Abstract
Spinal cord injury (SCI) is a devastating and complicated condition with no cure available. The initial mechanical trauma is followed by a secondary injury characterized by inflammatory cell infiltration and inhibitory glial scar formation. Due to the limitations posed by the blood–spinal cord barrier, systemic delivery of therapeutics is challenging. Recent development of various nanoscale strategies provides exciting and promising new means of treating SCI by crossing the blood–spinal cord barrier and delivering therapeutics. As such, we discuss different nanomaterial fabrication methods and provide an overview of recent studies where nanomaterials were developed to modulate inflammatory signals, target inhibitory factors in the lesion, and promote axonal regeneration after SCI. We also review emerging areas of research such as optogenetics, immunotherapy and CRISPR-mediated genome editing where nanomaterials can provide synergistic effects in developing novel SCI therapy regimens, as well as current efforts and barriers to clinical translation of nanomaterials.
Keywords: spinal cord injury, regenerative medicine, nanotechnology, nanomaterials
Graphical Abstract:

1. Introduction
This review article provides a summary of nanomaterial fabrication and therapeutic effects achieved by the nanoscale systems in pre-clinical studies of spinal cord injury (SCI). Specifically, we discuss strategies to 1) attenuate inflammatory response, 2) target inhibitory components, and 3) enhance axonal regeneration. In addition, efforts and barriers to clinical translation of nanomaterials for SCI repair are discussed. The majority of the nanomaterial studies covered in this review is published in the past 10 years. For the purpose of this review, we employ the United Kingdom House of Lords Science and Technology Committee definition of nanoscale, i.e., up to 1000 nm, or less than 1 μm (“sub-micron”), in size [1] in discussing various nanotechnology strategies developed to enhance SCI repair. Note that this review does not cover nanomaterials developed for delivery of mammalian cells, as these cells are micronsized [2] and therefore do not fit within our definition of nanoscale therapies. Readers are directed to other excellent reviews specifically for recent advances in cell transplantation therapies developed for SCI repair [3–7].
1.1. Spinal cord injury: introduction and current management
Spinal cord injury (SCI) is a debilitating condition as patients often suffer from neurological impairments and a reduction in quality of life such as partial or complete paralysis, respiratory distress, and bladder dysfunction [8]. Currently, there are over 280,000 patients with SCI and approximately 17,000 new cases every year in the United States alone [9]. The economic impact of SCI on patients is huge as the estimated lifetime cost of treatment per patient is well over one million dollars and may become over four million dollars depending on the age of the patient and the severity of the injury [10].
Despite advances in medicine, most treatments for SCI are palliative with minimal functional recovery. After diagnosis and initial stabilization, the patient is given a large systemic dose of 30 mg/kg bolus injection followed by a 5.4 mg/kg·h infusion over 24 hours of methylprednisolone (MP), a synthetic corticosteroid [11,12]. Studies have demonstrated that early administration of MP within the first 8 hours following SCI helps in reducing acute inflammatory responses [13,14]. Improvement in neurologic recovery in patients with MP administration was also shown in the report from the National Acute Spinal Cord Injury Study II. However, current method of MP administration is largely inefficient as the high dose is associated with severe side effects such as pneumonia, infections, corticosteroid myopathy and gastric bleeding [15,16].
1.2. Pathophysiology: primary and secondary injuries
The pathological progression of SCI can be categorized into primary and secondary injuries [17–19]. The primary injury is the result of the initial trauma caused by a physical force, which may take the form of compression, contusion, laceration, or stretch [20]. This initial trauma causes damage to the small vessels carrying blood to the tissue, thus creating a hypoxic environment and oxidative stress [21]. The mechanical insult also causes axonal membrane disruption and subsequent release of inhibitory breakdown products of the myelin sheath: Nogo-A, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp) [22]. Finally, inflammatory cells such as neutrophils and pro-inflammatory M1 macrophages migrate to the injury site because of increased permeability of the blood-spinal cord barrier [23–26]. These cells release pro-inflammatory cytokines such as interleukin (IL)-1 alpha, IL-1 beta, IL-6, CD95 ligand and tumor necrosis factor (TNF)-alpha and lead to death of many resident neuronal and glial cell populations in the spinal cord [17,24,25,27].
This cascade of biochemical events causes the spread of the injury into adjacent tissue, resulting in the secondary injury [28]. Secondary injury begins a few hours after the initial injury and could last for a few weeks. Distortion in the local microenvironment following SCI causes glial cells, such as astrocytes and microglia, to undergo dramatic genetic and morphological changes, transforming into reactive phenotypes [29]. These cells deposit excessive amounts of neuro-inhibitory chondroitin sulfate proteoglycans (CSPGs) in the extracellular matrix (ECM) [30,31]. This connective tissue around the lesion boundary is known as a glial scar, and it acts to physically block axon regeneration near the lesion site [32]. Studies have shown that bacterial enzyme chondroitinase ABC (ChABC) effectively removes glial scar and promotes axonal regeneration as well as functional recovery after SCI in animal models [33–36]; however, intrathecal ChABC injection without a proper delivery vehicle poses significant limitations in clinical translation because of thermal instability of ChABC at body temperature [37]. Additionally, reactive astrocytes also have been shown to contribute to prolonged disruption of the blood-spinal cord barrier at the lesion periphery, whereas the blood-spinal cord barrier is restored at the epicenter 2-3 weeks after injury [38]. Overall, the presence of CSPG-rich glial scar and the heterogeneity of blood-spinal cord barrier stability pose challenges in successful delivery of therapeutics to the lesion and improvements in patient outcome.
1.3. Benefits of nanotherapeutic strategies for SCI repair
As alluded to earlier, systemic administration of therapeutics, such as MP and ChABC, in both pre-clinical and clinical studies of SCI raises several complications such as high drug dose required to achieve therapeutic effects, high cost of treatment, waste of drugs, and systemic cytotoxicity and side effects [21,39,40]. To overcome these limitations and deliver therapeutics more efficiently, researchers have developed various nanocarriers that allow localized, slow, and sustained delivery of therapeutics at the site of injury [41,42]. These therapeutic nanocarriers can be encapsulated in injectable scaffolds to be directly delivered to the lesion site, or be specifically designed to extravasate the blood-spinal cord barrier and localize into the injured spinal cord tissues [43,44].
Numerous studies suggest that drugs delivered via nanocarriers could achieve similar therapeutic effects at a lower or the same dose in pre-clinical models, both at the cellular and behavioral levels [45]. In some cases, studies have shown improved patient outcomes in nanocarrier-assisted drug delivery approaches compared to the conventional systemic drug administration. In addition to drug delivery, providing sub-cellular nanotopography is also crucial in nerve repair strategies [46]. Yet, there currently exist no clinical trials designed to assess the effects of nanomaterials specifically for SCI repair [47]. Interestingly, no clinical trials exist for microcarriers in SCI applications either. The only microscale materials listed on ClinicalTrials.gov are micro-electrode array implants to control a prosthesis, mainly for peripheral neural injury treatment [48].
2. Nanomaterials Fabrication
To encourage wide utilization of nanomaterials for SCI repair, we briefly review the synthesis and SCI applications of the following nanomaterials: nanoparticles, nanofibers, carbon nanotubes, and quantum dots (Figure 1). Nanomaterials type, base material, shape, dimensions, and method of synthesis are also summarized in Table 1.
Figure 1.
Representative images of nanomaterials and fabrication overview schematics. A: SEM images of PLGA nanoparticles [191]. Scale bar: 5 μm. B: Field-emission scanning electron microscopy (SEM) image of electrospun silk nanofibers [74]. Scale bar: 5 μm. C: SEM images of self-assembled peptide nanofibers [77]. Scale bar: 300 nm. Schematic of IKVAV self-assembling peptides are from [87]. D: SEM image of multi-walled carbon nanotubes. Scale bar: 250 μm (inset: 25 μm). Image and schematic adapted from [90]. E: Transmission electron microscopy (TEM) images of CdTe quantum dot [113]. Quantum dots with multiple shell layers can be fabricated by ultraviolet light irradiation. Scale bar: 20 nm. Top right: High-resolution TEM images. Scale bar: 2 nm. Copyright permissions: A from Elsevier, B and C (schematic) from John Wiley and Sons, C (SEM image) from Royal Society of Chemistry. D is open access. E (TEM image) from American Chemical Society.
Table 1.
