Abstract
Objective:
To determine whether systemic medical factors, such as vascular risk factors, metabolic and inflammatory markers contribute to cognitive decline in Parkinson’s disease (PD); if confirmed to determine whether a clinically applicable risk factor model can predict the conversion from normal cognition (NC) to mild cognitive impairment (MCI).
Methods:
58 patients who met the UK Brain Bank Criteria for PD underwent clinical and laboratory assessment at study entry; 47 patients were re-assessed after two years. Medical history, vascular risk (QRISK2), blood metabolic and inflammatory factors, brain vessel examinations, activity of daily living, and neuropsychological testing were performed.
Results:
Forty patients had NC and 18 patients had MCI at baseline. Patients with MCI had higher level of interleukin 6, folic acid below normal range and higher L-dopa equivalent dose compared to cognitive normal patients at baseline. Patients with NC at baseline were classified into two groups: patients who remained cognitively normal (non-converters, n=23) and patients who progressed to MCI (converters, n=11). MCI converters were older at baseline and had higher QRISK2 than the non-converters. Patients with higher QRISK2, lower uric acid level and lower activity of daily living scale at baseline had a higher risk of converting from NC to MCI with a sensitivity of 72.2%, a specificity of 87%, and an overall accuracy of 82.4%.
Conclusion:
Systemic medical factors are associated with cognitive impairment in PD both cross-sectionally and longitudinally. A risk factor model predicting the decline from NC to MCI could be constructed.
Keywords: vascular risk factors, inflammation, metabolic factors, cognitive impairment, Parkinson’s disease
Introduction
Cognitive impairment in PD is probably linked to the spread of synucleinopathy-associated neurodegeneration. Medical co-morbidities, including vascular impairment (Rektor et al.2009) and metabolic (Schlesinger 2008) and inflammatory factors (Dufek et al. 2009, Veselý et al. 2018) may also contribute to the cognitive decline. Our previous study (Rektor et al. 2009) showed significant correlations between the presence of vascular factors, including intima-medial thickness (IMT), an indicator of large vessel impairment, and pulsatility and resistance indices that indicate small vessel impairment, and cognitive and clinical status. Another study provided evidence that co-morbid atherosclerosis and otherwise subclinical impairment of brain vessels may increase the risk of mortality in PD patients (Rektor et al. 2012). Inflammation may also contribute to cognitive decline in PD (Veselý et al. 2018, Dufek et al. 2009, Bonifati et al. 2007). However, the extent to which the contribution of these medical co-morbidities is clinically relevant and may modify the clinical course of the disease is unclear. Unlike α-synucleinopathy, medical co-morbidities provide more opportunities for therapeutic interventions at the present time. Such systemic medical treatment may have the potential to prevent or slow cognitive decline in patients with PD. As Parkinson’s disease mild cognitive impairment (PD-MCI) is considered to be a transitional state to PD dementia (PDD), treatments are urgently needed to slow its progression (Litvan et al.2018). Availability of systemic medial risk factors models that could identify patients at high risk of cognitive decline is therefore urgently needed in clinical practice. Because of the disease heterogeneity of PD and the complexity of underlying causes for cognitive impairment in PD, a single biomarker is not likely to diagnose or predict cognitive decline in PD. Therefore, an algorithm combining different biomarkers may prove to be more useful.
Methods
A total of 58 patients were assessed during baseline visits (Table 1). From this initial cohort, 47 patients were re-assessed after two years. None of the patients had systemic inflammatory disease or other serious co-morbidity. None of the patients were treated with antidepressants, anticholinergics, acetylcholinesterase inhibitors, statins, uric acid lowering medications, vitamin B12, or folic acid supplementation therapy. All patients gave their written informed consent. The ethics committee of the Faculty Hospital Nitra, Slovak Republic, approved the study protocol.
Table 1.
Descriptive summary of the patients’ data
| All subjects (n=58) | Patients with normal cognition at baseline with 2-yr follow-up data (n=34) |
||
|---|---|---|---|
| Gender | F | 24 (41.4%) | 13 (38.2%) |
| M | 34 (58.6%) | 21 (61.8%) | |
| Education | Basic | 10 (17.2%) | 3 (8.8%) |
| High school | 34 (58.6%) | 21 (61.8%) | |
| University | 14 (24.1%) | 10 (29.4%) | |
| Motor phenotype | Intermediate | 11 (19.0%) | 7 (20.6%) |
| PIGD | 34 (58.6%) | 20 (58.8%) | |
| TD | 13 (22.4%) | 7 (20.6%) | |
| Age at baseline (years) | 64.0 ± 8.1, 65.0 (45.0–82.0) |
63.1 ± 7.9, 64.0 (50.0–82.0) |
|
| Age at onset of PD (years) | 58.4 ± 8.4, 60.0 (40.0–76.0) |
57.7 ± 8.1, 59.0 (40.0–76.0) |
|
| Duration of PD at baseline (years) | 5.6 ± 4.1, 4.0 (1.0–20.0) |
5.3 ± 3.4, 4.5 (1.0–15.0) |
|
| Daily L-dopa equivalent | 503.8 ± 365.2, 412.5 (27.0–2010.0) |
402.1 ± 291.9, 362.0 (54.0–1115.0) |
|
Legend: FUP (follow-up), PD (Parkinson’s disease), PIGD (postural instability and gait disorder), TD (tremor-dominant)
The clinical status of PD patients was assessed using the Unified Parkinson’s disease rating scale (UPDRS part I, II, III) (2003), activity of daily living (ADL) scale (Harrison et al. 2009), and Barthel index (BI) (Mahoney and Barthel 1965). A detailed medical history, including assessment of medical co-morbidities and vascular risk factors was obtained from each patient. Laboratory tests were performed at the Faculty Hospital in Nitra, Slovakia. Normative data based on values obtained in healthy blood donors. All patients had blood tests for the serum level of uric acid (Dimension Vista®), folic acid (Dimension Vista®), vitamin B12 (Dimension Vista®), lipoprotein (Dimension Vista®), cholesterol (Dimension Vista®), and homocysteine (Dimension Vista®). We also measured interleukin 6 (IL-6) and C3, C4 complement serum levels. These factors were chosen as they displayed a clinical relevance in PD in our previous studies (Veselý et al. 2018, Dufek et al. 2009). The level of IL-6 was measured by an electrochemiluminescence immunoassay method (ECLIA) commercial test (Elecsys Cobas®). Factors of the complement system (C3, C4) were assessed by nephelometry (Dimension Vista® system Flex reagent® cartridge).
