) for 10 minutes at 37°C. The medium was then removed, and the cell lysates were prepared for Western blot.
Imaging and Quantitative Analysis
Samples were imaged using a transmitted light microscope (Leica DM LB, air objectives: ×2.5 with NA 0.07, ×5 with NA 0.12, ×10 with NA 0.25, and ×20 with NA 0.4), or a confocal microscope (Zeiss LSM 780, air objective: ×10 with NA 0.45 and ×20 NA 0.8, oil objective: ×40 with NA 1.3 and ×63 NA 1.4, Zeiss LSM 510 Meta, air objective: ×10 with NA 0.45, oil objective ×40 with NA 1.3, or Leica TCS CARS SP8, air objective: ×10 with NA 0.4, oil objective: ×40 with NA 1.1). Three-dimensional maximal projections were digitally constructed from confocal z-stacks. The images were edited using PhotoShop software (CS5, Adobe) to optimize visualization. Surface areas were quantified from microscopy images using Image J software (Image J 1.48v National Institutes of Health).
Immunohistochemistry and Immunofluorescence
After euthanizing the mice, tissues were immersed in 4% paraformaldehyde, washed in PBS, and then processed for staining. For analysis of the microvasculature, retinas were stained by direct immunostaining or by using biotinylated Griffonia simplicifolia lectin 4 (isolectin B4, iB4; Vector Laboratories)22 followed by immunostaining. Tracheas and ears were dissected, fixed in 4% paraformaldehyde, and whole-mount staining was performed as described previously.23 All fluorescently labeled samples were mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (Vector Laboratories). The following primary antibodies were used for immunostaining of mouse tissues: polyclonal goat anti-mouse VEGFR3 (AF743; 1:50–1:100, R&D Systems), monoclonal rat anti-mouse PECAM1 (platelet endothelial cell adhesion molecule 1; clone MEC 13.3, 553370, 1:500; BD Pharmingen), polyclonal rabbit anti-human fibrinogen (1:300; DAKO), rat anti-mouse CD144 (555289, 1:100; BD Pharmingen), rat anti-mouse CD144 (eBioV13, 1:100, eBioscience), rabbit anti-mouse Claudin-5 (34–1600, 1:500; Invitrogen), goat anti-mouse podocalyxin (R&D AF1556, 1:500), rabbit anti-mouse NG2 (Millipore AB5320, 1:1000) and rabbit antimouse LYVE1 (1:500).24 The primary antibodies were detected with the appropriate fluorescent Alexa 488, 594 or 647 secondary antibody conjugates (Molecular Probes/Invitrogen) or visualized by using the AEC chromogen system. For staining of mouse lung EC, cells were fixed in 4% paraformaldehyde for 10 minutes at room temperature and washed 3× in PBS. After permeabilization with 0.1% Triton X-100 (Fluka) in PBS for 5 minutes, cells were washed 3× in PBS and blocked with 1% BSA-PBS for 30 minutes at room temperature. Primary antibodies were diluted 1:100 in 1% BSA-PBS and incubated for 1 hour at room temperature. The following antibodies were used for staining of mouse LECs: polyclonal goat anti-mouse VEGFR3 (AF743; R&D Systems) and rat anti-mouse CD144 (555289; BD Pharmingen). Cells were washed 3× and incubated with the appropriate fluorescent Alexa 488 and 594 secondary antibody conjugates (Molecular Probes/Invitrogen) for 30 minutes in 1% BSA-PBS at room temperature. After 3 washes in PBS, coverslips were mounted with aqueous mounting media containing 4’,6-diamidino-2-phenylindole (Vectashield) and sealed (Cytoseal).
Immunoprecipitation and Immunoblotting
Lysates containing equal amounts of protein from lungs were separated in 7.5% Mini-PROTEAN TGX Precast gels (BIORAD). After blotting to polyvinylidene fluoride membranes (Immobilon-P PVDF; Millipore), the proteins were detected using goat anti-mouse VEGFR2 (AF644, 1:1000; R&D Systems), goat anti-mouse VEGFR3 (AF743, 1:1000; R&D Systems), mouse anti-HSC-70 (SC-7298, 1:5000; Santa Cruz Biotechnology), rabbit anti-human pVEGFR2 Tyr1175 (19A10, 1:1000; Cell Signaling), goat anti-human VEGFR2 (AF357, 1:500; R&D Systems), goat anti-human VEGFR3 (AF349, 1:1000; R&D Systems), or mouse anti-human VEGFR3 (9D9,25 1:1000)–specific primary antibodies. The blots were then probed with horseradish peroxidase–labeled secondary antibodies (Dako, Glostrup, Denmark), and the signal was visualized with the SuperSignal West Pico Chemiluminescent Substrate or the SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL).
Isolation of Mouse Retinal ECs
Retinas were dissected, minced, and incubated in Dulbecco modified Eagle medium (4.5 g/L glucose with L-glutamine) containing 200 U/mL collagenase IV (Invitrogen) and 2.4 U/mL Dispase (17105–041; Life technologies) for 30 minutes at 37°C on agitation. To obtain a single-cell suspension, cells were first passed through a 25-G needle, then filtered through a cell strainer (70-μm pore size; BD Falcon) centrifuged at 200g for 5 minutes at 4°C. Cells were then resuspended in cold washing buffer (0.1% BSA, 2 mmol/L EDTA pH 7.4 in PBS), and incubated with biotinylated iB4 (1:12.5; Vector Laboratories) for 30 minutes on ice with agitation and after that incubated with streptavidin-coupled magnetic beads (Dynabeads MyOne Streptavidin T1, 656.02; Dynal Biotech ASA) for 30 minutes on ice with gentle agitation. The beads were separated from the solution using a magnetic particle concentrator (Dynal MPC-S; Invitrogen), and the cells in the supernatant were kept as the non-EC fraction. Non-EC fraction and washed beads were pelleted at 200g for 5 minutes, and the supernatant was removed. The purified ECs and non-ECs were snap-frozen in liquid nitrogen and stored at −80°C until use.
Isolation of Mouse Lung ECs
Primary mouse lung EC isolation was performed as described previously.26 Briefly, minced lungs were digested for 1 hour at 37°C in 0.1% collagenase IV (Sigma), passed through a 70-μm pore size cell strainer (BD Falcon) and cell suspensions were plated onto tissue culture plates coated with 0.1% gelatin, 10 mg/mL human plasma fibronectin (Sigma), and 30 mg/mL bovine collagen (Nutacon). One negative and 2 consecutive positive cell sorting steps were performed using Dynabeads M-450 sheep anti-rat IgG (Invitrogen) and purified rat anti-mouse CD16/CD32 Fcγ receptor, rat anti-mouse CD102 (ICAM-2, catalog no 553326, 1:200), and rat anti-mouse CD31 (PECAM1, clone MEC 13.3, 553370, 1:500; all from BD Pharmingen). Cells were grown on mouse lung EC media, consisting of 1 part of DMEM and 1 part of Ham’s F12 medium, including 0.1 μg/mL heparin (Sigma), 100 μg/mL penicillin/streptomycin (Gibco/Invitrogen), 6 mmol/L l-glutamine, 20% heat inactivated Fetal Calf Serum (Biowest), and 50 μg/mL endothelial mitogens (Biogenesis/Serotec). The media was supplemented with 500 nmol/L 4-hydroxytamoxifen (Sigma) to induce Cre-mediated gene deletion in culture. EC purity and inducible Cre-mediated loss of gene expression, after 48 to 72 h of induction were evaluated by immunofluorescence.