Summary of nanomaterial type, shape, size, fabrication methods, and corresponding references. NP: nanoparticles, NF: nanofibers, CNT: carbon nanotubes, SWCNT: single-walled CNT, MWCNT: multi-walled CNT, QD: quantum dots, PLGA: poly(lactic-co-glycolic acid), PCL: poly(ε-caprolactone), PLLA: polylactic acid, PCLEEP: poly(caprolactone-co-ethyl ethylene phosphate).
| Type | Base Material | Shape | Size (nm) | Synthesis Method | References |
|---|---|---|---|---|---|
| NP | PLGA | Sphere | 150 - 400 | Solvent evaporation, Spray drying | [46,48,186–189] |
| Chitosan | Sphere | 150 - 400 | Ionotropic gelation | [58,59,155] | |
| Silica | Sphere | 50 - 300 | Tetramethylorthosilicate based synthesis, water-in-oil-microemulsion | [61,64] | |
| NF | PCL | N/A | 548.89 | Electrospun | [233,262] |
| PLGA | N/A | 250(aligned), 360(random) | Electrospun | [234,236] | |
| PCL/PLGA | N/A | 592 | Electrospun | [84] | |
| PLLA | N/A | 300 - 1500 | Electrospun | [146,231] | |
| Silk | N/A | 330 | Electrospun | [74] | |
| Collagen-I | N/A | 208.2 | Electrospun | [75] | |
| Gelatin | N/A | 300 - 1300 | Electrospun | [232] | |
| PCLEEP | N/A | 740 - 820 | Electrospun | [195,235] | |
| RADA16-I | N/A | 15 - 50 | Self-assembly | [81,82,85,246] | |
| RAD16-II | N/A | 10 - 20 | Self-assembly | [80] | |
| LDLK12 | N/A | 12 | Self-assembly | [247,250] | |
| IKVAV | N/A | N/A | Self-assembly | [79,251] | |
| CNT | Carbon | SWCNT | 0.8 - 1.5 | High-pressure carbon monoxide conversion | [95,237] |
| SWCNT | 1.5 | Chemical vapor deposition (CVD) | [97,243] | ||
| MWCNT | 10 - 400 | CVD | [94,239,243,244] | ||
| MWCNT | 15 - 37 | Catalytic CVD | [99,241] | ||
| QD | CdE-ZnS (E=Se, Te, S) | Sphere | ~12 | Precursor pyrolysis, precipitation in MeOH | [107,108] |
| CdSe, CdTe, CdTe-ZnTe | Sphere | ~3 | Precursor hydrolysis, mercaptopropionic acid stabilizer | [109,110] | |
| CdTe-CdS-ZnS | Sphere | 2 - 5 | Microwave/UV irradiation layer-by-layer assembly | [114,115] | |
| CdSeTeS | Sphere | 4 - 5 | Microwave irradiation | [116] |
2.1. Nanoparticles
Nanoparticles are increasingly being studied in experimental models for SCI treatment. The composition of these nanoparticles is extremely diverse including polymers, metals, metal oxides, silica, and biological molecules [49–51]. Because of their biocompatibility, polymeric nanoparticles are among the most extensively used for drug delivery to the spinal cord [52]. Most nanoparticles are synthesized by single oil in water or double water in oil in water emulsion techniques [53]. For drug encapsulations, drugs are first dissolved in water and later added to the polymer solution in an organic solvent [53,54]. Other methods include 1) nanoprecipitation, where the base material and the drug are dissolved in an organic solvent, added to water dropwise, solvent evaporated and NP collected by centrifugation [54] and spraydrying technique, where a liquid feedstock, suspension or emulsion of the compound is atomized into a spray of fine droplets and then dried by a hot air-stream to evaporate moisture [55]. As the moisture evaporates, the NPs are formed and are readily recovered from the drying gas. Readers are directed to other excellent articles for synthesis of poly(lactic-co-glycolic acid) (PLGA, Figure 1A) [53–56], chitosan [57–59], and silica [60–65] nanoparticles that have been utilized within pre-clinical SCI models.
As the field is progressing, more nanoparticles are being fabricated [66], which will provide insights into benefits and functions of nanoparticles for various SCI treatment applications. Careful selection of a particular nanoparticle type should be made based on the specific requirements of the study. This is especially true in the spinal cord due to potential problems associated with the nanoparticle crossing the blood-spinal cord barrier and the structural complexity of the central nervous system.
2.2. Electrospun nanofibers
In contrast to drugs, topographical cues in the implanted scaffolds at the lesion site can serve as physical guides for the extension of new axons [67–69]. Electrospinning of nanofibers is advantageous for tissue engineering as this method can produce three-dimensional, highly porous scaffolds with a large surface area, which favors cell adhesion [70]. Most groups that utilize electrospinning use very similar systems. Typically, a high-voltage power supply is applied to a polymer solution loaded within a syringe and once electrostatic forces from the field are strong enough to overcome the surface tension of the polymer, a jet forms and polymer is pulled towards a grounded collector. As the polymer travels to the collector, dry fibers are formed upon evaporation of the solvent. Fibers can be collected on flat surfaces for random orientation or on rotating mandrels for aligned fiber morphology (Figure 1B). This method is relatively simple, inexpensive and offers high tunability. Parameters of the electrospinning system and polymer solution such as viscosity, flow rate, electric field strength, rotation speed, and temperature may be tuned to control fiber size, density, and morphology [71].
Aligned electrospun scaffolds can partially mimic the neural ECM environment as the nanofibers can form sub-micron topography, which could influence axonal growth and orientation [71–73]. Numerous electrospun scaffolds have been generated with natural biomaterials for neural regeneration. Aligned silk electrospun nanofibers can be prepared by collecting fibers of silk and polyethylene oxide onto a spinning aluminum wheel with interspaced glass squares glued to the outside circumference of the wheel [74]. Electrospun collagen scaffolds can be generated for use in SCI by collecting nanofibers on rotating aluminum foils [75].
2.3. Self-assembled nanofibers
Nanofibrous hydrogels may also be formed by spontaneous self-assembling peptides. These materials are composed of natural amino acid sequences and as such, they are nonimmunogenic, nontoxic, and biodegradable [76]. Self-assembling peptides are also advantageous as they can undergo gelation in physiological conditions and have a morphology that mimics in vivo ECM. Many common self-assembling peptides form nanofibrous structures by means of their ionic complementarity. Some peptides may have alternating positively and negatively charged amino acids on the hydrophilic domains of their surfaces [77]. Plus, in aqueous solution, these peptides may quickly assemble into β-sheets that form nanofibers ranging from 5 to 200 nm [78]. Another mechanism by which peptides form nanofibers is through an amphiphilic assembly. These self-assembling peptides, known as peptide amphiphiles, are composed of hydrophobic domains conjugated to peptide sequences that promote β-sheet formation, and an end domain oligopeptide sequence that often introduces bioactivity [78]. A common end sequence in peptide amphiphiles is the laminin-derived peptide sequence isoleucine-lysine-valine-alanine-valine (IKVAV), which can form fibers approximately 6-8 nm in diameter. This peptide amphiphile has also been studied for use in SCI [79].
One of the most widely studied self-assembling peptides with ionic complementarity is RADA16-I, which can form networks with fibers of approximately 10 nm in diameter. RADA16-I has been investigated in several studies as a treatment for SCI [80–83], Although these peptides have many advantages as biologic scaffolds for regeneration, many are inert and do not exhibit much bioactivity. As such, RADA16-I self-assembling peptides have been modified by linking short bioactive sequences to the peptide backbone. RADA16-I scaffolds have been modified for use in the spinal cord by linking biological motifs, such as IKVAV (Figure 1C), arginine-glycine-aspartic acid (RGD), and bone marrow homing peptide 1 [82,84–87].
2.4. Carbon nanotubes
Carbon nanotubes are carbon allotropes made of one or more single-atom sheets of graphene rolled into hollow cylinders [88]. Single-walled carbon nanotubes have diameters ranging from 0.4 to 2 nm, however, multi-walled carbon nanotubes can exhibit diameters as large as 100 nm (Figure 1D) [89]. Carbon nanotubes can be synthesized through various means such as chemical vapor deposition and high-pressure carbon monoxide [90]. Chemical vapor deposition is a common method that is used for large scale production of carbon nanotubes. With this method, a flowing hydrocarbon gas decomposes at high temperatures on either a silica catalyst or zeolite support. To control carbon nanotube properties such as the number of walls, diameter, and length, characteristics of the catalyst can be altered. Although this technique is cost-effective, carbon nanotubes produced by chemical vapor deposition often have a high density of defects. High-pressure carbon monoxide can produce high quality carbon nanotubes also on a large scale. With high-pressure carbon monoxide, carbon nanotubes form from the carbon of CO gas flowing through high-pressure, high-temperature reactors [91]. Metal catalyst clusters, such as iron, act as nucleating sites for carbon nanotubes to grow.
Carbon nanotube nanostructures have demonstrated promise in neural regeneration applications because of their size, which closely mimics that of ECM proteins, and high surface area [92,93]. For synthesis of carbon nanotube islands, Nick et al. heated silicon substrates in a chemical vapor deposition reactor while introducing hydrogen, argon, ethylene, and water vapor [94]. Carbon nanotubes may also be modified on their surfaces, as in a study where single-walled carbon nanotubes produced with high-pressure carbon monoxide conversion were functionalized with carboxyl groups through acid treatment [95].
In another study, polyethyleneimine, a permissive substrate for neurite outgrowth [96], was grafted to single-walled carbon nanotubes for in vitro experiments [97]. Here, carboxylic acid functionalized single-walled carbon nanotubes were sequentially reacted with oxalyl chloride and polyethyleneimine to form single-walled carbon nanotube-polyethyleneimine. The same researchers later functionalized carboxylated single-walled carbon nanotubes with polyethylene glycol (PEG) to produce net-uncharged carbon nanotubes [98]. In a separate study, multi-walled carbon nanotubes were functionalized with a 1,3-dipolar cycloaddition [99]. A thermally sensitive carbon nanotube-based hydrogel can also be fabricated by adding N-isopropylacrylamide to solutions of single-walled carbon nanotubes and PEG-dodecylamineaminoethyl piperazine crosslinker to prevent carbon nanotube aggregation in SCI [100].
