Abstract
Nitidine chloride (NC), a quaternary ammonium alkaloid, exhibits multiple biological activities, including antimalarial, antifungal, and antiangiogenesis. Recently, NC has been characterized to perform antitumor activity in a variety of malignancies. NC has been identified to suppress cell proliferation, stimulate apoptosis, and induce cell cycle arrest, retard migration, invasion and metastasis. Moreover, NC is reported to sensitize cancer cells to chemotherapeutic drugs. In this review article, we describe the functions of NC in human cancers and discuss the molecular insight into NC-involved antitumor feature. This review article will stimulate the deeper investigation for using NC as a potent agent for the management of cancer patients.
Keywords: Nitidine chloride, Cancer, Therapy, Target, Antitumor
Introduction
Nitidine, a phytochemical alkaloid, is mainly extracted from the root of Zanthoxylum nitidum that belongs to the family of Rutaceae, which is often distributed in Northeastern Asia 1 (Figure 1). Nitidine has been characterized as a folk medicine because it is helpful for reducing pain, enhancing blood circulation and cleaning blood stasis 1. Some studies have revealed that nitidine has effects on protection of tumor, inflammation, and HIV infection 2, 3. NC exhibits multiple biological activities including antimalarial 4, antifungal 5, and antiangiogenesis 6. Moreover, NC has been identified to inhibit cell proliferation, induce apoptosis, trigger cell cycle arrest, and sensitize cancer cells to chemotherapeutic drugs. Some researchers have discovered the underlying antitumor mechanisms of NC in human cancers 1, 7. In this review article, we describe the functions of NC in human cancers and highlight the molecular insight of NC-induced antitumor feature (Figure 2).
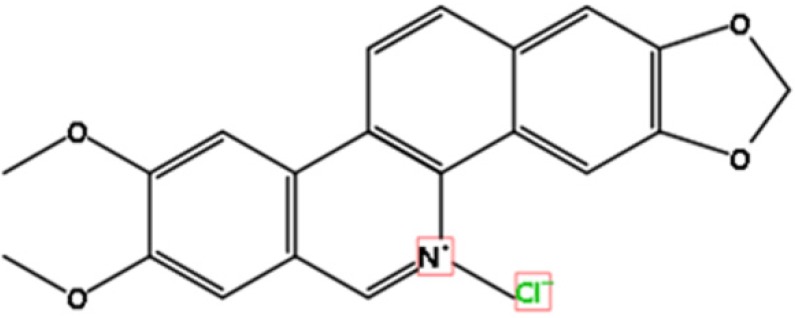
Figure 1.
The structure of Nitidine chloride is illustrated. The molecular formula is C21H18CLNO4.
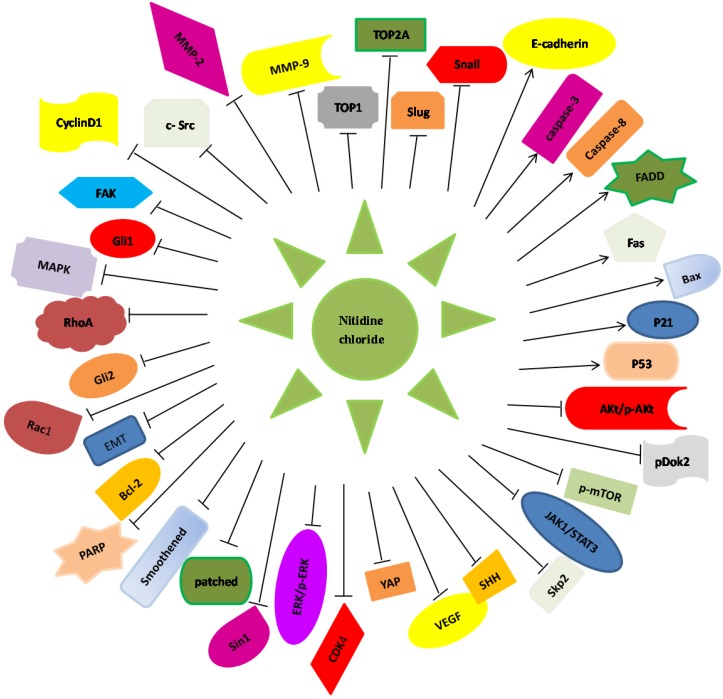
Figure 2.
Illustration of Nitidine chloride -regulated targets and signaling pathways in human cancers. Nitidine chloride exerts its anti-tumor function via regulating the expression of these downstream genes in human malignancies.
