Abstract
Lung cancer is the leading cause of cancer death worldwide. Cigarette smoking is the most common risk factor for lung carcinoma; other risks include genetic factors and exposure to radon gas, asbestos, secondhand smoke, and air pollution. Nicotine, the primary addictive constituent of cigarettes, contributes to cancer progression through activation of nicotinic acetylcholine receptors (nAChRs), which are membrane ligand-gated ion channels. Activation of nicotine/nAChR signaling is associated with lung cancer risk and drug resistance. We focused on nAChR pathways activated by nicotine and its downstream signaling involved in regulating apoptotic factors of mitochondria and drug resistance in lung cancer. Increasing evidence suggests that several sirtuins play a critical role in multiple aspects of cancer drug resistance. Thus, understanding the consequences of crosstalk between nicotine/nAChRs and sirtuin signaling pathways in the regulation of drug resistance could be a critical implication for cancer therapy.
Keywords: nicotinic acetylcholine receptor, drug resistance, mitochondria, sirtuin, lung cancer
Introduction
Globally, lung cancer is greatest cause of cancer-related deaths. Lung cancer accounts for 14% and 12% of all cancers in men and women, respectively, and represents 24.6% of all cancer-related deaths 1. Because of its extraordinary disease burden and international variability in trends of population growth, aging, and smoking behaviors, the global epidemiology of lung cancer requires continual monitoring 2. Two main subtypes of lung cancer are small-cell lung carcinoma (SCLC) and non-SCLC (NSCLC), respectively accounting for 15% and 85% of all lung cancers 3. NSCLC is further classified into three types: squamous cell carcinoma (SCC), adenocarcinoma, and large-cell carcinoma. SCC is the subtype of NSCLC strongly correlated with cigarette smoking 4. NSCLC is a complex heterogenous disease with interpatient, intratumor, and inter-/intrametastatic heterogeneity at the subtype level 5. Both epithelial growth factor receptor (EGFR) mutation and gene amplification status may be notable in determining chemoresistance in NSCLC 6. Several studies have revealed that never-smokers with lung cancer are more responsive to EGFR-tyrosine kinase inhibitor (EGFR-TKI) therapy than smokers 7, 8. Tumor progression and chemoresistance mediated by nicotine and its metabolites have been partly attributed to phosphoinositide 3-kinase (PI3K)/AKT, nuclear factor-κB (NF-κB), and mitochondrial signaling pathways 7, 9, 10. Therefore, resistance is an inevitable barrier limiting lung cancer therapy effectiveness and reducing enthusiasm, making it today's pervasive challenge for long-term disease control.
The biochemical and physiological properties of Nicotine
Chemicals in cigarette smoke enter the bloodstream and affect the body; thus, smoking causes many diseases including cardiovascular disease, chronic obstructive pulmonary disease (COPD), and lung cancer 11. Furthermore, nicotine mediates therapeutic resistance, survival and antiapoptosis, and self-renewal of cancer stem cells and modulates many immune properties of cancer 12. Cigarette smoke contains several carcinogens including benzo[a]pyrene (BaP), polycyclic aromatic hydrocarbons (PAH), nicotine, and nitrosamines 4, 13-15. Nicotine can promote cancer progression by activating cell-surface receptors, particularly nicotinic acetylcholine receptors (nAChRs) and β-adrenergic receptors (β-AR) 16-18. Tobacco-specific N-nitrosamines is formed by the N-nitrosation of tobacco alkaloids (Fig. 1).
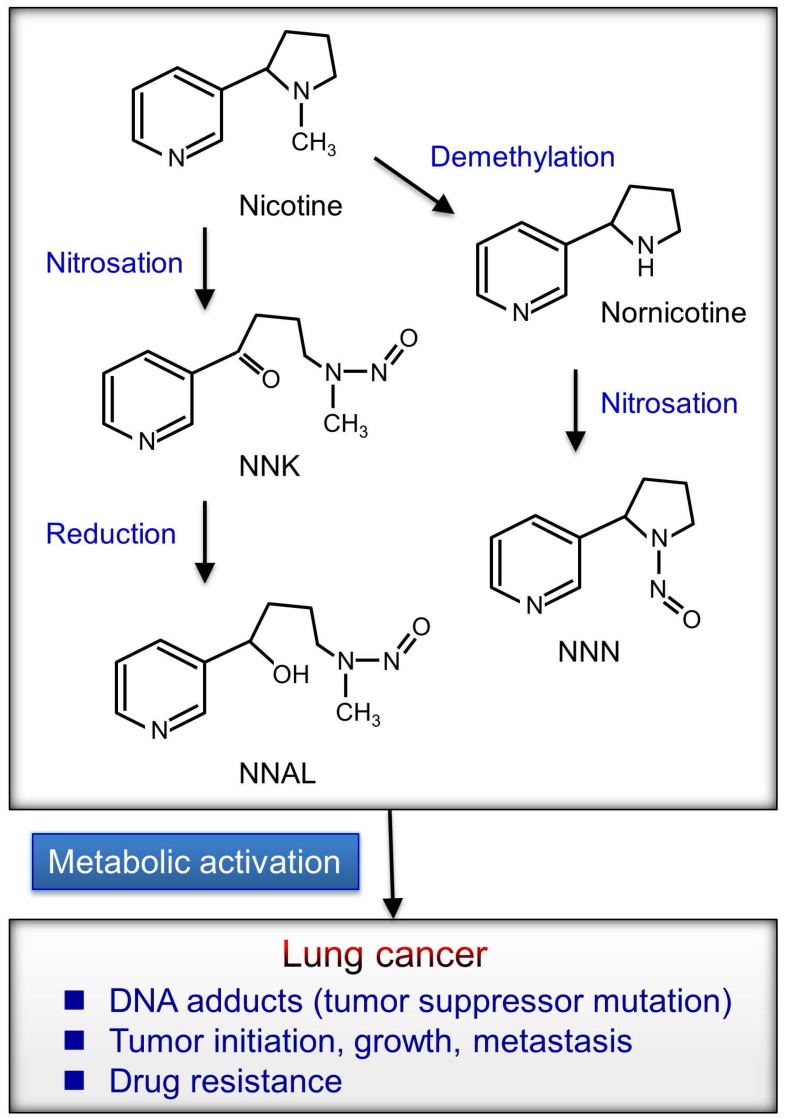
Figure 1.
Tobacco-specific N-nitrosamines are formed by the N-nitrosation of nicotine. NNN (N′-nitrosonornicotine) and NNK (4-(metylnitrosamino)-1-(3-pyridyl)-1-butanon) are the most potential carcinogens formed by nicotine from cigarette smoke. NNAL (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) is a metabolite from the reduction of NNK and total NNAL (NNAL and its glucuronides) in urine and can be used to examine the possible role of N-nitrosamines metabolites in tumor development. Nicotine metabolites carcinogens may induce multiple mutations in critical genes, such as p53, KRAS, p16, and Rb. A permanent mutation occurs in these critical genes that can contribute to activation of the oncogene or blockade of the tumor suppressor gene. Multiple aberrant events can continue to cause cells with abnormal regulation and eventually lung cancer progression.
In addition to nicotine, its oncogenic derivatives 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN), present in tobacco smoke, can activate nAChRs signaling and stimulate multiple cancer-promoting signaling 18, 19. Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), an NNK metabolite, is associated with the lung cancer risk in smokers 20. These oncogenic metabolites may induce the formation of DNA adducts that leads to mutations of tumor suppressor genes including Rb and p53 21. Nicotine or NNK signaling may contribute to cancer progression 18, 22. Nicotine was implicated in promoting the self-renewal of stem-like side-population cells from lung cancers. The subpopulation of cancer stem-like cells was implicated in tumor initiation, generation of heterogeneous tumor populations, metastasis, dormancy, and drug resistance 23. Furthermore, nicotine can inhibit apoptosis induced by opioids, etoposide, cisplatin, and UV irradiation in lung cancer cells 24-26. Therefore, the activation of nicotine signaling might be associated with drug resistance in lung cancer.
The potential mechanisms of Nicotine on lung carcinogenesis
Cigarette smoke is associated with an increased risk of all histological types of lung cancer 27. In general, the lungs retain on average 60%-80% of mainstream smoke particulate matter and 90%-100% of nicotine after cigarette smoke inhalation 28. The nicotine-derived metabolites including NNK and NNN are potent carcinogens because they bind to α7nAChR 14. These metabolites and DNA interactions may be the primary cause of lung cancer in smokers. The binding activity of NNK to α7nAChR was 1,300 times greater than that of nicotine 29. Different nAChR subunits were expressed in NSCLC cells of smokers and nonsmokers 30. Nicotine present in the plasma of average smokers enhanced α7nAChR expression in human SCC cells 31. SCC was most strongly associated with cigarette smoke, and adenocarcinoma, the most common lung cancer in never-smokers, has the weakest association. The higher expression of all affected nAChR subunits (α7, dupα7, α5, and α9) in smokers than in nonsmokers indicated that tobacco components upregulate the expression of a few nAChR subunit genes in SCC histologic type 32. The literature has also indicated that only two subunits (α7 and α5) demonstrated significantly increased expression in SCC with a poor prognosis 32. Furthermore, patients with SCC who died presented significantly higher α7nAChR expression than patients with SCC who survived. For early-stage lung cancers, smoking cessation was associated with a large reduction in mortality risk 33. α5-nAChR was associated with lung cancer risk and onset, with exposure to a primary cancer etiologic factor (smoking duration and amount), and with the effects of a preventive action (smoking cessation) 34-37. Chronic exposure to nicotine and derivatives can lead to the upregulation of all nAChRs in smokers. Activation of nicotine-nAChR signaling promotes cancer progression 18. Upregulation of α7nAChR in lung cancer cells involved in the nicotine-induced tumor progression 38. Homomeric α7nAChR was implicated as the primary receptor facilitating nicotine- and NNK-mediated cell proliferation 21. The nicotine-α7nAChR axis can initiate cell invasion and the epithelial-to-mesenchymal transition (EMT) in NSCLC 39. nAChRs, β-AR, and EGFR often coexpress on human lung cancer cells and airway epithelial or endothelial cells that might lead to cancer progression 40. Nicotine signaling triggers the production of β-AR ligands, such as adrenaline and noradrenaline, which contribute to the development of lung cancer 21.
Survival analysis of a Cancer Genome Atlas (TCGA) lung cancer dataset demonstrated that high expression of acetylcholine receptors (AChRs) gene family such as CHRM2, CHRM3, CHRNA1, CHRNA2, CHRNA6, CHRNB3, or CHRNE is associated with favorable prognosis in NSCLC adenocarcinoma, but that of CHRNA5/α5nAChR or CHRNA7/α7nAChR is associated with an unfavorable prognosis 41. Among these proteins, only α7nAChR in the AChR family could affect prognosis in both lung adenocarcinoma and SCC 41. High CHRNA1 expression is associated with reduced survival in early-stage lung adenocarcinoma after complete resection 42. Notably, the levels of α7nAChR expression in SCC are higher than those in adenocarcinoma among patients with lung cancer, particularly in smokers 32. Thus, α7nAChR is a potential target for lung cancer treatment.
Single nucleotide polymorphisms (SNPs) located on chromosome 15q25, which contains the nAChR subunits encoding by the CHRNA5, CHRNA3, and CHRNB4, are associated with lung cancer risk 43. Lung cancer risk was more than fivefold higher among individuals with both a family history of lung cancer and two copies of high-risk alleles rs8034191 or rs1051730 located in the 15q24-25.1 locus 44. The change in rs6495309T>C on 15q25 may affect the activity of OCT1 binding to the CHRNA3 promoter, which contributes to CHRNA3 overexpression. These results confirm that 15q25 is a susceptibility region for lung cancer in Chinese individuals 45. The gene cluster CHRNA5-CHRNA3-CHRNB4 contributes to nicotine dependence risk in African-American and European-American individuals 46. The missense SNP rs16969968 of CHRNA5 is significantly associated with both nicotine dependence and increased risk of lung cancer 47, 48. The genome-wide associations of CHRNA3 and CHRNA5 on 15q25.1 were confirmed in familial SCC 49. The CHRNA3 polymorphism functions as a genetic modifier of lung adenocarcinoma risk in the Chinese population, particularly in nonsmoking women 50. CHRNA5 polymorphism is associated with lung adenocarcinoma risk in a population-based series of lung adenocarcinoma patients and healthy controls. An analysis of a family-based series of nonsmoker lung cancer cases and healthy controls indicated a similar trend. In addition, the same D398N variation correlated with CHRNA5 mRNA levels in the lungs of patients with adenocarcinoma 51. Acetylcholine/nAChRs and muscarinic acetylcholine receptors can promote cancer cell and lung fibroblasts or myofibroblast proliferation, respectively 52, 53. Chronic nicotine inhalation or endogenous acetylcholine released in the lungs may play a role in lung disease including COPD and lung cancer. Nicotine/nAChRs stimulates several signaling pathways conferring lung cancer cell survival, as described in the next section (Fig. 2).