The patients completed a cognitive test battery consisting of the Mini-mental state examination (MMSE) (Folstein et al. 1975), Addenbrooke’s cognitive examination (ACE) (Hsieh et al. 2015, Matias-Guiu et al. 2017), Benton visual retention test (BVRT) (Barrash et al. 2010), the clock drawing test (CDT) (De Pandis et al. 2010), the verbal fluency test (VFT) (Auriacombe et al. 1993, and immediate and delayed recall subtests of the Wechsler Memory Scale III (WMS III) (Wechsler 1997). Patients were classified as normal cognition (NC) or MCI. MCI was defined using level 1 MDS criteria (Litvan et al. 2012).
We used the QRISK2 scale, which encompasses risk factors including age, gender, elevated cholesterol, blood pressure/treatment, diabetes, smoking status, body mass index, and chronic kidney disease; the scale is appropriate in patients who have not had a prior vascular event (https://qrisk.org/2016/) to be correlated with clinical findings. All patients underwent ultrasonographic (USG) examination of the intimal-medial thickness (IMT) of the common carotid artery (CCA) on both sides. Most of the patients also underwent USG examination of the pulsatility index (PI) (40 patients on the right side and 42 on the left side) and the resistance index (RI) (41 patients) of the medial cerebral artery (MCA) on both sides. In other patients, PI and RI were not measured for technical reasons (not all the patients had temporal bone window).
Statistics
Categorical data were presented as proportions, and their associations with cognitive status at baseline and with cognitive decline over a two-year period were tested using the Fisher’s exact test. Quantitative data were described as mean±SD and median (minimum-maximum), their correlations were assessed using Spearman’s rank correlation coefficient, and between-group comparisons were analysed using the Mann-Whitney U test because the data were not normally distributed according to the Shapiro-Wilk test. For significant variables (p<0.05), receiver operating characteristic (ROC) analyses were performed to determine an area under the curve (AUC) expressing the discriminatory ability of the variables to distinguish PD NC from MCI patients at baseline and MCI converters from non-converters over the two-year period. To identify most robust associations with cognitive status and discard any possible false positive results due to multiple testing, multivariate stepwise logistic regression analyses based on significant variables were performed.
In addition, changes in the quantitative data over the two-year period were examined using paired Wilcoxon tests. The level of significance was set at p<0.05. The calculated p-values were corrected for multiple comparisons using Benjamini-Hochberg false discovery rate (FDR) correction. To predict the decline from NC to MCI over a two-year period, multivariate stepwise logistic regression model was created based on stepwise selection from all variables at baseline. The rationale for usage of all variables instead of the statistically significant ones is that a variable which is insignificant in univariate analysis can substantially aid in prediction when it is combined with other variables in a multivariate model. The model enabled calculation of the probability of cognitive decline for each patient, computation of odds ratio (OR) with confidence intervals (CI) for each variable and estimation of sensitivity, specificity and accuracy of the model. The statistical analyses were performed using IBM SPSS Statistics software (version 25) and MATLAB R2018a.
Results
Cognitive status at baseline and at 2-year follow-up
At baseline, 40 patients were classified as NC and 18 patients as MCI. At the follow-up visit, 11 patients from the baseline NC group had converted to MCI; 23 NC patients remained cognitively normal; and 6 NC patients were lost to follow-up care. In the MCI group, 5 patients were lost to follow-up care; the remaining 13 MCI patients did not progress to dementia over the two years.
Comparison between NC vs. MCI patients at baseline
The comparison of the clinical data, vascular risk factors, metabolic and immunology factors and brain vessel pathology at the baseline visit between NC and MCI patients indicated that the patients in the MCI group had lower levels of education (p=0.012), higher age (p=0.017), higher L-dopa equivalent (p=0.010), higher values of IMT of right carotid artery (p=0.030), higher levels of the C3 complement factor (p=0.033), and higher levels of IL-6 than the patients in the NC group (p=0.009; Table 2). ROC analysis revealed AUC values for age of 0.697 (CI=0.543–0.850, p=0.017), L-dopa equivalent dose of 0.713 (CI=0.573–0.852, p=0.010), right carotid IMT of 0.678 (CI=0.529–0.828, p=0.031), C3 of 0.676 (CI=0.531–0.822, p=0.033), and IL-6 of 0.715 (CI=0.566–0.864, p=0.009). In addition, 44.4% of the subjects in the MCI group had folic acid levels below the normal range compared to 12.5% in the NC group (p=0.014).
Table 2.
