Abstract
Aims:
The aims of this study are to confirm disparities in diabetes mortality rates based on race, determine if race predicts combinations of diabetes and multiple chronic conditions (MCC) that are leading causes of death (LCD), and determine if combinations of diabetes plus MCC mediate the relationship between race and mortality.
Methods:
We performed a retrospective cohort study of 443,932 Medicare beneficiaries in the State of Michigan with type 2 diabetes mellitus and MCC. We applied Cox proportional hazards regression to determine predictors of mortality. We applied multinomial logistic regression to determine predictors of MCC combinations.
Results:
We found that race influences mortality in Medicare beneficiaries with Type 2 diabetes mellitus and MCC. Prior to adjusting for MCC combinations, we observed that Blacks and American Indian/Alaska Natives have increased risk of mortality compared to Whites, while there is no difference in mortality between Hispanics and Whites. Regarding MCC combinations, Black/African American beneficiaries experience increased odds for most MCC combinations while Asian/Pacific Islanders and Hispanics experience lower odds for MCC combinations, compared to Whites. When adjusting for MCC, mortality disparities observed between Whites, Black/African Americans, and American Indians/Alaska Natives persist.
Conclusions:
Compared to Whites, Black/African Americans in our cohort had increased odds of most MCC combinations, and an increased risk of mortality that persisted even after adjusting for MCC combinations.
Keywords: type 2 diabetes, race, mortality, CHF, CKD, COPD
1. INTRODUCTION
Diabetes is one of the most common chronic diseases, affecting 30 million people in the United States, with Type 2 diabetes mellitus (hereinafter referred to as diabetes) being the most common [1]. Its prevalence increases every year [2] and over the past two decades has reached epidemic proportions, being a major cause of morbidity and mortality in the United States [3, 4]. Many studies describe disparities in diabetes prevalence, incidence, and mortality among different race and ethnicity groups, with increased rates observed in racial and ethnic minority groups [5–8]. While disparities due to diabetes alone are well-described, less understood are disparities related to other chronic conditions associated with diabetes, including combinations of these comorbidities. Using claims data from a cohort of Medicare beneficiaries in the State of Michigan, the aims of this study are three-fold:
Confirm disparities in diabetes mortality rates based on race;
Determine if race is predictive of combinations of diabetes and multiple chronic conditions (MCC) that are leading causes of death (LCD); and
Determine if combinations of diabetes plus MCC mediate the relationship between race and mortality.
Several studies provide evidence that racial and ethnic minorities experience diabetes-related mortality at higher rates compared to whites. For instance, Golden et al. [9] found that Black, Native Americans and Alaskan Natives, and Hispanics exhibit 2.3, 1.9, and 1.5 times greater mortality related to diabetes, respectively, than their White counterparts. Other studies [10–12] report similar increases in mortality among Blacks explained by economic inequality and segregation [10], as well as socioeconomic status [11]. These studies suggest that access to health care, which is a key disadvantage for minority populations, does not solely account for the increased diabetes-only mortality rates among racial and ethnic minorities. However, a review of the literature shows an incomplete understanding of the relationship between race/ethnicity and diabetes-only mortality, especially when accounting for various confounding factors that could contribute to such disparities.
Diabetes is comorbid with MCC that are LCD in the United States, including cardiovascular disease (CVD), chronic kidney disease (CKD), cancer, chronic obstructive pulmonary disease (COPD), Alzheimer’s disease (AD), and stroke [13]. Certain combinations of diabetes and chronic conditions, such as diabetes and CHF, or diabetes and CKD, have been well studied, and thus there is extensive literature on racial and ethnic disparities in the development of these chronic conditions in patients with diabetes. Racial and ethnic minorities, particularly Hispanics and Blacks, exhibit increased rates of diabetes-related CKD compared to Whites [9,14]. In addition, Hispanics and African Americans with diabetes have a higher prevalence of early CKD compared to Whites, with some evidence that Blacks experience a more rapid decline in renal function compared to Whites once they develop proteinuria [15, 16].
While racial and ethnic minorities experience greater rates of CKD with diabetes, the combination of diabetes and congestive heart failure (CHF) is more prevalent in Whites. Whites with diabetes are more likely to develop CHF or other cardiovascular complications compared to racial and ethnic minority populations [14], as one study demonstrated that increasing levels of HbA1c, used as a measure of progressing diabetes, increased the risk of developing CHF in Whites compared to racial and ethnic minorities [17]. Wong et al. [18] reported that Hispanic and Black populations with diabetes are at lower risk of developing cardiovascular disease than Whites with diabetes.
The risks of comorbid cancer, COPD, Stroke and AD are less well described. A few studies report that Blacks and Hispanics with diabetes are at increased risk for liver or colorectal cancer [19–21], while breast cancer is not more prevalent in any racial or ethnic subgroup [22]. A systematic review by Gläser et al. [23] found that diabetes can worsen the progression and prognosis of COPD, while Davis et al. [24] reported that among adults aged 60–79, Blacks had twice the prevalence of diabetes plus COPD as other racial and ethnic populations. Data regarding stroke in combination with diabetes is not well described, with some studies reporting higher rates of stroke among Blacks and Hispanics, but they leave open the possibility that this could be due to minority populations exhibiting higher rates of stroke and diabetes in general [5, 25, 26]. Another study found that racial and ethnic minority populations were at lower risk of developing all forms of diabetic cardiovascular disease, including stroke [27]. Finally, there is an even larger gap in the understanding of the relationship between diabetes and AD. One study demonstrates that uncontrolled diabetes increases the risk of AD, however it did not discuss racial or ethnic disparities related to risk [28]. Another study demonstrated that diabetes led to increases in cognitive decline in old age, but there was no difference in cognitive decline between Blacks and Whites [29].
The literature summarized here describes disparities between racial and ethnic groups in the context of the relationship between diabetes and individual chronic conditions. However, little is known about disparities between racial and ethnic groups in diabetes plus MCC (i.e. more than one additional chronic condition). One study found that minority diabetic patients are more likely to be diagnosed with end-stage diabetic nephropathy, but they have fewer other conditions such as CVD, COPD and cancer [30]. Young et al. [30] found that the risk of CVD-related mortality in non-Hispanic whites with diabetes was higher than that of minorities. Davis et al. [24] reported that the co-occurrence of diabetes, CVD and cancer amongst Hispanics, non-Hispanic blacks and Whites increased with age; however, non-Hispanic Whites had lower prevalence of these comorbidities compared to minorities.