2.5. Quantum dots
To the best of the authors’ knowledge, no studies have examined the use of quantum dots for delivering therapeutics into the lesioned spinal cords. Nevertheless, quantum dots are gaining attention as a novel drug delivery vehicle because of their excellent bioimaging parameters and versatility in surface modification, as well as the ability to replace toxic metals in the core with other biocompatible drug carriers [101,102]. In the future, advances in synthesis methods will allow biocompatible, noncytotoxic quantum dots that will be suitable for localized and systemic drug delivery without inducing side effects [101].
Quantum dots are semiconductor crystalline nanoparticles, typically between 2 and 10 nm in diameter. Quantum dots are typically composed of a heavy metal core, an unreactive intermediate shell to improve optical properties and an outer coating that can be tailored to specific applications [103,104]. For biomedical applications, various outer coating materials from biocompatible synthetic polymers to natural ECM components have been used. For example, a study has shown that covalent conjugation with biomolecules such as antibodies or peptides to improve neuron interfacing via integrins on the cell surfaces [105]. Furthermore, tethered quantum dot films can be used to electrically stimulate neural cells [106].
Quantum dots can be synthesized by pyrolysis [107,108] or hydrolysis of precursors [109–113] with multiple shell layers [114–116] added to the core (Figure 1E). Quantum dots can also be synthesized using biological organisms such as bacteria, virus, worms, and cancer cells, as reviewed by Zhou et al. [117]. Finally, to prevent cytotoxcitiy from release of toxic metal ions in the core and reactive oxygen species that lead to cell death [118–121], carbon nanodots have been synthesized for biomedical applications [122–131]. So far, no studies exist that utilized carbon nanodots in spinal cord applications. With excellent biocompatibility and stable fluorescence, carbon nanodots may be more widely investigated for drug delivery and bioimaging applications for SCI repair.
3. Nanoscale Drug Delivery Strategies
In the following sections, we provide an overview of nanoscale drug delivery strategies to achieve three major therapeutic targets: targeting inflammatory response, scavenging inhibitory components, and promoting axonal regeneration (Figure 2). Furthermore, we discuss a few emerging areas that show potential to revolutionize SCI repair strategies, and current efforts and barriers to clinical translation of nanoscale drug delivery strategies.
Figure 2.
Nanomaterials improve cell, tissue, and behavior outcomes in rat models of SCI. A: Injection of dextran sulfate (DS)-minocycline hydrochloride (MH) complexes in agarose gel into the lesion i) reduce reactive glial cells 6 weeks after injury (red: CD68), ii) decrease the ratio of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages, and iii) improve behavioral outcomes [162]. Scale bar: 1 mm. * denotes significant difference from untreated control; + from blank gel, and # from intraperitoneal (IP) injection of MH. B: Flavopiridol-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles result in improved tissue sparing (H&E images), higher BBB scores, and lower errors in grid walking compared to blank PLGA nanoparticles [191]. Scale bar: 500 μm. C: Left: spinal cord neurons interacting with a multi-walled carbon nanotube (MWCNT) substrate. Red arrows indicate tight contacts between MWCNT and axonal membrane. Scale bar: 500 nm. Right: Total number of identified growth cones normalized to the number of detected fibers on multi-walled carbon nanotubes (solid circle) and control conditions (open circle) [99]. *p < 0.05. D: Top: luxol fast blue (LFB) and H&E images of spinal cord injected with saline (control) or QL6 self-assembled peptides. Scale bar: 300 μm. Middle: Reactive astrocyte staining with GFAP 8 weeks after injury. Scale: 250 μm. Bottom: Basso, Beattie and Bresnahan (BBB) locomotor score indicating improved functional recovery after QL6 injection. [253]. Copyright permissions: A from Elsevier, B from Elsevier, C from American Chemical Society, D is open access.
3.1. Nanotechnologies to attenuate inflammatory response after SCI
As discussed earlier, secondary injury cascades after SCI include increased inflammatory response and the rise of reactive glial cells. Researchers have developed various methods of nanotechnology-mediated delivery of anti-inflammatory molecules to the lesion site to address such secondary injury responses. A key feature of this strategy is to locally deliver a high dose of anti-inflammatory therapeutics in nano-carriers that can achieve slow, sustained release of the drugs over a long period of time. This approach overcomes limitations of naked systemic delivery of therapeutics, such as drug toxicity from high doses required to achieve therapeutic effects, high cost of treatment, and wasted drugs [21,132]. In this section, we discuss various nanocarrier strategies to locally deliver therapeutics in a sustained manner to minimize inflammation.
3.1.1. Microscale and systemic strategies to reduce inflammatory response after SCI
Research strategies to minimize inflammatory response without nanomaterials have also been developed. One such method is systemic administration of therapeutics in saline. As mentioned in Section 1.2., CD95 ligand is a pro-inflammatory molecule that induces apoptosis of cells at the lesion and in the surrounding tissue after SCI [27]. Intraperitoneal injection of CD95 ligand-neutralizing antibody reduced the number of apoptotic cells at the lesion and in the surrounding tissue in a mouse model of transection SCI at T8/9. This also resulted in improved behavioral performance[133]. Similarly, in a mouse model of T9 contusion SCI, systemic injection of anti-inflammatory drug minocycline or CD25 antibody resulted in improved locomotor function recovery despite no difference in inflammatory marker levels [134]. A different anti-inflammatory molecule, IL-4, was administered into T8 contused rat SCI models [135]. The authors observed elevated anti-inflammatory IL-10 levels in blood, reduced macrophage/microglia coverage at the lesion and improved locomotor function. In a different study, acute inflammatory response was attenuated by systemic administration of atorvastatin in rat T10 contusion SCI models [136]. Reduced apoptosis and improved locomotor function were observed in both acute (4 hours) and chronic (4 weeks) SCI rats. Finally, anti-inflammatory effects of curcumin in rat compression SCI at T8-T9 was reported [137]. Intraperitoneal injection of curcumin reduced edema after SCI, improved locomotor function, reduced apoptosis and pro-inflammatory cytokine concentrations in spinal cord.
Another approach involves reducing the presence of pro-inflammatory macrophages. Examples include generation of anti-inflammatory M2 macrophages by incubating homologous macrophages with peripheral nerve segments prior to transplantation [138], reducing macrophage and microglia infiltration into the lesion by transplantation of degradation-resistant high-molecular weight hyaluronic acid hydrogels [139], and transplanting mesenchymal stem cells to promote M2 phenotype polarization of macrophages in the lesion [140]. Interestingly, transplantation of autologous mesenchymal stem cells and stimulated macrophages was explored in clinical trials [141,142]. Stimulated macrophages were obtained by incubating them with a piece of dermis ex vivo prior to transfusion. Both studies showed that these autologous cell transplantation therapies were well tolerated and also improved clinical outcomes in a fraction of the patients. However, all these studies utilized saline or media to deliver cells, which leads to poor cell survival after transplantation [143].
In clinic, an interesting strategy to target inflammation after SCI is to treat the patients with anti-inflammatory diet by eliminating common intolerable and inflammation-inducing foods and supplementing anti-inflammatory diet such as omega-3 and antioxidants. This diet intervention clinical trial of 20 SCI patients resulted in decreased sensory neuropathic pain and circulating pro-inflammatory cytokines [144] as well as improved mood in SCI patients, with potential mechanism being reduced serum IL-1 β levels [145].
Although clinical trials of cell transplantation and diet intervention have been investigated, all these non-nanoscale strategies to attenuate inflammatory response after SCI pose limitations in exerting full clinical potential. All the systemic injection studies discussed here delivered a high dose of tens of milligrams of the drug per rat, and many reported repeated daily injection of the therapeutics over a long period of time to minimize inflammation. In addition, cell transplantation in liquid results in poor cell survival, thereby necessitating a biomaterial carrier to prolong cell survival at the lesion. It is important to note that biomaterials with nanoscale fibers increase in vitro neurite extension and proliferation of neural stem cells compared to microscale fibers [146,147]. Finally, anti-inflammatory diets cannot be generalized to many SCI patients as a quarter to over half of the SCI patient population suffer from gastrointestinal complications including dysphagia, or difficulty in swallowing [148,149]. These limitations call for increased research and development of nanoscale materials to deliver anti-inflammatory molecules, cells, and nutrients to target inflammation after SCI and to improve patient outcome.
3.1.2. Methylprednisolone delivery in nanomaterials
Methylprednisolone (MP) is a synthetic glucocorticoid that has been widely investigated for its anti-oxidative and anti-inflammatory effects and is the only FDA approved drug to mitigate effects of SCI [150]. MP has been shown to up-regulate the expression and activation of the β-catenin signaling pathway, including low-density lipoprotein receptor related protein-6 (LRP) pho-sphorylation, β-catenin, and glycogen synthase kinase-3β (GSK) phosphorylation (Figure 3) [151]. This signaling cascade prevents cellular apoptosis and reduces autophagy thus promoting restoration of neurological function after SCI. The clinical administration of MP consists of a systemic injection at 30 mg/kg and imposes various side effects such as gastric bleeding, sepsis, and pneumonia [12,14]. Thus, several studies have been designed to develop nanoparticles for localized, sustained delivery of MP. A study in 2008 developed PLGA-based nanoparticles to encapsulate MP [152]. MP was released from MP-nanoparticles for 6 days, and MP-nanoparticles were then embedded in agarose hydrogel and placed on top of the lesion, above dura mater of the contused T9. A denser agarose gel layer was placed on top for stability. The authors observed diffusion of MP into the lesion site 2 and 7 days after the MP-nanoparticle application, and reduced staining intensity of ED-1, calpain, and iNOS. The follow-up study compared systemic injection of MP versus local delivery of MP ± nanoparticle in a T9 hemisection rat spinal cord injury model with behavioral assessment at 1, 2, and 4 weeks post injury and immunohistochemistry at 24 hours, and 2 and 4 weeks [153]. A sustained in vitro MP release profile was established over 96 hours. The study demonstrated that localized, sustained MP-nanoparticle delivery led to not only anti-inflammatory and anti-apoptotic effects similar to the initial study, but also significantly reduced lesion volume and improved walking of the rat subjects after injury. Although delayed delivery of the therapeutics was not explored, both studies exhibited therapeutic effects from localized delivery of MP at a much lower dosage (156 μg/200-230 g and 200 μg/230-260 g, respectively).