Comparison of clinical and metabolic, inflammatory and vascular data between CN and MCI patients at baseline
| CN patients (n=40) | MCI patients (n=18) | p¥ | |
|---|---|---|---|
| Gender, F / M | 15 (37.5%) / 25 (62.5%) | 9 (50.0%) / 9 (50.0%) | 0.402 |
| Education, basic / high school / university | 3 (10.7%) / 25 (89.3%) / 12 (32.4%) | 7 (43.8%) / 9 (56.3%) / 2 (18.2%) | 0.012* |
| Clinical phenotype, PIGD / other | 22 (64.7%) / 18 (45.0%) | 12 (85.7%) / 6 (33.3%) | 0.566 |
| Age (years) | 62.8 ± 7.9, 64.0 (48.0–82.0) | 66.8 ± 8.2, 68.0 (45.0–79.0) | 0.017* |
| Duration of PD (years) | 5.1 ± 3.4, 4.0 (1.0–15.0) | 6.7 ± 5.4, 4.5 (1.0–20.0) | 0.431 |
| Daily L-dopa equivalent dose | 417.3 ± 292.1, 383.0 (27.0–1115.0) | 696.1 ± 441.4, 652.5 (200.0–2010.0) | 0.010* |
| IMT right | 0.7 ± 0.3, 0.6 (0.3–1.6) | 0.8 ± 0.3, 0.8 (0.3–1.6) | 0.030* |
| IMT left | 0.7 ± 0.3, 0.7 (0.3–1.8) | 0.8 ± 0.3, 0.7 (0.3–1.4) | 0.270 |
| PI right¤ | 1.1 ± 0.9, 0.9 (0.2–5.0) | 0.8 ± 0.2, 0.8 (0.3–1.1) | 0.317 |
| PI left¤ | 1.0 ± 0.5, 0.8 (0.2–2.1) | 0.8 ± 0.2, 0.8 (0.4–1.1) | 0.184 |
| RI right¤ | 0.6 ± 0.4, 0.6 (0.1–2.4) | 0.5 ± 0.1, 0.5 (0.2–0.7) | 0.430 |
| RI left¤ | 0.6 ± 0.3, 0.6 (0.1–1.3) | 0.5 ± 0.2, 0.6 (0.3–0.8) | 0.447 |
| QRISK2 | 13.6 ± 8.9, 11.5 (1.5–40.9) | 16.2 ± 9.3, 15.8 (2.3–42.5) | 0.190 |
| Uric acid (μmol/l) | 264.7 ± 70.9, 261.0 (122.0–420.0) | 259.9 ± 74.4, 242.5 (147.0–430.0) | 0.614 |
| Homocystein (μmol/l) | 12.4 ± 3.0, 11.7 (8.0–20.7) | 13.3 ± 3.3, 13.0 (7.9–17.8) | 0.233 |
| B12 (pg/ml) | 308.5 ± 127.1, 287.0 (121.0–694.0) | 291.0 ± 91.3, 251.5 (172.0–527.0) | 0.807 |
| Folic acid (ng/ml) | 7.1 ± 2.5, 6.7 (3.8–14.1) | 7.8 ± 5.1, 4.9 (3.1–18.0) | 0.313 |
| C3 (g/l) | 1.1 ± 0.3, 1.1 (0.7–2.9) | 1.2 ± 0.2, 1.2 (0.8–1.5) | 0.033* |
| C4 (g/l) | 0.3 ± 0.2, 0.2 (0.2–1.3) | 0.3 ± 0.1, 0.3 (0.1–0.8) | 0.108 |
| Interleukin 6 (pg/ml) | 3.1 ± 1.7, 3.0 (0.2–8.4) | 5.5 ± 3.8, 4.7 (1.5–16.8) | 0.009* |
| UPDRS total | 31.6 ± 16.6, 27.0 (9.0–76.0) | 36.4 ± 13.4, 34.5 (7.0–61.0) | 0.116 |
| UPDRS I | 1.6 ± 2.4, 1.0 (0.0–13.0) | 2.2 ± 2.0, 1.5 (0.0–8.0) | 0.092 |
| UPDRS II | 8.2 ± 5.4, 7.5 (0.0–21.0) | 9.9 ± 5.8, 9.0 (2.0–18.0) | 0.292 |
| UPDRS III | 21.7 ± 11.4, 20.5 (5.0–56.0) | 24.9 ± 8.7, 25.0 (3.0–43.0) | 0.110 |
| ADL | 7.0 ± 2.0, 8.0 (1.0–8.0) | 6.4 ± 1.8, 7.0 (2.0–8.0) | 0.068 |
| Barthel index | 96.8 ± 8.7, 100.0 (60.0–100.0) | 93.1 ± 9.9, 100.0 (70.0–100.0) | 0.072 |
| PI right above normal¤ | 12 (40.0%) | 1 (10.0%) | 0.124 |
| PI left above normal¤ | 12 (37.5%) | 2 (20.0%) | 0.451 |
| RI right above normal¤ | 1 (3.2%) | 0 (0.0%) | 1.000 |
| RI left above normal¤ | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Uric acid below normal | 8 (20.0%) | 4 (22.2%) | 1.000 |
| Homocysteine above normal | 26 (65.0%) | 13 (72.2%) | 0.764 |
| B12 below normal | 4 (10.0%) | 1 (5.6%) | 1.000 |
| Folic acid below normal | 5 (12.5%) | 8 (44.4%) | 0.014* |
Statistically significant result p<0.05
Values not known in all patients (specifically, PI right, PI left, RI right and RI left was evaluated for 10 MCI patients and 30, 32, 31 and 31 CN patients, respectively)
p-values calculated using Fisher’s exact test and Mann-Whitney U test, as appropriate
Legend: ADL (activity of daily living score), CN (cognitive normal), IMT (intimomedial thickness), PD (Parkinson’s disease), PI (pulsatility index), PIGD (postural instability and gait disorder), RI (resistance index), UPDRS (Unified Parkinson’s disease rating scale)
Table 2 lists the results of the univariate analyses of the clinical and laboratory variables between the two cognitive groups. As there can be false positive results among the variables due to multiple testing, we also performed multivariate stepwise regression analysis to identify which of the significant variables from Table 2 have true and most robust associations with cognitive status at baseline (NC vs. MCI). The following variables were identified as significant regressors in the model: IL-6 (OR=1.42, CI=1.02–1.99, p=0.041), low folic acid level (OR=6.33, CI=1.17–34.13, p=0.032) and L-dopa equivalent dose (OR=1.003, CI=1.001–1.005, p=0.014). None of the other variables met the entry criteria into the model mostly because they were correlated with the selected variables.