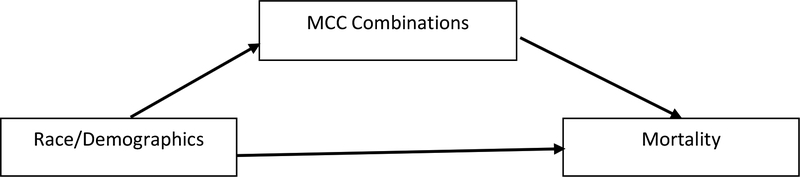
Little research exists that focuses on the relationship between diabetes and multiple chronic conditions and mortality. Individuals with diabetes have an increased risk of developing other chronic conditions, and vice versa, that can lead to death at a more rapid rate than those without these conditions [31, 32]. Determining whether there is a racial component and how other factors affect diabetes-related mortality is essential for a more complete understanding of diabetes-related mortality. Using Medicare claims data for Michigan beneficiaries with Type 2 diabetes mellitus, we aim to determine if race and various demographic characteristics influence mortality and combinations of diabetes and various chronic conditions, and whether the presence of multiple chronic conditions mediates the relationship between race/ethnicity and mortality (see Figure 1 for our conceptual model).
Figure 1:
Model of Race, other Demographics, Multiple Chronic Conditions, and Mortality. Theoretical Model.
2. SUBJECTS, MATERIALS and METHODS
2.1. Data:
We used 2012 claims data from the Centers for Medicare and Medicaid Services (CMS) for all Medicare beneficiaries in the State of Michigan. The Master Beneficiary Segment Files (MBSF) include four separate databases. The Base Segment database provides basic beneficiary demographic information. The Chronic Conditions Warehouse (CCW) database includes variables that code for the presence of any of 27 chronic conditions tracked by CMS. The Cost and Use database provides information about total annual costs for outpatient services, acute inpatient services, skilled nursing, and testing and imaging services, among others. The Part D file contains information about Part D covered drug use and costs. We merged files to create one database that contains claims data for 1,851,328 Michigan Medicare beneficiaries. We eliminated 351,002 beneficiaries (remaining n=1,500,326) under the age of 65 because a beneficiary is eligible for Medicare before age 65 if they qualify for Social Security disability and we wanted to eliminate early disability as a confounder.
The CCW data includes a variable that identifies people with diabetes (n=511,120, 34.1% of beneficiaries age 65 and over), but it includes both beneficiaries with Type 1 and Type 2 diabetes. Our goal is to only study beneficiaries with Type 2 diabetes mellitus, so we eliminated beneficiaries with Type 1 diabetes diagnosis codes (ICD-9 codes 250.x1, 250.x3) or secondary diabetes codes (ICD-9 codes 249.xx) in Part B Carrier, Home Health, Hospice, and Outpatient Claims data. Finally, we removed 4,475 beneficiaries with race designated as Other or Unknown due to the uncertain nature of these groupings. After eliminating beneficiaries under the age of 65, with uncertain race, and with only Type 1 or secondary diabetes codes, our final sample size is 443,932 (29.6% of beneficiaries age 65+).
We analyzed the following variables to describe our sample and create groups for comparison: Age in years (at death, or as of 2012), female Sex (reference: male), urban residency (reference : rural residency), and Race (coded as Black/African American (black), Asian/Pacific Islander (A/PI), Hispanic, and American Indian/Alaska Native (AI/AN), with non-Hispanic White as the reference category). We also analyzed the length of time that had elapsed since a beneficiary was diagnosed with diabetes. One of the limitations of the CCW data is that the algorithm that CMS uses starts in 1999, with this being the earliest date of diagnosis available. If a beneficiary had diabetes before 1999, the date of diagnosis in the database is still listed as 1999. We analyzed data from 2012, so we do not know whether someone actually was diagnosed in 1999 and had diabetes for the 13 years from 1999 to 2012, or if someone was diagnosed before 1999 and had diabetes for longer than 13 years. Instead of using the length of time from diagnosis as a continuous measure, we instead include it as a categorical measure of duration of diabetes diagnosis coded as 0–2 years (reference category), 3–7 years, 8–12 years, and over 13 years.
Finally, we included a Part D subsidy variable as a proxy measure for income for Medicare beneficiaries. According to CMS [33], “this variable indicates the beneficiary’s Part D low-income subsidy cost sharing group for a given month. The Part D benefit requires enrollees to pay both premiums and cost-sharing, but the program also has a low-income subsidy that covers some or all of those costs for certain low-income individuals, including deductibles and cost-sharing during the coverage gap.” Each beneficiary receives a code for each month of the year which indicates whether they received any level of the subsidy or not. We coded this variable as 0 = Full Subsidy (received the subsidy in all 12 months), indicating lowest income, 1 = Partial Subsidy (received subsidy in 1 to 11 months), indicating moderate income, and 2 = No subsidy (did not receive subsidy in any month) indicating highest income.
We also included various combinations of MCC as an independent categorical variable. The leading causes of death that are chronic conditions in the State of Michigan [34] are: 1. Heart Disease (we use the CCW database indicator for Congestive Heart Failure - CHF), 2. Cancer, 3. Chronic Lower Respiratory Disease (we use the CCW variable for COPD), 4. Stroke, 5. Alzheimer’s disease, 6. Diabetes, and 7. CKD. There are several variables that code for cancer in the CCW database. We use a combined measure of ever being diagnosed with one of the following cancers for our Cancer variable: breast, colorectal, endometrial, lung, and prostate. Finally, we use the CCW variables for Stroke, AD, CKD as indicators of those conditions.
Because our aim is to establish that disparities in mortality, as well as combinations of MCC that are LCD, exist based on race, we created a new variable that identified beneficiaries as having diabetes alone, as well as any of the 63 combinations of diabetes and MCC that exist. Instead of including all 64 combinations of disease, we chose a cross-section of combinations representing diabetes plus, one, two, three, and four additional MCC (generally based on highest prevalence), including: Type 2 diabetes mellitus alone (Reference category), Diabetes plus CHF, Diabetes plus Cancer, Diabetes plus COPD, Diabetes plus Stroke, Diabetes plus CKD, Diabetes plus CHF/COPD, Diabetes plus CHF/CKD, Diabetes plus CHF/COPD/CKD, Diabetes plus CHF/COPD/Stroke/CKD, and Diabetes plus all other MCC combinations.