Lentivirus Constructs Design and Silencing Experiments
VEGFR3 mRNA in cultured ECs was silenced with shRNA expressed by lentivirus. The most efficient shRNA construct (clone TRCN0000000636) was determined among several constructs tested from the TRC shRNA library (Broad Institute, provided via FuGU, University of Helsinki) and was used for further experiments. The control lentivirus encoded for scrambled shRNA, and the vector plasmid contained a puromycin resistance gene. We used a standard lentivirus production protocol (3-plasmid transfection in 293FT host cells). HUVECs were transfected with lentivirus-containing cell culture supernatant for 24 hours, followed by a 2-day selection using puromycin (2 μg/mL). The cells were then directly used for biochemical analyses.
Mice
The Committee for Animal Experiments of the District of Southern Finland approved the animal experiments. The mice were housed in conventional conditions, in individually ventilated cages. We used female and male mice in pure C57BL/6J genetic background that were backcrossed for at least 8 generations. Cre-negative littermates have been used as controls in all experiments. Mouse strains are summarized in Table 2. The number of mice analyzed in each experiment is indicated in the figures.
Table 2.
Mouse Lines Used in This Study
| Mouse Line | Description | References | Source |
|---|---|---|---|
| PdgfbiCreERT2 | Inducible Cre recombinase under the control of the Pdgfb gene promoter | 27 | Dr Marcus Fruttiger |
| R26iCreERT2 | Inducible Cre recombinase under the control of the ROSA26 locus | 28 | The Jackson Laboratory, Stock No 008463 |
| Vegfr3flox/flox | Vegfr3 conditional mouse | 29 | Our laboratory |
| Vegfr2flox/flox | Vegfr2 conditional mouse | 30 | Dr Erwin Wagner |
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA from mouse tissues or HUVECs was isolated using the NucleoSpin RNA II Kit (Macherey-Nagel). Homogenization was performed using rotor-stator homogenization, followed by on-column DNase digestion (RNase-Free DNase Set, 79254). Quality control of samples was performed using a Nanodrop ND-1000 spectrophotometer. RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer’s instructions. Three real-time quantitative reverse transcription polymerase chain reactions (RT-qPCR) were performed from every in vitro transcription reaction using TaqMan Gene Expression Assays (Applied Biosystems) and the iQ Supermix Kit (Bio-Rad). RT-qPCR was performed using a BIO-RAD C1000 Thermal cycler according to a standardized protocol. The TaqMan Gene Expression Assays used for mouse mRNA were as follows: Gapdh (4352932E), Cadh5 (Mm00486938_m1), Pdgfrb (Mm00435546_ m1), Vegfr2 (Mm01222419_m1), Vegfr3 (Mm01292608_m1), Vegf (Mm00437304_m1), Fga (Mm00802584_m1), and for human mRNA were: GAPDH (Hs99999905_m1), VEGFR2 (Hs00911700_ m1), VEGFR3 (Hs01047677_m1), HEY1 (Hs01114113_m1), HES1 (Hs00172878_m1), and DLL4 (Hs01117332_g1).
Targeted Gene Deletions in Mice
Newborn PdgfbiCreERT2;Vegfr3flox/flox (Vegfr3iΔEC) and control littermate Vegfr3flox/flox pups were injected intragastrically with 2 μL of 4-hydroxytamoxifen (25 mg/mL; Sigma) dissolved in 97% ethanol, on postnatal day (P) 3 and P4 using a 10 μL Hamilton syringe. The pups were euthanized at P5, and the eyes were harvested for analysis. Adult (>8 weeks old) R26iCreERT2;Vegfr3flox/flox (Vegfr3iΔ) and control littermate Vegfr3flox/flox mice were treated with intraperitoneal injections of tamoxifen (Sigma) dissolved in sterile corn oil (100 μL, 2 mg per day, for 5 consecutive days), or with tamoxifen in corn oil via a feeding needle (2 mg per day, for 3 consecutive days). Cre activity in adult (>8 weeks old) Vegfr3iΔEC and control littermate Vegfr3flox/flox adult mice was induced with tamoxifen in corn oil via a feeding needle (2 mg per day, for 3 consecutive days). For the tumor experiments, Cre activity was induced by subcutaneous implantation of sustained tamoxifen-release pellets (25 mg; Innovative Research Inc., Sarasota, FL), 1 day before tumor cell inoculation. Mice were analyzed at the timepoints indicated in the results.
Transduction of the Mouse Ear Skin With Adenoviral Gene Transfer Vectors
Adenoviral vectors encoding human VEGF165, VEGFCΔNΔC or LacZ were injected intradermally into the ears of mice (2.5×108 plaque-forming units of AdVEGF165 and AdLacZ, and 2×109 plaque-forming units of AdVEGFCΔNΔC and AdLacZ, in a volume of 40 or 50 μL). The Miles assay was performed 48 hours after the adenoviral injection, and the ears were harvested and processed as described above.
Transmission Electron Microscopy
Samples from kidneys and lungs from Vegfr3 deleted (n=7) and control mice (n=7) were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 mol/L phosphate buffer, pH 7.4, then postfixed in 1% osmium tetroxide, dehydrated in acetone, and embedded in Epon LX112 (Ladd Research Industries, VT). One-micrometer sections were stained with toluidine blue to select the area of interest. Eightynanometer sections were cut with a Leica Ultracut UCT microtome and were examined in a Tecnai Spirit transmission electron microscope (Fei Europe, Eindhoven, The Netherlands). Images were captured with a Quemesa CCD camera (Olympus Soft Imaging Solutions GMBH, Munster, Germany).
Statistical Analysis
All data are presented as mean±SEM. Statistical analyses were performed using the IBM SPSS Statistics 20 or Graphpad Prism V6.0 for MacOSX. Means of 2 groups with equal variances were compared with a 2-tailed Student t test. Means of ≥3 groups were analyzed with 1-way ANOVA with Tukey post hoc test. P<0.05 was considered as statistically significant.