Figure 3.
Pro-inflammatory responses via β-catenin signal transduction pathway. Binding of methylprednisolone induces lipoprotein receptor related protein-6 (LRP-6) phosphorylation, which in turn induces glycogen synthase kinase (GSK) phosphorylation and induces dissociation of β-catenin from a protein complex consisting of adenomatous polyposis coli (APC), Axin, and GSK. Subsequently, β-catenin is translocated into the nucleus where it binds to the transcription factors and regulate cell apoptosis. Adapted from [151].
In addition to PLGA, carboxymethylchitosan/polyamidoamine (CMCht/PAMAM) dendrimer nanoparticles have been developed to preferentially deliver MP to glial cells in vitro and hemisectioned rat spinal cord lesions [154]. MP-encapsulated CMCht/PAMAMs were 109 nm in diameter, and MP was sustainably released for 14 days. MP-CMCht/PAMAM uptake by microglia was faster compared to astrocytes and oligodendrocytes in vitro, and microglia viability was significantly reduced in an MP dose-dependent manner. In vivo, MP-CMCht/PAMAM internalization was detected at the lesion site three hours after nanoparticle administration, and correspondingly rat subjects demonstrated improved Basso, Beattie and Bresnahan (BBB) locomotor scale score over 4 weeks of time when MP-nanoparticles were delivered locally. In addition, an interesting study was reported wherein chitosan-MP conjugate nanoparticles, of 150 - 350 nm in size, were developed as non-viral gene delivery carriers [155]. The authors took advantage of the fact that MP is a glucocorticoid whose receptors are also nuclear receptors in the cytoplasm of the mammalian cells. They showed that compared to chitosan-plasmid DNA (pDNA) conjugate, chitosan-MP-pDNA composite was more effective in gene delivery into cells cultured in vitro as well as into T9-compressed rat spinal cords in vivo when injected into the epicenter. Also, the presence of MP in the composite exhibited anti-inflammatory and anti-apoptotic effects in the injured spinal cord tissues.
3.1.3. Minocycline delivery in nanomaterials
In addition to MP, other anti-inflammatory drugs have been developed and shown preclinical success for SCI repair. One example is minocycline, with its anti-oxidative, anti-apoptotic, and mitochondria-stabilizing properties [156–158]. Recently, nanoparticles composed of PEGylated poly-ε-caprolactone were developed as a minocycline delivery vehicle to modulate pro-inflammatory microglia/macrophages in vitro and in vivo [159]. Nanoparticles were about 100 nm in diameter, and clathrin-mediated endocytosis of nanoparticles by activated microglia/macrophages in vitro was detected within 24 hours after treatment, and TNF-α and IL-1β secretions were reduced. Next, minocycline-loaded nanoparticles at 1 μg/mg reversed activated microglia/macrophages in vitro. In vivo response was assessed by injecting minocycline-loaded nanoparticles in a hydrogel carrier at T12 of mice spinal cords. After 3 and 15 days post injection, the authors observed specific internalization of nanoparticles by activated microglia/macrophages in response to the injury caused by hydrogel injection. In a follow-up study, the authors utilized the same drug-nanoparticle complex at the same concentration for an in vivo compression SCI model [160]. The authors observed highly selective uptake of nanoparticles by activated microglia and macrophages with subsequent reductions in inflammatory response by these cells, as well as increased infiltration of M2 macrophages to the lesion; however, only the immediate injection showed therapeutic effects as evidenced by Basso Mouse Scale.
Another group developed dextran sulfate-minocycline hydrochloride complexes via Ca2+ or Mg2+ cation-assisted self-assembly [161]. These complexes are easy to create by mixing dextran sulfate dissolved in CaCl2 or MgCl2 with minocycline hydrochloride, ranging from 50 to 100 nm in diameter, and exhibit slow, sustained release of minocycline hydrochloride over 40 days. The same group then assessed the effect of this complex for SCI repair using a rat unilateral C5 cervical contusion model [162]. Agarose hydrogel was mixed into the complexes for localized injection of therapeutics, and release rate was controlled by varying the ratio of dextran sulfate to minocycline hydrochloride (Figure 2A). Compared to IP injection of minocycline hydrochloride, minocycline hydrochloride gel injection into the lesion combined with application onto the dura mater resulted in higher retention of minocycline hydrochloride at the lesion over 21 days, reduced lesion volume 6 weeks after the injury, reduced CD68-expressing reactive microglia/macrophages, and decreased pro-inflammatory to anti-inflammatory macrophage phenotype ratio. Furthermore, minocycline hydrochloride gel improved behavioral outcomes and increased myelination of neurons around the lesion site.
3.1.4. Other anti-inflammatory drugs in nanomaterials
Other drugs that were encapsulated in nanoparticles to attenuate the inflammatory response after SCI include Chicago Sky Blue [163] and estrogen [164]. Chicago Sky Blue, also known as p425, was recently discovered as an allosteric inhibitor for macrophage migration inhibitory factor (MIF) by interfering with the interaction of MIF with its receptor CD74 [165]. According to the authors of the Chicago Sky Blue delivery study, Chicago Sky Blue is less cytotoxic than MP at a high concentration of 10 μM. Furthermore, whereas MP has a short therapeutic window of 48 hours post injury, Chicago Sky Blue can exert its effects for up to 96 hours after SCI [163]. The authors then determined that nanoparticles of up to 200 nm in size can be systemically delivered and extravasated into the contused spinal cord lesion site up to 96 hours post injury. To enhance localization of Chicago Sky Blue to the lesion site, the authors encapsulated Chicago Sky Blue in "stealth liposomes" of 110 nm in size that are PEGylated to help evade the reticuloendothelial system, cross the blood-spinal cord barrier and localize to the lesion site. Although in vitro cell response to Chicago Sky Blue released from liposomes was not characterized, the authors showed improved white matter sparing, preservation of vascular structure and stability after tail vein injection of Chicago Sky Blue-liposome 96 hours after the contusion SCI at T9. Transcription levels of not only anti- but also pro-inflammatory cytokines were upregulated, which the authors attributed to the timing of therapeutic delivery. Although other therapeutic outcome measurements such as neural regeneration and behavioral assessments were not performed in this study. This study nevertheless showed the potential of using liposomes and Chicago Sky Blue to localize delivery of SCI therapeutics via systemic injection.
The effects of localized delivery of estrogen in PLGA nanoparticles on SCI repair were assessed with the premise that estrogen is anti-inflammatory, anti-apoptotic and neurotrophic [164]. Estrogen was encapsulated in PEGylated PLGA nanoparticles, embedded in agarosebased gel plugs, and placed on the contused spinal cord at T9 immediately after the injury. Although nanoparticle size was not directly reported, previous work by the authors indicated that these nanoparticles are of 100 nm in diameter [166]. The authors harvested whole blood, cerebrospinal fluid, and injured spinal cord tissues 6 hours after nanoparticle application for cytokine analysis. Estrogen levels in tissues were twice as much as those in plasma in both 2.5 μg and 25 μg estrogen doses, suggesting successful localized estrogen delivery. Further, 27 pro- and anti-inflammatory cytokines were profiled from plasma, cerebrospinal fluid, and tissues. Notably, key markers of SCI such as IL-6, GFO-KC, MCP-1, and S100β levels dropped in all three sample groups after localized estrogen treatment. Overall, this study suggests the possibility of estrogen therapy for SCI repair, and the impact of this study would be further potentiated by complementary immunohistochemistry and behavioral analyses.
Finally, inflammation can be attenuating by scavenging pro-inflammatory monocytes from blood circulation via immune-modifying nanoparticles that bind to macrophage receptor with collagenous structure (MARCO) present on the surfaces of monocytes [167,168]. Systemic injection of the immune-modifying nanoparticles into mice sustaining contusion spinal cord injury 1) showed a significant decrease in pro-inflammatory cell profiles at the lesion site, 2) decreased deposition of fibronectin, collagen IV, and CSPG, 3) increased axonal regeneration and serotonin secretion, and 4) improved functional recovery as measured by Basso Mouse Scale assessment [168].