Role of NC in human cancers
Breast cancer
Breast cancer is one of the leading causes of cancer-mediated deaths in female 8. Currently, the treatments of breast cancer include surgery, radiotherapy, and chemotherapy. However, due to metastasis and drug resistance, breast cancer patients have poor prognosis 8. NC has been reported to inhibit the metastasis of breast cancer cells via inactivation of the c-Src/FAK-associated pathway 3. Moreover, NC decreased the MMP-9 and MMP-2 formation and their proteolytic activity in mammary cancer cells 3. Mechanistically, NC reduced PDGF-triggered phosphorylation of c-Src, FAK, MAPK, and inactivated the activity of RhoA, Rac1, and AP 3. Another study revealed that NC exposure led to inhibition of cell proliferation and induction of cell cycle arrest through elevation of multiple gene expressions such as p53, p21, Bax, and active forms of caspase-3, caspase-9, and cleaved PARP, and downregulation of Bcl-2 9. Notably, NC sensitizes cell sensitivity to doxorubicin for cell proliferation in breast cancer 9.
Accumulating evidence suggest that EMT plays an essential role in tumor metastasis due to that mesenchymal cells acquire more migratory feature 8. EMT is a programme that epithelial cells transfer to mesenchymal cells, which is often happened in multiple biological processes such as fibrosis, wound healing, and tumor metastasis 10. The cell phenotype is changed from apical-basal polarity and tight junctions to elongated and spindle-shape cells and loose interaction, leading to increased migration and invasion 11. EMT molecular markers are changed from loss of epithelial markers such as E-cadherin to acquired mesenchymal molecules such as Slug, Snail, Vimentin, Zeb1, and Zeb2 12. Various EMT inducers led to EMT in cells, which have CSC characters 13, 14. CSC have been identified in multiple types of cancers, which is associated with tumor metastasis, drug resistance, and tumor reoccurrence 15. CD44+/CD24- has been characterized as a marker for breast CSC, and CSC are involved in tumor metastasis and radiotherapy resistance 16, 17. Recently, Sun et al. identified that NC inhibited migratory and invasive capability due to suppression of EMT and CSC-like phenotype by suppression of HH pathway in breast cancer cells 18. Specifically, NC downregulated the expression of several molecules in HH pathway including Gli1 and Gli2, suppressed the expression level of mesenchymal markers such as Zeb1, Slug, and Snail, leading to EMT reversal. Strikingly, NC attenuated the expression of Nanog, Nestin, Oct-4 and CD44 through HH pathway in breast cancer cells 18. These reports indicate that NC exerts its tumor suppressive function in breast cancer cells.
Liver cancer
LC is one of the common malignancies, with HCC as a main subtype 19. The pathogenesis of HCC and its development process are complex and are related to a variety of signal transduction pathways, including STAT3, SHH, and ERK 20. One study reported that the NC repressed HCC proliferation though JAK1/STAT3 pathway due to enhancement of apoptosis and increased level of p21 and Bax, and decreased cyclinD1, CDK4 and Bcl-2 in HCC 21. Similarly, another study also found that NC reduced tumor volume and tumor weight in mice via suppression of ERK, STAT3, and SHH pathways, and regulation of Bcl-2, Bax, CyclinD1, VEGF pathway 20. These genes are involved in cell proliferation, apoptosis, and tumor angiogenesis in liver cancer 20.
In addition, Ou et al also found that NC affected cell proliferation, apoptotic death, and cell cycle by elevation of p53, Bax, caspase-3 and p21, but inhibition of Bcl-2 in liver cancer 1. Furthermore, a supramolecular formulation of NC has been developed and alleviated hepatotoxicity and improved anticancer activity 22. Interestingly, NC was found to be a candidate substrate of OCT1 and OCT3 that transfer NC into hepatocytes. Moreover, MATE1 makes NC leave from hepatocytes, while CYPs contributed to NC metabolism such as CYP3A4 and attenuated the hepatic toxicity of NC 23. TOP1 and TOP2A are key tumor drivers in liver cancer 24, suggesting that TOP1 and TOP2A might be promising targets for treating malignances 25. One group has shown that the NC exposure attenuated the TOP1 and TOP2A expression levels 26. Recently, NC was found to suppress the expression of eight genes that are highly expressed in liver cancer 27. The analogues of nitidine have been synthesized and exhibited extraordinary inhibition of cell proliferation in liver cancer cells 28. Similarly, supramolecular formulation of NC reduced its toxicity to liver and promoted tumor suppressive activity in liver cancer cells 29. However, further experiments are necessary to define the molecular mechanism of NC in HCC.