Finally, our dependent variable is age at death, where the ages of individuals who were still alive in 2012 were treated as right-censored.
2.2. Statistical Analysis:
We used IBM SPSS 24.0 [35] to analyze the data. We first examined the basic demographic characteristics of our sample. We also calculated mortality rates per 1,000 person-years to examine differences in mortality between groups based on duration of diabetes diagnosis, sex, race, Part D subsidy, patient location, and MCC combinations. Next, we fitted a Cox proportional hazards regression model to our right-censored age at death measure to determine the relationships of our independent socio-demographic variables with the hazard of mortality (Model 1–Table 2). Then, we fitted a multinomial logistic regression model to the categorical dependent variable defined by the 10 combinations of diabetes and MCC, to determine if disparities based on race are evident in diabetes plus MCC combinations when adjusting for the other socio-demographic characteristics (Table 3). Finally, we fitted a second Cox regression model (Model 2–Table 2) to determine if simultaneous inclusion of socio-demographic variables along with our categorical variable indicating MCC combinations in the Cox model influences the relationship of race with the hazard of mortality.
Table 2:
Cox Proportional Hazards Regression Models Predicting Hazard of Mortality
| Model 1 | Model 2 | |
|---|---|---|
| Independent Variables | Hazard ratio (95% CI) | Hazard ratio (95% CI) |
| Duration of Diabetes Diagnosis (Reference: 0–2 years) | ||
| 3–7 years | 0.765 (0.740 – 0.790)*** | 0.759 (0.735–0.785)*** |
| 8–12 years | 0.752 (0.729 – 0.775)*** | 0.725 (0.704–0.748)*** |
| 13+ years | 0.508 (0.488 – 0.528)*** | 0.471 (0.453–0.496)*** |
| Female Sex | 0.605 (0.592 – 0.619)*** | 0.632 (0.618–0.647)*** |
| Race (Reference: Non-Hispanic White) | ||
| Black | 1.149 (1.113 – 1.187)*** | 1.096 (1.061 – 1.131)*** |
| Asian/Pacific Islander | 0.859 (0.749 – 0.985)* | 0.949 (0.828 – 1.089) |
| Hispanic | 1.065 (0.971 – 1.169) | 1.098 (1.000 – 1.205) |
| American Indian/Alaska Native | 1.333 (1.074 – 1.654)** | 1.314 (1.059 – 1.630)* |
| Part D Subsidy (Reference: Full Subsidy) | ||
| Partial Subsidy | 29.794 (27.680 – 32.070)*** | 27.757 (25.787 – 29.877)*** |
| No Subsidy | 5.104 (4.750 – 5.485)*** | 5.718 (5.321 – 6.146)*** |
| Urban Patient Location | 0.989 (0.953 – 1.027) | 0.958 (0.923 – 0.995)* |
| Diabetes MCC Combinations (Reference: Diabetes Alone) | ||
| Diabetes and CHF | 1.382 (1.261 –1.515)*** | |
| Diabetes and Cancer | 1.460 (1.309 – 1.629)*** | |
| Diabetes and COPD | 1.397 (1.257 – 1.553)*** | |
| Diabetes and Stroke | 1.480 (1.310 – 1.673)*** | |
| Diabetes and CKD | 1.757 (1.597 – 1.933)*** | |
| Diabetes and CHF/COPD | 2.389 (2.199 – 2.596)*** | |
| Diabetes and CHF/CKD | 3.283 (3.048 – 3.537)*** | |
| Diabetes and CHF/COPD/CKD | 5.345 (5.006 – 5.707)*** | |
| Diabetes and CHF/COPD/Stroke/CKD | 5.572 (5.204 – 5.966)*** | |
| Diabetes and all other MCC Combinations | 3.547 (3.433 – 3.764)*** |
p<0.05,
p<0.01,
p<0.001
Table 3:
Multinomial Logistic Regression Model Predicting Combinations of Diabetes and Multiple Chronic Conditions
| Reference Outcome Category: Diabetes Alone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetes and CHF | Diabetes and Cancer | Diabetes and COPD | Diabetes and Stroke | Diabetes and CKD | Diabetes and CHF/COPD | Diabetes and CHF/CKD | Diabetes and CHF/COPD/CKD | Diabetes and CHF/COPD/Stroke/CKD | Diabetes and All Other MCC Combinations | |
| Independent Variables | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) |
| Intercept - β (S.E) | −1.151 (0.031)*** | −1.893 (0.041)*** | −0.655 (0.030)*** | −2.043 (0.046)*** | −1.324 (0.033)*** | −0.691 (0.31)*** | −1.409 (0.035)*** | −0.551 (0.029)*** | −1.367 (0.038)*** | 0.811 (0.019) |
| Duration of Diabetes Diagnosis (Reference: 0–2 years) | ||||||||||