Results
Genetic Deletion of Vegfr3 Increases Vascular Leakage
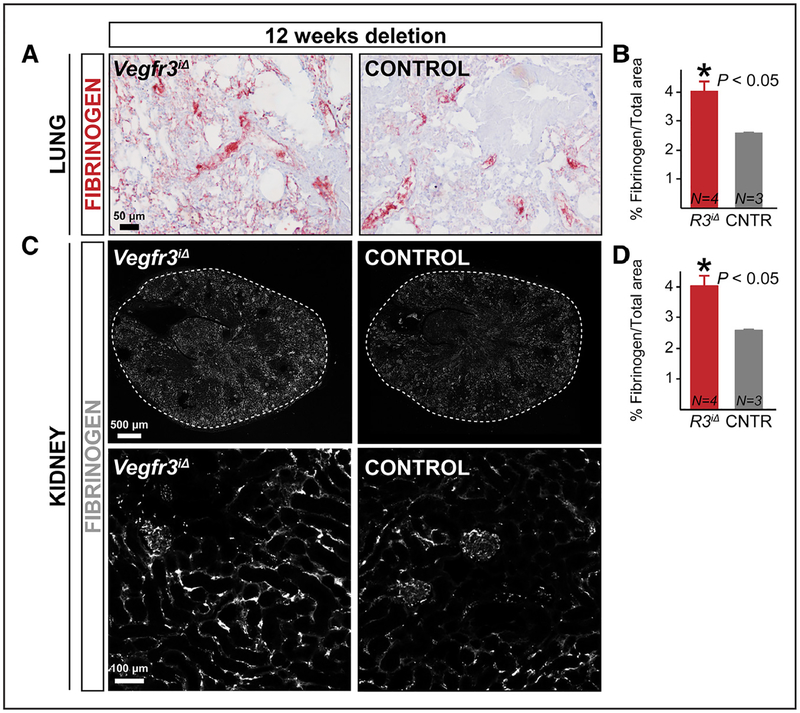
One of the most prominent functions of VEGF and VEGFR2 in blood vessels is the regulation of solute and macromolecule extravasation from the vessels.31,32 We analyzed vascular permeability in quiescent blood vessels of R26iCreERT2;Vegfr3flox/flox mice (Vegfr3iΔ),28,29 in which Vegfr3 is globally deleted (Online Figure IA). In conditions of increased vascular permeability, fibrinogen, a 340-kDa plasma glycoprotein, extravasates into tissues, where it is proteolytically cleaved and deposited as fibrin.33 Surprisingly, we observed increased staining of fibrinogen in the lungs of adult Vegfr3iΔ mice 12 weeks after tamoxifen administration (Figure 1A and 1B). Liver fibrinogen expression was not changed, indicating that increased production was not the underlying cause of excessive fibrinogen extravasation (Online Figure IB). Quantification of vascular density in various tissues indicated that blood vessel size or density was not altered in the Vegfr3iΔ mice, suggesting that the increased leakage was not associated with angiogenesis (Online Figure IC through 1J). Fibrinogen may be produced also by lung ECs,34 raising the possibility of increased local fibrinogen production by the Vegfr3iΔ endothelium in the lungs. We thus analyzed the kidneys from the same mice, where we also found increased fibrinogen deposition (Figure 1C and 1D), further confirming the increased vessel leakage.
Figure 1. Global long-term deletion of Vegfr3 results in vascular leakage.
A–D, Immunostaining and quantification of % fibrinogen area in Vegfr3iΔ (R3iΔ) and control (CNTR; Vegfr3flox/flox) adult mouse lungs (A and B) and kidneys (C and D). Mice were euthanized 12 wk after tamoxifen induction. *P<0.05. Error bars, SEM. Numbers (N) are indicated on the bars.
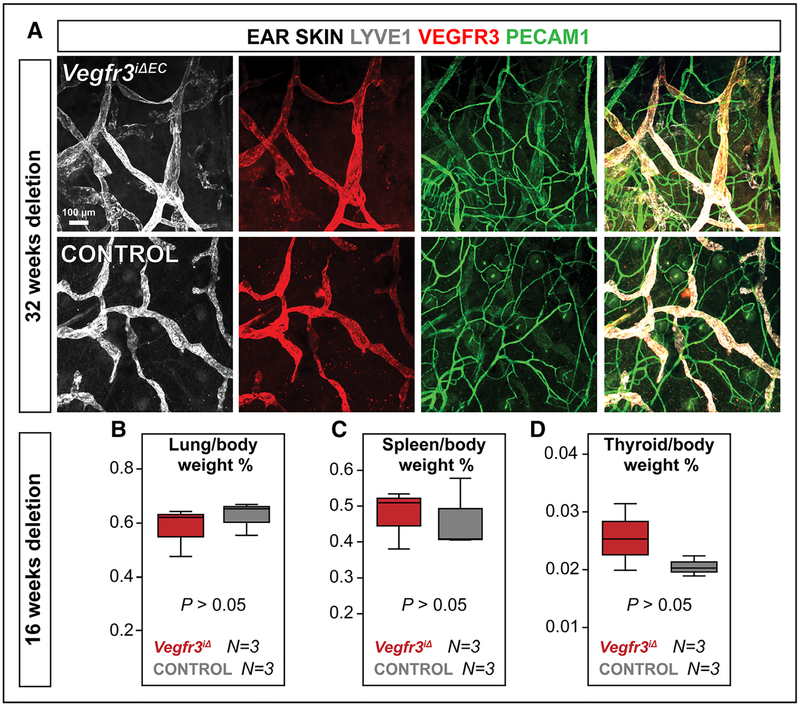
To determine if increased vessel permeability correlated directly with loss of Vegfr3 expression in adult blood vascular ECs, we analyzed mice with endothelial-specific Vegfr3 deletion (Vegfr3iΔEC), using the PdgfbiCreERT2 mice.27 To confirm that this deletion does not affect lymphatic ECs, we treated Vegfr3iΔEC and control mice with tamoxifen shortly after birth and analyzed their lymphatic vessels 32 weeks after the deletion. VEGFR3 staining in the skin was indistinguishable between Vegfr3iΔEC and control adult mice (Figure 2A). Furthermore, the mice had no tissue edema or fluid retention to indicate that they would have malfunctional lymphatic vessels (Figure 2B through 2D). Unlike in control mice, local overexpression of VEGF did not upregulate VEGFR3 in blood vessels of Vegfr3iΔEC mice, as shown previously,11 indicating that the recombination was otherwise successful in blood vascular ECs (Online Figure IIA).
Figure 2. PdgfbiCreERT2 activation does not lead to Vegfr3 deletion in lymphatic vasculature.
A, LYVE1 (gray), VEGFR3 (red), and PECAM1 (green) staining of ear skin of Vegfr3iΔEC adult mice. The mice were treated with tamoxifen via gavage on postnatal days (P) 3 to 5 and were analyzed at 8 mo of age. B and C, No significant fluid retention in organs of adult Vegfr3iΔ mice. Weights of lung, spleen, and thyroid tissues normalized to body weight of Vegfr3iΔ and control mice 4 mo after tamoxifen administration. The mice were treated with tamoxifen via gavage for 3 consecutive days when they were 12 wk old and analyzed 16 wk later. P>0.05. Error bars, SEM. Numbers (N) are indicated below the bars.