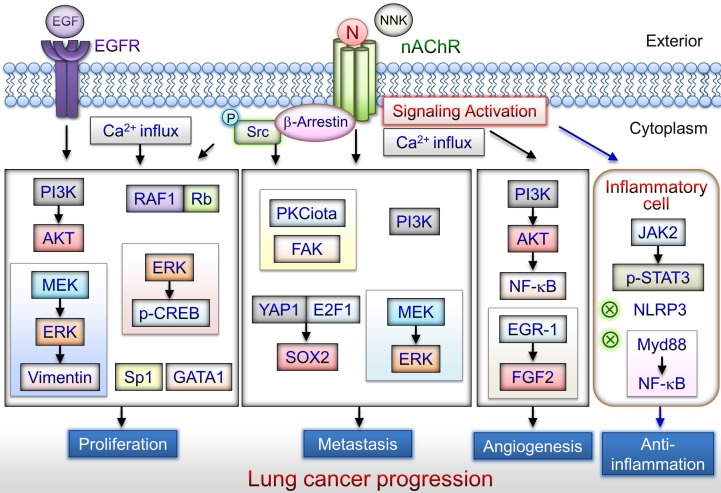
Figure 2.
nAChR-mediated signaling pathways that lead to lung cancer progression. Nicotine/α7nAChR mediates the proliferative effects through several pathways including PI3K/AKT, MEK/ERK, RAF1/Rb, and Sp1/GATA1 activation signaling in lung cancer cells. Cigarette smoking is associated with metastasis of lung cancer. Nicotine/α7nAChR can induce NSCLC cell migration and invasion via the MEK/ERK signaling pathway. NNK enhances lung cancer cell migration via activation of ERK or the Src-PKCiota-FAK signaling axis. Nicotine/α7nAChR can enhance the metastasis of lung cancer cells through activation of the YAP1-E2F1 signaling axis. Moreover, α7nAChR may facilitate lung cancer progression including angiogenesis via the PI3K/AKT or ERG1/FGF2 signaling pathways. α7nAChR-mediated signaling may be a potential target for attenuating the production of inflammatory cytokines in inflammatory cells. Nicotine/nAChR can promote lung cancer development via different signaling pathways. N: nicotine; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
The potential mechanisms of Nicotine on lung cancer progression
Nicotine may induce α7nAChR expression in human SCLC cells via the Sp1/GATA regulation signaling pathway 31. α7nAChR expression levels are elevated in SCC compared with adenocarcinoma of the lung, particularly in smokers 32, 54. High α7nAChR expression levels in lung cancer cells may be involved in the nicotine-induced tumorigenesis 32, 54. α7nAChR levels in patients with SCC who are active smokers are correlated with their smoking history 31. The function of α7nAChR-mediated lung cancer progression including in proliferation 31, 54-65, angiogenesis 66, and metastasis 39, 67-69, has been revealed (Fig. 2). The tumor-promoting effect is mediated by α7nAChR signaling pathways described in subsequent subsections.
Cell proliferation
α7nAChR mediates the proliferative effects of nicotine in lung cancer cells 70. Nicotine/α7nAChR signaling enhances NSCLC cell proliferation by scaffolding protein β-arrestin-mediated activation of the Src and Rb-RAF protooncogene serine/threonine-protein kinase (RAF-1) pathways 55. Moreover, continuous exposure to nicotine in SCC of the lungs results in α7nAChRs upregulation, which may enhance tumor growth 31. Nicotine stimulates tumor growth and ERK activation in a murine orthotopic model of lung cancer 54. A blockade of α7nAChRs suppresses nicotine-induced lung cancer cell growth and vimentin expression through the MEK/ERK signaling pathway 63. Consistent with these results, nicotine increased expression of nAChR and stimulated proliferation of SCC cell line 65. α7nAChR may mediate the proliferative activity of nicotine in poorly differentiated NSCLC 56. Nicotine-induced α7nAChR and α4nAChR expression in NSCLC cells, along with p-CREB and p-ERK1/2 activation accompanied by increased noradrenaline, leading to cell proliferation 57. Nicotine/α7-nAChR promoted proliferation in human SCLC cells via the Sp1/GATA regulation signaling pathway 31. NNK/α7nAChR may increase cell growth in SCLC cells via an influx of Ca2+ 60. Nicotine stimulates NSCLC cell proliferation and PPARβ/δ expression through activation of PI3K/mTOR signals that suppress AP-2α binding activity to PPARβ/δ promoter 61. α7nAChRs or α9nAChRs mediated nicotine-induced cell proliferation and activation of the AKT and ERK signaling pathways 64. The activated cell-membrane α7nAChRs formed complexes with EGFR, whereas activated mitochondrial α7nAChRs was physically associated with the intramitochondrial protein kinases PI3K and Src that increased expression of cyclin D1 and activation of ERK1/2 lead to lung cancer proliferation 71. α-Cobratoxin, a high-affinity α7-nAChR antagonist reduced tumor growth in nude mice orthotopically engrafted with NSCLC cells 59. An α7nAChR inhibitor (APS8) may suppress NSCLCs proliferative effects of nicotine 58. These studies revealed that nicotine/α7nAChR signals mediate proliferation in lung cancer.
Metastasis
Cigarette smoke status and history are associated with lung cancer metastasis 72, 73. α7nAChRs may mediate cancer cell growth depending on NSCLC differentiation status 56. α7nAChR and heteromeric nAChRs can promote tumor invasion in NSCLC 56. Nicotine/α7-nAChR can induce NSCLC cell migration and invasion via the MEK/ERK signaling pathway 39. NNK promotes lung cancer cell migration and contactin-1 expression via the α7nAChR-mediated ERK signaling pathway 67. Moreover, NNK enhances lung cancer cell migration and invasion via activation of the c-Src-PKCiota-FAK signaling axis 68. β-Cryptoxanthin may repress lung cancer cell motility through the downregulation of α7nAChR/PI3K signaling 69. Nicotine/α7nAChR signaling enhance migration and the expression of SOX2 in NSCLC cell lines through the YAP-E2F1 signaling axis 23. These studies suggest that α7nAChR can enhance lung cancer cell metastasis through the activation of different signaling pathways.
Angiogenesis
α7nAChR enhances angiogenesis via the PI3K/AKT pathway and NF-κB activation, which is partially dependent on vascular endothelial growth factor (VEGF) 74. Nicotine/α7nAChR signaling mediates proangiogenic effects through angiogenesis and EMT 75. MG624, an α7nAChR antagonist, reduces nicotine-induced early growth response gene 1 (Egr-1) binding activity to the fibroblast growth factor 2 (FGF2) promoter that inhibits angiogenic effects in SCLC 66. Thus, α7nAChR may facilitate lung cancer progression including angiogenesis; however, its detailed effect requires investigation.
Anti-Inflammation
α7nAChR attenuates ventilator-induced lung injury and plays an anti-inflammatory role in several inflammatory diseases 76-78. Activation of inflammation‐related receptors, such as toll-like receptors, enhances NF-κB signaling pathways in both acute and chronic inflammation that can considerably increase cancer risk 79. Choline/α7nAChR signaling modulates TNF release via the inhibition of NF‐κB activation 80. Moreover, nicotine/α7nAChR mediates anti-inflammatory action on macrophages via recruitment and activation of JAK2, initiating the STAT3 and SOCS3 signaling cascade 81. Nicotine/α7nAChR suppresses TNF-α expression in human airway epithelial cells by inhibiting MyD88 and NF-κB activity 82. An anti-inflammation study revealed that α7nAChR signaling inhibits NLRP3 inflammasome activation by preventing mitochondrial DNA release 83. Accumulating evidence suggests that the vagus nerve may modulate lung infection and inflammation through the α7nAChR signaling pathway 84. Thus, α7nAChR may be a potential target for attenuating inflammatory cytokine production in lung diseases 85.
Exposure to nicotine adversely affects dendritic cells, a cell type that has an important role in anticancer immunosurveillance 86. Nicotine may suppress anticancer immunity by increasing or reducing number of regulatory T cells and T helper 17 cells, respectively 87. Moreover, nicotine inhibits the cytotoxic activity of natural killer (NK) cells, and the effect of nicotine on NK cells can be abolished by β2nAChR deficiency 88. However, to understand immune regulation and progression in lung cancer, the roles of nicotine-mediated pathways in distinct immune cells warrant investigation.
The potential mechanisms of sirtuins on cancer progression
Sirtuins play diverse roles in controlling the cell cycle and proliferation in response to stress, thus promoting cell survival, apoptosis, or senescence 89. Sirtuin 1 (SIRT1) has been involved in decision making over cellular senescence or apoptosis in lung diseases including COPD and cancer 53, 90. Cellular senescence is caused by replicative and stress-related senescence with p53 and p16 activation, respectively, leading to p21 activation and cell-cycle arrest. SIRT1 can function as p53 deacetylase that blocks p53-dependent pathways, thereby regulating cell-cycle and inactivating apoptotic process 91. SIRT1 and p53 play vital roles in maintaining genomic stability and integrity, which favor cell survival and protect against tumorigenesis. Increased DNA damage and cellular senescence may contribute to accelerated lung aging and COPD pathogenesis 53. SIRT6 can significantly suppress TGF-β-induced senescence of human bronchial epithelial cells via p21 degradation 92. Moreover, SIRT6 may play a pivotal role in inhibiting fibrosis. SIRT1 and SIRT6 prevent telomere dysfunction during DNA replication 93. SIRT1 and SIRT6 loss may promote aging and arise from oxidative stress through PI3K-mTOR signaling activation 94.
Sirtuins are responsible for cellular metabolic reprogramming and drug resistance by inactivating cell death pathways and promoting uncontrolled proliferation 90. SIRT1 overexpression is associated with poor prognosis in patients with lung cancer 95. SIRT1-mediated survival of cancer cells contributes to chemoresistance in tumors. Furthermore, SIRT1 is considerably expressed in the brain metastastic tissues of patients with NSCLC 96. SIRT1 may promote breast cancer progression through modulating AKT activity 97. SIRT1 was significantly overexpressed in human prostate cancer cells, and SIRT1 inhibition contributes to suppress cancer cell growth 98. SIRT1 limits prostatic intraepithelial neoplasia in SIRT1-knockout mice. In A/J mice, β-cryptoxanthin, strongly associated with reduced lung cancer risk, restores nicotine-reduced lung SIRT1 levels to that normal and inhibits nicotine-promoted lung tumorigenesis and emphysema 99. In addition, SIRT1 levels decrease in some human cancers including glioma, bladder, and ovarian cancer 100. SIRT1 also acts as a tumor suppressor via the c-Myc-SIRT1 feedback loop that regulates cell growth and transformation 101. Thus, the tumor suppressor or promoter role of sirtuins in cancer progression may depend on their tissue- and cancer-specific expression and the examined conditions. Similarly, SIRT6 plays functions of both tumor promoter and suppressor in the development of different cancer, potentially depending on specific tissue 90.
The SIRT1 inhibitor sirtinol induces senescence-like growth arrest through impaired activation of RAS-mitogen-activated protein kinase (MAPK) signaling in human lung cancer cells 102. The combination of inauhzin (SIRT1 inhibitor) and cisplatin or doxorubicin can considerably reduce NSCLC cell growth in a p53-dependent manner 103. Two SIRT2 inhibitors AEM1 and AEM2 can induce p53-dependent proapoptotic activity in NSCLC cells 104. Moreover, AEM2 demonstrates similar inhibition of SIRT2 as AC-93253. SIRT1/2 inhibitor, salermide, can increase death receptor 5 expression via the ATF4-ATF3-CHOP axis and contribute to NSCLC cell apoptosis 105. Therefore, sirtuins play notable roles in both lung cancer development and the nicotine/nAChR-regulated signaling (Fig. 3). Although several sirtuins can function as both tumor promoters and suppressors, SIRT1/3/5-7 blockade may aid in effective chemotherapy, as described in the next section.