| 3–7 years | 0.544 (0.525–0.565)*** | 0.663 (0.637–0.690)*** | 0.634 (0.613–0.655)*** | 0.569 (0.540–0.600)*** | 0.633 (0.611–0.656)*** | 0.496 (0.477–0.517)*** | 0.456 (0.435–0.477)*** | 0.433 (0.416–0.450)*** | 0.387 (0.365–0.409)*** | 0.442 (0.433–0.452)*** |
| 8–12 years | 1.629 (1.575–1.686)*** | 1.332 (1.279–1.387)*** | 1.180 (1.140–1.222)*** | 1.531 (1.458–1.608)*** | 1.408 (1.358–1.459)*** | 1.767 (1.705–1.83)*** | 2.099 (2.021–2.180)*** | 2.117 (2.047–2.189)*** | 2.575 (2.467–2.688)*** | 2.114 (2.070–2.159)*** |
| 13+ years | 2.274 (2.161–2.392)*** | 1.437 (1.346–1.535)*** | 1.074 (1.011–1.141)*** | 1.919 (1.782–2.066)*** | 2.122 (2.013–2.237)*** | 2.124 (2.011–2.244)*** | 4.363 (4.152–4.585)*** | 3.991 (3.812–4.177)*** | 5.657 (5.361–5.970)*** | 3.858 (3.734–3.986)*** |
| Female Sex | 1.035 (1.007–1.065)* | 0.764 (0.740–0.789)*** | 0.972 (0.946–1.000) | 1.072 (1.029–1.116)** | 0.872 (0.848–0.897)*** | 0.977 (0.948–1.007) | 0.892 (0.864–0.920)*** | 0.767 (0.746–0.789)*** | 0.854 (0.824–0.884)*** | 0.827 (0.813–0.840)*** |
| Race (Reference: Non-Hispanic White) | ||||||||||
| Black | 1.219 (1.169–1.271)*** | 1.245 (1.185–1.307)*** | 0.952 (0.910–0.995)* | 0.998 (0.936–1.065) | 1.322 (1.267–1.380)*** | 1.163 (1.113–1.216)*** | 1.468 (1.405–1.533)*** | 1.291 (1.241–1.343)*** | 1.710 (1.633–1.790)*** | 1.505 (1.467–1.543)*** |
| Asian/Pacific Islander | 0.767 (0.688–0.854)*** | 0.765 (0.666–0.878)*** | 0.443 (0.390–0.503)*** | 0.649 (0.546–0.771)*** | 0.636 (0.562–0.720)*** | 0.379 (0.329–0.436)*** | 0.569 (0.497–0.651)*** | 0.285 (0.247–0.329)*** | 0.303 (0.252–0.364)*** | 0.319 (0.296–0.344)*** |
| Hispanic | 0.866 (0.787–0.953)*** | 0.678 (0.596–0.771)*** | 0.727 (0.658–0.803)*** | 0.904 (0.788–1.037) | 0.879 (0.796–0.971)*** | 0.620 (0.554–0.694)*** | 0.775 (0.695–0.865)*** | 0.550 (0.495–0.611)*** | 0.598 (0.524–0.683)*** | 0.574 (0.540–0.610)** |
| American Indian/Alaska Native | 0.696 (0.539–0.900)** | 0.632 (0.460–0.869)** | 0.989 (0.799–1.225) | 0.880 (0.630–1.229) | 1.106 (0.888–1.378) | 0.930 (0.735–1.177) | 0.962 (0.749–1.234) | 0.952 (0.767–1.181) | 1.123 (0.865–1.457) | 0.758 (0.658–0.873)*** |
| Part D Subsidy (Reference: Full Subsidy) | ||||||||||
| Partial Subsidy | 0.817 (0.728–0.916)** | 0.802 (0.673–0.955)* | 0.654 (0.583–0.734)*** | 1.005 (0.854–1.182) | 0.937 (0.829–1.058) | 0.763 (0.686–0.849)*** | 1.374 (1.240–1.523)*** | 1.556 (1.434–1.688)*** | 2.281 (2.091–2.487)*** | 1.985 (1.868–2.108)*** |
| No Subsidy | 0.607 (0.582–0.633)*** | 1.213 (1.140–1.291)*** | 0.500 (0.480–0.520)*** | 0.717 (0.672–0.765)*** | 0.772 (0.737–0.809)*** | 0.343 (0.329–0.357)*** | 0.516 (0.493–0.540)*** | 0.314 (0.302–0.326)*** | 0.284 (0.272–0.297)*** | 0.368 (0.359–0.378)*** |
| Urban Patient Location | 1.098 (1.050–1.148)** | 1.076 (1.023–1.133)** | 0.961 (0.921–1.002) | 0.991 (0.931–1.055) | 1.093 (1.044–1.144)*** | 0.969 (0.926–1.105) | 1.177 (1.118–1.238)*** | 1.131 (1.082–1.182)*** | 1.199 (1.131–1.272)*** | 1.287 (1.253–1.323)*** |
p<0.05,
p<0.01,
p<0.001
3. RESULTS
Table 1 includes descriptive statistics for our entire sample as well as the mortality rates per 1,000 person-years. The average age of our sample is 77.2 years old, and consistent with this age group and U.S. census data, our sample is mostly female (56.1%) and mostly white (82.0%). A little over 89% live in counties designated as urban, and about 18% received some Part D subsidy during the year, indicating incomes low enough to meet the requirements for receiving the subsidy. Close to 65% of beneficiaries had a duration of diabetes diagnosis of three to seven years or eight to 12 years. While close to 25% of beneficiaries had a diagnosis of diabetes alone, CHF and COPD (both at 5.8%) were the MCCs with the highest prevalence for diabetes plus one additional chronic condition. The most prevalent MCC combination is diabetes plus CHF/COPD/CKD (6.2%). Finally, 7.3% of beneficiaries with diabetes plus any MCC died in 2012.