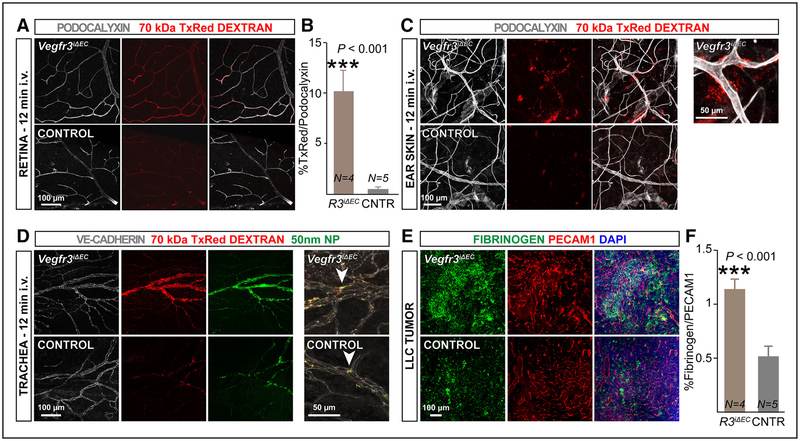
Basal vascular permeability was assessed in adult PdgfbiCreERT2;Vegfr3flox/flox mice ten days after tamoxifen treatment. Intravenous administration of 70-kDa Texas red dextran, which has a leakage profile similar to that of albumin,17 followed by high-resolution imaging of the retinal and skin vasculature, revealed more extravasation of the tracer in Vegfr3iΔEC mice than in littermate controls (Vegfr3flox/flox; Figure 3A through 3C). We also observed enhanced extravasation in the tracheal blood vessels of Vegfr3iΔEC mice after systemic injection of 70-kDa Texas red dextran or fluorescent nanoparticles (50-nm diameter; Figure 3D). There was no difference in blood vessel area after short-term endothelial-specific deletion of Vegfr3 (Online Figure III), indicating that the deregulation of vascular permeability was not a consequence of vessel caliber changes.
Figure 3. Short-term Vegfr3 deletion in the blood vessel endothelium results in increased vascular permeability in retina, trachea, ear skin, and in a tumor isograft.
A–C, Eight-week-old Vegfr3iΔEC (R3iΔEC) and control (CNTR) mice were analyzed 7 d after tamoxifen administration. Representative images of 70-kDa Texas red dextran extravasation in baseline conditions, in retinal (A) and ear skin (C) blood vessels (stained for podocalyxin in gray), 12 min after dextran injection. B, Quantification of 70-kDa Texas red dextran colocalization with retinal blood vessels 30 min after intravenous administration. D, Representative images of 70-kDa Texas red dextran and 50-nm dragon green nanoparticle extravasation from tracheal blood vessels (podocalyxin, gray) 12 min after administration. E, Sections from Lewis lung carcinoma (LLC) tumors grown in Vegfr3iΔEC or control mice stained for fibrinogen (green) and PECAM1 (red). F, Quantification of fibrinogen signal intensity normalized to total PECAM1 pixel density. ***P<0.001. Error bars, SEM. Numbers (N) are indicated on the bars.
To study whether Vegfr3 deletion exacerbates endothelial barrier dysfunction during angiogenesis, we analyzed fibrinogen staining in tumors grown in Vegfr3iΔEC mice, after normalization to PECAM1 staining to compensate for increased blood vessel numbers in Vegfr3iΔEC tumors.12 Our analysis showed that Lewis lung carcinoma isografts in Vegfr3iΔEC mice have more fibrinogen deposition than tumors grown in control mice, indicating that loss of Vegfr3 increases capillary leakage also during sprouting angiogenesis (Figure 3E and 3F).
Vegfr3-Deleted Mice Display Normal Blood Vessel Structure
Mural cells are critical regulators of vascular permeability and vessel stability.35 We examined vessel pericyte coverage by NG2 staining in retinas of postnatal and adult Vegfr3iΔEC mice. Pericyte coverage appeared normal at both time-points (Online Figure IVA through IVC). Consistent with the unaltered pericyte numbers, there were no changes in platelet-derived growth factor receptor-β mRNA expression in the lungs of adult mice 1 or 2 weeks after global Vegfr3 deletion (Online Figure IVD). We conclude that VEGFR3 does not regulate vascular permeability by affecting perivascular cells.
Various types of vascular damage may compromise endothelial integrity, leading to increased vascular leakage.36 We therefore analyzed the ultrastructure of blood capillaries by transmission electron microscopy. We treated 7- to 10-week-old R26iCreERT2;Vegfr3flox/flox and control mice with tamoxifen and analyzed them 15 to 18 weeks later. There was no indication of apoptosis, necrosis, endothelial retraction, or hemorrhage in lung and kidney endothelia of Vegfr3iΔ or control mice (Online Figure VA through VH). The kidney fenestrae appeared normal in number and morphology (Online Figure VA through VD), and there was no detectable proteinuria that could signify EC injury37 (Online Figure VI and VJ). These results indicated that the increased vascular permeability observed in the Vegfr3iΔ mice is not because of EC damage.
Vegfr3 Deletion Decreases VE-Cadherin at EC Junctions In Vivo and In Vitro
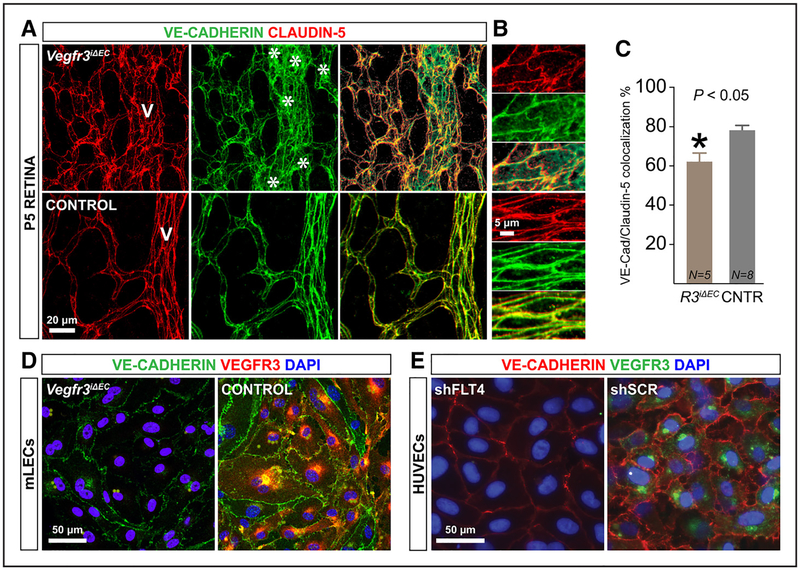
Adherens and tight junctions that connect ECs are important regulators of vascular permeability.1,38 In control mice at postnatal day (P) 5, both the adherens junction protein VE-Cadherin and the tight junction protein Claudin-5 were localized at the EC junctions. In the retinas of Vegfr3iΔEC mice, VE-Cadherin staining was mainly intracellular, whereas the expression of Claudin-5 was unaltered, resulting in a 25% reduction in the colocalization of the 2 proteins (Figure 4A through 4C). In addition, the ECs of the Vegfr3iΔEC retinal vessels lost their normal elongated shape, appearing more rounded than control retinal ECs (Figure 4B). Importantly, the VE-Cadherin mRNA expression levels remained unaltered in the Vegfr3-deficient mice at various time-points after global gene deletion (Online Figure VIA). These findings were reproduced in cultured ECs isolated from the lungs of Vegfr3iΔEC mice. In vitro administration of 4-hydroxy tamoxifen followed by immunostaining for VE-Cadherin confirmed a significant decrease of VE-Cadherin junctional localization in Vegfr3-deleted ECs (Figure 4D). In addition, HUVECs transduced with a lentiviral vector expressing VEGFR3 shRNA (shFLT4) showed a similar loss of junctional VE-Cadherin staining compared with the control-treated cells (Figure 4E).