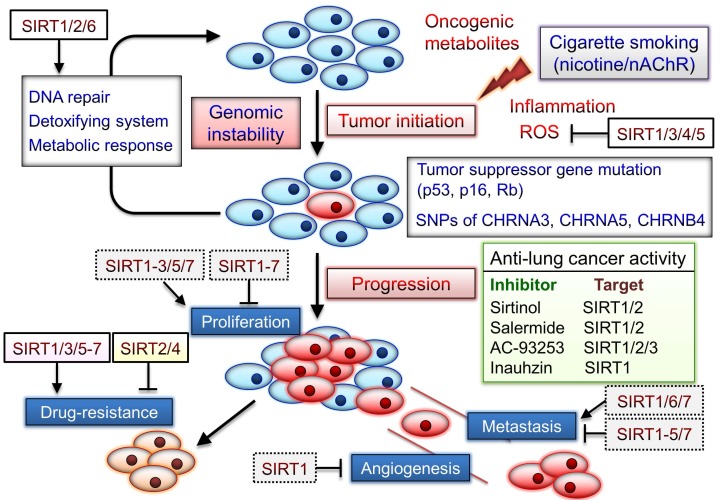
Figure 3.
Roles of sirtuins may be involved in nicotine/nAChR-mediated signaling pathway. Cigarette smoke form carcinogens, including polycyclic aromatic hydrocarbons and the nicotine-derived nitrosamines 4-(methylnitrosamino)-1-(3-pyrydyl)-1-butanone (NNK) and N-nitrosonornicotine (NNN). Tumor suppressor mutations caused by these carcinogens may initiate carcinogenesis. NNK and NNN also significantly contribute to tumor development via activation of nAChRs signaling. SNPs located in a region of chromosome region 15q25 that contains nAChR subunits (CHRNA5, CHRNA3, and CHRNB4) are significantly associated with lung cancer risk. Sirtuins can exert their capacity to respond to environmental changes, and their expression is often altered in cancer. However, the tumor suppressor or promoter role of sirtuins in cancer progression may depend on their tissue- and cancer-specific expression and examined conditions. Several sirtuin inhibitors can suppress lung cancer development and blockade of sirtuins may be a potential anticancer strategy. The dotted box indicated the roles of sirtuins in other cancer types.
The potential mechanisms of Nicotine and sirtuins on drug resistance
Nicotine may reduce the cytotoxic effects of chemotherapy and radiotherapy that cause poor therapeutic response 87, 106. Nicotine-mediated tumor-promoting effects are apparently mediated by nAChRs expressed on cell membranes and by mitochondria 87. Additionally, mitochondria are critical mediators of cancer progression, as this process requires flexibility to adapt to cellular and environmental alterations in addition to cancer therapies 107. Nicotine-impaired metabolism and mitochondrial defects were critical in metabolic responses to cancer progression 108. Activation of cell-membrane and mitochondrial nAChRs produces a combination of growth-promoting and antiapoptotic signals that implemented the tumor-promoting action of nicotine in lung cells 71. Furthermore, nAChRs were identified to control either CaKMII or Src-dependent signaling pathways in mitochondria that protect cells from apoptosis 109.
Nicotine also permeated cells and activated mitochondrion-nAChRs coupling to inhibit mitochondrial permeability transition pore (mPTP) opening, preventing apoptosis 62. Nicotine-induced survival may occur by a mechanism of multisite phosphorylation of BAD, which may lead to human lung cancer and/or chemoresistance development 110. Activation of nicotine-α7nAChR signaling can trigger membrane depolarization, which activates voltage-gated calcium channels and subsequently activates the MAPK pathway, possibly increasing B-cell lymphoma-2 (Bcl-2) expression and apoptosis downregulation 111. Nicotine prevents cisplatin-mediated apoptosis by regulating α5nAChR/AKT signaling and several mitochondria proteins including Bcl-2, Bax, survivin, and caspase 3 in gastric cancer cells 112. Long-term nicotine exposure-induced chemoresistance is mediated by STAT3 activation and ERK1/2 downregulation via nAChR and β-AR in bladder cancer cells 113. Emerging evidence suggests that feedback activation of STAT3 signaling is a common cause of drug resistance to receptor tyrosine kinase-targeted therapies and conventional chemotherapy 114. Long-term exposure to NNK combined with arecoline activated EGFR/AKT signaling is involved in antiapoptosis, cancer stem cell properties, and cisplatin resistance in head and neck SCC (HNSCC) cells 115. Nicotine/α9nAChR-PPM1F signaling can attenuate p-p53 (Ser-20)- and p-BAX (Ser-184)-induced proapoptotic pathways 116. Therefore, nAChRs may be a promising molecular target to arrest lung cancer progression and reopen mitochondrial apoptotic pathways. Nicotine can induce erlotinib resistance via the crosstalk between α1nAChR and EGFR/AKT/ERK signaling pathways in NSCLC 117. A recent study suggested that smoking containing nicotine causes resistance to erlotinib therapy in NSCLC 118.
Sirtuins can exert their capacity to respond to environmental changes and their expression is often altered in cancer 89. SIRT1, SIRT3, SIRT4, and SIRT7 are strongly expressed in lung adenocarcinoma, whereas SIRT5 is highly expressed in SCC 119. Analysis of the TCGA NSCLC dataset revealed that high expression levels of SIRT2/6 were associated with longer overall survival (OS) 119. However, high SIRT6 expression was associated with poor OS in 98 patients with NSCLC 120. Nicotine enhanced oxidative stress and activates NF-κB 121. Several sirtuins are critical in inhibiting excessive, damaging levels of ROS that drive cancer drug resistance 122. Nuclear SIRT1 promotes ROS stress resistance via the deacetylation of several transcriptional regulators, including p53, forkhead homeobox type O (FOXO) proteins, PGC-1α, heat shock factor protein 1 (HSF1), and nuclear erythroid factor 2-related factor 2 (NRF2), and this contributes to antioxidant production 123. Studies have suggested that mitochondrial-sirtuins (SIRT3, SIRT4, and SIRT5) are members of a family of NAD+-dependent deacetylases and are implicated in the oxidative stress response through the regulation of mitochondrial metabolism and antioxidant mechanisms 124-126. Mitochondrial SIRT3 may coordinate ROS; SIRT5 also limits ROS by activating SOD1 and NRF2 to maintain cellular redox homeostasis 122, 126, 127. Thus, sirtuins may promote cancer cell survival by limiting ROS that would lead to cancer drug resistance. Several recent studies have supported the presence of sirtuins-mediated drug resistance with human cancers (Table 1). Some sirtuins regulate lung cancer progression, as described in the next subsection, and signaling molecules are associated with drug resistance.
Table 1.
Effects of sirtuins on cancer drug resistance
| Types of sirtuin | Types of cancer | Drug | Effect of cancer drug resistance | Year of publication | Ref |
|---|---|---|---|---|---|
| SIRT1 | CRC | TRAIL | miR-128 suppressed SIRT1 expression to sensitize TRAIL-induced apoptosis | 2018 | 177 |
| SIRT1 | Breast cancer | Paclitaxel | A SIRT1-PRRX1-KLF4-ALDH1 circuitry regulates breast cancer stemness and metastasis. KLF4 inhibitor Kenpaullone sensitizes breast cancer cells | 2018 | 178 |
| SIRT1 | Cervical cancer | Paclitaxel | Knockdown of SIRT1 promotes apoptosis of paclitaxel-resistant human cervical cancer cells | 2018 | 179 |
| SIRT1 | ATC | Doxorubicin | 6-phosphogluconate dehydrogenase (6PGD) was critically involved in ATC resistance to doxorubicin. Decreased enzymatic activity of SIRT1 in response to 6PGD inhibition in doxorubicin-resistant ATC cells | 2018 | 180 |
| SIRT1 | Cervical cancer | Doxorubicin | β2-AR activation induces chemoresistance by modulating p53 acetylation through upregulating Sirt1 in cervical cancer cells | 2017 | 181 |
| SIRT1 | Gastric cancer | Cisplatin | miR-132 regulated SIRT1/CREB/ABCG2 signaling pathway contributes to cisplatin resistance | 2017 | 182 |
| SIRT1 | HCC | Oxaliplatin | LncRNA HULC triggered autophagy by stabilizing SIRT1 and attenuates chemosensitivity of HCC cells | 2017 | 183 |
| SIRT1/SIRT3 | Breast and Cervical cancer | Etoposide | Cancer cells with low SIRT1 levels maintained their resistance and survival by increasing SIRT3 expression | 2017 | 148 |
| SIRT1 | Prostate cancer | Docetaxel | The UCA1-miR-204-SIRT1 axis modulates docetaxel sensitivity of prostate cancer cells | 2016 | 184 |
| SIRT1 | Breast cancer | Tamoxifen | Brachyury mediates tamoxifen resistance by regulating SIRT1 | 2016 | 185 |
| SIRT1 | Bladder cancer | Capsaicin | Capsaicin inhibited multiple bladder cancer cells by inhibiting tumor-associated NADH oxidase (tNOX) and SIRT1 | 2016 | 186 |
| SIRT1 | ATL | Etoposide | SIRT1 inhibition enhances chemosensitivity and survival of ATL cells by reducing DNA double-strand repair | 2015 | 187 |
| SIRT1 | Endometrial carcinoma | Cisplatin and paclitaxel | SIRT1 overexpression significantly enhanced drug resistance. Selective SIRT1 inhibitor (EX527) significantly increased chemosensitivity | 2015 | 188 |
| SIRT1 | CML | Hsp90 inhibitors (17-AAG and AUY922) | SIRT1 inhibitors (amurensin G and EX527) effectively potentiated sensitivity of Hsp90 inhibitors | 2015 | 189 |
| SIRT1 | ESCC | Cisplatin | Overexpression of SIRT1 may cause resistance of ESCC cells to cisplatin through Noxa expression | 2015 | 190 |
| SIRT1 | PC | 5-fluorouracil (5-FU) and gemcitabine | Overexpression of miR-494 inhibited chemoresistance of PC by downregulating SIRT1 and c-Myc | 2015 | 191 |
| SIRT1 | CRC | 5-FU | SIRT1/PGC1α-dependent increase in oxidative phosphorylation leads to CRC drug resistance | 2015 | 192 |
| SIRT1 | CML | Imatinib | Divalproex sodium enhances antileukemic effects of imatinib in CML through SIRT1 | 2015 | 193 |
| SIRT1 | AML | TKI (Quizartinib, AC220) | Inhibition of SIRT1 by SIRT1 inhibitor Tenovin-6 (TV6) enhanced TKI-mediated sensitivity | 2014 | 194 |
| SIRT1 | Thyroid cancer | Etoposide | SIRT1-Foxp3 signaling confers drug resistance | 2014 | 195 |
| SIRT1 | Breast cancer | TRAIL | Metformin mediates miR-34a to suppress the SIRT1/PGC-1α/NRF2 pathway and increases drug sensitivity | 2014 | 196 |
| SIRT1 | CML | TKIs (imatinib, nilotinib or dasatinib) | All-trans-retinoic acid (ATRA) effectively blocked acquisition of BCR-ABL mutations and resistance. ATRA inhibited NAD+-dependent SIRT1 deacetylase via CD38 expression | 2014 | 197 |
| SIRT2 | RCC | 5-FU | SIRT2+ cells mediates RCC drug resistance | 2018 | 198 |
| SIRT2 | AML | Daunorubicin, arabinocytidine | SIRT2 mediates multidrug resistance in AML cells via ERK1/2 signaling pathway | 2016 | 199 |
| SIRT2 | Melanoma | Doxorubicin | AC-93253, a SIRT2 inhibitor increases drug sensitivity | 2015 | 200 |
| SIRT3 | Synovial sarcoma | Pazopanib | Knockdown of SIRT3 confers increased resistance to chemotherapeutic agents | 2018 | 201 |
| SIRT3 | HCC | Sorafenib | SIRT3 protein expression was significantly higher in patients treated with metformin | 2017 | 202 |
| SIRT3 | Glioma | Linalool | Overexpression of SIRT3 significantly inhibited a linalool-induced increase of mitochondrial ROS production and apoptotic cell death | 2017 | 203 |
| SIRT3 | Breast cancer | Cisplatin | SIRT3 silencing sensitizes breast cancer cells to cytotoxic treatments through ROS production | 2017 | 124 |
| SIRT4 | CRC | 5-FU | SIRT4 increased the sensitivity of CRC cells to 5-FU | 2016 | 204 |
| SIRT5 | NSCLC | CDDP, 5-FU or bleomycin | SIRT5 facilitates cancer cell growth and drug resistance in NSCLC cells | 2014 | 127 |
| SIRT6 | HCC | Doxorubicin | SIRT6 increased doxorubicin resistance via FOXO3 activity | 2018 | 205 |
| SIRT6 | NSCLC | Gefitinib | Astragaloside IV sensitizes NSCLC cells to gefitinib potentially via regulation of SIRT6 | 2017 | 206 |
| SIRT6 | PC | Gemcitabine | Quinazolinedione SIRT6 inhibitors sensitize cancer treatment | 2015 | 207 |
| SIRT6 | NSCLC | Paclitaxel | SIRT6 knockdown NSCLC cells improved drug sensitivity | 2015 | 120 |
| SIRT6 | Breast cancer | Trastuzumab (Herceptin) | MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes drug resistance | 2014 | 208 |
| SIRT7 | NSCLC | Gemcitabine | Depletion of SIRT7 promoted drug sensitivity | 2018 | 151 |
| SIRT7 | Breast cancer, osteosarcoma, and ovarian cancer | Cisplatin, Doxorubicin | SIRT7 inhibition significantly increases stress resistance and modulates insulin/IGF-1 signaling pathways | 2014 | 209 |
CRC, Colorectal cancer; ATC, Anaplastic thyroid carcinoma; HCC, Hepatocellular carcinoma; ATL, Adult T-cell leukemia-lymphoma; CML, Chronic myeloid leukemia; ESCC, Esophageal squamous cell carcinoma; PC, Pancreatic cancer; AML, Acute myeloid leukemia; RCC, Renal cell carcinoma; and NSCLC, Non-small-cell lung carcinoma.