Evaluation of longitudinal change in data over a two-year follow-up period
The assessment of longitudinal change in the acquired data revealed that there were statistically significant increases in the values of IMT, PI, RI, QRISK2, and UPDRS (in total as well as in its subscores) and significant decreases in the values of B12, ADL, and the Barthel index over a two-year period in all 47 patients (Table 3). The evaluation was also performed separately for 34 NC patients and 13 MCI patients who had follow-up data. The results in the subgroups were consistent with the ones based on all patients with the exception of ADL in both NC and MCI patients, and QRISK2, B12, and UPDRS I in MCI patients, which were not statistically significant. Additionally, there was a significant decrease in folic acid in the MCI group. All results remained statistically significant after FDR correction for multiple comparison except for B12 in all patients and NC patients, Barthel index in NC patients, and folic acid in MCI patients. Uric acid levels decreased in all studied subgroups; however, the difference was not significant. Detailed summaries of baseline and longitudinal data are given in Supplementary tables A–C.
Table 3.
Evaluation of longitudinal change in clinical, metabolic, inflammatory and vascular data over a 2-year period in all, CN and MCI patients
| All patients with 2-yr follow-up data (n=47) |
CN patients with 2-yr follow-up data (n=34) |
MCI patients with 2-yr follow-up data (n=13) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) of pts with worsening§ |
Median of change over 2 yrs† |
p¥ | N (%) of pts with worsening§ |
Median of change over 2 yrs† |
p¥ | N (%) of pts with worsening§ |
Median of change over 2 yrs† |
p¥ | |
| IMT right | 44 (93.6%) | 0.21 | <0.001** | 32 (94.1%) | 0.23 | <0.001** | 12 (92.3%) | 0.16 | 0.002** |
| IMT left | 45 (95.7%) | 0.16 | <0.001** | 32 (94.1%) | 0.16 | <0.001** | 13 (100.0%) | 0.10 | 0.001** |
| PI right¤ | 33 (97.1%) | 0.25 | <0.001** | 25 (96.2%) | 0.25 | <0.001** | 8 (100.0%) | 0.24 | 0.012** |
| PI left¤ | 33 (97.1%) | 0.21 | <0.001** | 26 (100.0%) | 0.19 | <0.001** | 7 (87.5%) | 0.26 | 0.018** |
| RI right¤ | 34 (100.0%) | 0.11 | <0.001** | 26 (100.0%) | 0.11 | <0.001** | 8 (100.0%) | 0.13 | 0.012** |
| RI left¤ | 32 (94.1%) | 0.10 | <0.001** | 25 (96.2%) | 0.10 | <0.001** | 7 (87.5%) | 0.11 | 0.018** |
| QRISK2 | 30 (63.8%) | 0.50 | 0.005** | 22 (64.7%) | 0.85 | 0.014** | 8 (61.5%) | 0.10 | 0.135 |
| Uric acid (μmol/l) | 29 (61.7%) | −13.00 | 0.108 | 20 (58.8%) | −10.50 | 0.270 | 9 (69.2%) | −13.00 | 0.162 |
| Homocysteine (μmol/l) | 23 (48.9%) | −0.10 | 0.623 | 16 (47.1%) | −0.10 | 0.791 | 7 (53.8%) | 1.30 | 0.600 |
| B12 (pg/ml) | 33 (70.2%) | −25.00 | 0.017** | 23 (67.6%) | −23.50 | 0.038* | 10 (76.9%) | −31.00 | 0.196 |
| Folic acid (ng/ml) | 28 (59.6%) | −0.30 | 0.062 | 18 (52.9%) | −0.20 | 0.462 | 10 (76.9%) | −0.90 | 0.021** |
| C3 (g/l) | 26 (55.3%) | 0.02 | 0.346 | 21 (61.8%) | 0.06 | 0.351 | 5 (38.5%) | 0.00 | 0.789 |
| C4 (g/l) | 17 (36.2%) | −0.01 | 0.225 | 14 (41.2%) | −0.01 | 0.771 | 3 (23.1%) | −0.02 | 0.068 |
| Interleukin 6 (pg/ml) | 14 (29.8%) | −0.40 | 0.067 | 9 (26.5%) | −0.30 | 0.150 | 5 (38.5%) | −1.30 | 0.124 |
| UPDRS total | 34 (72.3%) | 9.00 | <0.001** | 21 (61.8%) | 7.50 | 0.014** | 13 (100.0%) | 12.00 | 0.001** |
| UPDRS I | 27 (57.4%) | 1.00 | 0.007** | 20 (58.8%) | 1.00 | 0.013** | 7 (53.8%) | 1.00 | 0.235 |
| UPDRS II | 30 (63.8%) | 3.00 | 0.002** | 19 (55.9%) | 1.50 | 0.044* | 11 (84.6%) | 4.00 | 0.004** |
| UPDRS III | 31 (66.0%) | 5.00 | <0.001** | 19 (55.9%) | 4.00 | 0.031* | 12 (92.3%) | 9.00 | 0.002** |
| ADL | 12 (25.5%) | 0.00 | 0.036* | 6 (17.6%) | 0.00 | 0.219 | 6 (46.2%) | 0.00 | 0.067 |
| Barthel index | 19 (40.4%) | 0.00 | <0.001** | 10 (29.4%) | 0.00 | 0.026* | 9 (69.2%) | −5.00 | 0.007** |
Statistically significant result p<0.05 (which did not remain significant after FDR correction)
Statistically significant result p<0.05 (which remained significant even after FDR correction for multiple comparisons)
Worsening is defined as increase of values in IMT, PI, RI, QRISK2, homocysteine, C3, C4, interleukin 6, UPDRS and decrease of values in uric acid, B12, folic acid, ADL and Barthel index.