Figure 4. Vegfr3 deletion increases VE-Cadherin internalization in endothelial cells.
A and B, VE-Cadherin (green) and Claudin-5 (red) staining of P5 retinas of Vegfr3iΔEC mice. Note the nonjunctional green staining indicating VE-Cadherin internalization (asterisks).C, Quantification of VE-Cadherin/Claudin-5 colocalization. *P<0.05. Error bars, SEM. Numbers (N) are indicated on the bars (N pooled together from 2 independent experiments). D and E, Immunofluorescence staining of VEGFR3 (green), VE-Cadherin (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue) in cultured lung endothelial cells (ECs) from Vegfr3iΔEC mice treated with 4-hydroxytamoxifen in vitro (D), and in human umbilical vein endothelial cells (HUVECs) after transduction with lentivirus expressing shFLT4 or scrambled control vector (E). mLEC indicates mouse lung EC.
Increased Vascular Leakage in Vegfr3-Deleted Mice Is VEGF/VEGFR2 Dependent
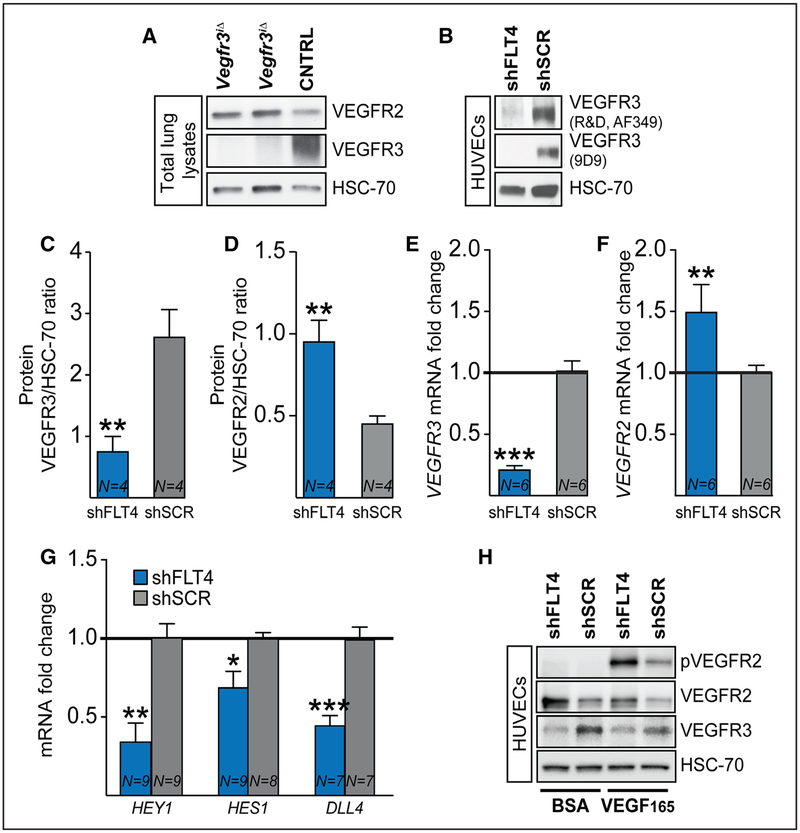
VEGFR2 associates with VE-Cadherin at EC junctions, where the complex regulates EC barrier integrity and vascular permeability. In response to VEGF, VE-Cadherin becomes phosphorylated and adherens junctions are transiently disassembled and internalized.39 We have shown previously that Vegfr3 deletion during developmental angiogenesis in newborn mice results in Vegfr2 upregulation at the mRNA and protein levels.13 This raised the possibility that the increased activity of the VEGF/VEGFR2 signaling axis is responsible for the vessel leakage observed in the Vegfr3-deleted adult mice. Indeed, VEGFR2 protein was increased in the lungs of mice 16 weeks after global Vegfr3 deletion (Figure 5A). In contrast, the Vegf mRNA levels were not altered in the lungs of these mice (Online Figure VIB). Furthermore, we detected increased Vegfr2 mRNA in ECs in the Vegfr3iΔEC retinas (Online Figure VIC through VIE). Although this difference was not statistically significant, it may be partially masked by the high levels of VEGFR2 expressed by retinal neurons.40 We also confirmed our findings in cultured HUVECs transduced with a VEGFR3 shRNA lentivirus (Figure 5B). VEGFR3 silencing increased VEGFR2 protein and mRNA levels significantly in the cells (Figure 5C through 5F). This was associated with downregulation of the NOTCH1 target genes HEY1 and HES1 and the NOTCH1 ligand DLL4 (Figure 5G). As expected, VEGFR2 phosphorylation after stimulation with VEGF165 was increased in the VEGFR3-silenced cells when compared with the cells transduced with control lentivirus (Figure 5H), reflecting enhanced VEGF sensitivity because of VEGFR2 upregulation.
Figure 5. Vegfr3 deletion increases VEGFR2 levels.
A, Western blotting of VEGFR2, VEGFR3 and heat shock protein 70 in the lungs of Vegfr3iΔ and control mice. B, Validation of vascular endothelial growth factor receptor (VEGFR) 3 silencing in human umbilical vein endothelial cells (HUVECs) after transduction with lentivirus expressing shFLT4 or scrambled control, using 2 different primary antibodies against VEGFR3. C–F, Quantification of VEGFR3 and VEGFR2 protein (C and D) and mRNA levels (E and F) in HUVECs transduced with lentivirus expressing shFLT4 or scrambled control. G, mRNA levels of NOTCH1 target genes HEY1, HES1 and DLL4 in VEGFR3-silenced HUVECs (4 independent experiments). H, Western blotting of VEGFR2 and pVEGFR2 from transduced HUVECs, stimulated with VEGF165 for 10 min before lysis. *P<0.05, **P<0.01, ***P<0.001, Error bars, SEM. Numbers (N) are indicated on the bars.