Table 1:
Descriptive Statistics for Medicare Beneficiaries with Type 2 Diabetes Mellitus, n=443,932
| Independent Variables | n (%) | Mortality rate/1000 person-years (95% CI) |
|---|---|---|
| Age - mean (SD) | 77.19 (7.88) | |
| Duration of Diabetes Diagnosis | ||
| 0–2 years | 103,470 (23.3) | 57 (56–59) |
| 3–7 years | 153,225 (34.5) | 59 (58–60) |
| 8–12 years | 134,435 (30.3) | 96 (94–98) |
| 13+ years | 52,802 (11.9) | 84 (81–86) |
| Sex | ||
| Male | 195,073 (43.9) | 76 (75–78) |
| Female | 248,859 (56.1) | 70 (69–71) |
| Race | ||
| Non-Hispanic White | 364,156 (82.0) | 74 (73–75) |
| Black/African American | 64,475 (14.5) | 71 (69–73) |
| Asian/Pacific Islander | 5,564 (1.3) | 37 (32–43) |
| Hispanic | 8,185 (1.8) | 55 (50–61) |
| American Indian/Alaska Native | 1,552 (0.3) | 53 (43–66) |
| Part D Subsidy | ||
| Full Subsidy | 68,048 (15.3) | 11 (10–12) |
| Partial Subsidy | 14,733 (3.3) | 580 (567–592) |
| No Subsidy | 361,151 (81.4) | 64 (63–65) |
| Patient Location | ||
| Rural | 46,882 (10.6) | 66 (64–69) |
| Urban | 397,050 (89.4) | 74 (73–75) |
| Combinations of Diabetes and MCC | ||
| Diabetes | 109,901 (24.8) | 11 (10–12) |
| Diabetes and CHF | 25,534 (5.8) | 29 (27–31) |
| Diabetes and Cancer | 17,667 (4.0) | 25 (23–27) |
| Diabetes and COPD | 25,927 (5.8) | 19 (17–20) |
| Diabetes and Stroke | 10,700 (2.4) | 31 (28–34) |
| Diabetes and CKD | 23,790 (5.4) | 27 (25–30) |
| Diabetes and CHF/COPD | 21,623 (4.9) | 49 (46–52) |
| Diabetes and CHF/CKD | 20,185 (4.5) | 84 (81–89) |
| Diabetes and CHF/COPD/CKD | 27,335 (6.2) | 138 (134–142) |
| Diabetes and CHF/COPD/Stroke/CKD | 15,408 (3.5) | 183 (177–190) |
| Diabetes and other MCC Combinations | 145,862 (32.9) | 131 (129–133) |
| Dependent Variable | ||
| Mortality | ||
| Living at end of 2012 | 411,592 (92.7) | |
| Died in 2012 | 32,340 (7.3) |
Regarding the mortality rates per 1,000 person-years, there was no difference between Black (71, 95% CI: 69–73) and White (74, 95% CI: 73–75) beneficiaries. However, the rates for A/PIs (37, 95% CI: 32–42), Hispanics (55, 95% CI: 50–61), and AI/ANs (53, 95% CI: 43–66) significantly differed from each other and from the White and Black rates. The mortality rate per 1,000 person years for beneficiaries with a duration of diagnosis from eight to 12 years (96, 95% CI: 94–98) was significantly higher than each of the other groups based on duration of diagnosis. Finally, as might be expected, increasing numbers of MCC were associated with higher mortality rates. For instance, the mortality rate for beneficiaries with diabetes plus CHF/COPD/CKD (the most common MCC combination) was 138 (95% CI: 134–142) compared to 11 (95% CI: 10–12) for those with diabetes alone.
The results of the first Cox regression model using race and other socio-demographic variables to predict the hazard of mortality highlight several significant relationships (Table 2–Model 1). First, we observed no difference in the risk of mortality between Whites and Hispanics (HR = 1.07, 95% CI:0.971–1.17. However, A/PIs had a 14% lower risk (HR = 0.859, 95% CI:0.749–0.985) compare to Whites. Finally, Blacks had a 15% greater risk (HR = 1.15, 95% CI: 1.11–1.19) and AI/ANs had 33% greater risk (HR = 1.33, 95% CI: 1.07–1.65) of mortality compared to Whites. Compared with beneficiaries with a duration of diabetes diagnosis of zero to two years, beneficiaries with diabetes from three to seven years had 24% lower risk (HR = 0.765, 95% CI:0.740–0.790), eight to 12 years had 25% lower risk (HR = 0.752, 95% CI:0.729–0.775), and 13 or more years had a 49% lower risk (HR=0.508, 95% CI:0.488–0.528) of mortality. These results are contradictory to the mortality rates per 1000 person-years and we expand on this in greater detail in the Discussion section. Compared to males, females had a 30% lower risk of dying (HR = 0.605, 95% CI:0.592–0.619), while there was no difference in the risk of death between beneficiaries living in urban counties compared to rural counties (HR = 0.989, 95% CI:0.953–1.027). Finally, the relationship of the Part D Subsidy, as a measure of low income, with mortality was not consistent with expectations. Compared to beneficiaries who received the full subsidy (778 deaths from 68,048 beneficiaries), those who received a partial subsidy (8,538 deaths from 14,733 beneficiaries; HR = 29.79, 95% CI: 27.68–32.07), and no subsidy (23,024 deaths from 361,151 beneficiaries; HR = 5.10, 95% CI: 4.75–5.49), had greatly increased risks of mortality, perhaps indicating that the subsidy acts to remove barriers to care for the lowest income beneficiaries and provides intermittent coverage to those with moderate income who only qualify for the subsidy for limited parts of the benefit year. We expand on this in the Discussion section.
Next, Table 3 includes the results of the multinomial logistic regression model that we fitted to determine the influence of race on the MCC combinations. There are some notable highlights for all variables. First, Black Medicare beneficiaries had increased odds of all diabetes-MCC combinations (except for diabetes plus COPD and diabetes plus Stroke), compared to diabetes alone, compared to Whites. The increased odds ranged from 16% greater for diabetes plus CHF/COPD (OR = 1.163, 95% CI:1.113–1.216) to 71% greater odds for diabetes plus CHF/COPD/Stroke/CKD (OR = 1.710, 95% CI:1.633–1.790). In addition, Blacks are at 29% increased odds for diabetes plus CHF/COPD/CKD (OR = 1.291, 95% CI:1.241–1.343), which is the most common MCC combination observed in this cohort. Conversely, A/PIs are at lower odds for all diabetes MCC combinations, compared to diabetes alone, relative to Whites. For instance, the decreased odds range from 23% lower odds for diabetes plus Cancer (OR = 0.765, 95% CI:0.666–0.878) to 71% lower odds for diabetes plus CHF/COPD/CKD (OR = 0.285, 95% CI:0.247–0.329). In addition, Hispanics have about 13% to 45% lower odds for several combinations compared to diabetes alone, most notably diabetes plus CHF/COPD/CKD (OR = 0.550, 95% CI:0.495–0.611), compared to Whites. Finally, there are very few differences in odds of MCC combinations compared to diabetes alone between AI/ANs and Whites, with significantly lower odds related to Diabetes plus CHF (OR = 0.696, 95% CI:0.539–0.900),Cancer (OR = 0.632, 95% CI:0.460–0.869), and Diabetes plus All Other MCC Combinations (OR = 0.758, 95% CI:0.658–0.873).
In addition to race, we also observed several important relationships between diabetes plus MCC and duration of diabetes diagnosis, sex, Part D Subsidy, and patient location (Table 3). For instance, after 7 years, the increased duration of diagnosis significantly increases the odds of having various MCC combinations, as one would expect. In most cases of MCC combinations, females are at lower odds for any combination compared to diabetes alone, relative to males. For instance, females are at about 11% lower odds for diabetes and CHF/CKD (OR = 0.892, 95% CI:0.864–0.920), and 23% lower odds for diabetes plus CHF/COPD/CKD (OR = 0.0.767, 95% CI:0.746–0.789). However, females have slightly increased odds for cardiovascular related outcomes including diabetes plus CHF (OR = 1.03, 95% CI: 1.007–1.065), and diabetes plus Stroke (OR = 1.072, 95% CI: 1.029–1.116). Urban dwellers consistently experience increased odds for most MCC combinations, most notably 29% increased odds for Diabetes plus All Other MCC Combinations (OR =1.287, 95% CI: 1.253–1.323).