Ovarian cancer
OC is one type of malignancies in females. The Fas/FasL system is involved in carcinogenesis 30. Fas can bind to FasL and initiate activation of caspase-8 and caspase-3 to exert apoptosis 31. Fas expression was remarkably downregulated in OC patient clinical samples 32, 33. Therefore, overexpression of Fas could become a potential therapeutic target for OC 34. NC was found to elevate the level of FADD, caspase-8 and caspase-3 in OC cells 34. Skp2 as an oncoprotein is the key adaptor of the SCF type of E3 ligase 35-37. NC was reported to inhibit the Skp2 level in OC cells, while Skp2 upregulation abolished NC-induced antitumor property 38. Thus, suppression of Skp2 expression level by NC might be a useful approach for management of OC patients 38. There is one study showing that NC blocked cell migratory and invasive activity through downregulation of MMP-2 as well as MMP-9 via suppression of ERK pathway in OC cells 39. NC induced apoptosis and retarded cell proliferation in OC cells by reduced pAkt and modulation of Bcl-2 family expression 40. Moreover, NC sensitized OC cells to doxorubicin treatment, leading to a synergistic suppression of proliferation 40.
Renal cancer
RCC patients with distant metastasis have low five-year survival rate. The underlying molecular mechanisms of renal cancer development and progression are unclear. The higher expression of pAkt is exhibited in RCC, especially in later and metastatic RCC 41, 42. One study has shown that NC attenuated the cell invasion and metastasis of RCC cells through inhibition of Akt signaling pathway and MMP-2 and MMP-9 43. This group further found that NC inhibited phosphorylation of ERK and Akt, upregulated the protein level of p53, Bax, cleavage caspase-3 and PARP and downregulated Bcl-2 in RCC cells 44. Without a doubt, the detailed molecular insight into NC-induced antitumor activity in RCC is required to be further investigated.
Glioblastoma
GBM is one type of aggressive brain malignancy 45. Due to that GBM is highly proliferative, invasive and chemoresistant, the combined treatments of surgery, chemotherapy, and radiotherapy have not significantly improved the survival rate of GBM patients 46, 47. Liu et al dissected that NC impaired cell viability and motility of GMB cells via inactivation of pAkt and mTOR 48. Moreover, NC was reported to increase the expression of cleaved PARP and cleaved caspase 3, and to inhibit pDok2 in GBM cells 49. Similarly, NC prohibited cell proliferation and colony formation, induced cell cycle arrest at G2/M phase in glioma cells. Notably, NC suppressed GSK3-β pathway in glioma cells 49. Taken together, NC could be a potential anti-glioma agent, which is needed to further explore its antitumor activity.
Osteosarcoma
Osteosarcoma is the common bone malignant tumor in young adults and children 50-52. One study reported that NC prevented the growth of osteosarcoma cells and also stimulated the apoptosis via elevated cleaved forms of caspase-3 and caspase-9, and increased Bax, and down-regulation of Bcl-2 53. Another study revealed that NC inhibited EMT process and suppressed the invasive ability via targeting the Akt/GSK-3β/Snail signaling pathway 54. Specifically, NC treatment elevated E-cadherin expression and repressed the level of N-cadherin, vimentin, and fibronectn in osteosarcoma cells 54. Recently, Xu et al demonstrated that NC impeded proliferation, migration, and invasion, and stimulated apoptosis by inhibition of SIN1 in osteosarcoma cells, indicating that NC might be a useful agent to act as an inhibitor of SIN1 in osteosarcoma 55. Altogether, NC could be a potential agent for treating osteosarcoma.
Other cancers
CRC is one of the most commonly diagnosed malignancies. NC was identified to impede the proliferation of CRC cells and induce apoptosis 56. NC treatment in CRC cells promoted the protein level of Bax, p53, cleaved caspase-3 and -9, and decreased Bcl-2 level and reduced the phosphorylation of ERK, which could lead to cell proliferation inhibition and apoptosis 56. Elevation of STAT3 expression is observed and associated with metastasis in GC 57. NC inhibited STAT3 activation, leading to repression of its downstream targets such as cyclin D1, Bcl-xL, and VEGF in GC cells 6. This finding dissects that NC could be a potent STAT3 inhibitor in gastric cancer to treat this deadly disease. However, further research is necessary to define the mechanism of NC in suppression of growth and metastasis of GC cells.