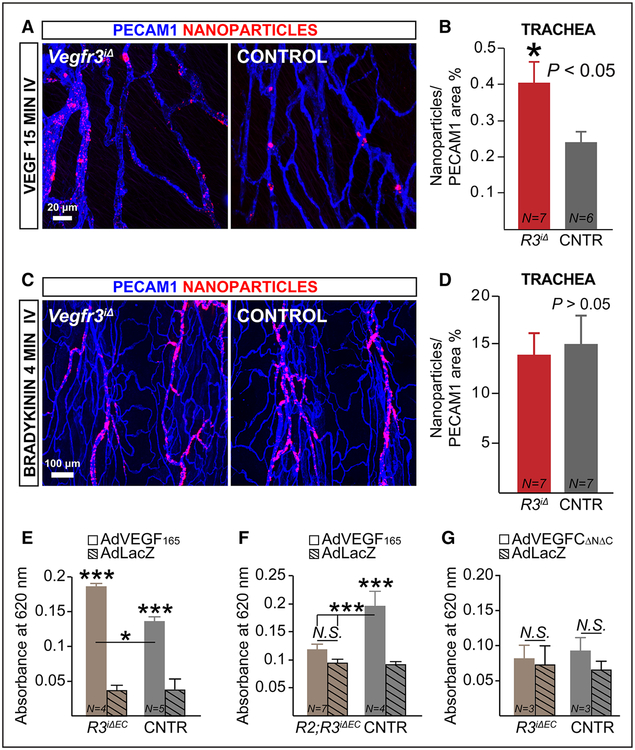
Next, we analyzed the extravasation of intravenously injected fluorescent nanoparticles from tracheal blood vessels 15 minutes after the administration of VEGF to globally Vegfr3-deleted mice. In wild-type mice, nanoparticles with a diameter of ≥50 nm extravasate when a permeability-inducing agent, such as VEGF, is included (Online Figure VIIA). Nanoparticle extravasation was significantly greater in the tracheas of Vegfr3iΔ mice than in those of control mice when normalized to the corresponding vascular area (Figure 6A and 6B), suggesting that VEGFR3 restricts vascular permeability stimulated by VEGF/VEGFR2 activation. Nanoparticles were particularly prominent in between the endothelium and endothelial basement membrane in postcapillary venules, as described previously.6,18–21 On the contrary, a similar amount of nanoparticle leakage was observed in the tracheas of Vegfr3iΔ and control mice 4 minutes after intravenous injection of bradykinin, which promotes vascular permeability via eNOS/iNOS-dependent endocytosis of phosphorylated VE-Cadherin41 (Figure 6C and 6D). Thus, the results suggest that the increased permeability by loss of Vegfr3 is specifically related to the VEGF/VEGFR2 pathway.
Figure 6. VEGFR3 restricts vascular permeability by suppressing VEGF/VEGFR2 signaling.
A and B, Representative whole-mount images and quantification of nanoparticle (FluoSpheres 0.1 μm) leakage in tracheal blood vessels of adult Vegfr3iΔ (R3iΔ) and control (CNTR) mice 2 wk after tamoxifen administration. The mice received vascular endothelial growth factor (VEGF) intravenously 15 min before termination. C and D, Vegfr3 loss of function does not affect bradykinin-induced permeability. Representative images and quantification of nanoparticle (0.1 μm) leakage in the tracheas of adult Vegfr3iΔ and CNTR mice 2 wk after tamoxifen administration. The mice received 1 mg/kg bradykinin intravenously, 4 min before termination. E–G, Miles assay of VEGF or VEGFC-induced vascular permeability in Vegfr3iΔEC (R3iΔEC) vs CNTR mice and in compound Vegfr2;Vegfr3iΔEC (R2;R3iΔEC) vs littermate CNTR (Vegfr2flox/flox;Vegfr3flox/flox) 48 h after transduction of the ear skin with the indicated adenoviral vectors. For C–G, the mice were induced at the age of 9 wk with tamoxifen via gavage and analyzed 1 wk thereafter. *P<0.05, ***P<0.001. Error bars, SEM. Numbers (N) are indicated on the bars.
We next overexpressed VEGF165 or β-galactosidase by intradermal injection of recombinant adenoviral vectors and performed the Miles permeability assay in Vegfr3iΔEC and control mice. This assay measures plasma extravasation because Evans blue binds to serum albumin.16 We analyzed the skin 2 days after vector administration. As expected, we observed increased Evans blue extravasation in AdVEGF165-injected ears; however, extravasation was significantly increased by Vegfr3 deletion (Figure 6E). We repeated this experiment after concurrently deleting Vegfr2, using compound Vegfr2;Vegfr3iΔEC mice30 (receptor deletion shown in Online Figure VIIB). Simultaneous loss of Vegfr2 prevented the increased extravasation of Evans blue in response to VEGF165 overexpression in the Vegfr3iΔEC mice (Figure 6F). On the contrary, administration of VEGFCΔNΔC did not increase vascular permeability under these conditions (Figure 6G). These data further support the hypothesis that excessive VEGF/VEGFR2 activity underlies the increased permeability of the Vegfr3-deleted vessels (Figure 7).
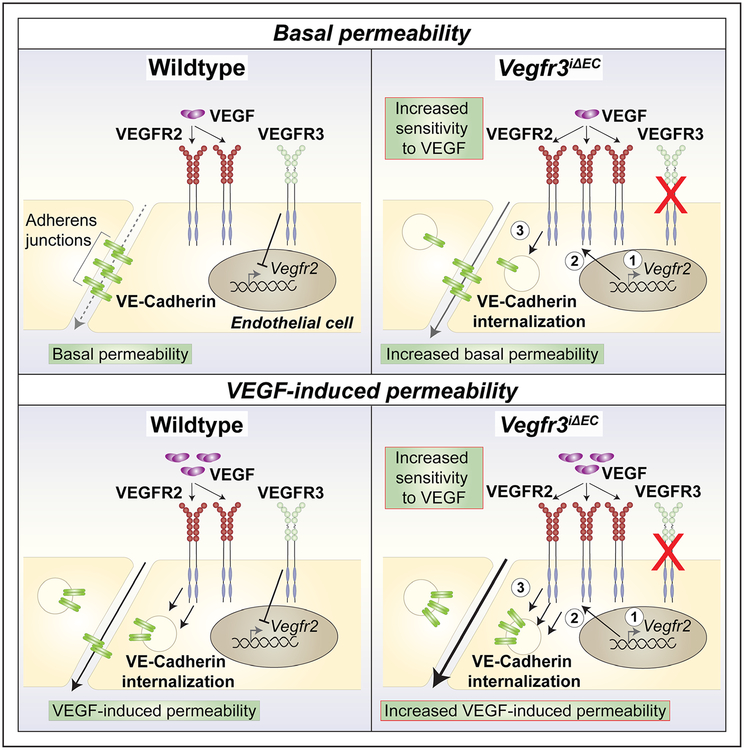
Figure 7. Schematic summarizing the effects of vascular endothelial growth factor receptor (VEGFR) 3 in VEGFR2-regulated vascular permeability in steady state and after VEGF stimulation.
