Abstract
Objective: To investigate the correlation between the BRAF V600E gene mutation and clinicopathological features and thyroid function after iodine-131 treatment in patients with papillary thyroid cancer (PTC).
Methods: A total of 128 PTC patients who underwent iodine-131 treatment after a total thyroidectomy from February 2015 to November 2016 at Hunan Cancer Hospital, China, were recruited. There were 25 males and 103 females. The age range was 11 to 73 years old. The BRAF V600E mutation in tumor tissues was detected by amplification-restriction mutation system polymerase chain reaction (ARMS-PCR), and the serum levels of Tg, TSH, Tg-Ab, and Tpo-Ab were measured by chemiluminescence after iodine-131 treatment. The BRAF V600E mutation was shown to be associated with clinicopathological characteristics and thyroid function indicators after iodine-131 treatment.
Results: BRAF V600E mutation was detected in 75 of the 128 patients (58.6%) and was observed more frequently in cases with elevated Tg levels (Tg>1.00) at 3, 6, 12, and 18 months after treatment compared with patients without any BRAF mutations (P<0.05). Patients with BRAF V600E mutation had significant lower level of Tg-Ab at 3 and 12 months after treatment with iodine-131 than patients without BRAF V600E mutation (P<0.05). Among the 75 BRAF V600E patients, no significant association was found between the levels of TSH and Tpo-Ab after iodine-131 treatment (P>0.05). The BRAF V600E mutation was closely associated with the high-risk and age of the patient (≥45 years old) (P<0.05), but there was no significant correlation with gender, clinical stage, and distant metastasis.
Conclusion: The BRAF V600E mutation is closely related to serum Tg elevation after treatment with iodine-131 in papillary thyroid cancer. These findings suggest that this BRAF mutation may be a predictor of the efficacy of iodine-131 treatment for papillary thyroid cancer.
Keywords: I -131 treatment, BRAF V600E, Thyroid function, papillary thyroid carcinoma
Introduction
Thyroid cancer is the most common malignant tumor of the endocrine system and the most common head and neck cancer. The incidence of thyroid cancer has increased significantly in the past two decades1. Thyroid cancer primarily includes the following four pathological types: papillary thyroid carcinoma, follicular thyroid carcinoma, undifferentiated carcinoma and medullary thyroid carcinoma. Among these types, papillary thyroid carcinoma is the most common, accounting for 85% to 90% of the total incidences. Due to the relatively inert biological behavior of PTC, the overall prognosis is relatively good, and the 10-year survival rate of patients is greater than 90%. However, many cases still have a poor prognosis, any many patients eventually die from postoperative recurrence and metastasis. Therefore, predicting the prognosis of papillary thyroid carcinoma and customizing individualized treatment programs have become topics of concern for clinicians.
At present, surgical resection is the preferred treatment for papillary thyroid carcinoma, and this treatment is widely used in clinical practice2. After thyroidectomy, adjuvant treatment is usually given, depending on the severity of the condition and the metastasis of the tumor. Among the various adjuvant treatments, I-131 adjuvant radiotherapy is the most common. Thyroid tissue-derived tumor cells usually retain the ability to take up iodine. I-131 treatment can effectively eliminate residual thyroid tissue and micrometastases; therefore, this treatment plays an important role in reducing the recurrence rate and improving survival3. Predictive evaluation of the therapeutic effect of I-131 treatment and the development of a corresponding degree of chemotherapy can effectively improve the prognosis of patients with PTC and prolong the recurrence-free survival of patients.
The BRAF gene is located on human chromosome 7q34 and encodes a serine/threonine protein kinase with a molecular weight of 67 kD-99 kDs4. This protein kinase is involved in regulating the transcriptional activity of cells during cell growth, division and differentiation, and it affects the regulation of cell morphology and the distribution of the cytoskeleton. Approximately 80% of the BRAF mutations are an A-T point mutation at the bp 1799, which is known as the BRAF V600E mutation. BRAF gene mutations are associated with the development of a variety of tumors, such as melanoma5, lung cancer6, colorectal cancer7 and thyroid cancer. In recent years, a large body of literature has shown that the BRAF V600E mutation is associated with a series of adverse clinical pathological factors in PTC, such as advanced disease, invasive characteristics, and others8-14. Many studies have shown that the BRAF V600E mutation may result in the activation of downstream genes of the MAPK pathway, which may lead to a slight downregulation of the sodium iodide symporter (NIS), thereby causing an inaccurate NIS localization wherein the NIS cannot be accurately localized to the cell membrane15, 16. This failure of proper localization leads to a decrease in the ability of tumor cells to take up iodine17, 18. I-131 adjuvant radiotherapy relies on the uptake of radioactive iodine by tumor cells; therefore, the BRAF V600E mutation may lead to a decrease in the therapeutic effect of iodine-131, resulting in a poor prognosis.
The clinical evaluation of the efficacy of I-131 treatment after total thyroid cancer surgery includes imaging examination and tests for thyroid function19. The evaluation of the efficacy of I-131 treatment in the thyroid index primarily includes thyroglobulin (Tg), thyroid stimulating hormone (TSH), free T3 (FT3), free T4 (FT4), thyroid peroxidase antibody (TPO-Ab) and thyroglobulin antibody (TG-Ab). Among these factors, dynamic monitoring of thyroglobulin (Tg) levels is important for postoperative follow-up and prognosis evaluation, and such monitoring has been widely used in clinical practice 20, 21. Thyroglobulin glycoprotein is derived from functional thyroid tissue. Under the condition of thyroidectomy, if other physical and chemical conditions are stable, the serum Tg level is directly proportional to the size of thyroid residual tissue.