Median of change over 2 yrs was calculated as median of difference in values of the respective variable after two years and at baseline
Values not known in all patients (specifically, PI right, PI left, RI right and RI left was evaluated for 34, 36, 35 and 35 out of 47 patients; 26, 28, 27 and 27 out of 34 CN patients; and 8 out of 13 MCI patients
p-values calculated using paired Wilcoxon test
Legend: ADL (activity of daily living score), CN (cognitive normal), IMT (intimomedial thickness), PI (pulsatility index), RI (resistance index), UPDRS (Unified Parkinson’s disease rating scale)
Comparison between cognitive converters vs. non-converters during the two-year follow-up period
Patients who converted to MCI status during the follow-up period were older at baseline (p=0.015) and had higher QRISK2 (p=0.008) than the non-converters (Table 4). The AUC calculated using the ROC analysis was 0.759 (CI=0.593–0.924, p=0.016) for age and 0.779 (CI=0.619–0.938, p=0.009) for QRISK2. Findings show that QRISK2 can discriminate between converters and non-converters better than age alone. When multivariate stepwise logistic regression model with age and QRISK2 was performed, only QRISK2 was identified as having most robust association with the cognitive deterioration (OR=1.12, CI=1.02–1.24, p=0.022). Age did not remain significant in the multivariate model as it was strongly correlated with QRISK2 (Spearman’s correlation coefficient ρ=0.80, p<0.001).
Table 4.
Comparison of clinical, metabolic, inflammatory and vascular data at baseline between non-converters (i.e. patients who remained cognitively normal after 2 years) and converters (i.e. patients that progressed to MCI after 2 years)
| Non-converters (n=23) | Converters (n=11) | p¥ | |
|---|---|---|---|
| Gender, females / males | 9 (39.1%) / 14 (60.9%) | 4 (36.4%) / 7 (63.6%) | 1.000 |
| Education, basic / high school / university | 1 (4.3%) / 14 (60.9%) / 8 (34.8%) | 2 (18.2%) / 7 (63.6%) / 2 (18.2%) | 0.399 |
| Motor phenotype, PIGD / other | 14 (60.9%) / 9 (39.1%) | 6 (54.5%) / 5 (45.5%) | 1.000 |
| Age (years) | 60.0 ± 7.0, 63.0 (50.0–72.0) | 69.0 ± 8.0, 65.0 (58.0–82.0) | 0.015* |
| Duration of PD (years) | 6.0 ± 3.0, 5.0 (1.0–12.0) | 5.0 ± 4.0, 4.0 (1.0–15.0) | 0.243 |
| IMT right | 0.7 ± 0.2, 0.6 (0.4–1.3) | 0.8 ± 0.3, 0.7 (0.5–1.6) | 0.344 |
| Daily L-dopa equivalent dose | 423.0 ± 283.0, 372.0 (105.0–1115.0) | 359.0 ± 319.0, 300.0 (54.0–940.0) | 0.403 |
| IMT left | 0.7 ± 0.2, 0.7 (0.4–1.2) | 0.8 ± 0.4, 0.7 (0.5–1.8) | 0.513 |
| PI right¤ | 0.9 ± 0.6, 0.9 (0.3–2.5) | 1.3 ± 1.5, 0.7 (0.2–5.0) | 0.672 |
| PI left¤ | 0.9 ± 0.4, 0.8 (0.2–2.0) | 1.1 ± 0.6, 1.0 (0.5–2.1) | 0.308 |
| RI right¤ | 0.5 ± 0.2, 0.5 (0.2–0.9) | 0.8 ± 0.7, 0.5 (0.1–2.4) | 0.781 |
| RI left¤ | 0.6 ± 0.3, 0.6 (0.1–1.2) | 0.7 ± 0.3, 0.7 (0.4–1.3) | 0.253 |
| QRISK2 | 10.7 ± 7.0, 8.8 (1.5–27.2) | 19.7 ± 11.3, 13.9 (7.7–40.9) | 0.008* |
| Uric acid (μmol/l) | 268.0 ± 79.7, 268.0 (122.0–420.0) | 250.4 ± 42.7, 244.0 (185.0–339.0) | 0.403 |
| Homocysteine (μmol/l) | 12.3 ± 3.0, 11.6 (8.4–20.7) | 12.0 ± 2.1, 11.4 (9.5–16.9) | 0.856 |