VEGFR3 suppresses VEGFR2 mRNA and protein levels, thus limiting VEGF-induced vascular permeability. On VEGFR3 loss of function, VEGFR2 expression levels increase, resulting in increased sensitivity to VEGF signals, enhanced VE-Cadherin internalization and excessive vascular leakage.
Discussion
Our present findings reveal that VEGFR3 limits VEGF/VEGFR2-induced vascular permeability. On deletion of Vegfr3, Vegfr2 mRNA and protein levels were upregulated in blood vessel endothelia, leading to increased sensitivity to VEGF-induced VEGFR2 activation. As a result, Vegfr3-deleted mice exhibited increased vascular leakage in normal tissues, tumors, and on acute VEGF administration, while retaining normal vascular morphology.
VEGFR3 was long regarded as a receptor involved primarily in lymphangiogenesis, but recent studies indicate that VEGFR3 also functions in sprouting angiogenesis.11–13,42 In the steady state conditions, VEGFR3 has been detected in high endothelial venules and fenestrated endothelia, as well as in various nonvascular cells such as some neurons, macrophages, and megakaryocyte progenitors.14,15 Some expression of VEGFR3 has also been reported in retinal vessels of adult mice, where little VEGFR2 is expressed.3 To study the function of Vegfr3 in blood vessels, we induced its long-term gene deletion in adult mice. We observed increased fibrinogen deposition in all tissues analyzed, without changes in blood vessel structure that could account for increased leakage. To exclude the possibility that lymphatic vessel defects could be responsible for the tissue edema, we analyzed mice deleted of Vegfr3 in blood vascular ECs, using a validated endothelial-specific Cre line (PdgfbiCreERT2). Increased permeability was detected in retinal, skin, and tracheal blood vessels already 7 days after Vegfr3 deletion in blood vascular endothelium. Quantification of fibrinogen deposition in syngeneic tumors grown in Vegfr3iΔEC mice confirmed increased vascular leakage also in angiogenic endothelia, which express high amounts of VEGFR3.11 After conditional deletion of Vegfr3, we observed increased VE-Cadherin internalization in postnatal retinal vessels, as well as reduced junctional VE-Cadherin localization in cultured ECs. Our results indicate disrupted endothelial barrier function on Vegfr3 deletion. Because there were no signs of EC pathology, we propose that both quiescent and angiogenic blood vascular ECs depend on VEGFR3 signaling to limit vascular permeability at least in the trachea, lungs, kidneys, retinas, and skin. However, at present, we cannot rule out the possibility that this regulatory mechanism is lacking in some other vascular beds.
In neonatal mice, conditional deletion of Vegfr3 suppresses downstream Notch signaling, leading to upregulation of Vegfr2 mRNA and protein levels.13 We observed a similar accumulation of VEGFR2 protein in the lungs of adult Vegfr3iΔ mice, suggesting that the inhibitory effect of VEGFR3 signaling on VEGFR2 expression persists in adult mice. VEGFR3 induced NOTCH signaling and suppressed VEGFR2 expression also in cultured ECs, supporting a cell-autonomous mechanism. Our further in vivo studies showed that the vascular hyperpermeability in the Vegfr3-deleted mice is specific to the VEGF/VEGFR2 pathway, as it was not triggered by the general vasodilator bradykinin, and as it could be eliminated by concurrent genetic deletion of Vegfr2. Because loss of Vegfr3 did not affect Vegf expression levels, we concluded that Vegfr3iΔ ECs are more sensitive to VEGF signals because they express higher amounts of VEGFR2.
VEGFR2 upregulation did not affect the glomerular ultrastructure or kidney filtration function even after prolonged gene deletion despite the fact that VEGF/VEGFR2 signaling levels in the kidney glomeruli are tightly regulated.43 Surprisingly, we did not observe increased vascular density in any of the tissues analyzed despite the fact that a hyperspouting phenotype has been reported in Vegfr3iΔEC mutant mice during development and in angiogenic conditions.12 Different thresholds of VEGF are required to produce distinct EC responses, such as migration or proliferation,22,44,45 and it is possible that the lack of hypersprouting in the Vegfr3-deleted endothelia is because of the relatively low VEGF levels in unchallenged adult mice. Furthermore, overexpression of VEGFC did not increase leakage in Vegfr3iΔEC or control mouse skin. As the protein isoform used in our experiments represents the mature, proteolytically processed form of VEGFC, which is able to activate also VEGFR2,46 our results suggest that neither VEGFC/VEGFR3 nor the VEGFC/VEGFR2 pathways regulate blood vascular permeability in these conditions.47
In conclusion, VEGFR3 expression in adult blood vessels limits vascular permeability, by keeping VEGF/VEGFR2 signals in check. An analogous finding has been reported for VEGFR1, which functions as a decoy receptor decreasing endothelial sensitivity to VEGF/VEGFR2 stimulation. The Vegfr1-deleted mice show increased angiogenesis during postnatal development and after myocardial infarction.48 Our results highlight the existence of another regulatory circuit within the VEGFR family, which fine-tunes the function of the main VEGF-signaling receptor, VEGFR2. Mechanistic understanding of this circuit and the downstream targets of VEGFR3 is of paramount importance, as disturbed EC barrier function accompanies multiple pathological conditions, such as inflammation, wound healing, tumor metastasis, ischemia, pulmonary injury, and sepsis.49,50
Supplementary Material
Novelty and Significance.
What Is Known?
Vascular endothelial growth factor (VEGF) receptor-3 (VEGFR3) is expressed in lymphatic endothelial cells and controls lymphangiogenesis.
VEGFR3 is weakly expressed in blood vessel endothelia, except in fenestrated endothelium where the expression is higher.
VEGFR2 is the main regulator of angiogenesis and vascular permeability in blood vessel endothelium.
What New Information Does This Article Contribute?
VEGFR3 signaling prevents excessive vascular leakage in blood vascular endothelium.
Loss of Vegfr3 increases vascular permeability without affecting blood vessel density or integrity.
VEGFR3 limits permeability in adult blood vessels by suppressing VEGFR2 expression levels.