We primarily investigated the relationship among the BRAF V600E gene mutation, the clinicopathological features of PTC patients, and the levels of the thyroid function indicators Tg, TSH, Tg-Ab and Tpo-Ab after iodine-131 treatment.
Materials and Methods
Materials
A cohort of 128 patients with PTC who underwent I-131 treatment following total thyroidectomy at Hunan Cancer Hospital from February 2015 to November 2016 were recruited. Patients were eligible for enrolment into the cohort if they had primary papillary thyroid carcinoma and received total thyroidectomy and postoperative iodine-131 treatment. The main exclusion criteria were a diagnosis of double primary cancer and poor physical condition. Written informed consents were obtained from all patients.
There were 25 male and 103 female patients with an age range of 11 to 73 years and a median age of 40 years. According to the International Anti-Cancer Alliance/United States Cancer Society (UICC/AJCC) 2008 staging criteria, 68 cases were stage I, 8 cases were stage II, 8 cases were stage III, and 38 cases were stage IV; the stages of the remaining 6 cases were unknown.
Methods
Total thyroidectomy and iodine-131 treatment were performed in all cases, and TSH inhibition was performed according to the ATA treatment guidelines. The BRAF V600E mutation was detected by amplification-blocking mutation system polymerase chain reaction (ARMS-PCR). The serum levels of Tg, TSH, Tg-Ab and Tpo-Ab were detected by chemiluminescence after the administration of iodine-131 treatment. All testing procedures were performed in compliance with quality control standards.
Data analysis
The statistical software SPSS for Windows was used for the statistical analyses. The Pearson's chi-square test or a t-test were used to analyze the relationships among the presence of the BRAF mutation, the clinicopathological features of patients and the results of the thyroid function tests. When P<0.05, differences were considered statistically significant.
Results
Relationship between the BRAFV600E mutation and clinicopathological features
Among all the patients, 75 cases were positive for the BRAF V600E mutation, and 53 patients carried the wild type BRAF gene, thus the mutation rate was 58.6%. Among the patients with the BRAF V600E mutation, there were 17 males and 58 females; among the patients with wild-type BRAF, there were 8 males and 47 females (P=0.25). The median age of patients with the BRAF mutation was 41 years, while the median age of patients with wild-type BRAF was 38 years (P=0.12). The BRAF V600E mutation was closely associated with the high-risk age (≥45 years) (P=0.025). There was no significant correlation between the BRAF V600E mutation and lymph node metastasis (P=0.059), distant metastasis (P=0.76), or clinical stage (P=0.26) (Table 1).
Table 1.
Comparison of BRAF V600E mutation status and clinicopathological features of 128 patients with PTC.