NC inhibited cell viability via induction of apoptosis by hindering STAT3 pathway in oral cancer cell lines and a tumor xenograft model 58. NC treatment did not have liver or kidney toxicity in mouse model 58. This group further identified that NC suppressed Mcl-1 protein level via lysosome-dependent degradation in oral squamous cell carcinoma 59. These reports suggest that NC might be a putative agent against oral cancer 58. NC exposure exhibited growth inhibition of AML via induction of cell cycle arrest and apoptosis 60. NC elevated the expression of p27 and Bax, and reduced the level of Cyclin B1, CDK1 and Bcl-2, as well as inactivated PARP in AML cells 60. Moreover, NC-induced cell growth inhibition in AML cells is partly due to inactivation of the phosphorylation of Akt and ERK 60. Similarly, NC induced erythroid differentiation and apoptosis in CML via regulation of c-Myc-miRNAs axis 61. In addition, NC repressed cell proliferation and enhanced apoptotic death through elevation of p53 in NPC cells 62. Recently, NC was reported to inhibit cell proliferation and invasion through suppression of YAP expression in prostate cancer cells 63. We believe the tumor suppressive activity of NC will be exhibited in a range of human malignancies.
Conclusions
NC has been characterized as a potent anti-tumor agent in a wide spectrum of human cancers via various molecular mechanisms (Table 1). In this regard, NC exhibits tumor suppressive functions through targeting numerous signaling pathways. It is noted that NC could have nephrotoxicity due to high-affinity of OCT2 and MATE1 to NC in kidney and have its hepatotoxicity 29, 64. However, multiple questions should be answered before NC will be used in clinical trial. For example, NC is absolutely safe for using in human cancer patients? How can NC be delivered to specific organs with tumors? How to improve the NC bio-solubility in human body? What are the detailed and specific molecular mechanisms of NC in different types of human cancers? We believe that answering these questions will promote NC to be used in clinic trials in the near future.
Table 1.
Summary of the functions of nitidine chloride in human cancers.
| Cancer type | Function | Targets | Reference |
|---|---|---|---|
| Breast cancer | Inhibits growth and induces cell cycle arrest; reduces migration and invasion; inhibits metastasis. | Downregulates c-Src/FAK; MMP-9, MMP-2, c-Src, FAK, MAPK, RhoA, Rac1, AP, Bcl-2, smoothened, patched, Gli1, Gli2, Snail, Slug, Zeb1, Nanog, Nestn, Oct-4, and CD44; upregulates p53, p21, Bax, cleaved caspase-9 and -3, and PARP. | 3, 9, 18 |
| Liver cancer | Inhibits cell growth and induces apoptosis and cell cycle arrest. | Decreases JAK1/STAT3, cyclinD1, CDK4, Bcl-2, ERK, SHH, TOP1 and TOP2A; upregulates p21, p53, Bax, and caspase-3 and p21. | 20, 21, 26 |
| Ovarian cancer | Inhibits proliferation, migration, invasion, and induced apoptosis. | Downregulates Skp2, MMP-2, MMP-9, ERK, pAkt, Bcl-2. Increases the expression of Fas, FADD, caspase-8 and caspase-3. | 31, 32, 38-40 |
| Renal cancer | Suppresses invasion and metastasis; Triggers apoptosis. | Inhibits AKT signaling pathway, down-regulates MMP-2 and MMP-9. inhibited phosphorylation of ERK and Akt, upregulates p53, Bax, cleavage caspase-3, and cleavage PARP, and downregulates Bcl-2, caspase-3 and PARP, | 43, 44 |
| Glioblastoma | Inhibits cell viability, migration and invasion, and induces cell cycle arrest. | Suppresses pAkt, mTOR, pDok2, GSK3-b; increases cleaved PARP and cleaved caspase 3. | 48, 49 |
| Osteosarcoma | Inhibits proliferation, migration, invasion and EMT, induces the apoptosis. | Upregulates cleaved caspase-3, cleaved caspase-9, E-cadherin and Bax. Downregulates pro-caspase-3, pro-caspase-9, Bcl-2; Akt/GSK-3/Snail, SIN1, N-cadherin, vimentin, and fibronectn. | 53-55 |
| Colorectal cancer | Inhibits proliferation, enhances apoptosis. | Increases Bax, p53, cleaved caspase-3 and -9. Decreases Bcl-2, and pERK. | 56 |
| Gastric cancer | Inhibits angiogenesis and metastasis. | Inhibits STAT3 activation, cyclin D1, Bcl-xL, and VEGF. | 6 |
| Oral cancer | Inhibits cell viability, and enhanced apoptosis. | Downregulates STAT3 signaling pathway, suppresses Mcl-1 level. | 58, 59 |
| Acute myeloid leukemia | Inhibits cell growth, induces cell cycle arrest and apoptosis; | Increases p27, Bax; Decreases Cyclin B1, CDK1, Bcl-2, pAkt, and ERK. | 60 |
| Chronic myeloid leukemia | Induces erythroid differentiation and apoptosis. | Targets c-Myc-miRNAs axis. | 61 |
| Nasopharyngeal carcinoma | Inhibits proliferation, and enhances apoptosis. | Upregulates p53. | 62 |
| Prostate cancer | Inhibits cell proliferation and invasion. | Suppresses YAP. | 63 |
Acknowledgments
This work was supported by the Major project from the Natural Science Foundation of Education Department of Anhui Province (KJ2019ZD27).