SIRT1
Nicotine can upregulate SIRT1 expression in a time- and concentration-dependent manner 128. BaP, a carcinogen in cigarette smoke, can induce SIRT1 in human bronchial epithelial cells 129. SIRT1 is involved in BaP-induced transformation associated with TNF-α-β-catenin axis activation and is a potential therapeutic target for lung cancer 129. The SIRT1-PARP-1 axis plays a critical role in the regulation of cigarette smoke-induced autophagy and has notable implications for understanding the mechanisms of cigarette smoke-induced cell death and senescence 130. α7nAChR-SIRT1 axis activation alleviates angiotensin II-induced VSMC senescence 131. Recent studies have focused on the biological functions of SIRT1 in metabolic diseases, cancer, aging and cellular senescence, inflammatory signaling in response to environmental stress, and cell survival 132-135. SIRT1 expression was a strong predictor for poor OS and progression-free survival in patients with NSCLC who underwent platinum-based chemotherapy 136. Silencing of SIRT1 could significantly enhance the chemosensitivity of lung cancer cells to cisplatin treatment 136. SIRT1 was negatively associated with proapoptotic factors BAD, BAX, and BID in TCGA NSCLC patients 119. SIRT1 suppression sensitizes lung cancer cells to WEE1 inhibitor-induced DNA damage and apoptosis 137. SIRT1 deacetylates and inactivates p53, allowing cells to bypass apoptosis 138. The transcription factor FOXO 3 alpha (FOXO3a) may induce the expression of several antioxidant genes, including Mn superoxide dismutase (MnSOD), catalase, peroxiredoxins 3 and 5 (Prx3 and Prx5, respectively), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TR2), and uncoupling protein 2 (UCP-2) 139. SIRT1-mediated deacetylation of FOXO3a increases cell survival in response to oxidative stress 140. Moreover, SIRT1 may play a role in the acquisition of aggressiveness and chemoresistance in ovarian cancer and have potential as a therapeutic target for ovarian cancer 141.
SIRT2-SIRT7
SIRT3, located in mitochondria, is correlated with NSCLC malignancy 142. α7nAChRs activation inhibits platelet-derived growth factor‐induced cells migration by activating the mitochondrial deacetylase SIRT3, implying a critical role for α7nAChRs in mitochondrial biology and PDGF‐related diseases 143. The activity of SIRT3 can protect cancer cells from chemotherapy-induced oxidative stress 144, 145. Additionally, SIRT3 promotes the activation of AKT signaling pathways in NSCLC 142. SIRT3 promoted p53 degradation in PTEN-deficient NSCLC cell lines via the ubiquitin-proteasome pathway 146. SIRT3 can also mediated FOXO3a nuclear translocation that activates MnSOD and catalase expression 147. Notably, SIRT1 knockdown cells can increase SIRT3 expression and cell survival and have relatively high resistance to H2O2 or etoposide treatment 148. SIRT3 might be a therapeutic target for breast cancer, improving the effectiveness of cisplatin and tamoxifen treatments 124. SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via FOXO3 deacetylation 127. Besides, SIRT5 knockdown makes lung cancer cells more sensitive to drug (cisplatin, 5-fluorouracil or bleomycin) treatment 127. SIRT5 depletion suppresses the expression of NRF2 and its downstream drug-resistance genes 127.
Patients with high cytosol expression but low nuclear expression of SIRT6 can have poor clinical outcomes of lung cancer 120. Furthermore, SIRT6 knockdown in NSCLC cell lines can improve paclitaxel sensitivity by reducing NF-κB and Beclin1 (autophagy mediator) levels 120. Cigarette smoke increases SIRT6 expression in vivo (mice) and in vitro (rheumatoid arthritis synovial fibroblasts) 149. Reduced SIRT6 expression mediates the augmentation of radiation-induced apoptosis via cAMP signaling in lung cancer cells 150. A recent report revealed that SIRT7 depletion promotes gemcitabine-induced cell death 151. Functioning as an oncogene, SIRT7 can be suppressed by miR-3666, which could increase NSCLC cell apoptosis 152.
Thus, the aforementioned studies together have demonstrated tumor progression modulated by the SIRT1, SIRT3, and SIRT5-7, along with the tumor-suppressive effects of SIRT2 and SIRT4. SIRT2 mediates the ROS production and p27 levels, leading to lung cancer cell apoptosis and cell-cycle arrest 153. SIRT2 overexpression increases NSCLC cells' sensitivity to cisplatin treatment 153. Moreover, recent findings suggest that SIRT4 inhibits lung cancer progression through mitochondrial dynamics mediated by the ERK-Drp1 pathway 154. At present, one clinical trial (NCT02416739) is studying the combinatorial effects of the human sirtuin inhibitor (nicotinamide) and EGFR-TKI in NSCLC. The discovery of specific SIRT regulation and EGFR-TKI treatment would help elucidate the roles of sirtuins in lung cancer development. Although sirtuin clearly is critical in carcinogenesis, the crucial mechanisms by which the nicotine-mediated signaling or specific sirtuin pathways in different cell context lead to drug resistance require elucidation.
Cell-membrane nAChRs implement upregulation of proliferative and survival genes 62. Nicotine can promote oral precancerous growth through suppression of apoptosis by upregulating α7nAChR and peroxiredoxin 155. α7nAChR-mediated cell protection, through JAK2/PI3K/AKT/signal transducer and activator of transcription 3(STAT3)/NF-κB activation, leads to Bcl-2 production 156. Nicotine binds to nAChRs and stimulates secretion several factors including epidermal growth factor (EGF), VEGF, and neurotransmitters 157. Nicotine/nAChRs mediates EGF secretion and subsequent EGFR signaling activation, thus contributing to antiapoptosis 18. Nicotine and NNK also bind to β-ARs and promote survival signaling cascades 18, 30. Moreover, tissue-specific expression of α7β2, α3β2, α3β4, and α4β2 nAChRs located in the mitochondria outer membrane with anion channels that regulate the release of proapoptotic cytochrome c or ROS production has been observed 78, 158, 159. nAChR signaling in mitochondria is stimulated and engages PI3K/AKT kinases, similar to those activated by plasma membrane nAChRs. Nicotine contributes to progression and erlotinib resistance in an NSCLC xenograft model through the nAChR-EGFR cooperation 117. The nicotine-mediated α5nAChR/AKT signaling pathway prevents cisplatin-induced cancer cell apoptosis 112. Blockade of α7nAChRs inhibited nicotine-induced tumor growth and vimentin expression in NSCLC through the RAS-RAF-MAPK kinase (MEK)-extracellular signal-regulated kinase (ERK) signaling pathway 63. The nicotine and derivatives may mediate oncogenic signaling via nAChR, β-AR, and EGFR and combined with the effects of antiapoptosis in mitochondria that contribute to cancer progression (Fig. 4). The nicotine/nAChR signaling crosstalk with SIRT1/3/5-7 may contribute to cancer drug resistance.
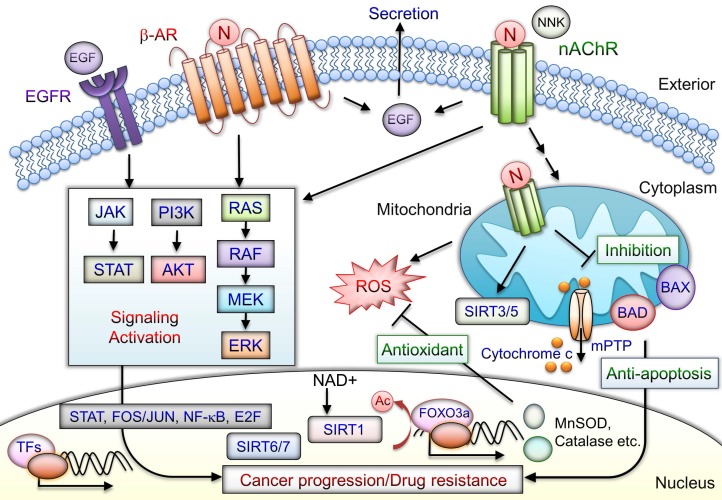
Figure 4.
Schematic of mediation of tumor-promoting actions by nicotine/nAChR. Nicotine interacts with nAChR and stimulates activation and crosstalk with β-AR and EGFR downstream, signaling to promote cancer progression. Activation of nAChRs and β-AR mediates EGF secretion to further transactivate EGFRs. In cancer cells, the signaling pathways downstream of nAChRs promote drug resistance and antiapoptosis by activating the transcription factors including STAT, NF-κB, Jun/Fos, and E2F through JAK, PI3K/AKT, RAS, RAF, and the MAPK signaling cascade. Mitochondrial nAChRs trigger phosphatidyl-inositol-3-kinase (PI3K) and AKT signaling pathways that prevent mPTP opening and cytochrome c release. Nicotine-induced antiapoptosis and drug resistance may include several mechanisms involved in overexpression of sirtuin proteins, phosphorylation of BAD, and blockade of BAX translocation, leading to tumor cell development. SIRT3 and SIRT5 are mitochondrial proteins. SIRT6 and SIRT7 are localized in the nucleus. SIRT1-mediated deacetylation of FOXO3a can induce expression of antioxidant enzymes including MnSOD and catalase that increase cell survival during cellular oxidative stress. Consequently, nicotine/nAChR mediates antiapoptotic pathways and concurrently crosstalks with β-AR or EGFR signaling activation may lead to cancer progression. N: nicotine; Ac: acetylation; NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
Conclusions and Future Directions
Genome-wide association studies have indicated a strong link between nicotine/nAChRs and lung cancer risk 34, 38. Nicotine might lead to suppressed apoptosis and cisplatin resistance via α5nAChR/AKT signaling 112. In addition, α7nAChR may be implicated in the NAD+/SIRT1 pathway, which promotes chemotherapeutic drug resistance 131. Nicotine/α9nAChR signaling can reduce apoptotic pathways 116. Nicotine-mediated cancer progression is mediated by nicotine/nAChRs signaling. By contrast to the tumor-suppressing role of SIRT2/4, the roles of SIRT1/6/7 and mitochondrial SIRT3/5 are strongly associated with drug resistance in lung cancer 119, 120, 124, 135, 142, 144, 146, 150-154. However, SIRT1/2 inhibitor, sirtinol, can increase anticancer potential and chemosensitivity in several cancer cells 102, 160, 161. SIRT1 may have contradictory roles in promoting or suppressing in different cell contexts or types of tumor development 162. Moreover, shikonin causes apoptosis in some lung cancer cell lines via the FOXO3a/EGR1/SIRT1 signaling pathway activation 163. In contrast to the tumor-suppressing role of SIRT1, the role of shikonin involves promoting liver cancer cell death by downregulating the SIRT1-MDR1/P-gp signaling pathway 164. However, emerging evidence suggests that SIRT1 can promote cancer drug resistance (Table 1). Thus, an investigation to unravel cancer cellular contexts in which ROS are beneficial or harmful, with sirtuins having tumor-suppressing or promoting functions, would contribute to the literature. Thus, a series of more comprehensive studies are necessary to validate the underlying molecular mechanisms of the nicotine-mediated pathway with specific sirtuin and related targets in different types of cancer.