| Variables | No. of patients | BRAF mutation | P value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Age, year | ||||
| <45 | 82 | 42 | 40 | 0.025 |
| ≥45 | 46 | 33 | 13 | |
| Gender | ||||
| Male | 25 | 17 | 8 | 0.25 |
| Female | 103 | 58 | 47 | |
| Clinical stage | ||||
| I | 68 | 38 | 30 | 0.26 |
| II | 8 | 3 | 5 | |
| III | 8 | 5 | 3 | |
| IV | 38 | 26 | 12 | |
| NA | 6 | 3 | 3 | |
| I+ III | 76 | 41 | 35 | 0.14 |
| III+IV | 46 | 31 | 15 | |
| Lymphovascular Invasion | ||||
| No | 7 | 2 | 5 | 0.059 |
| Yes | 121 | 73 | 48 | |
| Metastasis | ||||
| No | 117 | 69 | 48 | 0.76 |
| Yes | 11 | 6 | 5 | |
BRAFV600E mutation and thyroid function following iodine-131 treatment
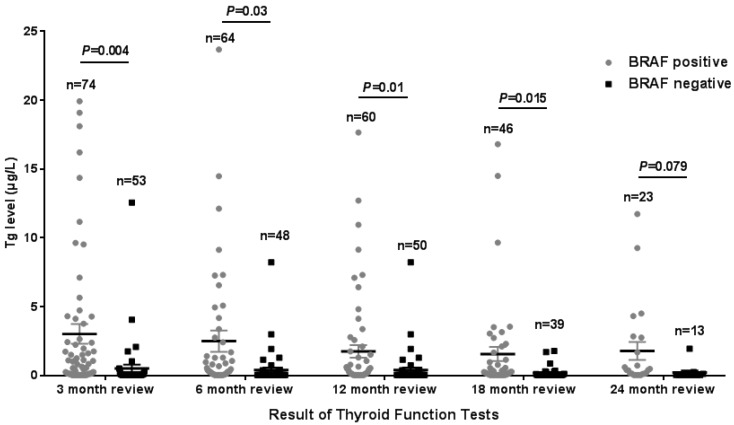
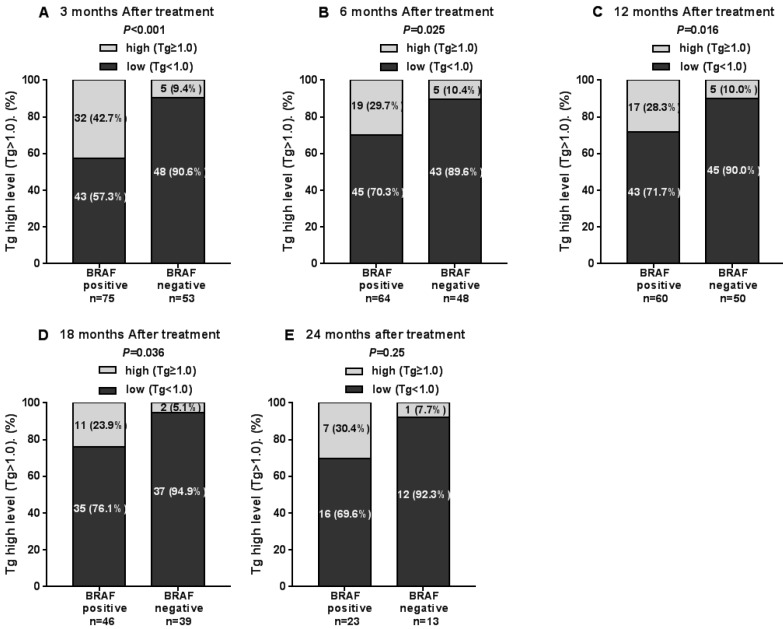
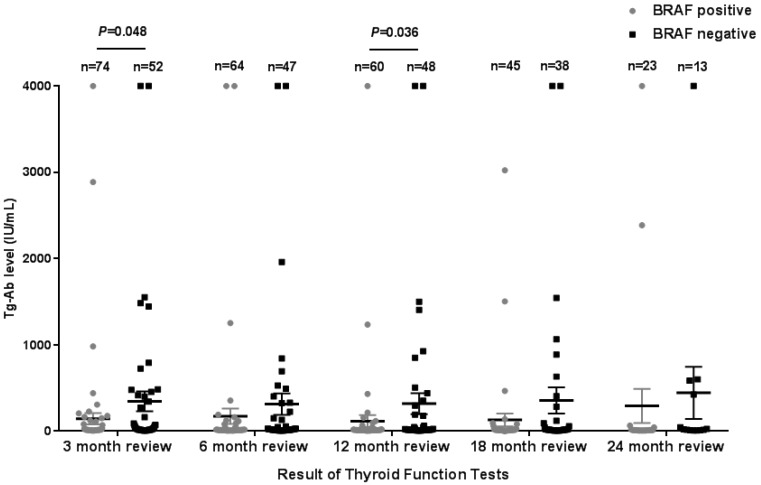
The Tg levels at 3, 6, 12, and 18 months after iodine-131 treatment in patients with the BRAF mutation were significantly higher compared with those in patients with wild-type BRAF (P<0.05) (Table 2, Fig. 1). BRAF V600E-mutation-patients compared with BRAF V600E-WT-patients, under 3 months treatment, the mean Tg level was 3.02 μg/L vs 0.54 μg/L, and the median Tg level was 0.54 μg/L vs 0.04 μg/L (P=0.004); at 6 months after treatment, the mean Tg level was 2.50 μg/L vs 0.51 μg/L, and the median Tg level was 0.30 μg/L vs 0.04 μg/L (P=0.03); at 12 months after treatment, the mean Tg level was 3.65 μg/L vs 0.39 μg/L, and the median Tg level was 0.26 μg/L vs 0.04 μg/L (P=0.010); and at 18 months after treatment, the mean Tg level was 1.74 μg/L vs 0.18 μg/L, and the median Tg level was 0.28 μg/L vs 0.04 μg/L (P=0.015). The rate of elevated Tg levels (Tg>1.00) at 3, 6, 12, and 18 months after treatment was significantly higher in patients with the BRAF mutation than in patients with wild-type BRAF (44.5% vs 15.2%, 29.7% vs 10.4%, 27.8% vs 11.1%, 27.6% vs 5.1%) (P<0.05) (Table 2, Fig. 2). The level of Tg-Ab at 3 and 12 months after treatment with iodine-131 in patients with the BRAF mutation was significantly lower than in patients with wild-type BRAF (P<0.05) (Table 2, Fig. 3). The mean level of Tg-Ab was 94.10 IU/mL vs 274.85 IU/mL, and the median Tg-Ab level was 16.31 IU/mL vs 26.93 IU/mL (P=0.048). At 12 months after treatment, the mean Tg-Ab level was 52.74 IU/mL vs 241.31. IU/mL, and the median Tg-Ab level was 13.47 IU/mL vs 17.46 IU/mL (P=0.036). Due to the tracking time limit, there were only 36 cases that could be traced to 24 months after treatment, including 23 cases of BRAF-positive patients and 13 cases of patients with wild-type BRAF. No significant differences were detected between BRAF mutational status and thyroid function parameters, which may be due to the low number of cases (Tg mean=1.78 μg/L vs 0.21 μg/L, median=0.40 μg/L vs 0.04 μg/L, P=0.079). There was no correlation between BRAF V600E mutational status and the levels of TSH and Tpo-Ab after iodine 131-treatment (P>0.05) (Table 2).
Table 2.