| B12 (pg/ml) | 317.3 ± 141.8, 292.0 (121.0–694.0) | 324.6 ± 119.6, 311.0 (135.0–571.0) | 0.490 |
| Folic acid (ng/ml) | 7.2 ± 2.9, 6.4 (3.8–14.1) | 7.2 ± 2.4, 7.2 (3.9–11.4) | 0.800 |
| C3 (g/l) | 1.2 ± 0.4, 1.1 (0.8–2.9) | 1.1 ± 0.2, 1.1 (0.8–1.5) | 0.344 |
| C4 (g/l) | 0.3 ± 0.1, 0.2 (0.2–0.6) | 0.3 ± 0.1, 0.3 (0.2–0.4) | 0.612 |
| Interleukin 6 (pg/ml) | 2.8 ± 1.7, 2.2 (0.2–7.4) | 3.3 ± 1.0, 3.6 (1.5–5.0) | 0.201 |
| UPDRS total | 30.0 ± 15.0, 27.0 (9.0–67.0) | 37.0 ± 19.0, 29.0 (16.0–76.0) | 0.258 |
| UPDRS I | 1.0 ± 2.0, 1.0 (0.0–6.0) | 2.0 ± 1.0, 2.0 (0.0–3.0) | 0.403 |
| UPDRS II | 8.0 ± 5.0, 8.0 (0.0–21.0) | 9.0 ± 7.0, 7.0 (2.0–18.0) | 0.971 |
| UPDRS III | 21.0 ± 11.0, 17.0 (5.0–48.0) | 27.0 ± 13.0, 25.0 (9.0–56.0) | 0.143 |
| ADL | 7.0 ± 2.0, 8.0 (2.0–8.0) | 6.0 ± 3.0, 8.0 (1.0–8.0) | 0.274 |
| Barthel index | 97.0 ± 9.0, 100.0 (60.0–100.0) | 95.0 ± 11.0, 100.0 (65.0–100.0) | 0.403 |
| PI right above normal¤ | 6 (35.3%) | 3 (33.3%) | 1.000 |
| PI left above normal¤ | 6 (31.6%) | 4 (44.4%) | 0.677 |
| RI right above normal¤ | 0 (0.0%) | 1 (11.1%) | 0.333 |
| RI left above normal¤ | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Uric acid below normal | 5 (21.7%) | 1 (9.1%) | 0.638 |
| Homocysteine above normal | 14 (60.9%) | 8 (72.7%) | 0.705 |
| B12 below normal | 2 (8.7%) | 1 (9.1%) | 1.000 |
| Folic acid below normal | 3 (13.0%) | 2 (18.2%) | 1.000 |
Statistically significant result p<0.05
Values not known in all patients (specifically, PI right, PI left, RI right and RI left was evaluated for 9 MCI patients and 17, 19, 18 and 18 CN patients, respectively)
p-values calculated using Fisher’s exact test and Mann-Whitney U test, as appropriate
Legend: ADL (activity of daily living score), CN (cognitive normal), IMT (intimomedial thickness), PD (Parkinson’s disease), PI (pulsatility index), PIGD (postural instability and gait disorder), RI (resistance index), UPDRS (Unified Parkinson’s disease rating scale)
Post-hoc analysis: Multivariate prediction of conversion to MCI
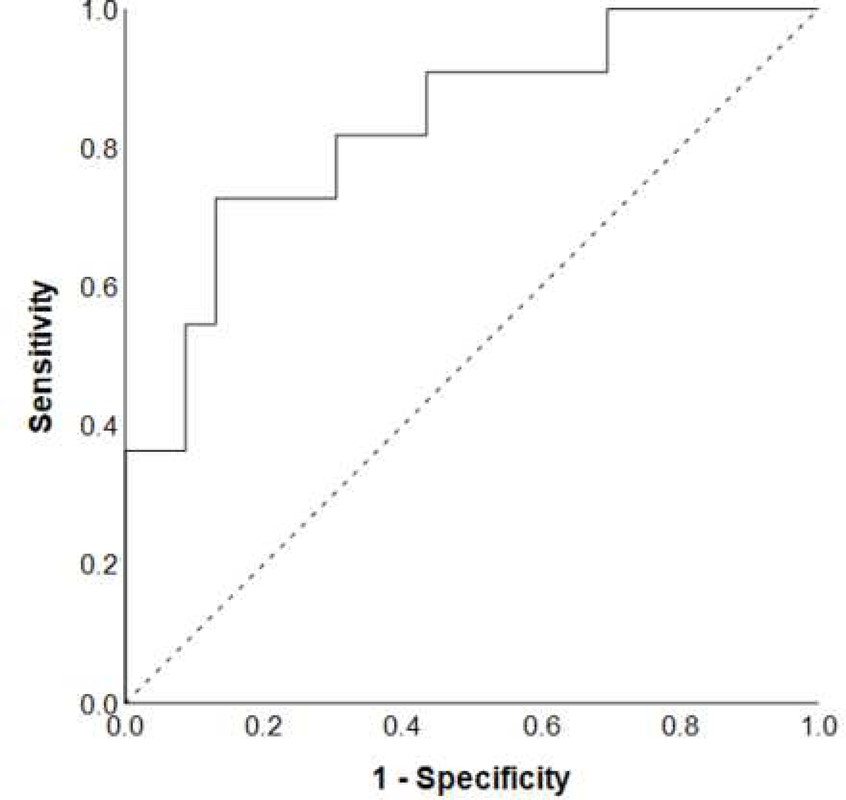
As combination of different factors may have higher predictive value then when tested in isolation, we performed a complementary multivariate stepwise logistic regression analysis using all variables at baseline. The final model consisted of QRISK2, uric acid and activity of daily living scale and enabled prediction of cognitive deterioration with a sensitivity of 72.2%, a specificity of 87%, and an overall accuracy of 82.4%. ROC curve for the model is shown in Figure 1. The logistic regression analysis showed that patients with higher QRISK2 (OR=1.16, CI=1.03–1.31, p=0.014), lower uric acid (OR=0.99, CI=0.97–1.00, p=0.133), and lower activity of daily living scale (OR=0.68, CI=0.46–1.02, p=0.064) at baseline had a higher risk of converting from NC to MCI in two years. An example calculation of probability of converting to MCI is shown in Supplement D.
Figure 1.
The ROC curve for the final multivariate logistic regression model for prediction of cognitive deterioration over a two-year period.