Nicotine activates α7nAChR and β2nAChR, and these two factors are associated with EGF and VEGEF receptors, respectively. However, activated mitochondrial α7nAChRs and β4nAChR interacted with the PI3K and Src 62. This interaction leads to increased cell proliferation and ROS-mediated apoptosis resistance through ERK signaling and mPTP inhibition, respectively 62. The same group of researchers demonstrated a nicotine-mediated growth-promoting effect via interaction of α7nAChR with that EGF, α3nAChR with VEGF, α4nAChR with insulin-like growth factor I (IGF-I) and VEGF, whereas α9nAChR was with EGF, IGF-I, and VEGF. Similar to the binding affinity of cell-membrane nAChRs, nicotine can bind to mitochondrial nAChRs. Mitochondrial nAChRs coupled with the inhibition of mPTP opening causes tumor progression 71. These results indicate that nicotine-mediated activation of the cell-membrane or mitochondrial nAChRs leads to tumor-promoting and antiapoptotic signals in tumor development. Therefore, nAChRs may be a potential molecular target to suppress lung cancer progression and trigger mitochondrial apoptotic pathways.
An accumulating body of evidence is demonstrating that long noncoding RNAs (lncRNAs) have various biological functions, including modulation of growth, cell differentiation, drug resistance, and cancer progression 165-167. MALAT-1 is highly expressed and mediates poor progression in lung cancer 168. LncRNA-SCAL1 (smoke and cancer-associated lncRNA1) can alleviate CS-mediated oxidative stress in airway epithelial cells 169. Although lncRNAs were to be dysregulated in various human diseases, how lncRNAs connect with environmental exposures remains largely unknown. GAS5 may be proapoptotic and increase sensitivity to cell death due to environmental stressors 170, 171. HOTAIR mediates EMT because of cigarette smoke extracts 172, and similar studies have indicated that MALAT1 is involved in cigarette smoke extract-induced EMT and malignant transformation 172. Circulating extracellular vesicles (EVs) can transfer biomolecules, including ligands, cytokines, and genetic information, to recipient cells to enact functional changes 173. EVs can contain relatively stable RNA species, including small noncoding RNAs and lncRNAs 174. LncARSR is highly expressed in sunitinib-resistant renal cancer cells, and these drug-resistant cancer cells can transfer lncARSR to drug-sensitive recipient cells and acquire chemoresistance via exosomes 175. Exosomes mediated transfer of lncRNA UCA1 can considerably increase tamoxifen resistance in estrogen receptor (ER)-positive breast cancer cells 176. Thus, lncRNAs may cause cancer-acquired chemoresistance via exosomal pathway. However, whether nicotine promotes cancer drug resistance via the tumor environment communication remains unclear. Thus far, nicotine-mediated drug resistance induced by lncRNAs in lung cancer remains unclear. Therefore, nicotine-mediated drug resistance through lncRNA regulation in NSCLC warrants further exploration.
Over the past few years, nAChRs have been found to be selectively overexpressed in various cancers, including lung cancer. Targeting nAChRs signaling pathways can significantly attenuate nicotine-associated drug resistance. Several α7nAChR antagonists are potential anticancer drugs 38. Collectively, studies have suggested that nAChR-induced antiapoptotic effects may play notable roles in drug resistance in lung cancer. Elucidation of the consequences of nicotine/nAChRs signaling crosstalk with other pathways and specific sirtuins may be crucial to therapeutic implications. These studies may facilitate the design of useful therapeutic strategies to reverse chemoresistance in patients with different types of cancer.
Implication
Nicotine and derived metabolites are associated with lung cancer risk in smokers. The α7nAChR is significantly upregulated in NSCLC and correlates with its unfavorable prognosis. Activation of nicotine/α7nAChR signaling leads to lung cancer progression. Studies have suggested that nicotine/nAChR axis and SIRT1/3/5-7 mediates cancer drug resistance. Blockade of the signaling mediated by nicotine/nAChR and specific sirtuins may enhance the efficacy of chemotherapy.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of the Republic ofChina (MOST 107-2314-B-038-114 and 108-2314-B-038-063-MY3), Ministry of Education of the Republic of China (DP2-107-21121-T04), Taipei Medical University (TMU107-AE1-B04), Taipei Medical University and Shuang Ho Hospital (106TMU-SHH-18 and 107TMU-SHH-09).
Authors' Contributions
WLC, KYC, KYL, PHF and SMW contributed to writing the manuscript and revising it critically for important intellectual content. All authors read and approved the manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Abbreviations
- SCLC
small-cell lung carcinoma
- NSCLC
non-small-cell lung carcinoma
- EGFR
epithelial growth factor receptor
- EGFR-TKI
EGFR-tyrosine kinase inhibitor
- NF-κB
nuclear factor-κB
- PI3K
phosphoinositide 3-kinase
- BaP
benzo[a]pyrene
- PAH
polycyclic aromatic hydrocarbons
- nAChRs
nicotinic acetylcholine receptors
- β-AR
β-adrenergic receptors
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N-nitrosonornicotine
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- SNP
single nucleotide polymorphism
- RAF-1
proto-oncogene serine/threonine-protein kinase
- MEK
mitogen-activated protein kinase kinase
- ERK
extracellular signal-regulated kinases
- MAPK
mitogen-activated protein kinase
- EMT
epithelial-to-mesenchymal transition
- SOX2
sex-determining region Y-box 2
- VEGF
vascular endothelial growth factor
- Egr-1
growth response gene 1
- FGF2
fibroblast growth factor 2
- STAT3
signal transducer and activator of transcription 3
- NLRP3
nucleotide-binding domain and leucine-rich repeat containing protein 3
- ASC
adult stem cell
- ROS
reactive oxygen species
- NAC
N-acetyl-cysteine
- mPTP
mitochondrial permeability transition pore
- Bcl2
B-cell lymphoma-2
- HNSCC
head and neck squamous cell carcinoma
- SIRT1
Sirtuin 1
- TAM
tumor-associated macrophage
- FOXO3
Forkhead box 3
- NRF2
nuclear factor (erythroid-derived 2)-like 2
- OS
overall survival
- RFS
recurrence-free survival
- and NRF2
nuclear factor erythroid-2-related factor
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Cheng TY, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The International Epidemiology of Lung Cancer: Latest Trends, Disparities, and Tumor Characteristics. J Thorac Oncol. 2016;11:1653–71. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furrukh M. Tobacco Smoking and Lung Cancer: Perception-changing facts. Sultan Qaboos Univ Med J. 2013;13:345–58. doi: 10.12816/0003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosa Iglesias V, Giuranno L, Dubois LJ, Theys J, Vooijs M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front Oncol. 2018;8:267. doi: 10.3389/fonc.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bethune G, Bethune D, Ridgway N, Xu Z. Epidermal growth factor receptor (EGFR) in lung cancer: an overview and update. J Thorac Dis. 2010;2:48–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Condoluci A, Mazzara C, Zoccoli A, Pezzuto A, Tonini G. Impact of smoking on lung cancer treatment effectiveness: a review. Future Oncol. 2016;12:2149–61. doi: 10.2217/fon-2015-0055. [DOI] [PubMed] [Google Scholar]
- 8.Garces YI, Yang P, Parkinson J, Zhao X, Wampfler JA, Ebbert JO. et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–41. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 9.Nishioka T, Luo LY, Shen L, He H, Mariyannis A, Dai W. et al. Nicotine increases the resistance of lung cancer cells to cisplatin through enhancing Bcl-2 stability. Br J Cancer. 2014;110:1785–92. doi: 10.1038/bjc.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Q, Yu S, Zhao W, Qin S, Chu Q, Wu K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol Cancer. 2018;17:53. doi: 10.1186/s12943-018-0793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojewski AM, Tanner NT, Dai L, Ravenel JG, Gebregziabher M, Silvestri GA. et al. Tobacco Dependence Predicts Higher Lung Cancer and Mortality Rates and Lower Rates of Smoking Cessation in the National Lung Screening Trial. Chest. 2018;154:110–8. doi: 10.1016/j.chest.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaal C, Chellappan S. Nicotine-Mediated Regulation of Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Adenocarcinoma by E2F1 and STAT1 Transcription Factors. PLoS One. 2016;11:e0156451. doi: 10.1371/journal.pone.0156451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 14.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 15.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 16.Schuller HM. Beta-adrenergic signaling, a novel target for cancer therapy? Oncotarget. 2010;1:466–9. doi: 10.18632/oncotarget.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2007;20:629–41. doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011:456743. doi: 10.1155/2011/456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 20.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le CT, Zhang Y. et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–6. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis R, Rizwani W, Banerjee S, Kovacs M, Haura E, Coppola D. et al. Nicotine promotes tumor growth and metastasis in mouse models of lung cancer. PLoS One. 2009;4:e7524. doi: 10.1371/journal.pone.0007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaal CM, Bora-Singhal N, Kumar DM, Chellappan SP. Regulation of Sox2 and stemness by nicotine and electronic-cigarettes in non-small cell lung cancer. Mol Cancer. 2018;17:149. doi: 10.1186/s12943-018-0901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai H, May WS, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:1886–91. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- 25.Maneckjee R, Minna JD. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A. 1990;87:3294–8. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM. et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker RR, Dixon M. The retention of tobacco smoke constituents in the human respiratory tract. Inhal Toxicol. 2006;18:255–94. doi: 10.1080/08958370500444163. [DOI] [PubMed] [Google Scholar]
- 29.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem Pharmacol. 1998;55:1377–84. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 30.Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S. et al. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638–47. doi: 10.1158/0008-5472.CAN-06-4628. [DOI] [PubMed] [Google Scholar]
- 31.Brown KC, Perry HE, Lau JK, Jones DV, Pulliam JF, Thornhill BA. et al. Nicotine induces the up-regulation of the alpha7-nicotinic receptor (alpha7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J Biol Chem. 2013;288:33049–59. doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bordas A, Cedillo JL, Arnalich F, Esteban-Rodriguez I, Guerra-Pastrian L, de Castro J. et al. Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget. 2017;8:67878–90. doi: 10.18632/oncotarget.18948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Park JY, Drobes DJ. et al. Variation in the alpha 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J. 2014;14:70–6. doi: 10.1038/tpj.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LS, Baker T, Hung RJ, Horton A, Culverhouse R, Hartz S. et al. Genetic Risk Can Be Decreased: Quitting Smoking Decreases and Delays Lung Cancer for Smokers With High and Low CHRNA5 Risk Genotypes - A Meta-Analysis. EBioMedicine. 2016;11:219–26. doi: 10.1016/j.ebiom.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall FS. Genetic Risk for Lung Cancer and the Benefits of Quitting Smoking. EBioMedicine. 2016;11:19–20. doi: 10.1016/j.ebiom.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA. et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–46. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Hu Y. alpha7 nicotinic acetylcholine receptors in lung cancer. Oncol Lett. 2018;16:1375–82. doi: 10.3892/ol.2018.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Ding XP, Zhao QN, Yang XJ, An SM, Wang H. et al. Role of alpha7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget. 2016;7:59199–208. doi: 10.18632/oncotarget.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179–89. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 41.Ma G, Ji D, Qu X, Liu S, Yang X, Wang G. et al. Mining and validating the expression pattern and prognostic value of acetylcholine receptors in non-small cell lung cancer. Medicine (Baltimore) 2019;98:e15555. doi: 10.1097/MD.0000000000015555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang PM, Yeh YC, Chen TC, Wu YC, Lu PJ, Cheng HC. et al. High expression of CHRNA1 is associated with reduced survival in early stage lung adenocarcinoma after complete resection. Ann Surg Oncol. 2013;20:3648–54. doi: 10.1245/s10434-013-3034-2. [DOI] [PubMed] [Google Scholar]