Regarding the Part D Subsidy, for beneficiaries with no subsidy (i.e. highest incomes) we observed lower odds of any MCC combination (except diabetes plus Cancer), ranging from 23% lower odds for diabetes plus CKD (OR = 0.772, 95% CI:0.737–0.809) to 72% lower odds for diabetes plus CHF/COPD/Stroke/CKD (OR = 0.284, 95% CI:0.272–0.297), compared to those with the full subsidy (i.e. lowest income). Results for the partial subsidy (moderate income) are equivocal. Compared to those with the full subsidy, beneficiaries with the partial subsidy had 35% lower odds for diabetes plus COPD (OR = 0.654, 95% CI:0.583–0.734), and 128% increased odds for diabetes plus CHF/COPD/Stroke/CKD (Table 3).
Finally, results from the Cox proportional hazards regression model that included MCC as a covariate (Table 2–Model 2) are quite similar to Model 1 without MCC. First, duration of diabetes diagnosis and female sex are associated (with similar magnitude) with lower risk of mortality, while less than a full Part D Subsidy is associated with (again with similar magnitude) increased risk of mortality. In other words, even when adjusting for the presence of MCC combinations, females are still at lower risk for mortality, and beneficiaries without a Part D Subsidy are still at increased risk of dying (with very similar differences between these groups).
Some results related to groups based on race are also similar. There is still no difference in the odds of mortality between Hispanics and Whites. In addition, compared to Whites, A/PIs are still at lower risk of mortality (5% lower odds, HR = 0.949, 95% CI: 0.828–1.089), although this difference is not significant. Finally, when adjusting for diabetes plus MCC, Blacks had 10% higher risk (HR = 1.10, 95% CI:1.061–1.131) of mortality, and AI/AN’s had 31% higher risk (OR=1.31, 95% CI:1.059–1.630) of mortality, compared to whites. As expected, the presence of MCC in addition to diabetes increased the risk of mortality in all cases by almost 40% (Diabetes plus CHF, HR = 1.38, 95% CI: 1.26–1.52) to 460% (Diabetes plus CHF/COPD/Stroke/CKD, HR = 5.57, 95% CI: 5.20–5.97).
4. DISCUSSION
The aim of this study was to determine if there are differences in mortality rates, and combinations of diabetes and MCC, based on race and other socio-demographics among Medicare beneficiaries in the state of Michigan with diabetes and other chronic conditions. Our results indicate that race does influence overall mortality in this cohort. Contrary to other literature [36, 37], we observed lower risk (A/PIs) or no difference in risk (Hispanics) of all-cause mortality compared to Whites. In addition, Blacks had increased risk of mortality (even after adjusting for MCC) compared to Whites, which is in agreement with other literature that shows increased mortality for racial and ethnic groups [10]. We observed that those living in urban areas have lower risk of mortality compared to their rural counterparts, while females have lower risk compared to males. Finally, Part D Subsidy, as a measure of low income, was protective against mortality, with those not receiving the full subsidy at higher risk of mortality. This, too, is contrary to most literature regarding the inverse relationship between income or poverty and poorer health outcomes [38]. However, although eligibility for the subsidy is income-based, we recognize that the Part D subsidy might not be a true measure of income and the decreased risk of mortality associated with receiving having Part D drug insurance is supported by other literature that reports similar findings [39, 40].
Finally, although we observed higher mortality rates per 1,000 person-years with increasing duration of diabetes, we also observed decreased hazards of mortality with increased duration of diabetes. On the face this seems contradictory. However, mortality rates are expressed as unadjusted rates, while hazard ratios were generated in the context of a fully saturated Cox model using right-censored age at death as the time measure and accounting for several covariates. The mean age at death is significantly lower for those with a duration of diabetes of zero to two years (78.81, 95% CI:78.56–79.05) compared to beneficiaries with durations of three to seven years (80.49, 95% CI:80.31–80.67), eight to 12 years (83.43, 95% CI:83.30–83.56), and 13+ years (84.93, 95% CI: 84.74–85.12). Our results support other studies reporting that onset of type 2 diabetes in the elderly has little effect on lifespan and in fact, the reduction in life expectancy declines with increasing age [41]. Once people have diabetes for a longer time, their life expectancy approaches normal and their risk of dying from diabetes actually decreased, but the possibility exists that for elderly people who are newly diagnosed their risk is heightened [42] until they learn to manage their diabetes. The mortality rates per 1000 person years reflect the fact that older people die at greater rates so if people have diabetes for a longer period of time, they are probably older with a higher mortality rate. But the relationship between age and newly diagnosed (who die at earlier ages) may lead to contradictory hazard ratios and mortality rates for these groups.
Regarding the analyses with diabetes and MCC as the dependent variable, we observed increased odds for Blacks having any of the MCC combinations compared to having diabetes alone, relative to being White. In this cohort, Blacks have increased odds of 16% to 71% of having combinations of diabetes and MCC that are LCD; and, when adjusting for these combinations, Blacks have 10% higher risk of mortality than Whites. In general, where the relationships were significant, A/PIs, Hispanics, and AI/ANs had lower odds for diabetes and MCC combinations, compared to having diabetes alone and being White. Females had lower odds of MCC combinations compared to males with the notable exceptions of diabetes plus CHF and diabetes plus Stroke, while residents of urban counties had higher odds for MCC combinations. Increased duration of a diabetes diagnosis was generally associated with MCC combinations, but only after seven years. Contrary to the results for Part D Subsidy and mortality, for beneficiaries with no subsidy (higher income), the odds for diabetes plus MCC combinations were lower in most cases with the exception being Diabetes plus Cancer). For the group with a partial subsidy, lower odds for MCC combinations were generally observed for diabetes and one or two other chronic conditions, while increased odds were observed for diabetes and greater than two other chronic conditions. There is some evidence [43] that those who receive the full low-income Part D subsidy have higher rates of comorbidity, which is in agreement with our finding that beneficiaries with the partial subsidy or no subsidy generally have lower odds of MCC combinations. Clearly, there are race and ethnic disparities related to the presence of MCC in addition to diabetes. In every instance where race is a significant predictor of the different diabetes and MCC combinations, Black race significantly increases odds of these combinations, consistent with previous literature, while A/PIs, Hispanics, and AI/ANs generally experience significantly lower odds of these combinations. Our observation that Blacks have increased odds of diabetes and CHF is contrary to findings from Lewis et al. [14] that Whites with diabetes are more likely to develop CHF or other cardiovascular complications compared to diabetic racial and ethnic minority populations. However, Blacks have higher odds of developing diabetes and CKD, supporting the results of previous studies [9,14–16], yet the lower odds of CKD related combinations in Hispanics is contrary to Golden et al. [9] and Lewis et al. [14]. Finally, the lower odds of CHF in Hispanics in our cohort supports results of other studies [18].