Correlation between BRAF mutation status and thyroid function in 128 patients with papillary thyroid carcinoma.
| Test items | 3 months review | P value | 6 months review | P value | 12 months review | P value | 18 months review | P value | 24 months review | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRAF+ | BRAF- | BRAF+ | BRAF- | BRAF+ | BRAF- | BRAF+ | BRAF- | BRAF+ | BRAF- | ||||||
| Tg Average (μg/L) | 3.02 | 0.54 | 0.004 | 2.50 | 0.51 | 0.03 | 3.65 | 0.39 | 0.010 | 1.74 | 0.18 | 0.015 | 1.78 | 0.21 | 0.079 |
| Tg Median (μg/L) | 0.54 | 0.04 | 0.30 | 0.04 | 0.26 | 0.04 | 0.28 | 0.04 | 0.40 | 0.04 | |||||
| Rate of elevated Tg (≥1.00) | 42.7% | 9.4% | <0.001 | 29.7% | 10.4% | 0.025 | 28.3% | 10.0% | 0.016 | 27.6% | 5.1% | 0.036 | 30.4% | 7.7% | 0.25 |
| TSH Average (IU/mL) | 0.930 | 0.780 | 0.45 | 0.370 | 0.290 | 0.68 | 1.098 | 1.555 | 0.65 | 0.222 | 0.281 | 0.60 | 0.398 | 0.107 | 0.60 |
| TSH Median (IU/mL) | 0.114 | 0.082 | 0.072 | 0.036 | 0.066 | 0.082 | 0.0545 | 0.0505 | 0.101 | 0.042 | |||||
| Tg-Ab Average (IU/mL) | 94.10 | 274.85 | 0.048 | 115.05 | 233.95 | 0.29 | 52.74 | 241.31 | 0.036 | 131.49 | 257.77 | 0.09 | 293.31 | 445.07 | 0.66 |
| Tg-Ab Median (IU/mL) | 16.31 | 26.93 | 14.68 | 22.05 | 13.47 | 17.46 | 14.08 | 18.99 | 14.15 | 21.85 | |||||
Figure 1.
Tg levels in the 3rd, 6th, 12th, 18th, and 24th months after thyroid cancer treatment with iodine-131 in 128 patients from Hunan Cancer Hospital (error bar: mean with SEM).
Figure 2.
The rate of elevated Tg levels in the 3rd, 6th, 12th, 18th and 24th months after thyroid cancer treatment with iodine-131.
Figure 3.
Tg-Ab levels in the 3rd (A), 6th (B), 12th (C)and 18th (D) month after thyroid cancer treatment with iodine-131in 128 patients from Hunan Cancer Hospital (error bar: mean with SEM).
Clinicopathological features and thyroid function following iodine-131 treatment
Based on our sample size and follow-up time, there was no correlation between thyroid function and patient's age, gender, clinical stage, lymphovascular invasion, and metastasis (P>0.05).
Discussion
Thyroid cancer is a common endocrine tumor with an increasing prevalence in China year by year22-24. The four primary histological types include papillary carcinoma, medullary carcinoma, undifferentiated carcinoma, and follicular carcinoma 25, among which papillary thyroid cancer (PTC) is the most common, accounting for 85% to 90% of the total incidence. The clinical biological characteristics of thyroid cancer with different pathological types are also different. Papillary thyroid carcinoma is one of the less malignant thyroid cancers, which shows low lymph node metastasis rate and distant metastasis rate. Clinically, in addition to the general clinical features, most papillary thyroid carcinoma patients have considerable variable clinical manifestations. This situation may be caused by many factors, including age, gender, living environment, genetic background, and gene mutation.
In recent years, with advances in gene sequencing technology, molecular diagnosis has been widely used for the early diagnosis of cancer, the determination of radiotherapy and chemotherapy, and the postoperative management of patients. In the molecular diagnosis of thyroid cancer, the related molecular markers include TERT, BRAF, PAX8/ PPARγ, RAS and RET/PTC26. The BRAF V600E gene mutation is one of the most common mutations in PTC27, 28 and is closely related to the occurrence, development, biological behavior and prognosis of PTC29, 30. Studies have shown that an assessment of the BRAF V600E mutational status can be combined with cell morphological analysis to aid in the diagnosis of PTC in cytologically uncertain thyroid nodules31. Therefore, we selected 128 patients with PTC who underwent iodine-131 treatment after total thyroidectomy in the Cancer Hospital of Hunan Province from February 2015 to November 2016. We investigated the relationships among the BRAF V600E mutation, clinical pathological features and the efficacy of iodine-131 treatment. Among the 128 patients with PTC included in this study, the BRAF V600E mutation rate was 58.6%. In a previous meta-analysis of BRAF V600E mutation in 25241 cases with PTC, 15290 (60.6%) were positive for BRAF mutation and 9,951 (39.4%) were tested negative for BRAF mutation32. The inclusion and exclusion criteria for that meta-analysis are basically similar to ours. And the BRAF V600E mutation rate was also similar to our finding.
In this study, we compared the clinical characteristics between patients with the BRAF mutation and patients with wild-type BRAF and found that the BRAF mutation was significantly more frequent in patients of the high-risk age (>45 years old). In the current prognosis evaluation system of thyroid cancer, the patient's age is a recognized risk factor for death; various clinical guidelines and risk assessment models include the patient's age as the primary risk factor in the management and prognostic evaluation of thyroid cancer. The presence of the BRAF mutation is closely related to the patient's age; thus, there may be synergistic effects leading to a poor prognosis33. Therefore, in the risk assessment of PTC patients, the BRAF gene mutation and the patient age should be considered as factors associated with a poor prognosis. This finding has a certain reference value for the diagnosis and treatment of patients of a high-risk age (>45 years old). However, due to the small sample size and short follow-up time, there was no significant correlation between the BRAF mutation and gender, lymphatic vessel invasion, distant metastasis or clinical stage in this study.
At present, there are few studies on the correlation between BRAF mutational status and iodine-131 treatment, and the results are controversial. According to the guidelines, the current strategies for the treatment of thyroid cancer are primarily surgical treatment, radioactive iodine treatment and TSH inhibition treatment; molecular-targeted therapy has gradually entered the field of vision as an emerging treatment program34. The clinical evaluation of the efficacy of iodine-131 treatment typically includes imaging and tests for thyroid function35. The key indicators of thyroid function are the levels of thyroglobulin (Tg), thyroid stimulating hormone (TSH), and thyroglobulin antibody (Tg-Ab)36. We focused on the relationship between gonadotropin function and BRAF V600E mutational status after iodine-131 treatment.