- 43.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D. et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–7. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 44.Liu P, Vikis HG, Wang D, Lu Y, Wang Y, Schwartz AG. et al. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J Natl Cancer Inst. 2008;100:1326–30. doi: 10.1093/jnci/djn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu C, Hu Z, Yu D, Huang L, Jin G, Liang J. et al. Genetic variants on chromosome 15q25 associated with lung cancer risk in Chinese populations. Cancer Res. 2009;69:5065–72. doi: 10.1158/0008-5472.CAN-09-0081. [DOI] [PubMed] [Google Scholar]
- 46.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF. et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–56. doi: 10.1158/0008-5472.CAN-09-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA. et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandey N, Pal S, Sharma LK, Guleria R, Mohan A, Srivastava T. SNP rs16969968 as a Strong Predictor of Nicotine Dependence and Lung Cancer Risk in a North Indian Population. Asian Pac J Cancer Prev. 2017;18:3073–9. doi: 10.22034/APJCP.2017.18.11.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byun J, Schwartz AG, Lusk C, Wenzlaff AS, de Andrade M, Mandal D. et al. Genome-wide association study of familial lung cancer. Carcinogenesis. 2018;39:1135–40. doi: 10.1093/carcin/bgy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He P, Yang XX, He XQ, Chen J, Li FX, Gu X. et al. CHRNA3 polymorphism modifies lung adenocarcinoma risk in the Chinese Han population. Int J Mol Sci. 2014;15:5446–57. doi: 10.3390/ijms15045446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falvella FS, Galvan A, Frullanti E, Spinola M, Calabro E, Carbone A. et al. Transcription deregulation at the 15q25 locus in association with lung adenocarcinoma risk. Clin Cancer Res. 2009;15:1837–42. doi: 10.1158/1078-0432.CCR-08-2107. [DOI] [PubMed] [Google Scholar]
- 52.Pieper MP, Chaudhary NI, Park JE. Acetylcholine-induced proliferation of fibroblasts and myofibroblasts in vitro is inhibited by tiotropium bromide. Life Sci. 2007;80:2270–3. doi: 10.1016/j.lfs.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 53.Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011;81:265–84. doi: 10.1159/000324601. [DOI] [PubMed] [Google Scholar]
- 54.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D. et al. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell Prolif. 2008;41:936–59. doi: 10.1111/j.1365-2184.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E. et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–17. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medjber K, Freidja ML, Grelet S, Lorenzato M, Maouche K, Nawrocki-Raby B. et al. Role of nicotinic acetylcholine receptors in cell proliferation and tumour invasion in broncho-pulmonary carcinomas. Lung Cancer. 2015;87:258–64. doi: 10.1016/j.lungcan.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Al-Wadei HA, Al-Wadei MH, Schuller HM. Cooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLoS One. 2012;7:e29915. doi: 10.1371/journal.pone.0029915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zovko A, Viktorsson K, Lewensohn R, Kolosa K, Filipic M, Xing H. et al. APS8, a polymeric alkylpyridinium salt blocks alpha7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar Drugs. 2013;11:2574–94. doi: 10.3390/md11072574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grozio A, Paleari L, Catassi A, Servent D, Cilli M, Piccardi F. et al. Natural agents targeting the alpha7-nicotinic-receptor in NSCLC: a promising prospective in anti-cancer drug development. Int J Cancer. 2008;122:1911–5. doi: 10.1002/ijc.23298. [DOI] [PubMed] [Google Scholar]
- 60.Sheppard BJ, Williams M, Plummer HK, Schuller HM. Activation of voltage-operated Ca2+-channels in human small cell lung carcinoma by the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Int J Oncol. 2000;16:513–8. doi: 10.3892/ijo.16.3.513. [DOI] [PubMed] [Google Scholar]
- 61.Sun X, Ritzenthaler JD, Zhong X, Zheng Y, Roman J, Han S. Nicotine stimulates PPARbeta/delta expression in human lung carcinoma cells through activation of PI3K/mTOR and suppression of AP-2alpha. Cancer Res. 2009;69:6445–53. doi: 10.1158/0008-5472.CAN-09-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chernyavsky AI, Shchepotin IB, Grando SA. Mechanisms of growth-promoting and tumor-protecting effects of epithelial nicotinic acetylcholine receptors. Int Immunopharmacol. 2015;29:36–44. doi: 10.1016/j.intimp.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C, Yu P, Zhu L, Zhao Q, Lu X, Bo S. Blockade of alpha7 nicotinic acetylcholine receptors inhibit nicotine-induced tumor growth and vimentin expression in non-small cell lung cancer through MEK/ERK signaling way. Oncol Rep. 2017;38:3309–18. doi: 10.3892/or.2017.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mucchietto V, Fasoli F, Pucci S, Moretti M, Benfante R, Maroli A. et al. alpha9- and alpha7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br J Pharmacol. 2018;175:1957–72. doi: 10.1111/bph.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J. et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown KC, Lau JK, Dom AM, Witte TR, Luo H, Crabtree CM. et al. MG624, an alpha7-nAChR antagonist, inhibits angiogenesis via the Egr-1/FGF2 pathway. Angiogenesis. 2012;15:99–114. doi: 10.1007/s10456-011-9246-9. [DOI] [PubMed] [Google Scholar]
- 67.Hung YH, Hung WC. 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) enhances invasiveness of lung cancer cells by up-regulating contactin-1 via the alpha7 nicotinic acetylcholine receptor/ERK signaling pathway. Chem Biol Interact. 2009;179:154–9. doi: 10.1016/j.cbi.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 68.Shen J, Xu L, Owonikoko TK, Sun SY, Khuri FR, Curran WJ. et al. NNK promotes migration and invasion of lung cancer cells through activation of c-Src/PKCiota/FAK loop. Cancer Lett. 2012;318:106–13. doi: 10.1016/j.canlet.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iskandar AR, Miao B, Li X, Hu KQ, Liu C, Wang XD. beta-Cryptoxanthin Reduced Lung Tumor Multiplicity and Inhibited Lung Cancer Cell Motility by Downregulating Nicotinic Acetylcholine Receptor alpha7 Signaling. Cancer Prev Res (Phila) 2016;9:875–86. doi: 10.1158/1940-6207.CAPR-16-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–8. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Chernyavsky AI, Shchepotin IB, Galitovkiy V, Grando SA. Mechanisms of tumor-promoting activities of nicotine in lung cancer: synergistic effects of cell membrane and mitochondrial nicotinic acetylcholine receptors. BMC Cancer. 2015;15:152. doi: 10.1186/s12885-015-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15:e568–80. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shenker RF, McTyre ER, Ruiz J, Weaver KE, Cramer C, Alphonse-Sullivan NK. et al. The Effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer Med. 2017;6:944–52. doi: 10.1002/cam4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pillai S, Chellappan S. alpha7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Curr Drug Targets. 2012;13:671–9. doi: 10.2174/138945012800398847. [DOI] [PubMed] [Google Scholar]
- 76.Bregeon F, Xeridat F, Andreotti N, Lepidi H, Delpierre S, Roch A. et al. Activation of nicotinic cholinergic receptors prevents ventilator-induced lung injury in rats. PLoS One. 2011;6:e22386. doi: 10.1371/journal.pone.0022386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galle-Treger L, Suzuki Y, Patel N, Sankaranarayanan I, Aron JL, Maazi H. et al. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat Commun. 2016;7:13202. doi: 10.1038/ncomms13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hajiasgharzadeh K, Sadigh-Eteghad S, Mansoori B, Mokhtarzadeh A, Shanehbandi D, Doustvandi MA. et al. Alpha7 nicotinic acetylcholine receptors in lung inflammation and carcinogenesis: Friends or foes? J Cell Physiol. 2019;234:14666–79. doi: 10.1002/jcp.28220. [DOI] [PubMed] [Google Scholar]
- 79.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 80.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH. et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ. et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 82.Li Q, Zhou XD, Kolosov VP, Perelman JM. Nicotine reduces TNF-alpha expression through a alpha7 nAChR/MyD88/NF-kB pathway in HBE16 airway epithelial cells. Cell Physiol Biochem. 2011;27:605–12. doi: 10.1159/000329982. [DOI] [PubMed] [Google Scholar]
- 83.Lu B, Kwan K, Levine YA, Olofsson PS, Yang H, Li J. et al. alpha7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014;20:350–8. doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu H, Li L, Su X. Vagus nerve through alpha7 nAChR modulates lung infection and inflammation: models, cells, and signals. Biomed Res Int. 2014;2014:283525. doi: 10.1155/2014/283525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamada M, Ichinose M. The cholinergic anti-inflammatory pathway: an innovative treatment strategy for respiratory diseases and their comorbidities. Curr Opin Pharmacol. 2018;40:18–25. doi: 10.1016/j.coph.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Sanner T, Grimsrud TK. Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment - A Review. Front Oncol. 2015;5:196. doi: 10.3389/fonc.2015.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14:419–29. doi: 10.1038/nrc3725. [DOI] [PubMed] [Google Scholar]
- 88.Hao J, Shi FD, Abdelwahab M, Shi SX, Simard A, Whiteaker P. et al. Nicotinic receptor beta2 determines NK cell-dependent metastasis in a murine model of metastatic lung cancer. PLoS One. 2013;8:e57495. doi: 10.1371/journal.pone.0057495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodriguez RM, Fraga MF. Aging and cancer: are sirtuins the link? Future Oncol. 2010;6:905–15. doi: 10.2217/fon.10.57. [DOI] [PubMed] [Google Scholar]
- 90.Carafa V, Altucci L, Nebbioso A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front Pharmacol. 2019;10:38. doi: 10.3389/fphar.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK. et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 92.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y. et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18:447–76. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barnes PJ, Baker J, Donnelly LE. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am J Respir Crit Care Med. 2019;200:556–64. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 95.Wang C, Yang W, Dong F, Guo Y, Tan J, Ruan S. et al. The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis. Oncotarget. 2017;8:66343–51. doi: 10.18632/oncotarget.18494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han L, Liang XH, Chen LX, Bao SM, Yan ZQ. SIRT1 is highly expressed in brain metastasis tissues of non-small cell lung cancer (NSCLC) and in positive regulation of NSCLC cell migration. Int J Clin Exp Pathol. 2013;6:2357–65. [PMC free article] [PubMed] [Google Scholar]