3.2. Nanotechnologies to overcome inhibitory effects following SCI
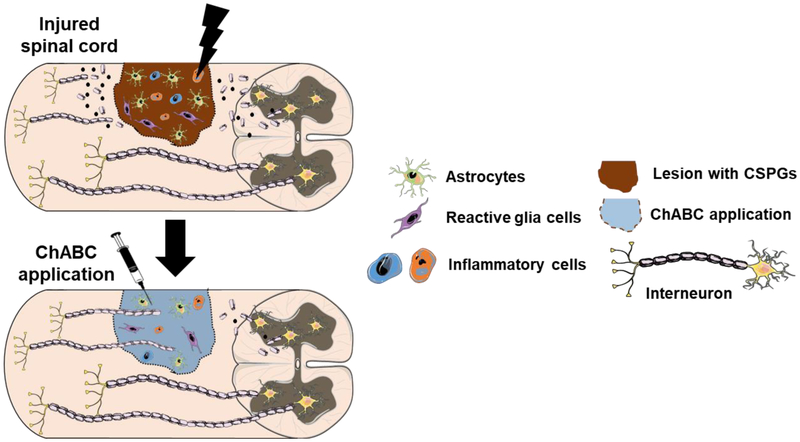
The regenerative capacity of damaged axons in adult CNS is limited. However, seminal work by Aguayo has demonstrated that axons from transected peripheral nerves can successfully regenerate over long distances [169]. The three known breakdown products of myelin sheath are Nogo-A, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein (OMgp) [22]. It has been shown that the Nogo-A is the most commonly expressed isoform present in the CNS and in contrast absent in the PNS [170]. Additionally, genetic deletion of MAG and OMgp in separate studies did not improve the neurite outgrowth after SCI. These findings suggest Nogo-A as a dominant player in the inhibition of axonal regeneration while MAG and OMgp provide secondary contributions [171,172]. All three myelin-derived inhibitors bind to the Nogo-66 receptor (NgR) primarily expressed on the surface of injured axons and prevent the regeneration of damaged axons through the Rho-dependent pathway (Figure 4) [173]. Furthermore, migration of astrocytes, glial cells, macrophages and microglia to the injury site causes the formation of a glial scar that blocks the advancement of the axonal growth cone [33,139,174]. Usually, the glial scar is considered a favorable process as it initiates a wound healing response that prevents the further spread of damage to uninjured parts and limits the spread of inflammatory reaction [175]. However, in the case of injury to the nervous system, it can act to physically block nerve regeneration near the lesion site due to the dense meshwork of reactive glial cells [32]. One of the main therapeutic approaches for overcoming the CSPG inhibition is through the application of the bacterial enzyme ChABC derived from bacteria Proteus vulgaris [176]. Research shows that the use of ChABC enzyme can help in preventing glial scar formation by enzymatic breakdown of inhibitory GAG side-chains from the CSPG protein core (Figure 5) [177].
Figure 4.
Pathway for signaling from p75–Nogo receptor complex to RhoA. Binding of myelin derived inhibitors (Nogo, MAG, and OMgp) on the NgR causes strengthening of p75 and Rho GDI-Rho GTP complex resulting in actin stabilization and inhibition of neurite outgrowth. B) Binding of anti-Nogo Ab or competitive antagonist for the Nogo-66 receptor such as NEP 1-40 have shown to decrease myelin induced growth cone collapse and promote neurite outgrowth.
Figure 5.
Glial scar mediated neurite inhibition. Glial scar acts as a barrier and axonal regeneration can be achieved after bacterial enzyme chondroitinase ABC (ChABC) treatment. Reactive glial cells, astrocytes, inflammatory cells such as microglia migrate to the lesion area following injury. They synthesize and secrete CSPGs around the lesion area and prevents regenerating axons to surpass. Treatment with ChABC removes the CSPGs in both the glial scar which facilitates the regenerating axons to pass through the lesion core to connect to the distal target. Figure adapted from Servier Medical Art.
3.2.1. Microscale and systemic strategies to target inhibitory environment after SCI
Many microscale technologies have been developed to locally deliver therapeutics to target both myelin-derived inhibitors and glial scar in pre-clinical models of SCI. For instance, Lee et al. used lipid microtubes (500 nm × 40 μm) to obtain a sustained release of biologically active, thermostabilized ChABC over 2 weeks [178]. Sustained and localized release of thermostabilized ChABC significantly improved CSPG digestion in glial scar and enhanced axonal sprouting and functional recovery after T10 hemisection injury in adult rats. Similarly, Wilems et al. used a combination of PLGA microparticles (approximately 9.1 μm) and fibrin hydrogel to achieve slow and sustained release of Nogo-66 receptor antagonist peptide, NEP1-40 [179]. The authors also obtained sustained release of ChABC from lipid microtubes over 2 weeks. These release formulations decreased CSPG deposition and increased axon growth in rat compression SCI at T8. The same research group also further modified the same platform by adding embryonic stem cell-derived progenitor motor neurons and heparin-bound growth factors (NT-3 and platelet derived growth factor-AA) [180]. When transplanted into hemisectioned rat spinal cords at T7-9, the authors observed lower CSPG content in the lesion and neuronal differentiation of progenitor motor neurons. However, the combination of progenitor motor neurons, ChABC and NEP1-40 reduced the transplanted cell survival and increased macrophage infiltration, warranting further investigation on the combinatorial therapy approach. Additionally, Xia et al. designed poly(propylene carbonate) electrospun micron-scale fibers as a platform for the sustained delivery of dibutyryl cyclic adenosine monophosphate (dbcAMP) to the hemisected spinal cord [181]. Results demonstrate that the sustained delivery of dbcAMP reduced glial scar formation, promoted axonal sprouting, and promoted functional recovery in contrast to the fibers without dbcAMP. Similarly, Rooney et al. used PLGA microspheres to deliver dbcAMP to the transected spinal cord using oligo[(polyethylene glycol) fumarate] hydrogels as scaffolds [182].
In contrast to the localized delivery mechanisms, Li et al. demonstrated the efficacy of systemic therapy by performing the subcutaneous treatment with NEP1-40 [183]. The authors argued that the local delivery initiated at the time of SCI may be logistically difficult for the treatment of many SCI patients. Dorsal hemisection was performed at T6 in adult female mice and the peptide was injected 7 days after SCI. Results indicate that delayed systemic delivery did not limit the degree of axon sprouting and functional recovery. However, authors acknowledged the importance of delivering therapeutics within a time window before reformation of the blood-spinal cord barrier.
In addition to therapeutic molecules, cell delivery has also been explored. For example, Nishimura et al. administered human neural stem cells encoding interferon-β gene intravenously to target glial scar in adult mice 1 week after T10 dorsal hemisection injury [184]. Previous study from the same group suggested that the administration of interferon-β induced functional and structural recovery in injured spinal cord [185]. Results showed that the animals exhibited suppression of glial scar formation and regeneration of damaged nerves in the lesioned spinal cord. The authors concluded that the exceptional migratory ability of neural stem cells makes intravenous injection a promising tool for delivering therapeutic genes in the treatment of SCI.
Release of myelin-derived inhibitors and formation of glial scar could begin as early as a few hours following injury and could last for weeks [40]. This calls for a sustained presence of therapeutics to effectively target inhibitory components in the spinal cord lesions. The presence of the blood-spinal cord barrier is a significant impediment to pharmaceutical intervention in the spinal cord, it inadvertently prevents the efficient delivery of the vast majority of therapeutics [40]. Because of this reason, nanocarriers are increasingly being studied to facilitate passage through the blood-spinal cord barrier for extended therapeutic delivery and target myelin breakdown products and inhibit glial scar formation in experimental models of SCI [186,187].
3.2.2. Polymeric nanoparticle-based delivery vehicles
As mentioned earlier, PLGA is one of the most commonly researched biomaterials used for SCI repair applications [54]. It is biodegradable and FDA-approved as a drug carrier. Additionally, the degradation rate of PLGA can be controlled by varying the ratio of monomer units used in the polymerization [53]. Baumann et al. incorporated a combination of PLGA nanoparticles (252 nm) and hyaluronic acid (HA) based hydrogels into the intrathecal space of injured rat spinal cords. The composite biomaterial was well tolerated showing no increase in inflammation, scarring, or cavity volume relative to controls [188]. In a separate study, the anti-Nogo-A antibody was encapsulated in 300 nm PLGA nanoparticles [189]. Anti-Nogo-A is an antagonist of the myelin-derived inhibitor Nogo-A known to cause growth cone collapse and prevent neurite outgrowth. Results showed the sustained release of bioactive anti-Nogo-A over 4 weeks while maintaining its bioactivity. The nanoparticles helped in slowing the rate of drug release, while the HA-based hydrogel could potentially help in localizing the particles at the site of injury.
Another drug studied to prevent glial scar-mediated damage after SCI is flavopiridol. Flavopiridol is a cell cycle inhibitor used in SCI models to mitigate apoptosis. SCI leads to the upregulation of cell cycle-related molecules, which cause post-mitotic cell death of neurons and oligodendrocytes and also initiate mitotic cell proliferation and activation of microglia, macrophages, and astrocytes [190]. Ren et al. used PLGA nanoparticles to obtain stable release of flavopiridol while avoiding the strong side effects of its systemic injection in a rat righthemisection injury model at T10 (Figure 2B) [191]. Results from the study show that blank PLGA nanoparticles lacking flavopiridol did not affect the proliferation or survival of astrocytes and neurons. Moreover, PLGA-flavopiridol nanoparticles resulted in decreased expression of inflammatory and cell cycle genes, such as TNF-alpha and caspase-3 at day 3 post-injury. Histological analysis showed fewer degenerating neurons and reactive astrocytes. Animals treated with flavopiridol nanoparticles exhibited improved motor recovery compared to those treated with blank nanoparticles [191].