Patients who were positive for the BRAF V600E mutation had significantly higher Tg levels compared with patients with wild-type BRAF. Tg is a glycoprotein that is derived from functional thyroid tissue, accounting for 75% of all thyroid proteins. Tg is a macromolecular glycoprotein secreted by thyroid follicular epithelial cells and consists of two identical monomeric peptide chains with a molecular weight of 660 kDa37. Under normal circumstances, Tg circulates within thyroid cells, and only a very small portion of the Tg is secreted into the blood through the thyroid lymphatic vessels. Under normal conditions, the residual tissue size of the thyroid is proportional to the serum levels of Tg. Therefore, after iodine-131 treatment following PTC total resection, the serum levels of Tg directly reflect the size of the residual thyroid tissue and micrometastases, and an increase in these levels indicate an increase in the residual thyroid tissue and micrometastases38. The Tg level of patients who were positive for the BRAF V600E mutation was significantly increased, suggesting that the therapeutic effect and prognosis of iodine-131 treatment are relatively poor in these patients. Other studies suggest that Tg level is a key predictor of iodine-131 treatment and PTC prognosis. After iodine-131 treatment following total thyroidectomy, the serum level of thyroglobulin was less than 1 μg/L, which is used as the standard for radioactive iodine ablation in patients with low-risk thyroid papillary carcinoma39. A Tg level higher than 1 μg/L indicates that the treatment effect of iodine-131 is not good. The rate of elevated Tg levels (>1.0 μg/L) was significantly increased in patients who were positive for the BRAF mutation (P<0.05). This finding indicates that the treatment effect of iodine-131 was worse in these patients than in patients with wild-type BRAF. Recent studies indicate that the BRAF V600E mutation can lead to a downregulation of iodine metabolism because the BRAF V600E mutation leads to the continuous activation of the downstream genes of the MAPK pathway, resulting in the decreased expression and abnormal localization of the sodium iodide symporter (NIS). In the case of wild-type BRAF, NIS is primarily located in the thyroid follicular membrane; however, under conditions of the BRAF V600E mutation, NIS cannot be accurately localized to the follicular membrane and is more dispersed in the cytoplasm40. Therefore, patients with the BRAF V600E mutation exhibit poor iodine absorption during iodine-131 treatment, which renders the treatment less effective41, leading to a poor prognosis. The findings of this study suggest that BRAF mutation can be used as a predictor of the efficacy of iodine-131 treatment.
Tg-Ab is a common autoantibody in the serum of patients with autoimmune thyroid disease. This antibody is primarily composed of IgG1, IgG2 and IgG4, and a small portion is IgA and IgM. TG-Ab is generally considered to cause no damage to the thyroid gland. At 3 months and 12 months after iodine-131 treatment, the Tg-Ab levels in patients with the BRAF mutation were significantly lower than in patients with wild-type BRAF. Regarding the role of Tg-Ab in the development of PTC, current research is sparse and contradictory. According to Rakib et al., the trend of postoperative Tg-Ab elevation can be used as a rough estimate of prognosis42, 43. According to Velsen et al., the continued increase in Tg-Ab may suggest tumor recurrence44. Smooke-Praw et al. conclude that the level of Tg-Ab cannot predict the disease state of PTC45, whereas Wu et al. state that the serum levels of Tg-Ab can be used as an independent predictor of PTC46. The results of the present study suggest that the BRAF mutation may reduce the levels of Tg-Ab in the serum, but the specific mechanism warrants further exploration.
Studies have suggested that patients with the BRAF V600E mutation should be overtreated to some extent47, because BRAF mutations are an independent risk factor for lymph node metastasis and are closely related to tumor size and invasiveness48-57. Both the clinical studies and the basic medical studies performed here suggest that the presence of the BRAF mutation may lead to a decrease in the efficacy of iodine-131 treatment. Therefore, we suggest that a higher level of radioactive iodine-131 treatment should be considered for patients who are positive for this mutation.
In recent years, individualized treatment has gradually gained attention, and the individualized treatment of cancer has been increasingly promoted58-63. As the gene with the highest mutation rate in PTC, the BRAF gene is of great significance for the development of PTC and is widely used in clinical practice. Testing for the BRAF V600E mutation is important, as this mutation is involved in the following: early diagnosis of thyroid cancer with FNC, independent risk factor prediction of PTC high-risk age, independent risk factor prediction of PTC lymph node vascular invasion and lymph node metastasis, prognosis evaluation of PTC, and posttreatment management64-70. The results of this study suggest that BRAF mutations should be used as an important indicator when developing individualized treatment options for patients with PTC. According to the results of this study, the BRAF V600E gene mutation is closely related to serum Tg elevation after PTC iodine-131 treatment, suggesting that this mutation may be used as a predictor of the efficacy of PTC I-131 treatment.
Acknowledgments
This study was supported by the Hunan provincial natural science fund (2018JJ3313), the Hunan provincial health committee research project (C20180370) and the Sister Institution Network Fund of MD Anderson Cancer Center (2016).