Abbreviations
- AP
activator protein
- AML
acute myeloid leukemia
- Bcl-2
B-cell lymphoma 2
- CDK4
cyclin-dependent kinase 4
- CML
chronic myeloid leukemia
- CRC
colorectal cancer
- CSC
cancer stem cells
- CYPs
cytochrome P450 enzymes
- EMT
epithelial mesenchymal transition
- ERK
extracellular regulated protein kinases
- FADD
Fas-associating protein with a novel death domain
- FAK
focal adhesion kinase
- FasL
Fas ligand
- GBM
Glioblastoma multiforme
- GC
gastric cancer
- Gli
glioma-associated oncogene homolog
- GSK-3β
Glycogen synthase kinase-3β
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency viruses
- HH
hedgehog
- JAK1
Janus kinase 1
- LC
Liver cancer
- MAPK
mitogen-activated protein kinase
- MATE1
multidrug and toxin extrusion 1
- MCL1
myeloid cell leukemia 1
- MMP
Matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NC
Nitidine chloride
- NPC
nasopharyngeal carcinoma
- OC
Ovarian cancer
- OCT1
organic cation transporter 1
- PARP
poly ADP-ribose polymerase
- PDGF
Platelet derived growth factor
- RCC
renal cell carcinoma
- SCF
Skp1-Cullin1-F-box complex
- SHH
sonic hedgehog
- Skp2
S-phase kinase associated protein 2
- STAT3
signal transducer and activator of transcription 3
- TOP1
topoisomerase 1
- VEGF
vascular endothelial growth factor
- ZEB1
Zinc finger E-box-binding homeobox 1
- AP
activator protein
- AML
acute myeloid leukemia
- Bcl-2
B-cell lymphoma 2
- CDK4
cyclin-dependent kinase 4
- CML
chronic myeloid leukemia
- CRC
colorectal cancer
- CSC
cancer stem cells
- CYPs
cytochrome P450 enzymes
- EMT
epithelial mesenchymal transition
- ERK
extracellular regulated protein kinases
- FADD
Fas-associating protein with a novel death domain
- FAK
focal adhesion kinase
- FasL
Fas ligand
- GBM
Glioblastoma multiforme
- GC
gastric cancer
- Gli
glioma-associated oncogene homolog
- GSK-3β
Glycogen synthase kinase-3β
- HCC
hepatocellular carcinoma
- HIV
human immunodeficiency viruses
- HH
hedgehog
- JAK1
Janus kinase 1
- LC
Liver cancer
- MAPK
mitogen-activated protein kinase
- MATE1
multidrug and toxin extrusion 1
- MCL1
myeloid cell leukemia 1
- MMP
Matrix metalloproteinase
- mTOR
mammalian target of rapamycin
- NC
Nitidine chloride
- NPC
nasopharyngeal carcinoma
- OC
Ovarian cancer
- OCT1
organic cation transporter 1
- PARP
poly ADP-ribose polymerase
- PDGF
Platelet derived growth factor
- RCC
renal cell carcinoma
- SCF
Skp1-Cullin1-F-box complex
- SHH
sonic hedgehog
- Skp2
S-phase kinase associated protein 2
- STAT3
signal transducer and activator of transcription 3
- TOP1
topoisomerase 1
- VEGF
vascular endothelial growth factor
- ZEB1
Zinc finger E-box-binding homeobox 1
References
- 1.Khan H, Hadda TB, Touzani R. Diverse Therapeutic Potential of Nitidine, A Comprehensive Review. Curr Drug Metab. 2018;19:986–91. doi: 10.2174/1389200219666180628165643. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Jiang W, Zhang Z, Qian M, Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012;144:145–50. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 3.Pan X, Han H, Wang L, Yang L, Li R, Li Z. et al. Nitidine Chloride inhibits breast cancer cells migration and invasion by suppressing c-Src/FAK associated signaling pathway. Cancer Lett. 2011;313:181–91. doi: 10.1016/j.canlet.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bouquet J, Rivaud M, Chevalley S, Deharo E, Jullian V, Valentin A. Biological activities of nitidine, a potential anti-malarial lead compound. Malar J. 2012;11:67. doi: 10.1186/1475-2875-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Poeta M, Chen SF, Von Hoff D, Dykstra CC, Wani MC, Manikumar G. et al. Comparison of in vitro activities of camptothecin and nitidine derivatives against fungal and cancer cells. Antimicrob Agents Chemother. 1999;43:2862–8. doi: 10.1128/aac.43.12.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Wang J, Lin L, He L, Wu Y, Zhang L. et al. Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther. 2012;11:277–87. doi: 10.1158/1535-7163.MCT-11-0648. [DOI] [PubMed] [Google Scholar]