- 97.Jin X, Wei Y, Xu F, Zhao M, Dai K, Shen R. et al. SIRT1 promotes formation of breast cancer through modulating Akt activity. J Cancer. 2018;9:2012–23. doi: 10.7150/jca.24275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung-Hynes B, Nihal M, Zhong W, Ahmad N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284:3823–32. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iskandar AR, Liu C, Smith DE, Hu KQ, Choi SW, Ausman LM. et al. beta-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila) 2013;6:309–20. doi: 10.1158/1940-6207.CAPR-12-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14:312–23. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan J, Minter-Dykhouse K, Lou Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;185:203–11. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ota H, Tokunaga E, Chang K, Hikasa M, Iijima K, Eto M. et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–85. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Zhang Q, Zeng SX, Hao Q, Lu H. Inauhzin sensitizes p53-dependent cytotoxicity and tumor suppression of chemotherapeutic agents. Neoplasia. 2013;15:523–34. doi: 10.1593/neo.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffmann G, Breitenbucher F, Schuler M, Ehrenhofer-Murray AE. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. J Biol Chem. 2014;289:5208–16. doi: 10.1074/jbc.M113.487736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu G, Su L, Hao X, Zhong N, Zhong D, Singhal S. et al. Salermide up-regulates death receptor 5 expression through the ATF4-ATF3-CHOP axis and leads to apoptosis in human cancer cells. J Cell Mol Med. 2012;16:1618–28. doi: 10.1111/j.1582-4934.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warren GW, Romano MA, Kudrimoti MR, Randall ME, McGarry RC, Singh AK. et al. Nicotinic modulation of therapeutic response in vitro and in vivo. Int J Cancer. 2012;131:2519–27. doi: 10.1002/ijc.27556. [DOI] [PubMed] [Google Scholar]
- 107.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166:555–66. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–46. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uspenska K, Lykhmus O, Obolenskaya M, Pons S, Maskos U, Komisarenko S. et al. Mitochondrial Nicotinic Acetylcholine Receptors Support Liver Cells Viability After Partial Hepatectomy. Front Pharmacol. 2018;9:626. doi: 10.3389/fphar.2018.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–44. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 111.Heusch WL, Maneckjee R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–6. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 112.Jia Y, Sun H, Wu H, Zhang H, Zhang X, Xiao D. et al. Nicotine Inhibits Cisplatin-Induced Apoptosis via Regulating alpha5-nAChR/AKT Signaling in Human Gastric Cancer Cells. PLoS One. 2016;11:e0149120. doi: 10.1371/journal.pone.0149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen RJ, Ho YS, Guo HR, Wang YJ. Long-term nicotine exposure-induced chemoresistance is mediated by activation of Stat3 and downregulation of ERK1/2 via nAChR and beta-adrenoceptors in human bladder cancer cells. Toxicol Sci. 2010;115:118–30. doi: 10.1093/toxsci/kfq028. [DOI] [PubMed] [Google Scholar]
- 114.Zhao C, Li H, Lin HJ, Yang S, Lin J, Liang G. Feedback Activation of STAT3 as a Cancer Drug-Resistance Mechanism. Trends Pharmacol Sci. 2016;37:47–61. doi: 10.1016/j.tips.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 115.Yang SH, Lee TY, Ho CA, Yang CY, Huang WY, Lin YC. et al. Exposure to nicotine-derived nitrosamine ketone and arecoline synergistically facilitates tumor aggressiveness via overexpression of epidermal growth factor receptor and its downstream signaling in head and neck squamous cell carcinoma. PLoS One. 2018;13:e0201267. doi: 10.1371/journal.pone.0201267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tu SH, Lin YC, Huang CC, Yang PS, Chang HW, Chang CH. et al. Protein phosphatase Mg2+/Mn2+ dependent 1F promotes smoking-induced breast cancer by inactivating phosphorylated-p53-induced signals. Oncotarget. 2016;7:77516–31. doi: 10.18632/oncotarget.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Wang S, Takayama K, Harada T, Okamoto I, Iwama E. et al. Nicotine induces resistance to erlotinib via cross-talk between alpha 1 nAChR and EGFR in the non-small cell lung cancer xenograft model. Lung Cancer. 2015;88:1–8. doi: 10.1016/j.lungcan.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 118.Imabayashi T, Uchino J, Osoreda H, Tanimura K, Chihara Y, Tamiya N. et al. Nicotine Induces Resistance to Erlotinib Therapy in Non-Small-Cell Lung Cancer Cells Treated with Serum from Human Patients. Cancers (Basel) 2019;11:282. doi: 10.3390/cancers11030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong J, Wang H, Lou W, Wang G, Tao H, Wen H. et al. Associations of sirtuins with clinicopathological parameters and prognosis in non-small cell lung cancer. Cancer Manag Res. 2018;10:3341–56. doi: 10.2147/CMAR.S166946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azuma Y, Yokobori T, Mogi A, Altan B, Yajima T, Kosaka T. et al. SIRT6 expression is associated with poor prognosis and chemosensitivity in patients with non-small cell lung cancer. J Surg Oncol. 2015;112:231–7. doi: 10.1002/jso.23975. [DOI] [PubMed] [Google Scholar]
- 121.Barr J, Sharma CS, Sarkar S, Wise K, Dong L, Periyakaruppan A. et al. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol Cell Biochem. 2007;297:93–9. doi: 10.1007/s11010-006-9333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.German NJ, Haigis MC. Sirtuins and the Metabolic Hurdles in Cancer. Curr Biol. 2015;25:R569–83. doi: 10.1016/j.cub.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kulkarni SR, Donepudi AC, Xu J, Wei W, Cheng QC, Driscoll MV. et al. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxid Redox Signal. 2014;20:15–30. doi: 10.1089/ars.2012.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Torrens-Mas M, Pons DG, Sastre-Serra J, Oliver J, Roca P. SIRT3 Silencing Sensitizes Breast Cancer Cells to Cytotoxic Treatments Through an Increment in ROS Production. J Cell Biochem. 2017;118:397–406. doi: 10.1002/jcb.25653. [DOI] [PubMed] [Google Scholar]
- 125.Jeong SM, Hwang S, Seong RH. SIRT4 regulates cancer cell survival and growth after stress. Biochem Biophys Res Commun. 2016;470:251–6. doi: 10.1016/j.bbrc.2016.01.078. [DOI] [PubMed] [Google Scholar]
- 126.Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB. et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. 2013;441:191–5. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 127.Lu W, Zuo Y, Feng Y, Zhang M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol. 2014;35:10699–705. doi: 10.1007/s13277-014-2372-4. [DOI] [PubMed] [Google Scholar]
- 128.Park GJ, Kim YS, Kang KL, Bae SJ, Baek HS, Auh QS. et al. Effects of sirtuin 1 activation on nicotine and lipopolysaccharide-induced cytotoxicity and inflammatory cytokine production in human gingival fibroblasts. J Periodontal Res. 2013;48:483–92. doi: 10.1111/jre.12030. [DOI] [PubMed] [Google Scholar]
- 129.Lu J, Zhang M, Huang Z, Sun S, Zhang Y, Zhang L. et al. SIRT1 in B[a]P-induced lung tumorigenesis. Oncotarget. 2015;6:27113–29. doi: 10.18632/oncotarget.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW. et al. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–9. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li DJ, Huang F, Ni M, Fu H, Zhang LS, Shen FM. alpha7 Nicotinic Acetylcholine Receptor Relieves Angiotensin II-Induced Senescence in Vascular Smooth Muscle Cells by Raising Nicotinamide Adenine Dinucleotide-Dependent SIRT1 Activity. Arterioscler Thromb Vasc Biol. 2016;36:1566–76. doi: 10.1161/ATVBAHA.116.307157. [DOI] [PubMed] [Google Scholar]
- 132.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haigis MC, Guarente LP. Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–21. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 134.Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21:1745–55. doi: 10.1210/me.2007-0079. [DOI] [PubMed] [Google Scholar]
- 135.Yang X, Yang Y, Gan R, Zhao L, Li W, Zhou H. et al. Down-regulation of mir-221 and mir-222 restrain prostate cancer cell proliferation and migration that is partly mediated by activation of SIRT1. PLoS One. 2014;9:e98833. doi: 10.1371/journal.pone.0098833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang T, Rong N, Chen J, Zou C, Jing H, Zhu X. et al. SIRT1 expression is associated with the chemotherapy response and prognosis of patients with advanced NSCLC. PLoS One. 2013;8:e79162. doi: 10.1371/journal.pone.0079162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen G, Zhang B, Xu H, Sun Y, Shi Y, Luo Y. et al. Suppression of Sirt1 sensitizes lung cancer cells to WEE1 inhibitor MK-1775-induced DNA damage and apoptosis. Oncogene. 2017;36:6863–72. doi: 10.1038/onc.2017.297. [DOI] [PubMed] [Google Scholar]
- 138.Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804:1684–9. doi: 10.1016/j.bbapap.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olmos Y, Sanchez-Gomez FJ, Wild B, Garcia-Quintans N, Cabezudo S, Lamas S. et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 2013;19:1507–21. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Voelter-Mahlknecht S, Mahlknecht U. The sirtuins in the pathogenesis of cancer. Clin Epigenetics. 2010;1:71–83. doi: 10.1007/s13148-010-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mvunta DH, Miyamoto T, Asaka R, Yamada Y, Ando H, Higuchi S. et al. SIRT1 Regulates the Chemoresistance and Invasiveness of Ovarian Carcinoma Cells. Transl Oncol. 2017;10:621–31. doi: 10.1016/j.tranon.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiong Y, Wang M, Zhao J, Wang L, Li X, Zhang Z. et al. SIRT3 is correlated with the malignancy of non-small cell lung cancer. Int J Oncol. 2017;50:903–10. doi: 10.3892/ijo.2017.3868. [DOI] [PubMed] [Google Scholar]
- 143.Li DJ, Tong J, Zeng FY, Guo M, Li YH, Wang H, Nicotinic ACh receptor alpha7 inhibits PDGF-induced migration of vascular smooth muscle cells by activating mitochondrial deacetylase sirtuin 3. Br J Pharmacol; 2018. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ansari A, Rahman MS, Saha SK, Saikot FK, Deep A, Kim KH. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell. 2017;16:4–16. doi: 10.1111/acel.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L, Ren X, Cheng Y, Huber-Keener K, Liu X, Zhang Y. et al. Identification of Sirtuin 3, a mitochondrial protein deacetylase, as a new contributor to tamoxifen resistance in breast cancer cells. Biochem Pharmacol. 2013;86:726–33. doi: 10.1016/j.bcp.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 146.Xiong Y, Wang L, Wang S, Wang M, Zhao J, Zhang Z. et al. SIRT3 deacetylates and promotes degradation of P53 in PTEN-defective non-small cell lung cancer. J Cancer Res Clin Oncol. 2018;144:189–98. doi: 10.1007/s00432-017-2537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 148.Carnevale I, Pellegrini L, D'Aquila P, Saladini S, Lococo E, Polletta L. et al. SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J Cell Physiol. 2017;232:1835–44. doi: 10.1002/jcp.25711. [DOI] [PubMed] [Google Scholar]
- 149.Engler A, Niederer F, Klein K, Gay RE, Kyburz D, Camici GG. et al. SIRT6 regulates the cigarette smoke-induced signalling in rheumatoid arthritis synovial fibroblasts. J Mol Med (Berl) 2014;92:757–67. doi: 10.1007/s00109-014-1139-0. [DOI] [PubMed] [Google Scholar]
- 150.Kim EJ, Juhnn YS. Cyclic AMP signaling reduces sirtuin 6 expression in non-small cell lung cancer cells by promoting ubiquitin-proteasomal degradation via inhibition of the Raf-MEK-ERK (Raf/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase) pathway. J Biol Chem. 2015;290:9604–13. doi: 10.1074/jbc.M114.633198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang Y, Han Z, Wang Y, Hao W. Depletion of SIRT7 sensitizes human non-small cell lung cancer cells to gemcitabine therapy by inhibiting autophagy. Biochem Biophys Res Commun. 2018;506:266–71. doi: 10.1016/j.bbrc.2018.10.089. [DOI] [PubMed] [Google Scholar]
- 152.Shi H, Ji Y, Zhang D, Liu Y, Fang P. MicroRNA-3666-induced suppression of SIRT7 inhibits the growth of non-small cell lung cancer cells. Oncol Rep. 2016;36:3051–7. doi: 10.3892/or.2016.5063. [DOI] [PubMed] [Google Scholar]
- 153.Li Z, Xie QR, Chen Z, Lu S, Xia W. Regulation of SIRT2 levels for human non-small cell lung cancer therapy. Lung Cancer. 2013;82:9–15. doi: 10.1016/j.lungcan.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 154.Fu L, Dong Q, He J, Wang X, Xing J, Wang E. et al. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36:2724–36. doi: 10.1038/onc.2016.425. [DOI] [PubMed] [Google Scholar]