Finally, there are very few published studies about the experience of A/PIs and the development of Diabetes plus MCC combinations. Across all combinations, A/PIs had lower odds of MCC combinations compared to Whites. Additional analyses indicate that 38.4% of A/PIs with diabetes have no other chronic condition we studied, statistically greater at P<0.05, than Whites (25.6%), Blacks (18.3%), Hispanics (30.2), and AIANs (27.5). In addition, while this same trend exists for A/PIs with diabetes plus one additional chronic condition (specifically CHF and Cancer), the prevalence of all other combinations of diabetes and MCC is significantly lower for this race group compared to all other groups based on race. Perhaps this indicates better use of prevention and intervention services, as well as adherence to dietary and medical management guidelines in the A/PIs.
We analyzed data for a large cohort of Medicare beneficiaries with Type 2 diabetes mellitus in the State of Michigan to determine the effects of race on MCC and mortality. A quick glance at our results indicates that most of the regression coefficients tested in these analyses are statistically significant. That is one of the limitations of analyzing data using large sample sizes. One of the natural concerns about these results is that the large sample sizes available to us provided additional power to detect small effect sizes. In our case, we note that we observed large effect sizes for many of the hypothesized relationships that would have been found as significant even with smaller samples. In addition, we were limited in the risk factors that we could include this study. As we used administrative claims data, we were limited to data detailing some diagnoses and basic beneficiary demographics such as those included here. We could not include proxy measures for other commonly accepted risk factors such as BMI, smoking status, measures of cholesterol or lipid profiles, and kidney function measures. Finally, there is a risk of over-adjustment in the relationship between mortality and race when adjusting for MCC. Although we do not include the results here, we did fit additional Cox models to create unadjusted regression coefficients for each independent variable. In all cases, the unadjusted hazard ratios for each level of each variable, including race, were remarkably similar to the model including all of the covariates, providing evidence against over-adjustment.
In summary, using a cohort of Medicare beneficiaries from the State of Michigan with Type 2 diabetes mellitus, our goal was to determine race-based differences in mortality and prevalence of MCC combinations. We found disparities in the presence of MCC combinations, as well as in mortality, after adjusting for these combinations. Future research in this area will focus on the effects of race and other demographics, while adjusting for MCC combinations, on the use of prevention and intervention services, as well as use of the emergency department and inpatient hospital stays, in this cohort of Medicare beneficiaries.
Acknowledgements:
Funding: This work was supported by the National Institutes of Health [Grant number: 1R15DK104260-01A1, 2015-2019].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose
REFERENCES
- [1].American Diabetes Association [Internet]. Statistics about Diabetes. [cited 2019 July 31]. Available from: http://www.diabetes.org/diabetes-basics/statistics/.
- [2].Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev 2002;18(Suppl. 3):S21–6. 10.1002/dmrr.289. [DOI] [PubMed] [Google Scholar]
- [3].Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther 2008;88(11):1254–64. 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7. 10.1038/414782a [DOI] [PubMed] [Google Scholar]
- [5].Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 2014;312(12):1218–26. https://doi:10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- [6].Insaf TZ, Strogatz DS, Yucel RM, Chasan-Taber L, Shaw BA. Associations between race, lifecourse socioeconomic position and prevalence of diabetes among US women and men: results from a population-based panel study. J Epi Community Health 2014. 68(4):318–25. 10.1136/jech-2013-202585. [DOI] [PubMed] [Google Scholar]
- [7].Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015;314(9):1021–9. https://doi:10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- [8].Shih M, Du Y, Lightstone AS, Simon PA, Wang MC. Stemming the tide: rising prevalence and ethnic subgroup variation among Asians in Los Angeles County. Prev Med 2014;63:90–5. 10.1016/j.ypmed.2014.03.016. [DOI] [PubMed] [Google Scholar]
- [9].Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: Biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. J Clin Endocrinol Metab 2014:97(9):E1579–1639. 10.1210/jc.2012-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosenstock S, Whitman S, West JF, Balkin M. Racial disparities in diabetes mortality in the 50 most populous US cities. J Urban Health 2014;91(5):873–85. https://doi:10.1007/s11524-013-9861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care 2005;28(9):2280–88. 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- [12].Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: An evidence review and map. Am J Public Health 2018;108(3): e1–e11. 10.2105/AJPH.2017.304246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].American Diabetes Association. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Supplement 1):S28–S37. https://DOI:10.2337/dc18-S003. [DOI] [PubMed] [Google Scholar]
- [14].Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJV, Solomon SD. et al. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. Am Heart J 2015;170(2):322–9. 10.1016/j.ahj.2015.05.008. [DOI] [PubMed] [Google Scholar]
- [15].Dreyer G, Hull S, Mathur R, Chesser A, Yaqoob MM. Progression of chronic kidney disease in a multi-ethnic community cohort of patients with diabetes mellitus. Diabetic Medicine: A Journal of the British Diabetic Association 2013;30(8):956–63. 10.1111/dme.12197. [DOI] [PubMed] [Google Scholar]