- 7.Kovacic P, Ames JR, Lumme P, Elo H, Cox O, Jackson H. et al. Charge transfer-oxy radical mechanism for anti-cancer agents. Anticancer Drug Des. 1986;1:197–214. [PubMed] [Google Scholar]
- 8.Ponde NF, Zardavas D, Piccart M. Progress in adjuvant systemic therapy for breast cancer. Nature reviews Clinical oncology. 2019;16:27–44. doi: 10.1038/s41571-018-0089-9. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Zhang N, Wang X, Cai C, Cun J, Li Y. et al. Nitidine chloride induces apoptosis, cell cycle arrest, and synergistic cytotoxicity with doxorubicin in breast cancer cells. Tumour Biol. 2014;35:10201–12. doi: 10.1007/s13277-014-2327-9. [DOI] [PubMed] [Google Scholar]
- 10.Stemmler MP, Eccles RL, Brabletz S, Brabletz T. Non-redundant functions of EMT transcription factors. Nat Cell Biol. 2019;21:102–12. doi: 10.1038/s41556-018-0196-y. [DOI] [PubMed] [Google Scholar]
- 11.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature reviews Clinical oncology. 2017;14:611–29. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 15.Lytle NK, Barber AG, Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nature reviews Cancer. 2018;18:669–80. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J. et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–73. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, Zhang N, Wang X, Li Y, Qi W, Zhang H. et al. Hedgehog pathway is involved in nitidine chloride induced inhibition of epithelial-mesenchymal transition and cancer stem cells-like properties in breast cancer cells. Cell Biosci. 2016;6:44. doi: 10.1186/s13578-016-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Shen A, Chen H, Liao J, Xu T, Liu L. et al. Nitidine chloride inhibits hepatic cancer growth via modulation of multiple signaling pathways. BMC Cancer. 2014;14:729. doi: 10.1186/1471-2407-14-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu DB. et al. Nitidine chloride inhibits hepatocellular carcinoma cell growth in vivo through the suppression of the JAK1/STAT3 signaling pathway. Int J Mol Med. 2013;32:79–84. doi: 10.3892/ijmm.2013.1358. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Tu M, Yang X, Sun S, Wu X, Zhou H. et al. The contribution of human OCT1, OCT3, and CYP3A4 to nitidine chloride-induced hepatocellular toxicity. Drug Metab Dispos. 2014;42:1227–34. doi: 10.1124/dmd.113.056689. [DOI] [PubMed] [Google Scholar]
- 24.Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. MiR-23a-mediated inhibition of topoisomerase 1 expression potentiates cell response to etoposide in human hepatocellular carcinoma. Mol Cancer. 2013;12:119. doi: 10.1186/1476-4598-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado JL, Hsieh CM, Chan NL, Hiasa H. Topoisomerases as anticancer targets. Biochem J. 2018;475:373–98. doi: 10.1042/BCJ20160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu LM, Xiong DD, Lin P, Yang H, Dang YW, Chen G. DNA topoisomerase 1 and 2A function as oncogenes in liver cancer and may be direct targets of nitidine chloride. Int J Oncol. 2018;53:1897–912. doi: 10.3892/ijo.2018.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu LM, Lin P, Yang H, Dang YW, Chen G. Gene profiling of HepG2 cells following nitidine chloride treatment: An investigation with microarray and Connectivity Mapping. Oncology reports. 2019;41:3244–56. doi: 10.3892/or.2019.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin SQ, Li LC, Song JR, Li HY, Li DP. Structurally Simple Phenanthridine Analogues Based on Nitidine and Their Antitumor Activities. Molecules; 2019. p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Yin H, Bardelang D, Xiao J, Zheng Y, Wang R. Supramolecular formulation of nitidine chloride can alleviate its hepatotoxicity and improve its anticancer activity. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2017;109:923–9. doi: 10.1016/j.fct.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 30.Rossin A, Miloro G, Hueber AO. TRAIL and FasL Functions in Cancer and Autoimmune Diseases: Towards an Increasing Complexity. Cancers (Basel); 2019. p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrahams VM, Kamsteeg M, Mor G. The Fas/Fas ligand system and cancer: immune privilege and apoptosis. Mol Biotechnol. 2003;25:19–30. doi: 10.1385/MB:25:1:19. [DOI] [PubMed] [Google Scholar]