VEGFR3 is important for lymphatic endothelial cell function and sprouting angiogenesis, yet the role of the receptor in quiescent blood vascular endothelial cells remains largely unknown. Here, we show that loss of VEGFR3 from endothelial cells increases vascular permeability in quiescent and VEGF stimulated blood endothelia and in angiogenic conditions. These effects were not accompanied by structural alterations of the blood vessels, and they could be rescued by concurrent deletion of VEGFR2. Our study reveals that VEGFR3 in quiescent blood vessels has a novel regulatory role, which specifically controls the function of VEGF/VEGFR2. These results lay the foundation for further studies on the importance of this mechanism in various tissues and on the identification of the downstream targets of VEGFR3 signaling in adult blood vascular endothelial cells. These mechanisms may be important in the pathogenesis of conditions where the endothelial barrier function is disrupted, such as sepsis, pulmonary injury, hematogenous metastasis, stroke, and edema associated with retinal diseases.
Acknowledgments
We thank Drs Marcus Fruttiger and Erwin Wagner for the PdgfbiCreERT2 and Vegfr2flox/flox mice; Dr Andrey Anisimov for lentiviral vector design, Dr Natalie Kofler for language editing, and Riitta Kauppinen, Tanja Laakkonen, Katja Salo, Ville Hyvönen, Tapio Tainola, and the personnel of the Laboratory Animal Center of the University of Helsinki for technical assistance. We also thank the AAV Gene Transfer and Cell Therapy Core Facility, the Protein Production and Purification Core Facility and the Molecular Imaging Unit at Biomedicum, University of Helsinki, and the Biocenter Oulu electron microscopy laboratory at University of Oulu for providing materials and services.
Sources of Funding
This study was funded by the Leducq Foundation (11CVD03), the Sigrid Juselius Foundation, the Cancer Society of Finland, the Academy of Finland (292816, 273817; all to K. Alitalo); the Academy of Finland Centre of Excellence Programs 2014 to 2019 (271845, 307366 to K. Alitalo) and 2012 to 2017 (136880 to L. Eklund), Ida Montini Foundation, Biomedicum Helsinki Foundation, Cancer Society of Finland, Finnish Foundation for Cardiovascular Research, Maud Kuistila Foundation (all to K. Heinolainen), K. Albin Johansson Foundation (to K. Heinolainen and G. Zarkada), and the Swiss National Science Foundation Advanced Postdoc.Mobility Grant (no: P300PB_164732, to S. Karaman).
Nonstandard Abbreviations and Acronyms
- ECs
endothelial cells
- HUVECs
human umbilical vein endothelial cells
- VE-cadherin
vascular endothelial cadherin
- VEGF(R)
vascular endothelial growth factor (receptor)
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.116.310477/-/DC1.
Disclosures
None.
References
- 1.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 2.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehling M, Adams S, Benedito R, Adams RH. Notch controls retinal blood vessel maturation and quiescence. Development. 2013;140:3051–3061. doi: 10.1242/dev.093351. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Padhan N, Sjöström EO, et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat Commun. 2016;7:11017. doi: 10.1038/ncomms11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mäkinen T, Veikkola T, Mustjoki S, Karpanen T, Catimel B, Nice EC, Wise L, Mercer A, Kowalski H, Kerjaschki D, Stacker SA, Achen MG, Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 9.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 10.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–949. [DOI] [PubMed] [Google Scholar]
- 11.Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 12.Tammela T, Zarkada G, Nurmi H, et al. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 2011;13:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci U S A. 2015;112:761–766. doi: 10.1073/pnas.1423278112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher CD, de Waal RM, Kaipainen A, Alitalo K. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol. 1998;153:395–403. doi: 10.1016/S0002-9440(10)65583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Stacker SA, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–2096. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 16.Radu M, Chernoff J. An in vivo assay to test blood vessel permeability. J Vis Exp. 2013:e50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egawa G, Nakamizo S, Natsuaki Y, Doi H, Miyachi Y, Kabashima K. Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci Rep. 2013;3:1932. doi: 10.1038/srep01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O’Brien S, Epstein DM, Koh GY, McDonald DM. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol. 2011;178:2897–2909. doi: 10.1016/j.ajpath.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 20.Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013;8:e70459. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Li X, Massena S, et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med. 2012;209:1363–1377. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmén C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 24.Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Ylä-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 25.Jussila L, Valtola R, Partanen TA, Salven P, Heikkilä P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–1604. [PubMed] [Google Scholar]
- 26.Reynolds LE, Hodivala-Dilke KM. Primary mouse endothelial cell culture for assays of angiogenesis. Methods Mol Med. 2006;120:503–509. [DOI] [PubMed] [Google Scholar]
- 27.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 28.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 29.Haiko P, Makinen T, Keskitalo S, Taipale J, Karkkainen MJ, Baldwin ME, Stacker SA, Achen MG, Alitalo K. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28:4843–4850. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. [DOI] [PubMed] [Google Scholar]
- 31.Nagy JA, Dvorak AM, Dvorak HF. Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med. 2012;2:a006544. doi: 10.1101/cshperspect.a006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF, Senger DR, Dvorak AM, Harvey VS, McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985;227:1059–1061. [DOI] [PubMed] [Google Scholar]
- 34.Guadiz G, Sporn LA, Simpson-Haidaris PJ. Thrombin cleavage-independent deposition of fihrombin cleavage-independent deposi Blood. 1997;90:2644–2653. [PubMed] [Google Scholar]
- 35.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Winn RK, Harlan JM. The role of endothelial cell apoptosis in inflammatory and immune diseases. J Thromb Haemost. 2005;3:1815–1824. doi: 10.1111/j.1538-7836.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri R. Proteinuria with and without renal glomerular podocyte effacement. J Am Soc Nephrol. 2006;17:2383–2389. doi: 10.1681/ASN.2006060628. [DOI] [PubMed] [Google Scholar]
- 38.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 39.Claesson-Welsh L. Vascular permeability–the essentials. Ups J Med Sci. 2015;120:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshikawa Y, Yamada T, Tai-Nagara I, Okabe K, Kitagawa Y, Ema M, Kubota Y. Developmental regression of hyaloid vasculature is triggered by neurons. J Exp Med. 2016;213:1175–1183. doi: 10.1084/jem.20151966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orsenigo F, Giampietro C, Ferrari A, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 43.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. [DOI] [PubMed] [Google Scholar]
- 45.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999;274:31047–31054. [DOI] [PubMed] [Google Scholar]
- 46.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumura K, Hirashima M, Ogawa M, Kubo H, Hisatsune H, Kondo N, Nishikawa S, Chiba T, Nishikawa S. Modulation of VEGFR-2-mediated endothelial-cell activity by VEGF-C/VEGFR-3. Blood. 2003;101:1367–1374. doi: 10.1182/blood-2002-05-1329. [DOI] [PubMed] [Google Scholar]
- 48.Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation. 2012;126:741–752. doi: 10.1161/CIRCULATIONAHA.112.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Nieuw Amerongen GP, van Hinsbergh VW. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul Pharmacol. 2002;39:257–272. [DOI] [PubMed] [Google Scholar]
- 50.Park-Windhol C, D’Amore PA. Disorders of vascular permeability. Annu Rev Pathol. 2016;11:251–281. doi: 10.1146/annurev-pathol-012615-044506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.