Abbreviations
- PTC
Papillary Thyroid Cancer
- Tg
thyroglobulin
- TSH
thyroid stimulating hormone
- Tg-Ab
thyroglobulin antibody
- Tpo-Ab
thyroid peroxidase antibody
- NIS
sodium iodide symporter
- ARMS-PCR
amplification-blocking mutation system polymerase chain reaction
References
- 1.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016;23:313–22. doi: 10.1530/ERC-15-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doubleday A, Sippel RS. Surgical options for thyroid cancer and post-surgical management. Expert Rev Endocrinol Metab. 2018;13:137–48. doi: 10.1080/17446651.2018.1464910. [DOI] [PubMed] [Google Scholar]
- 3.American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, Cooper DS, Doherty GM, Haugen BR, Kloos RT, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C. et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long GV, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V. et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377:1813–23. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 6.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A. et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–16. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 7.Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC. et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428–43. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu C, Zeng Z, Qi P, Li X, Guo C, Xiong F. et al. Identification of genomic alterations in nasopharyngeal carcinoma and nasopharyngeal carcinoma-derived Epstein-Barr virus by whole-genome sequencing. Carcinogenesis. 2018;39:1517–28. doi: 10.1093/carcin/bgy108. [DOI] [PubMed] [Google Scholar]
- 9.Tu C, Zeng Z, Qi P, Li X, Yu Z, Guo C, Genome-Wide Analysis of 18 Epstein-Barr Viruses Isolated from Primary Nasopharyngeal Carcinoma Biopsy Specimens. J Virol; 2017. p. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Wei F, Liang F, Li Q, Deng H, Tan S. et al. TSC22D2 identified as a candidate susceptibility gene of multi-cancer pedigree using genome-wide linkage analysis and whole-exome sequencing. Carcinogenesis. 2019;40:819–27. doi: 10.1093/carcin/bgz095. [DOI] [PubMed] [Google Scholar]
- 11.Bai Y, Guo T, Huang X, Wu Q, Niu D, Ji X. et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. 2018;472:779–87. doi: 10.1007/s00428-018-2357-6. [DOI] [PubMed] [Google Scholar]
- 12.Ebina A, Sugitani I, Fujimoto Y, Yamada K. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: is thyroid lobectomy the treatment of choice for low-risk patients? Surgery. 2014;156:1579–88. doi: 10.1016/j.surg.2014.08.060. discussion 88-9. [DOI] [PubMed] [Google Scholar]
- 13.Pitoia F, Jerkovich F, Urciuoli C, Schmidt A, Abelleira E, Bueno F. et al. Implementing the Modified 2009 American Thyroid Association Risk Stratification System in Thyroid Cancer Patients with Low and Intermediate Risk of Recurrence. Thyroid. 2015;25:1235–42. doi: 10.1089/thy.2015.0121. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JH, Lee HS, Kim EK, Moon HJ, Kwak JY. Malignancy Risk Stratification of Thyroid Nodules: Comparison between the Thyroid Imaging Reporting and Data System and the 2014 American Thyroid Association Management Guidelines. Radiology. 2016;278:917–24. doi: 10.1148/radiol.2015150056. [DOI] [PubMed] [Google Scholar]
- 15.Xia M, Zhang Y, Jin K, Lu Z, Zeng Z, Xiong W. Communication between mitochondria and other organelles: a brand-new perspective on mitochondria in cancer. Cell Biosci. 2019;9:27. doi: 10.1186/s13578-019-0289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Zhou Y, Oyang L, Han Y, Xia L, Lin J. et al. LPLUNC1 stabilises PHB1 by counteracting TRIM21-mediated ubiquitination to inhibit NF-kappaB activity in nasopharyngeal carcinoma. Oncogene. 2019;38:5062–75. doi: 10.1038/s41388-019-0778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagin JA, Wells SA Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med. 2016;375:1054–67. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riesco-Eizaguirre G, Gutierrez-Martinez P, Garcia-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–69. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Oriuchi N, Higuchi T, Zhang H, Endo K. PET in the follow-up of differentiated thyroid cancer. Br J Radiol. 2003;76:690–5. doi: 10.1259/bjr/31538331. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara S, Kikuchi M, Suehiro A, Kishimoto I, Harada H, Hino M. et al. Characteristics and prognosis of patients with thyroglobulin-positive and radioactive iodine whole-body scan-negative differentiated thyroid carcinoma. Jpn J Clin Oncol. 2015;45:427–32. doi: 10.1093/jjco/hyv021. [DOI] [PubMed] [Google Scholar]
- 21.Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G. et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81(Suppl 1):1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 22.Wei F, Wu Y, Tang L, Xiong F, Guo C, Li X. et al. Trend analysis of cancer incidence and mortality in China. Sci China Life Sci. 2017;60:1271–5. doi: 10.1007/s11427-017-9172-6. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y. et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62:640–7. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 24.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X. et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–e67. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 25.Chmielik E, Rusinek D, Oczko-Wojciechowska M, Jarzab M, Krajewska J, Czarniecka A. et al. Heterogeneity of Thyroid Cancer. Pathobiology. 2018;85:117–29. doi: 10.1159/000486422. [DOI] [PubMed] [Google Scholar]