- [16].Sinha SK, Shaheen M, Rajavashisth TB, Pan D, Norris KC, Nicholas SB. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care 2014;37(4):1060–8. 10.2337/dc13-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. HbA1c and coronary heart disease risk among diabetic patients. Diabetes Care 2014;37(2):428–35. 10.2337/dc13-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G. et al. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res 2012;9(2):146–52. https://doi.org/10.1177%2F1479164112436403. [DOI] [PubMed] [Google Scholar]
- [19].Cavicchia PP, Adams SA, Steck SE, Hussey JR, Liu J, Daguisé VG, et al. Racial disparities in colorectal cancer incidence by type 2 diabetes mellitus status. Cancer Causes Control 2013;24(2):277–85. 10.1007/s10552-012-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and Cancer: A Consensus Report. CA: A Cancer Journal for Clinicians 2010;60:207–21. 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- [21].Setiawan VW, Hernandez BY, Lu SC, Stram DO, Wilkens LR, Marchand LL, et al. Diabetes and racial/ethnic differences in hepatocellular carcinoma risk: The multiethnic cohort. J Natl Cancer Inst 2014;106(12):dju326. 10.1093/jnci/dju326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu AH, Kurian AW, Kwan ML, John EM, Lu Y, Keegan THM, et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: The California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomarkers Prev 2015;24(2):361–8. 10.1158/1055-9965.EPI-14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gläser S, Krüger S, Merkel M, Bramlage P, Herth FJF. Chronic obstructive pulmonary disease and diabetes mellitus: A systematic review of the literature. Respiration 2015;89:253–64. 10.1159/000369863. [DOI] [PubMed] [Google Scholar]
- [24].Davis J, Penha J, Mbowe O, Taira DA. Prevalence of single and multiple leading causes of death by race/ethnicity among US adults aged 60 to 79 years. Prev Chronic Dis 2017;14:E101 https://dx.doi.org/10.5888%2Fpcd14.160241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119(3):e21–181. [DOI] [PubMed] [Google Scholar]
- [26].Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation 2010;121(13):1492–1501. https://doi:10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- [27].Spanakis EK, Golden SH. Race/ethnic differences in diabetes and diabetic complications. Curr Diab Rep 2013;13(6):814–23. 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer’s disease: A population-based cohort study. Diabetologia 2009;52:1031–39. 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- [29].Rajan KB, Arvanitakis Z, Lynch EB, McAninch EA, Wilson RS, Weuve J, et al. Cognitive decline following incident and preexisting diabetes mellitus in a population sample. Neurology 2016;87:1681–7. 10.1212/WNL.0000000000003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Young BA, Maynard C, Boyko EJ. Racial Differences in Diabetic Nephropathy, Cardiovascular Disease and Mortality in a National Population of Veterans. Diabetes Care 2003;26:2392–9. 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- [31].Gimeno-Orna JA, Blasco-Lamarca Y, Campos-Gutierrez B, Molinero-Herguedas E, Lou-Arnal LM, García-García B. Risk of mortality associated to chronic kidney disease in patients with type 2 diabetes mellitus: a 13-year follow-up. Nefrologia 2015;35(5):487–92. https://doi:10.1016/j.nefro.2015.05.025. [DOI] [PubMed] [Google Scholar]
- [32].Lind M, Garcia Rodriguez LA, Booth GL, Cea-Soriano L, Shah BR, Ekeroth G, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population‐based study. Diabetologia 2013;56(12):2601–8. 10.1007/s00125-013-3063-1. [DOI] [PubMed] [Google Scholar]
- [33].ResDAC Research Data Assistance Center [Internet]. Monthly cost sharing group under Part D low-income subsidy. [cited 2019 July 30]. Available from: https://https://www.resdac.org/cms-data/variables/monthly-cost-sharing-group-under-part-d-low-income-subsidy-march.
- [34].2012 Geocoded Michigan Death Certificate Registry. Division for Vital Records & Health Statistics, Michigan Department of Health & Human Services; Population Estimate (latest update 6/2018), National Center for Health Statistics, U.S. Census Populations With Bridged Race Categories [cited 2019 May 29]. Available from: http://www.mdch.state.mi.us/pha/osr/deaths/causrankcnty.asp.
- [35].IBM Corp. IBM SPSS Statistics (24.0) for Windows, Released 2016. Armonk, NY: IBM Corp. [Google Scholar]
- [36].Kuo YF, Raji MA, Markides KS, Ray LA, Espino DV, Goodwin JS. Inconsistent use of diabetes medications, diabetes complications, and mortality in older Mexican Americans over a 7-year period: data from the Hispanic established population for the epidemiologic study of the elderly. Diabetes Care 2003;26(11):3054–60. 10.2337/diacare.26.11.3054. [DOI] [PubMed] [Google Scholar]
- [37].Liu JJ, Choo RWM, Liu S, Gurung RL, Wee SL, Lim SC. Cause-specific mortality in multiethnic South East Asians with type 2 diabetes mellitus. Asia Pac J Public Health 2019;31(4):306–14. https://doi.org/10.1177%2F1010539519849317. [DOI] [PubMed] [Google Scholar]
- [38].Saydah SH, Imperatore G, Beckles GL. Socioeconomic status and mortality: Contribution of health care access and psychological distress among U.S. adults with diagnosed diabetes. Diabetes Care 2013;36(1): 49–55. 10.2337/dc11-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Huh J and Reif J. Did Medicare Part D reduce mortality? J of Health Econ 2017;53:17–37. https://doi.org/10/1016/j.healeco.2017.01.005. [DOI] [PubMed] [Google Scholar]
- [40].Kaestner R, Schiman C, and Alexander GC. Effects of Prescription Drug Insurance on Hospitalization and Mortality: Evidence from Medicare Part D. Journal Risk and Insurance 2019; 86:595–628. doi: 10.1111/jori.12229. [DOI] [Google Scholar]
- [41].Panzram G Mortality and survival in type 2 (non-insulin dependent) diabetes mellitus. Diabetologia 1987;30:123 10.1007/BF00274216. [DOI] [PubMed] [Google Scholar]
- [42].Smith NL, Barzilay JI, Kronmal R, Lumley T, Enquobahrie D, and Psaty BM. New-onset diabetes and risk of all-cause and cardiovascular mortality. Diabetes Care 2006. 29(9):2012–17. 10.2337/dc06-0574. [DOI] [PubMed] [Google Scholar]
- [43].Stuart B, Yin X, Davidoff A, Simoni-Wastila L, Zuckerman I, Shoemaker JS, Doshi J. Impact of Part D low-income subsidies on medication patterns for Medicare beneficiaries with diabetes. Medical Care 2012;50(11):913–19. [DOI] [PubMed] [Google Scholar]