- 155.Wang C, Niu W, Chen H, Shi N, He D, Zhang M. et al. Nicotine suppresses apoptosis by regulating alpha7nAChR/Prx1 axis in oral precancerous lesions. Oncotarget. 2017;8:75065–75. doi: 10.18632/oncotarget.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marrero MB, Bencherif M. Convergence of alpha 7 nicotinic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT3 and NF-kappaB. Brain Res. 2009;1256:1–7. doi: 10.1016/j.brainres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 157.Pillai S, Rizwani W, Li X, Rawal B, Nair S, Schell MJ. et al. ID1 facilitates the growth and metastasis of non-small cell lung cancer in response to nicotinic acetylcholine receptor and epidermal growth factor receptor signaling. Mol Cell Biol. 2011;31:3052–67. doi: 10.1128/MCB.01311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lykhmus O, Gergalova G, Koval L, Zhmak M, Komisarenko S, Skok M. Mitochondria express several nicotinic acetylcholine receptor subtypes to control various pathways of apoptosis induction. Int J Biochem Cell Biol. 2014;53:246–52. doi: 10.1016/j.biocel.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 159.Chernyavsky A, Chen Y, Wang PH, Grando SA. Pemphigus vulgaris antibodies target the mitochondrial nicotinic acetylcholine receptors that protect keratinocytes from apoptolysis. Int Immunopharmacol. 2015;29:76–80. doi: 10.1016/j.intimp.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 160.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC. et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–55. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 161.Jin KL, Park JY, Noh EJ, Hoe KL, Lee JH, Kim JH. et al. The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J Gynecol Oncol. 2010;21:262–8. doi: 10.3802/jgo.2010.21.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Islam S, Abiko Y, Uehara O, Chiba I. Sirtuin 1 and oral cancer. Oncol Lett. 2019;17:729–38. doi: 10.3892/ol.2018.9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jeung YJ, Kim HG, Ahn J, Lee HJ, Lee SB, Won M. et al. Shikonin induces apoptosis of lung cancer cells via activation of FOXO3a/EGR1/SIRT1 signaling antagonized by p300. Biochim Biophys Acta. 2016;1863:2584–93. doi: 10.1016/j.bbamcr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 164.Jin YD, Ren Y, Wu MW, Chen P, Lu J. Effect of shikonin on multidrug resistance in HepG2: The role of SIRT1. Pharm Biol. 2015;53:1016–21. doi: 10.3109/13880209.2014.952836. [DOI] [PubMed] [Google Scholar]
- 165.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ. et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 167.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G. et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM. et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 169.Thai P, Statt S, Chen CH, Liang E, Campbell C, Wu R. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am J Respir Cell Mol Biol. 2013;49:204–11. doi: 10.1165/rcmb.2013-0159RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tani H, Torimura M, Akimitsu N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One. 2013;8:e55684. doi: 10.1371/journal.pone.0055684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tani H, Torimura M. Development of cytotoxicity-sensitive human cells using overexpression of long non-coding RNAs. J Biosci Bioeng. 2015;119:604–8. doi: 10.1016/j.jbiosc.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 172.Liu Y, Luo F, Xu Y, Wang B, Zhao Y, Xu W. et al. Epithelial-mesenchymal transition and cancer stem cells, mediated by a long non-coding RNA, HOTAIR, are involved in cell malignant transformation induced by cigarette smoke extract. Toxicol Appl Pharmacol. 2015;282:9–19. doi: 10.1016/j.taap.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 173.Lane RE, Korbie D, Hill MM, Trau M. Extracellular vesicles as circulating cancer biomarkers: opportunities and challenges. Clin Transl Med. 2018;7:14. doi: 10.1186/s40169-018-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y. et al. Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep. 2016;6:19413. doi: 10.1038/srep19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y. et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–68. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 176.Xu CG, Yang MF, Ren YQ, Wu CH, Wang LQ. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20:4362–8. [PubMed] [Google Scholar]
- 177.Lian B, Yang D, Liu Y, Shi G, Li J, Yan X. et al. miR-128 Targets the SIRT1/ROS/DR5 Pathway to Sensitize Colorectal Cancer to TRAIL-Induced Apoptosis. Cell Physiol Biochem. 2018;49:2151–62. doi: 10.1159/000493818. [DOI] [PubMed] [Google Scholar]
- 178.Shi L, Tang X, Qian M, Liu Z, Meng F, Fu L. et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene. 2018;37:6299–315. doi: 10.1038/s41388-018-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xia X, Zhou X. Knockdown of SIRT1 inhibits proliferation and promotes apoptosis of paclitaxel-resistant human cervical cancer cells. Cell Mol Biol (Noisy-le-grand) 2018;64:36–41. [PubMed] [Google Scholar]
- 180.Ma L, Cheng Q. Inhibiting 6-phosphogluconate dehydrogenase reverses doxorubicin resistance in anaplastic thyroid cancer via inhibiting NADPH-dependent metabolic reprogramming. Biochem Biophys Res Commun. 2018;498:912–7. doi: 10.1016/j.bbrc.2018.03.079. [DOI] [PubMed] [Google Scholar]
- 181.Chen H, Zhang W, Cheng X, Guo L, Xie S, Ma Y. et al. beta2-AR activation induces chemoresistance by modulating p53 acetylation through upregulating Sirt1 in cervical cancer cells. Cancer Sci. 2017;108:1310–7. doi: 10.1111/cas.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S. et al. Upregulated miR-132 in Lgr5(+) gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022–34. doi: 10.1002/mc.22656. [DOI] [PubMed] [Google Scholar]
- 183.Xiong H, Ni Z, He J, Jiang S, Li X, He J. et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36:3528–40. doi: 10.1038/onc.2016.521. [DOI] [PubMed] [Google Scholar]
- 184.Wang X, Yang B, Ma B. The UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate cancer cells. Cancer Chemother Pharmacol. 2016;78:1025–31. doi: 10.1007/s00280-016-3158-8. [DOI] [PubMed] [Google Scholar]
- 185.Li K, Ying M, Feng D, Du J, Chen S, Dan B. et al. Brachyury promotes tamoxifen resistance in breast cancer by targeting SIRT1. Biomed Pharmacother. 2016;84:28–33. doi: 10.1016/j.biopha.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 186.Lin MH, Lee YH, Cheng HL, Chen HY, Jhuang FH, Chueh PJ. Capsaicin Inhibits Multiple Bladder Cancer Cell Phenotypes by Inhibiting Tumor-Associated NADH Oxidase (tNOX) and Sirtuin1 (SIRT1) Molecules. 2016;21:849. doi: 10.3390/molecules21070849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li L, Ye S, Yang M, Yu W, Fan Z, Zhang H. et al. SIRT1 downregulation enhances chemosensitivity and survival of adult T-cell leukemia-lymphoma cells by reducing DNA double-strand repair. Oncol Rep. 2015;34:2935–42. doi: 10.3892/or.2015.4287. [DOI] [PubMed] [Google Scholar]
- 188.Asaka R, Miyamoto T, Yamada Y, Ando H, Mvunta DH, Kobara H. et al. Sirtuin 1 promotes the growth and cisplatin resistance of endometrial carcinoma cells: a novel therapeutic target. Lab Invest. 2015;95:1363–73. doi: 10.1038/labinvest.2015.119. [DOI] [PubMed] [Google Scholar]
- 189.Kim HB, Lee SH, Um JH, Kim MJ, Hyun SK, Gong EJ. et al. Sensitization of chemo-resistant human chronic myeloid leukemia stem-like cells to Hsp90 inhibitor by SIRT1 inhibition. Int J Biol Sci. 2015;11:923–34. doi: 10.7150/ijbs.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cao B, Shi Q, Wang W. Higher expression of SIRT1 induced resistance of esophageal squamous cell carcinoma cells to cisplatin. J Thorac Dis. 2015;7:711–9. doi: 10.3978/j.issn.2072-1439.2015.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu Y, Li X, Zhu S, Zhang JG, Yang M, Qin Q. et al. Ectopic expression of miR-494 inhibited the proliferation, invasion and chemoresistance of pancreatic cancer by regulating SIRT1 and c-Myc. Gene Ther. 2015;22:729–38. doi: 10.1038/gt.2015.39. [DOI] [PubMed] [Google Scholar]
- 192.Vellinga TT, Borovski T, de Boer VC, Fatrai S, van Schelven S, Trumpi K. et al. SIRT1/PGC1alpha-Dependent Increase in Oxidative Phosphorylation Supports Chemotherapy Resistance of Colon Cancer. Clin Cancer Res. 2015;21:2870–9. doi: 10.1158/1078-0432.CCR-14-2290. [DOI] [PubMed] [Google Scholar]
- 193.Wang W, Zhang J, Li Y, Yang X, He Y, Li T. et al. Divalproex sodium enhances the anti-leukemic effects of imatinib in chronic myeloid leukemia cells partly through SIRT1. Cancer Lett. 2015;356:791–9. doi: 10.1016/j.canlet.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 194.Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P. et al. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell. 2014;15:431–46. doi: 10.1016/j.stem.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kweon KH, Lee CR, Jung SJ, Ban EJ, Kang SW, Jeong JJ. et al. Sirt1 induction confers resistance to etoposide-induced genotoxic apoptosis in thyroid cancers. Int J Oncol. 2014;45:2065–75. doi: 10.3892/ijo.2014.2585. [DOI] [PubMed] [Google Scholar]
- 196.Do MT, Kim HG, Choi JH, Jeong HG. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic Biol Med. 2014;74:21–34. doi: 10.1016/j.freeradbiomed.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 197.Wang Z, Liu Z, Wu X, Chu S, Wang J, Yuan H. et al. ATRA-induced cellular differentiation and CD38 expression inhibits acquisition of BCR-ABL mutations for CML acquired resistance. PLoS Genet. 2014;10:e1004414. doi: 10.1371/journal.pgen.1004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wei R, He D, Zhang X. Role of SIRT2 in Regulation of Stemness of Cancer Stem-Like Cells in Renal Cell Carcinoma. Cell Physiol Biochem. 2018;49:2348–57. doi: 10.1159/000493835. [DOI] [PubMed] [Google Scholar]
- 199.Xu H, Li Y, Chen L, Wang C, Wang Q, Zhang H. et al. SIRT2 mediates multidrug resistance in acute myelogenous leukemia cells via ERK1/2 signaling pathway. Int J Oncol. 2016;48:613–23. doi: 10.3892/ijo.2015.3275. [DOI] [PubMed] [Google Scholar]
- 200.Karwaciak I, Gorzkiewicz M, Ryba K, Dastych J, Pulaski L, Ratajewski M. AC-93253 triggers the downregulation of melanoma progression markers and the inhibition of melanoma cell proliferation. Chem Biol Interact. 2015;236:9–18. doi: 10.1016/j.cbi.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 201.Shiozawa K, Shuting J, Yoshioka Y, Ochiya T, Kondo T. Extracellular vesicle-encapsulated microRNA-761 enhances pazopanib resistance in synovial sarcoma. Biochem Biophys Res Commun. 2018;495:1322–7. doi: 10.1016/j.bbrc.2017.11.164. [DOI] [PubMed] [Google Scholar]
- 202.Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F. et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer. 2017;86:106–14. doi: 10.1016/j.ejca.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 203.Cheng Y, Dai C, Zhang J. SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death. Acta Biochim Pol. 2017;64:343–50. doi: 10.18388/abp.2016_1438. [DOI] [PubMed] [Google Scholar]
- 204.Huang G, Cheng J, Yu F, Liu X, Yuan C, Liu C. et al. Clinical and therapeutic significance of sirtuin-4 expression in colorectal cancer. Oncol Rep. 2016;35:2801–10. doi: 10.3892/or.2016.4685. [DOI] [PubMed] [Google Scholar]
- 205.Hu JQ, Deng F, Hu XP, Zhang W, Zeng XC, Tian XF. Histone deacetylase SIRT6 regulates chemosensitivity in liver cancer cells via modulation of FOXO3 activity. Oncol Rep. 2018;40:3635–44. doi: 10.3892/or.2018.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dai PC, Liu DL, Zhang L, Ye J, Wang Q, Zhang HW. et al. Astragaloside IV sensitizes non-small cell lung cancer cells to gefitinib potentially via regulation of SIRT6. Tumour Biol. 2017;39:1010428317697555. doi: 10.1177/1010428317697555. [DOI] [PubMed] [Google Scholar]
- 207.Sociali G, Galeno L, Parenti MD, Grozio A, Bauer I, Passalacqua M. et al. Quinazolinedione SIRT6 inhibitors sensitize cancer cells to chemotherapeutics. Eur J Med Chem. 2015;102:530–9. doi: 10.1016/j.ejmech.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 208.Thirumurthi U, Shen J, Xia W, LaBaff AM, Wei Y, Li CW. et al. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci Signal. 2014;7:ra71. doi: 10.1126/scisignal.2005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aljada A, Saleh AM, Al Suwaidan S. Modulation of insulin/IGFs pathways by sirtuin-7 inhibition in drug-induced chemoreistance. Diagn Pathol. 2014;9:94. doi: 10.1186/1746-1596-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]