Discussion
Our findings imply a link between metabolic, vascular, and inflammatory systemic medical co-morbidities and cognitive decline in PD. Patients in the MCI group had a higher level of IL-6, level of folic acid below normal range and higher L-dopa equivalent than the patients in the NC group at baseline. These observations agree with a meta-analysis by Xie (Xie et al. 2017) showing that PD patients with cognitive dysfunction were more likely to have lower level of folic acid. This meta-analysis of 25 studies demonstrated higher systemic levels of interleukins in patients with PD than in healthy controls. Increased serum level concentrations of interleukins were found in idiopathic PD patients with and without vascular risk factors as well as in atypical parkinsonian syndromes (multiple system atrophy and progresive supranuclear palsy) as compared to the control healthy group. In our previous study we found that IL-6 elevation may be a marker of increased mortality in PD patients (Rektor et al. 2012). Higher L-dopa equivalent reflects a more advanced neurodegenerative process in PD. Our data are supported by the results of previous cross-sectional studies. We previously showed that subclinical vascular pathology influenced cognitive status in PD. This included a significant correlation between clinical and cognitive status and IMT, which is an indicator of large vessel impairment, and between clinical and cognitive status and the pulsatility index, an indicator of small vessel impairment (Rektor et al. 2009). Vascular co-morbidity was also significantly associated with cognitive impairment in patients with early PD. Furthermore, cognitive impairment was more prevalent in those with multiple vascular risk factors (Malek et al. 2016). Vascular risk factors (diabetes, hypertension, and hyperlipidemia) and white matter hyperintensity on MRI have been also associated with PD-MCI (Kandiah et al. 2013). A negative impact of inflammation on non-motor cognitive signs and quality of life in PD has also been reported (Veselý et al. 2018, Dufek et al. 2009, Bonifati et al. 2007). Our present study also showed that patients who converted to MCI status during the follow-up period were older at baseline and had higher QRISK2 than the non-converters. QRISK2 was significantly associated with cognitive decline, however the sensitivity of using it for prediction of conversion to MCI was low. As our aim was to construct a clinically applicable risk factor model we also tested other factors investigated in this study. The final model that enabled prediction of cognitive decline consisted of QRISK2, uric acid and activity of daily living scale rating. This cognitive predictor model is supported by several studies. In a longitudinal study (Swallow et al. 2016), increased vascular risk factors (using the QRISK2 tool) and cerebrovascular disease (CVD) were associated with older age, worse motor, and cognitive impairment. In a cross-sectional analysis, Annanmaki et al. 2008 showed that low plasma and low urine uric acid levels associated with decreased neuropsychological performance in PD patients. The prospective longitudinal follow-up of the same cohort after three years showed that low levels of uric acid correlated with further worsening of cognition, especially in the performance of a verbal fluency task (Annanmaki et al. 2011). In the longitudinal study of drug naïve early PD patients a low baseline level of uric acid was associated with the later occurrence of MCI (Pellecchia et al. 2016).
There are several possible mechanisms by which the co-morbidities investigated in this study may contribute to cognitive deterioration in PD: vascular pathology in PD includes fragmentation of capillaries and damage to the capillary network in multiple brain regions, particularly in the substantia nigra, middle frontal cortex, and brainstem nuclei (Malek et al. 2016) and it can lead to disruption of connectivity of cortico-basal ganglia-thalamocortical circuits; pro-inflammatory cytokines may generate symptoms of cognitive impairment in PD by direct effects on monoaminergic neurotransmission, the hypothalamic–pituitary–adrenal axis, and the kynurenine pathway of tryptophan degradation (Lindqvist et al. 2013). The pro-inflammatory cytokines and other factors secreted by reactive microglia may trigger a neurotoxic cascade that has detrimental effects within the CNS by exacerbating neuronal lesions (Lindqvist et al. 2013); uric acid, as a common antioxidant, is a scavenger of peroxyl radicals, hydroxyl radicals, and singlet oxygen and can inhibit the radicals generated by the decomposition of peroxynitrite. Uric acid can chelate metal irons such as iron and copper and convert them to less reactive forms that do not catalyze free-radical reactions. Both oxidative damage and the accumulation of unsequestered iron have been suggested as playing roles in the pathogenesis of PD (Cao et al. 2015), providing an explanation why uric acid might provide natural neuroprotection against PD (Annanmaki et al. 2011); folates are required for one-carbon transfer reactions, which are essential for the synthesis of DNA and RNA nucleotides, the metabolism of amino acids, and the occurrence of methylation reactions. The proposed mechanisms underlying that association include hyperhomocysteinemia, lower methylation reactions and tetrahydrobiopterin levels, and excessive mis-incorporation of uracil into DNA (Araújo et al. 2015). Folate deficiency may promote neuronal degeneration and sensitize neurons to amyloid and peptide toxicity (Dimopoulos et al. 2006).
We conclude that medical systemic factors or co-morbidities contribute to clinically relevant cognitive deterioration in PD. We found that different factors associated with baseline cognitive status versus interval changes. Baseline factors reflect cumulative effects of multiple morbid factors whereas interval predictors reflect specific progression changes where different factors may have a different temporal profile during the course of the disease. The main limitation of our study is the relatively small sample size and partial attrition during the follow-up period. Larger study cohorts are needed. It is possible that larger cohort studies would confirm importance of factors that were significantly different at the baseline visit between NC and MCI patients but didńt survive the correction for multiple comparisons. The two-year follow-up period is also relatively short; a longer observational period might reveal additional factors contributing to cognitive decline. Our post hoc analyses suggest that simplified predictor models may be useful in clinical practice but this will need to be reproduced and validated in independent cohorts. If confirmed, then this or a modified model could be used in everyday clinical practice. Our present results add to cumulative evidence from other studies that potentially treatable systemic medical factors or co-morbidities are clinically relevant for cognitive decline in PD. We propose that a careful general medical review should complement the neurological assessment in patients with PD.
Supplementary Material
Acknowledgments:
Support was provided by the project CEITEC 2020 (LQ1601) and grant AZV 16-33798A. We would like to thank Ján Necpal, M.D., from the Neurology Department, Zvolen, Slovak Republic for kindly referring patients for participation in this study. We also would like to thank to Anne Johnson for grammatical assistance and for reviewing the manuscript.
List of abbreviations:
- ACE
Addenbrooke’s cognitive examination
- ADL
activity of daily living
- AUC
area under the curve
- BI
Barthel index
- BVRT
Benton visual retention test
- CCA
common carotid artery
- CDT
the clock drawing test
- CI
confidence intervals
- IL-6
interleukin 6
- IMT
intimal-medial thickness
- MCA
medial cerebral artery
- MCI
mild cognitive impairment
- MMSE
Mini-mental state examination
- NC
normal cognition
- OR
odds ratio
- PD
Parkinson’s disease
- PDD
Parkinson’s disease dementia
- PD-MCI
Parkinson’s disease mild cognitive impairment
- PI
pulsatility index
- RI
resistance index
- ROC
receiver operating characteristic
- UPDRS
Unified Parkinson’s disease rating scale
- USG
ultrasonographic
- VFT
the verbal fluency test (VFT)
- WMS
Wechsler Memory Scale III
Footnotes
Compliance with ethical standards and disclosure of conflict of interest:
None of the authors report any conflict of interest or financial disclosure.