Although PLGA is among the most widely studied delivery vehicle, recent advances in polymer science have introduced several new and promising drug delivery tools. One such example is poly(methyl methacrylate) (PMMA) because of its extremely narrow size distribution and good mechanical stability [192]. In a study by Papa et al., PMMA-nanoparticles (approximately 100 nm in size) were fabricated and their ability to specifically target activated microglia and macrophages, both in vitro and in vivo, was examined [192]. Results showed that the PMMA-nanoparticles were internalized selectively by lipopolysaccharide-activated microglia, without any toxic effect. Moreover, in vitro and in vivo studies showed the selective release of a mimetic-drug, a far-red fluorophore To-Pro3 within the cell cytosol of activated microglia and macrophages. Therefore, nanoparticles could be used as delivery systems to control inflammatory reaction at the injury site, which may help in reducing glial scar formation.
3.2.3. Nanofiber-based delivery vehicles:
In addition to nanoparticles, nanofibers have been developed to deliver therapeutics against inhibitory molecules at the lesion site. For example, Liu et al. used electrospun collagen nanofibers for sustained protein delivery to treat SCI [193]. The previous study performed by the same group found that the nanofiber topography enhanced the maturation of human Schwann cells [194]. Additionally, electrospun collagen nanofibers were shown to have increased neurofilament sprouting with decreased astrocyte proliferation in a rat hemisection SCI model [75]. In this study, researchers crosslinked ChABC, neurotrophin-3 (NT-3) and heparin onto nanofibers [193]. Bioactive ChABC was detected for at least 32 days in vitro, however, its efficiency in vivo has yet to be determined. Moreover, dorsal root ganglion (DRG) outgrowth assay indicated sustained release of NT-3/heparin for 4 weeks. This study provided an alternative strategy for the sustained release of relatively unstable proteins/enzymes along with the synergistic effects of topographical cues from nanofibers.
In a separate study, Gelain et al. engineered neural prosthetics by assembling electrospun nanofibers made of PLGA and poly(ε-caprolactone) blended fibers and selfassembling peptides [84]. The average diameter of the fiber was 592 nm whereas the tube length was between 2 and 3 mm, depending on the size of the cavities to be filled. The scaffolds were encapsulated with the ChABC to target glial scar in combination with brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and vascular endothelial growth factor (VEGF) to obtain a controlled release of therapeutics during the chronic stage of SCI following contusion at T10. Moreover, mechanical and degradation properties of the scaffold and the release profile of the therapeutics can be tuned by varying the crosslinking density of peptides. Rats treated with the scaffolds showed a significant increase in the neuronal marker βIII-tubulin. Moreover, treated animals had higher BBB scores 6 months post-transplantation.
More recently, Nguyen et al. fabricated 3D aligned nanofiber-hydrogel scaffolds as a biomaterial platform to deliver proteins and nucleic acid therapeutics (small non-coding RNAs), along with synergistic contact guidance in a rat cervical (C5) hemisection SCI [195]. The scaffolds comprised aligned poly(caprolactone-co-ethyl ethylene phosphate) electrospun nanofibers that were distributed in a 3D configuration within a collagen hydrogel. Electrospinning enabled the incorporation of microRNA (miR)-222 and NT-3. miR-222 has been shown to modulate oligodendrocyte differentiation and remyelination in vitro [196], whereas NT-3 promotes neuronal survival and regeneration. The results show that a sustained release of NT-3 was obtained over a 3 month period. Moreover, nanofibers embedded with miR-222 promoted neurite extensions and supported remyelination of damaged axons. The results showed that aligned bio-functionalized scaffolding platform effectively provided bio-mimicking contact guidance, and allowed the controlled delivery of therapeutic biomolecules of different nature and molecular weights.
In a different therapeutic approach for inhibiting the glial scar, Tysseling-Mattiace et al. used a bioactive matrix without the encapsulation of exogenous proteins or cells in a mouse compression thoracic (T10) SCI model [79]. Peptide amphiphile molecules were self-assembled from aqueous solution into cylindrical nanofibers (6-8 nm in diameter) in vivo and displayed bioactive epitopes on their surface. A previous study from the same group reported negatively charged peptide amphiphiles incorporating the neuroactive pentapeptide epitope from laminin, isoleucine-lysine-valine-alanine-valine (IKVAV) [86]. The nanofibers containing the IKVAV epitope suppressed astrocytic differentiation of cultured neural progenitor cells. These led the authors to hypothesize that the injection of the bioactive amphiphile after SCI might reduce glial scar formation and thereby promote neuronal outgrowth. Results from the in vivo treatment showed reduced astrogliosis and cell death along with improved regeneration of both motor and sensory fibers through the injury site. These results demonstrate the significance of 3D nanoscale structures in limiting inhibitory glial scar formation, thereby promoting cellular recovery following SCI.
3.3. Nanotherapeutics for axonal regeneration
In addition to targeting inflammatory response and inhibitory molecules in the lesion, axonal regeneration after the injury is key to improving functional outcomes. Strategies to enhance neuronal regeneration include providing topographical cues to guide the outgrowth of axons, as well as delivering neurotrophic growth factors and other neuro-regenerative cytokines at the lesion [197]. These physicochemical cues can be provided using nanomaterials, such as nanoscale fibers for physical guidance and cytokines encapsulated in nanocarriers. In addition, we briefly discuss nanomaterial-mediated gene delivery strategies to enhance growth factor expression by the host cells at the lesion site.
3.3.1. Microscale and systemic strategies to enhance axonal regeneration after SCI
Delivery of neurotrophic factors in microparticles with micro- and macro-scale features has been widely investigated to promote axonal regeneration after SCI in vivo. For example, in situ delivery of neuregulin-1 in PLGA microparticles prolonged neuregulin-1 release enhanced neurite outgrowth and myelination in T7 compressed rat spinal cords [198]. Delivering neurotrophic factor-loaded microparticles in injectable hydrogels further enhanced axonal regeneration after SCI in vivo. For example, delivery of BDNF-encapsulating PLGA microparticles in hyaluronan and methylcellulose (HAMC) hydrogels promoted spinal learning in rat complete T2 transection SCI models [199]. BDNF binds to its receptor tropomyosin-related kinase B (TrkB) with high specificity and affinity to mediate most of its generally desirable effects in an injured spinal cord. TrkB is a tyrosine kinase receptor, which auto-phosphorylates intracellular tyrosine residues upon binding to BDNF and acts through four different but overlapping signaling pathways (Figure 6) [200–202]. Similarly, GDNF-loaded PLGA microspheres in alginate hydrogels supported axonal regeneration and behavioral recovery in rat T9/10 hemisection SCI models [203].
Figure 6.
BDNF-signaling pathway mechanism. Autophosphorylation tyrosine residues upon BDNF binding to TrkB acts through four different but overlapping intracellular signaling pathways. It sends pro-survival signals through phosphatidylinositol-3 (PI-3) kinase and serinethreonine-specific protein kinase B (Akt) pathway, stimulates axon growth through extracellular signal regulated kinase (ERK) and modulation of cyclic adenosine monophosphate (cAMP) pathway and influences synaptic plasticity and transmission through phospholipase C (PLC), inositol-3-phosphate (IP-3) and the calcium signaling pathway. Additionally, through the phosphorylation of N-methyl-D-aspartate (NMDA) receptor subunits, TrkB signaling may lead to an increase in calcium and sodium influx also contributing to the synaptic plasticity. Figure adapted from [312].
Strategies that employ the use of polymeric microstructures and cellular components have been extensively researched to improve axonal regeneration after spinal cord injury. For example, neuronal alignment and axonal extension was observed in 3D hydrogels with microtopographical cues generated by multiphoton excitation crosslinking [204–206] and sacrificial crystal templating [207–209]. Combining microtopography and electrically conducting polymers also enhanced neuronal responses in vitro [210]. Furthermore, several in vivo studies demonstrate the importance of microtopographical cues for axonal regeneration. For instance, a multiple channel poly(lactide-co-glycolide) bridge promoted axonal regeneration 8 weeks after rat and mouse thoracic spinal cord injuries [211]. Electrospun poly-L-lactic acid microfibers facilitated long distance axonal regeneration following implantation into thoracic rat spinal cord injuries [212]. Further, conjugating poly(lactide-co-glycolide) microfiber bridges with polysaccharides such as hyaluronan, heparin, or chitosan promoted axon growth and myelination, and prolonged lentivirus retention after injection into T9/10 hemisectioned mouse spinal cords [213]. Natural polymers have also been used to develop microstructured tissue scaffolds applied to SCI. Collagen scaffolds composed of filaments from bovine skin were implanted into thoracic spinal cord defects in rabbits [214]. Regenerated axons were found in the implants and significant functional recovery was demonstrated through BBB scoring.
Cell-based therapy has also been a promising strategy for promoting axon regeneration after SCI [215]. Stem cell delivery introduces the potential for transplanted cells to differentiate into axon sprouting neurons that can bridge lesions [216]. Stem cells have been studied extensively and have undergone investigation in several clinical trials for SCI, demonstrating some favorable results [216]. Schwann cells, which originate from the peripheral nervous system, have also shown potential for SCI recovery [217,218]. In addition to their role in myelination, these cells produce extracellular matrix elements which encourage axonal outgrowth [219].