- 26.Cabanillas ME, Ferrarotto R, Garden AS, Ahmed S, Busaidy NL, Dadu R. et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid. 2018;28:945–51. doi: 10.1089/thy.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur A, Moses W, Rahbari R, Khanafshar E, Duh QY, Clark O. et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer. 2011;117:4390–5. doi: 10.1002/cncr.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty A, Narkar A, Mukhopadhyaya R, Kane S, D'Cruz A, Rajan MG. BRAF V600E mutation in papillary thyroid carcinoma: significant association with node metastases and extra thyroidal invasion. Endocr Pathol. 2012;23:83–93. doi: 10.1007/s12022-011-9184-5. [DOI] [PubMed] [Google Scholar]
- 29.Pelizzo MR, Dobrinja C, Casal Ide E, Zane M, Lora O, Toniato A. et al. The role of BRAF(V600E) mutation as poor prognostic factor for the outcome of patients with intrathyroid papillary thyroid carcinoma. Biomed Pharmacother. 2014;68:413–7. doi: 10.1016/j.biopha.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Lee MY, Ku BM, Kim HS, Lee JY, Lim SH, Sun JM. et al. Genetic Alterations and Their Clinical Implications in High-Recurrence Risk Papillary Thyroid Cancer. Cancer Res Treat. 2017;49:906–14. doi: 10.4143/crt.2016.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beisa A, Kvietkauskas M, Beisa V, Stoskus M, Ostaneviciute E, Jasiunas E. et al. Significance of BRAF V600E Mutation and Cytomorphological Features for the Optimization of Papillary Thyroid Cancer Diagnostics in Cytologically Indeterminate Thyroid Nodules. Exp Clin Endocrinol Diabetes. 2019;127:247–54. doi: 10.1055/a-0588-4885. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Liu SZ, Zhang Q, Guan YX, Chen QJ, Zhu QY. Meta-Analyses of Association Between BRAF(V600E) Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell Physiol Biochem. 2016;38:763–76. doi: 10.1159/000443032. [DOI] [PubMed] [Google Scholar]
- 33.Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E. et al. Patient Age-Associated Mortality Risk Is Differentiated by BRAF V600E Status in Papillary Thyroid Cancer. J Clin Oncol. 2018;36:438–45. doi: 10.1200/JCO.2017.74.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chau NG, Haddad RI. Vandetanib for the treatment of medullary thyroid cancer. Clin Cancer Res. 2013;19:524–9. doi: 10.1158/1078-0432.CCR-12-2353. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Pan MZ, Huang JM, Xie P, Zhang F, Wei LG. Iodine-131: An Effective Method for Treating Lymph Node Metastases of Differentiated Thyroid Cancer. Med Sci Monit. 2016;22:4924–8. doi: 10.12659/MSM.899028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faix JD, Miller WG. Progress in standardizing and harmonizing thyroid function tests. Am J Clin Nutr. 2016;104(Suppl 3):913S–7S. doi: 10.3945/ajcn.115.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Netzel BC, Grebe SK, Carranza Leon BG, Castro MR, Clark PM, Hoofnagle AN. et al. Thyroglobulin (Tg) Testing Revisited: Tg Assays, TgAb Assays, and Correlation of Results With Clinical Outcomes. J Clin Endocrinol Metab. 2015;100:E1074–83. doi: 10.1210/jc.2015-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz RE, Veliz J, Wohllk N. [Preablative serum thyroglobulin as predictor of disease-free survival in differentiated thyroid cancer] Rev Med Chil. 2013;141:1506–11. doi: 10.4067/S0034-98872013001200002. [DOI] [PubMed] [Google Scholar]
- 39.Rosario PW, Mineiro Filho AF, Prates BS, Silva LC, Calsolari MR. Postoperative stimulated thyroglobulin of less than 1 ng/ml as a criterion to spare low-risk patients with papillary thyroid cancer from radioactive iodine ablation. Thyroid. 2012;22:1140–3. doi: 10.1089/thy.2012.0190. [DOI] [PubMed] [Google Scholar]
- 40.Tavares C, Coelho MJ, Eloy C, Melo M, da Rocha AG, Pestana A. et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocr Connect. 2018;7:78–90. doi: 10.1530/EC-17-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang N, Liang J, Lin YS. Unfavorable efficacy to (131)I ablation in BRAFV600E mutant papillary thyroid carcinoma with positive TgAb. Oncotarget. 2017;8:97407–15. doi: 10.18632/oncotarget.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakib SA, Sharif SB, Nahar A, Alam SM. Prediction of Thyroid Malignancies by Thyroid Auto Antibodies. Mymensingh Med J. 2018;27:585–95. [PubMed] [Google Scholar]
- 43.de Meer SGA, Vorselaars W, Kist JW, Stokkel MPM, de Keizer B, Valk GD. et al. Follow-up of patients with thyroglobulin-antibodies: Rising Tg-Ab trend is a risk factor for recurrence of differentiated thyroid cancer. Endocr Res. 2017;42:302–10. doi: 10.1080/07435800.2017.1319858. [DOI] [PubMed] [Google Scholar]
- 44.van Velsen EFS, Stegenga MT, van Kemenade FJ, Kam BLR, van Ginhoven TM, Visser WE. et al. Comparing the Prognostic Value of the Eighth Edition of the American Joint Committee on Cancer/Tumor Node Metastasis Staging System Between Papillary and Follicular Thyroid Cancer. Thyroid. 2018;28:976–81. doi: 10.1089/thy.2018.0066. [DOI] [PubMed] [Google Scholar]