References:
- Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K (2008) Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat Disord 14(7):576–578. [DOI] [PubMed] [Google Scholar]
- Annanmaki T, Pohja M, Parviainen T, Hakkinen P, Murros K (2011) Uric acid and cognition in Parkinson’s disease: a follow-up study. Parkinsonism Relat Disord 17(5):333–337. [DOI] [PubMed] [Google Scholar]
- Araújo JR, Martel F, Borges N, Araújo JM, Keating E. (2015) Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res Rev 22:9–19. [DOI] [PubMed] [Google Scholar]
- Auriacombe S, Grossman M, Carvell S, Gollomp S, Stern MB, Hurtig HI (1993) Verbal fluency deficits in Parkinson’s disease. Neuropsychology 7(2):182–192. [Google Scholar]
- Barrash J, Stillman A, Anderson SW, Uc EY, Dawson JD, Rizzo M (2010) Prediction of driving ability with neuropsychological tests: demographic adjustments diminish accuracy. J Int Neuropsychol Soc 16(4):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati DM, Kishore U (2007) Role of complement in neurodegeneration and neuroinflammation. Mol Immunol 44(5):999–1010. [DOI] [PubMed] [Google Scholar]
- Cao B, Wei QQ, Ou R, Yang J, Shang HF (2015) Association of serum uric acid level with cognitive function among patients with multiple system atrophy. J Neurol Sci 359(1–2):363–366. [DOI] [PubMed] [Google Scholar]
- De Pandis MF, Galli M, Vimercati S, Cimolin V, De Angelis MV, Albertini G (2010) A New approach for the quantitative evaluation of the clock drawing test: preliminary results on subjects with Parkinson’s disease. Neurol Res Int. 2010:283890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, et al. (2006) Association of cognitive impairment with plasma levels of folate, vitamin B12 and homocysteine in the elderly. In Vivo 20(6B):895–899. [PubMed] [Google Scholar]
- Dufek M, Hamanová M, Lokaj J, et al. (2009) Serum inflammatory biomarkers in Parkinson’s disease. Parkinsonism Relat Disord 15(4):318–320. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method forgrading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- Harrison MB, Wylie SA, Frysinger RC, Patrie JT, Huss DS, Currie LJ, Wooten GF (2009) UPDRS activity of daily living score as a marker of Parkinson’s disease progression. Mov Disord 24(2):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S, McGrory S, Leslie F, et al. (2015) The Mini-Addenbrooke’s Cognitive Examination: a new assessment tool for dementia. Dement Geriatr Cogn Disord 39(1–2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N, Mak E, Ng A, Huang S, Au WL, Sitoh YY, Tan LC (2013) Cerebral white matter hyperintensity in Parkinson’s disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat Disord 19(7):680–683. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O (2013) Cerebrospinal fluid inflammatory markers in Parkinson’s disease – Associations with depression, fatigue, and cognitive impairment. Brain Behav Immun 33:183–189. [DOI] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, et al. (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Kieburtz K, Tröster AI, Aarsland D (2018) Strengths and challenges in conducting clinical trials in Parkinson’s disease mild cognitive impairment: PD-MCI Clinical Trials. Mov Disord 33(4):520–527. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965; 14:61–65. [PubMed] [Google Scholar]
- Malek N, Lawton MA, Swallow DM, et al. (2016) Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease: Vascular Disease and Vascular Risk Factors in PD. Mov Disord 31(10):1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias-Guiu JA, Cortés-Martínez A, Valles-Salgado M, Rognoni T, Fernández-Matarrubia M, Moreno-Ramos T, Matías-Guiu J (2017) Addenbrooke’s cognitive examination III: diagnostic utility for mild cognitive impairment and dementia and correlation with standardized neuropsychological tests. Int Psychogeriatr 29(1):105–113. [DOI] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18(7):738–750. [DOI] [PubMed] [Google Scholar]
- Pellecchia MT, Savastano R, Moccia M, et al. (2016) Lower serum uric acid is associated with mild cognitive impairment in early Parkinson’s disease: a 4-year follow-up study. J Neural Transm 123(12):1399–1402. [DOI] [PubMed] [Google Scholar]
- QRISK®2–2016 risk calculator, downloaded on 31st January 2016 from https://qrisk.org/2016/ Rektor I, Goldemund D, Bednařík P, et al. (2012) Impairment of brain vessels may contribute to mortality in patients with Parkinson’s disease. Mov Disord 27(9):1169–1172. [DOI] [PubMed] [Google Scholar]
- Rektor I, Goldemund D, Sheardová K, Rektorová I, Michálková Z, Dufek M (2009) Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord 15(1):24–29. [DOI] [PubMed] [Google Scholar]
- Schlesinger I, Schlesinger N (2008) Uric acid in Parkinson’s disease. Mov Disord 23(12):1653–1657. [DOI] [PubMed] [Google Scholar]
- Swallow DM, Lawton MA, Grosset KA, et al. (2016) Statins are underused in recent-onset Parkinson’s disease with increased vascular risk: findings from the UK Tracking Parkinson’s and Oxford Parkinson’s Disease Centre (OPDC) discovery cohorts. J Neurol Neurosurg Psychiatry 87(11):1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselý B, Dufek M, Thon V, et al. (2018) Interleukin 6 and complement serum level study in Parkinson’s disease. J Neural Transm 125(5):875–881. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale the third edition, WMS-III. San Antonio, Texas, The Psychological Corporation, 1997. [Google Scholar]
- Xie Y, Feng H, Peng S, Xiao J, Zhang J (2017) Association of plasma homocysteine, vitamin B12 and folate levels with cognitive function in Parkinson’s disease: A meta-analysis. Neurosci Lett 636:190–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.