Many studies have combined the potential of tissue scaffolds and cell delivery for SCI. For example, agarose microchannels combined with bone marrow stromal cell-derived neurotrophic factors supported axonal regeneration in spinal cord transections in multiple studies [220,221]. Patel et al. improved Schwann cell survival and axonal ingrowth by suspending Schwann cells in ECM hydrogels, composed of collagen and laminin, and Matrigel® hydrogels [222]. In a similar study, Cerqueira et al. supported axon growth and improved functional recovery by delivering Schwann cells suspended in ECM hydrogels derived from decellularized peripheral nerve into thoracic contusions [223]. Transplantation of embryonic stem cell-derived neural progenitor cells in fibrin scaffolds containing NT-3 and platelet derived growth factor-AA also promoted survival and neuronal differentiation of the neural progenitor cells, and improved functional outcomes after mouse spinal cord hemisection at T9 [224]. However, more recent SCI research has moved towards the development of nanoscale structures, as these materials can more closely mimic native ECM components and provide a high surface area/volume ratio which make these structures a promising option for axonal regeneration [225].
3.3.2. Nanostructure
The physical features of the extracellular environment are important governing factors for cellular behavior in various tissues. For example, a study has shown that proliferation of rat pheochromocytoma cell PC-12 was enhanced when culture on coverglasses nanotextured via reactive ion etching [226]. Furthermore, axonal directionality of leech central nervous system derived neurons was enhanced on nanopatterns created by photolithography [227]. Extracellular protein fibers, such as collagen and laminin, are on the order of tens of nanometers in diameter and form structures that impact cellular behavior independent of chemical cues [228]. The influence of these features on cells, known as contact guidance, has been implicated in neuronal cell processes including migration, outgrowth, and alignment in the developing and injured nervous system [46,72,73,229], and in some cases contact guidance cues are preferred over chemical cues [230]. Neuronal projections, or neurites, sense their physical environment via growth cones, structures found at the end of elongating axons. Growth cones mediate contact guidance via sensation and transduction of physical cues within their environment to direct movement and extension of neurites [72].
Extensive research has leveraged contact guidance for the regeneration of neural tissue in the injured spinal cord. Researchers have demonstrated the ability to fabricate various nanotopographical features to influence axonal alignment and outgrowth. Common materials for fabrication over the past decade have included electrospun nanofibers, self-assembling peptide nanofibers, and carbon nanotubes. Here, these approaches will be discussed with regard to axon regeneration both in vitro and in vivo SCI models.
3.3.2.1. Electrospun nanofibers
Electrospun nanofibers have been developed to assess neural regeneration in both in vitro and in vivo. Several in vitro studies demonstrate the importance of nanoscale dimensions and fiber alignment in promoting neuronal growth and extension. For example, electrospun poly-L-lactic acid (PLLA) nanofibers (300 nm diameter) supported higher rates of neural stem cell differentiation and neurite outgrowth compared to microfibers (1.5 μm diameter) [146]. However, DRG neurite outgrowth was observed on larger PLLA electrospun nanofibers [231]. In another study, explanted DRGs cultured on randomly oriented gelatin extended neurites with greater length and abundance on fibers of larger diameter [232]. The discrepancies in these results from the initial study may not necessarily stem from nanofiber size but from material compositions, nanofiber alignment, cell types, and/or different time points of assessments.
In addition to PLLA, a nanocomposite scaffold composed of aligned poly(ε-caprolactone) fibers coated with PLGA core-shell nanospheres promoted proliferation and neurite extension of rat PC-12 cells [233]. Of note, alignment of fibers further enhanced neurite extension, as cells extended straight along aligned fibers and had a seemingly random distribution on unaligned fibers. A different study reported development of electrospun silk nanofibers, 330 nm in diameter and 1 nm in roughness, to deliver either BDNF, CNTF or both to rat retinal ganglion cells (RGCs) in vitro [74]. Although growth factors drastically increased axon growth from RGCs, silk fibers alone demonstrated the ability to promote axon outgrowth. Lee et al. demonstrated an enhanced effect of nanotopography combined with electrical stimulation by coating electrospun PLGA nanofibers with conductive polypyrrole [234]. Both PC-12 and embryonic hippocampal cells seeded onto scaffolds showed higher neurite length with aligned PLGA fibers, with synergistic effects observed from electrical stimulation.
Natural polymers such as collagen can also be utilized to develop electrospun nanofibers. Collagen nanofibers with an average diameter of 208 nm supported the outgrowth of DRG neurites the greatest when the fibers were aligned compared to randomly oriented and collagen coated glass [75]. In another study, the same research group enhanced the functionality of these scaffolds by incorporating NT-3 and ChABC for sustained delivery [193]. Interestingly, DRGs exhibited no neurite outgrowth on scaffolds unless NT-3 was present. Interestingly, in vivo implantation into complete unilateral C3 hemisections did not induce any significant axonal response to fiber orientation [75]. The authors postulate that the diameter of collagen fibers used may be too small, as larger fibers have been demonstrated superior axonal outgrowth [231].
Other studies have shown axonal regeneration along electrospun nanofibers in various rat models of SCI. In one study, electrospun poly(caprolactone-co-ethyl ethylene phosphate) nanofibers dispersed in collagen I hydrogels were implanted into C5 incisions of rat spinal cords [235]. At 12 weeks post injury, the implants parallel to the spinal cord length exhibited higher average neurite lengths than those implanted at an angle. The addition of NT-3 to parallel oriented implants demonstrated an even higher average neurite length. In a different study, Zamani et al. developed a two-piece electrospun PLGA nanofibrous scaffold where a core of aligned nanofibers surrounded by a nanorough cylindrical sheath [236]. Left lateral hemisections were performed at the T9-T10 level in rats and cylindrical nanofibrous scaffolds were implanted. At 8 weeks post implantation, axonal regeneration was observed along remaining scaffold fibers. Another group specifically targeted chronic SCI by developing hollow channels of poly(ε-caprolactone)/PLGA nanofibers functionalized with growth factors and self-assembling RADA16-I peptides to improve cell recruitment and adhesion [84]. Scaffolds were implanted into rats 1 month after T9-T10 contusion injuries. Structural recovery was detected 6 months post implantation in growth factor-loaded scaffolds, as regenerating nerve fibers were found along the full length of most channels in the presence of new vasculature. Functional improvements were also detected via BBB scoring and electrophysiology. Overall, these studies collectively demonstrate the importance of aligned electrospun nanofibers to promote axonal regeneration after SCI.
3.3.2.2. Carbon nanotubes
Axonal regeneration in both single-walled and multi-walled carbon nanotubes have been studied in vitro and in vivo. 2D in vitro studies include linear patterns of carbon nanotubes alternating with organometallic chemical octadecyltrichlorosilane to promote neurite extensions from hippocampal neurons [237], chitosan films aligned with multi-walled carbon nanotubes to promote alignment and neurite extension of hippocampal neurons [238], electrospun poly(ε-caprolactone) scaffolds coated in multi-walled carbon nanotubes to improve adhesion, proliferation and neurite length of PC-12 cells, [239], and carbon nanotube-polyethyleneimine (PEI) graft copolymer substrate for hippocampal neuron cultures [97]. Interestingly, carbon nanotube-PEI graft copolymer promoted favorable neuronal behavior when single-walled carbon nanotube was used, whereas multi-walled carbon nanotube substrates yielded the least favorable. The authors postulated that differences likely stem from the positive surface charge of PEI, whereas carbon nanotubes are negatively charged or zwitterionic [240].
Researchers have begun to incorporate carbon nanotubes within 3D scaffolds for axonal regeneration, as this approach can prevent the detachment and loss of carbon nanotubes that occurs with surface coating. For example, incorporation of single-walled carbon nanotubes into collagen type I-Matrigel® composite hydrogels enhanced neurite outgrowth from DRGs in vitro [95]. Lee et al. also added multi-walled carbon nanotubes to scaffolds by 3D printing amine functionalized multi-walled carbon nanotubes, between 15 and 37 nm in diameter, within poly(ethylene glycol) diacrylate hydrogels [241]. Neural stem cells extended significantly longer neurites on scaffolds with carbon nanotubes compared to cells cultured in the hydrogel alone. The increases in neurite length were attributed to the higher positive charge from amine groups on the carbon nanotubes, which may bind to a negatively charged neural membrane [242]. To better understand the mechanism of neural cell adhesion to nanoscale features, Sorkin et al. cultured either rat or locust neurons on carbon nanotube islands [243]. Neurons grew preferentially towards carbon nanotubes, and the authors suggested that cell entanglement with carbon nanotubes as an important mechanism for neurite-carbon nanotube adhesion. In a similar study, embryonic rat cortical neurons were cultured on a silicon substrate with regularly spaced carbon nanotube islands, either randomly oriented or vertically aligned [94]. Neurons were found to start accumulating on vertically aligned carbon nanotube pillars within 4 hours. At 14 and 21 days, cells had formed neural connections between pillars.
The impacts of carbon nanotubes on neural tissue explants have also been studied. Fabbro et al. fabricated films of pure multi-walled carbon nanotubes (15-20 nm) which were used as a substrate for co-culture of thin slices of the spinal cord and DRGs (Figure 2C) [99]. Favorable outgrowth of neurites on carbon nanotube substrate was mediated via formation of tight junctions between carbon nanotubes and cell membranes. This group later expanded on this study by fabricating 3D meshes of interconnected multi-walled carbon nanotubes to investigate their ability to guide neurites in-between segregated spinal cord explants in vitro [244]. Transverse spinal cord slices, separated by > 300 μm, were embedded in either multi-walled carbon nanotubes or gelled protein-rich plasma clot as a control. Although growth of neurite bundles occurred in both groups, neurites conformed to the complex 3D structure of the carbon nanotube network, extending in random directions. In contrast, neurites from control material formed into aligned thick bundles.