- 45.Smooke-Praw S, Ro K, Levin O, Ituarte PH, Harari A, Yeh MW. Thyroglobulin antibody levels do not predict disease status in papillary thyroid cancer. Clin Endocrinol (Oxf) 2014;81:271–5. doi: 10.1111/cen.12421. [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z. et al. Coexistence of thyroglobulin antibodies and thyroid peroxidase antibodies correlates with elevated thyroid-stimulating hormone level and advanced tumor stage of papillary thyroid cancer. Endocrine. 2014;46:554–60. doi: 10.1007/s12020-013-0121-x. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Chang JW, Jung SN, Park HS, Oh T, Lim YC. et al. Clinical implications of the extent of BRAF(V600E) alleles in patients with papillary thyroid carcinoma. Oral Oncol. 2016;62:72–7. doi: 10.1016/j.oraloncology.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Ghaznavi SA, Ganly I, Shaha AR, English C, Wills J, Tuttle RM. Using the American Thyroid Association Risk-Stratification System to Refine and Individualize the American Joint Committee on Cancer Eighth Edition Disease-Specific Survival Estimates in Differentiated Thyroid Cancer. Thyroid. 2018;28:1293–300. doi: 10.1089/thy.2018.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fan CM, Wang JP, Tang YY, Zhao J, He SY, Xiong F. et al. circMAN1A2 could serve as a novel serum biomarker for malignant tumors. Cancer Sci. 2019;110:2180–8. doi: 10.1111/cas.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong F, Deng S, Huang HB, Li XY, Zhang WL, Liao QJ. et al. Effects and mechanisms of innate immune molecules on inhibiting nasopharyngeal carcinoma. Chin Med J (Engl) 2019;132:749–52. doi: 10.1097/CM9.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bo H, Fan L, Gong Z, Liu Z, Shi L, Guo C. et al. Upregulation and hypomethylation of lncRNA AFAP1AS1 predicts a poor prognosis and promotes the migration and invasion of cervical cancer. Oncol Rep. 2019;41:2431–9. doi: 10.3892/or.2019.7027. [DOI] [PubMed] [Google Scholar]
- 52.Bo H, Fan L, Li J, Liu Z, Zhang S, Shi L. et al. High Expression of lncRNA AFAP1-AS1 Promotes the Progression of Colon Cancer and Predicts Poor Prognosis. J Cancer. 2018;9:4677–83. doi: 10.7150/jca.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng X, Xiong F, Li X, Xiang B, Li Z, Wu X. et al. Application of atomic force microscopy in cancer research. J Nanobiotechnology. 2018;16:102. doi: 10.1186/s12951-018-0428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Wei F, Tang L, Liao Q, Wang H, Shi L. et al. Herpesvirus acts with the cytoskeleton and promotes cancer progression. J Cancer. 2019;10:2185–93. doi: 10.7150/jca.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan C, Tu C, Qi P, Guo C, Xiang B, Zhou M. et al. GPC6 Promotes Cell Proliferation, Migration, and Invasion in Nasopharyngeal Carcinoma. J Cancer. 2019;10:3926–32. doi: 10.7150/jca.31345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang W, Zhou R, Wu Y, Liu Y, Su W, Xiong W. et al. PVT1 Promotes Cancer Progression via MicroRNAs. Front Oncol. 2019;9:609. doi: 10.3389/fonc.2019.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin K, Wang S, Zhang Y, Xia M, Mo Y, Li X. et al. Long non-coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis. Cell Mol Life Sci. 2019;76:4275–89. doi: 10.1007/s00018-019-03222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F. et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37:87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei F, Tang L, He Y, Wu Y, Shi L, Xiong F. et al. BPIFB1 (LPLUNC1) inhibits radioresistance in nasopharyngeal carcinoma by inhibiting VTN expression. Cell Death Dis. 2018;9:432. doi: 10.1038/s41419-018-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He Y, Jing Y, Wei F, Tang Y, Yang L, Luo J. et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis. 2018;9:235. doi: 10.1038/s41419-018-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mo Y, Wang Y, Zhang L, Yang L, Zhou M, Li X. et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J Cancer. 2019;10:3789–97. doi: 10.7150/jca.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F. et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mo Y, Wang Y, Xiong F, Ge X, Li Z, Li X. et al. Proteomic Analysis of the Molecular Mechanism of Lovastatin Inhibiting the Growth of Nasopharyngeal Carcinoma Cells. J Cancer. 2019;10:2342–9. doi: 10.7150/jca.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei F, Jing YZ, He Y, Tang YY, Yang LT, Wu YF. et al. Cloning and characterization of the putative AFAP1-AS1 promoter region. J Cancer. 2019;10:1145–53. doi: 10.7150/jca.29049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y. et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. 2019;18:29. doi: 10.1186/s12943-019-0956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B. et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 2019;18:10. doi: 10.1186/s12943-018-0928-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YA, Li XL, Mo YZ, Fan CM, Tang L, Xiong F. et al. Effects of tumor metabolic microenvironment on regulatory T cells. Mol Cancer. 2018;17:168. doi: 10.1186/s12943-018-0913-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fan C, Tang Y, Wang J, Wang Y, Xiong F, Zhang S. et al. Long non-coding RNA LOC284454 promotes migration and invasion of nasopharyngeal carcinoma via modulating the Rho/Rac signaling pathway. Carcinogenesis. 2019;40:380–91. doi: 10.1093/carcin/bgy143. [DOI] [PubMed] [Google Scholar]
- 69.Lian Y, Xiong F, Yang L, Bo H, Gong Z, Wang Y. et al. Long noncoding RNA AFAP1-AS1 acts as a competing endogenous RNA of miR-423-5p to facilitate nasopharyngeal carcinoma metastasis through regulating the Rho/Rac pathway. J Exp Clin Cancer Res. 2018;37:253. doi: 10.1186/s13046-018-0918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan C, Tang Y, Wang J, Xiong F, Guo C, Wang Y. et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J Cancer. 2018;9:2852–64. doi: 10.7150/jca.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]