- 32.Ma XY, He FX, Wu SF, Lu YP, Ma D. Expression of survivin in ovarian epithelial carcinoma and its correlation with expression of Fas and FasL. Ai Zheng. 2004;23:173–6. [PubMed] [Google Scholar]
- 33.Kang HJ, Moon HS, Chung HW. The expression of FAS-associated factor 1 and heat shock protein 70 in ovarian cancer. Obstet Gynecol Sci. 2014;57:281–90. doi: 10.5468/ogs.2014.57.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Yang L, Feng J. Nitidine chloride inhibits proliferation and induces apoptosis in ovarian cancer cells by activating the Fas signalling pathway. J Pharm Pharmacol. 2018;70:778–86. doi: 10.1111/jphp.12901. [DOI] [PubMed] [Google Scholar]
- 35.Chan CH, Morrow JK, Zhang S, Lin HK. Skp2: a dream target in the coming age of cancer therapy. Cell Cycle. 2014;13:679–80. doi: 10.4161/cc.27853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu HC, Yang YC, Chen YJ, Lin H, Ou YC, Chien CC. et al. Increased expression of SKP2 is an independent predictor of locoregional recurrence in cervical cancer via promoting DNA-damage response after irradiation. Oncotarget. 2016;7:44047–61. doi: 10.18632/oncotarget.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–47. doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mou H, Guo P, Li X, Zhang C, Jiang J, Wang L. et al. Nitidine chloride inhibited the expression of S phase kinase-associated protein 2 in ovarian cancer cells. Cell Cycle. 2017;16:1366–75. doi: 10.1080/15384101.2017.1327490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun X, Lin L, Chen Y, Liu T, Liu R, Wang Z. et al. Nitidine chloride inhibits ovarian cancer cell migration and invasion by suppressing MMP-2/9 production via the ERK signaling pathway. Mol Med Rep. 2016;13:3161–8. doi: 10.3892/mmr.2016.4929. [DOI] [PubMed] [Google Scholar]
- 40.Ding F, Liu T, Yu N, Li S, Zhang X, Zheng G. et al. Nitidine chloride inhibits proliferation, induces apoptosis via the Akt pathway and exhibits a synergistic effect with doxorubicin in ovarian cancer cells. Mol Med Rep. 2016;14:2853–9. doi: 10.3892/mmr.2016.5577. [DOI] [PubMed] [Google Scholar]
- 41.Horiguchi A, Oya M, Uchida A, Marumo K, Murai M. Elevated Akt activation and its impact on clinicopathological features of renal cell carcinoma. J Urol. 2003;169:710–3. doi: 10.1097/01.ju.0000038952.59355.b2. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda K, Horiguchi A, Sumitomo M, Asano T, Ito K, Hayakawa M. et al. Activated Akt prevents antitumor activity of gefitinib in renal cancer cells. Urology. 2009;74:209–15. doi: 10.1016/j.urology.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 43.Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W. et al. Nitidine chloride inhibits renal cancer cell metastasis via suppressing AKT signaling pathway. Food Chem Toxicol. 2013;60:246–51. doi: 10.1016/j.fct.2013.07.062. [DOI] [PubMed] [Google Scholar]
- 44.Fang Z, Tang Y, Jiao W, Xing Z, Guo Z, Wang W. et al. Nitidine chloride induces apoptosis and inhibits tumor cell proliferation via suppressing ERK signaling pathway in renal cancer. Food Chem Toxicol. 2014;66:210–6. doi: 10.1016/j.fct.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 45.Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379:1984–96. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 46.Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G, Group EGW. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii93–101. doi: 10.1093/annonc/mdu050. [DOI] [PubMed] [Google Scholar]
- 47.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M, Wang J, Qi Q, Huang B, Chen A, Li X. et al. Nitidine chloride inhibits the malignant behavior of human glioblastoma cells by targeting the PI3K/AKT/mTOR signaling pathway. Oncol Rep. 2016;36:2160–8. doi: 10.3892/or.2016.4998. [DOI] [PubMed] [Google Scholar]
- 49.Deshpande RP, Babu PP. pDok2, caspase 3 dependent glioma cell growth arrest by nitidine chloride. Pharmacol Rep. 2018;70:48–54. doi: 10.1016/j.pharep.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Huh WW, Holsinger FC, Levy A, Palla FS, Anderson PM. Osteosarcoma of the jaw in children and young adults. Head Neck. 2012;34:981–4. doi: 10.1002/hed.21850. [DOI] [PubMed] [Google Scholar]
- 51.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 53.Xu Q, Li ZX, Ye ZM. Nitidine chloride-induced apoptosis of human osteosarcoma cells and its mechanism. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:361–4. [PubMed] [Google Scholar]
- 54.Cheng Z, Guo Y, Yang Y, Kan J, Dai S, Helian M. et al. Nitidine chloride suppresses epithelial-to-mesenchymal transition in osteosarcoma cell migration and invasion through Akt/GSK-3beta/Snail signaling pathway. Oncol Rep. 2016;36:1023–9. doi: 10.3892/or.2016.4846. [DOI] [PubMed] [Google Scholar]
- 55.Xu H, Cao T, Zhang X, Shi Y, Zhang Q, Chai S. et al. Nitidine Chloride Inhibits SIN1 Expression in Osteosarcoma Cells. Mol Ther Oncolytics. 2019;12:224–34. doi: 10.1016/j.omto.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhai H, Hu S, Liu T, Wang F, Wang X, Wu G. et al. Nitidine chloride inhibits proliferation and induces apoptosis in colorectal cancer cells by suppressing the ERK signaling pathway. Mol Med Rep. 2016;13:2536–42. doi: 10.3892/mmr.2016.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cafferkey C, Chau I. Novel STAT 3 inhibitors for treating gastric cancer. Expert Opin Investig Drugs. 2016;25:1023–31. doi: 10.1080/13543784.2016.1195807. [DOI] [PubMed] [Google Scholar]
- 58.Kim LH, Khadka S, Shin JA, Jung JY, Ryu MH, Yu HJ. et al. Nitidine chloride acts as an apoptosis inducer in human oral cancer cells and a nude mouse xenograft model via inhibition of STAT3. Oncotarget. 2017;8:91306–15. doi: 10.18632/oncotarget.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang IH, Jung W, Kim LH, Shin JA, Cho NP, Hong SD. et al. Nitidine chloride represses Mcl-1 protein via lysosomal degradation in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:823–9. doi: 10.1111/jop.12755. [DOI] [PubMed] [Google Scholar]
- 60.Li P, Yan S, Dong X, Li Z, Qiu Y, Ji C. et al. Cell Cycle Arrest and Apoptosis Induction Activity of Nitidine Chloride on Acute Myeloid Leukemia Cells. Med Chem. 2018;14:60–6. doi: 10.2174/1573406413666170620091543. [DOI] [PubMed] [Google Scholar]
- 61.Liu N, Li P, Zang S, Liu Q, Ma D, Sun X. et al. Novel agent nitidine chloride induces erythroid differentiation and apoptosis in CML cells through c-Myc-miRNAs axis. PLoS One. 2015;10:e0116880. doi: 10.1371/journal.pone.0116880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang M, Ou H, Wang R, Liu W, Tang A. The effect of nitidine chloride on the proliferation and apoptosis of nasopharyngeal carcinoma cells. Journal of BUON. 2014;19:130–6. [PubMed] [Google Scholar]
- 63.Shi Y, Cao T, Sun Y, Xia J, Wang P, Ma J. Nitidine Chloride inhibits cell proliferation and invasion via downregulation of YAP expression in prostate cancer cells. Am J Transl Res. 2019;11:709–20. [PMC free article] [PubMed] [Google Scholar]
- 64.Li LP, Song FF, Weng YY, Yang X, Wang K, Lei HM. et al. Role of OCT2 and MATE1 in renal disposition and toxicity of nitidine chloride. Br J Pharmacol. 2016;173:2543–54. doi: 10.1111/bph.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]