Although application of carbon nanotubes in preclinical SCI models has not been investigated extensively, there have been several significant studies that demonstrated the benefits of carbon nanotubes. PEG-functionalized single-walled carbon nanotubes were injected into T9 transections in rats at either acute (5 minutes post injury) or delayed (1 week post injury) time points [98]. Delayed administration of single-walled carbon nanotube-PEG was found to increase neurofilament positive fibers in lesions. In another study, thermally sensitive single-walled carbon nanotube-poly(N-isopropylacrylamide) hydrogels were injected into C7 contused rat spinal cords [100]. β-III tubulin positive fibers were found migrating into the injury site two months post injury. Additionally, functional recovery and body weights were highest in single-walled carbon nanotube-poly(N-isopropylacrylamide) groups, although statistical significance was not achieved.
3.3.2.3. Self-assembled nanofibers
Self-assembling peptide nanofiber scaffolds have been studied extensively for neural regeneration both in vitro and in vivo. In an initial study in 2000, Holmes et al. investigated RAD16 peptide scaffolds as a substrate to support neurite outgrowth [80]. These peptides contain RAD tripeptide sequences, which are similar to RGD sequences that serve as attachment sites for integrins [245]. PC-12 cells cultured on RAD16-II scaffolds, with fiber diameters between 10 and 20 nm, extended neurites when either pre-primed with NGF or treated with NGF after being cultured on scaffolds [80]. Without NGF treatment, neurite extension was not seen, although these cells attached to scaffolds. Interestingly, the neurites were not linear, as found in cells cultured on rigid plastic substrates, but followed contours of the self-assembling peptide nanofiber scaffolds, mimicking neurons in vivo.
After a study demonstrated that self-assembling peptide nanofiber scaffolds could be used to restore function in the CNS by repairing an injured optical pathway [246], Guo et al. investigated the potential of self-assembling peptide nanofiber scaffolds in SCI [81]. In this study, RADA16-I peptides were used alone or with either Schwann cells or neural progenitor cells in rat spinal cord hemisection injuries at C6/7. Six weeks post implantation, scaffolds in the lesion supported cell infiltration and axonal regeneration. In addition, Schwann cell-containing scaffolds supported axonal regeneration the greatest compared to neural progenitor cell-containing scaffolds or scaffolds alone. RADA16-I was also studied in a T9-T10 contusion SCI by Cigognini et al. [82]. In these experiments, bone marrow homing peptide, which was previously shown to enhance neural stem cell survival and differentiation, was conjugated to RADA16-I peptides. RADA16-I scaffolds increased cellular infiltration, basement membrane deposition, and axonal sprouting with larger number of growth cones within the lesion. Functional recovery was also improved in RADA16-I groups.
While the RADA16-I scaffolds demonstrated potential for SCI repair, they are limited by their very low pH, which can damage host tissue upon injection. As such, many groups neutralize peptides before transplantation which causes formation of solid hydrogel and loss of injectability. To develop a more permissive injectable RADA16-I scaffold, Sun et al. modified peptides with RGD and IKVAV short sequences, as these two fibers are oppositely charged at physiological pH and form nanofibrous scaffolds when combined [85]. RADA16-RGD and RADA16-IKVAV sequences formed networks with fiber diameters ranging from 15 to 50 nm. These two peptides were blended together and injected into 2 mm T8 defects in rat spinal cord. After two months, many axons were found within the graft of modified scaffolds, whereas in the unmodified RADA16-I scaffolds axons only grew along the cavity surface, highlighting the importance of adhesive peptide sequences in promoting axonal extension.
Another self-assembling peptide, named Ac-FAQ, was developed by functionalizing LDLK12 peptide with another peptide sequence FAQRVPP [247]. LDLK12 is an ionic self-assembling peptide that has demonstrated favorable biocompatibility with stem cells in vitro and in vivo through implantation into rabbit skin [248,249], and the FAQRVPP sequence enhanced neural stem cell differentiation and viability in vitro [247]. Ac-FAQ significantly increased GAP-43+ fibers in rat T9–T10 contusions 8 weeks after injury and improved functional recovery. The same peptide nanofibers were also implemented into electrospun poly(ε-caprolactone)-PLGA scaffolds [250]. Implantation of Ac-FAQ incision sites in T9-T10 spinal cords of rats elicited a favorable immune response and axonal infiltration into the scaffolds.
A simpler injectable self-assembling peptide nanofiber scaffold was created with IKVAV peptides [79]. When injected into T10 compressed mouse spinal cords, IKVAV self-assembling scaffolds reduced astrogliosis, promoted motor and sensory fiber extension across the lesion, and partially improved locomotor function recovery. This group further studied the IKVAV peptide amphiphiles in a different mouse strain with the same injury as well as in a rat T13 contusion injury [251]. Functional recovery in mice was similar to the previous study, while rats recovered much faster, which the authors attributed to differences in the injury model. IKVAV peptide amphiphiles promoted regeneration of nerve fibers, which corresponded to improved functional recovery.
Finally, another injectable self-assembling peptide, K2(QL)6K2, or QL6, which self-assembles into β-sheets at neutral pH [252] was examined as a scaffold in T6-T7 compression injuries in rats (Figure 2D) [253]. QL6 scaffolds improved neural plasticity, axonal regeneration, and locomotor function. Wan et al. described the use of self-assembling peptide scaffolds for delivery of neural stem cells to T7 injured rat spinal cord [254]. After 8 weeks, axonal counting of neurofilament positive fibers showed increased axonal density in self-assembling peptide scaffolds and in scaffolds with neural stem cells. In addition, Basso Mouse Scale for locomotion (BMS) scores indicated improvements in both treatment groups.
3.3.3. Delivery of bioactive molecules for axonal regeneration
Neurotrophic growth factors are biologically active molecules native to the nervous system that play important roles in cellular interactions during development and neuronal processes such as axonal sprouting following injury [255]. Factors including NT-3, NGF, and glial-cell-line-derived neurotrophic factor (GDNF) have been found to enhance axonal regeneration in injured spinal cord tissue and as such have been widely studied in various systems as a therapy in SCI models [256,257]. Strategies to deliver growth factors to injured spinal cord have ranged from incorporating factors into implantable nanofiber scaffolds, hydrogels or by encapsulating factors in polymeric nanoparticles [4].
3.3.3.1. Delivery of neurotrophic factors
A common strategy for the delivery and sustained release of growth factors is to encapsulate those molecules in polymeric nanoparticles. In 2008, Wang et al. prepared PLGA nanoparticles via double emulsion solvent evaporation to deliver GDNF to injured spinal cord [258]. Nanoparticles approximately 224 nm in size were found to release GDNF in a sustained fashion for up to 7 days. GDNF loaded particles were studied in severe contusions at T9/T10 in rat spinal cords. BBB scoring of hindlimb function over 31 days suggested that release of GDNF improved locomotor recovery. Staining of neurofilament showed neuronal fibers within lesion centers of PLGA-GDNF injected spinal cords and smaller fragmented fibers in lesions of PLGA only injected spinal cords.
Stanwick et al. aimed to improve the release profile of neurotrophic factors from PLGA nanoparticles by dispersing particles within injectable hyaluronan and methylcellulose (HAMC) hydrogels [259]. Release of NT-3 from nanoparticles was investigated in vitro through a rat DRG bioassay. Neurite outgrowth was detected for up to 28 days in DRGs exposed to HAMC nanoparticles. This same group later investigated the efficacy of NT-3 release from PLGA/HAMC composites [260]. Hydrogels were injected into the intrathecal space or rat spinal cords following T1-2 clip compression injuries. Axonal counting demonstrated significantly improved axon regeneration in spinal cords treated with hydrogels containing NT-3. BBB scoring reflected this finding as NT-3 treated groups had higher scores. A similar delivery system and injury model was used again by these researchers to deliver fibroblast growth factor-2 [261]. Although blood vessel density was improved in the dorsal horns, axonal density and functional recovery were not enhanced. The authors believe this system could be improved by delivering a higher dose of growth factor or by including other beneficial biomolecules.
Another approach to deliver neurotrophic factors is through encapsulation in polymeric nanofibers that have many benefits for neural regeneration as described earlier. To improve the release profile of NGF from poly(ε-caprolactone) scaffolds, Valmikinathan et al. added bovine serum albumin (BSA) to the nanofibers to protect NGF during harsh fabrication conditions and to enhance NGF release by increasing porosity via BSA degradation [262]. NGF containing poly(ε-caprolactone) and poly(ε-caprolactone)-BSA nanofibers were electrospun to achieve fibers with an average diameter of 550 nm. Release studies confirmed a decreased initial burst and more controlled release of NGF from poly(ε-caprolactone)-BSA nanofibers over 4 weeks. Analysis of neurite growth from PC-12 cells cultured with release samples from nanofibers showed increased neurite growth and longer extension in poly(ε-caprolactone)-BSA groups. In another study, NGF-BSA was encapsulated in emulsion electrospun poly(l-lactic acid-co-ε-caprolactone) nanofibers ~600 nm in diameter [263]. Compared to traditional blending electrospinning, the emulsion method to fabricate nanofibers reduced initial burst and exhibited a more stable release of BSA over 12 days. PC-12 cells cultured in supernatant from NGF-containing fibers exhibited neurite outgrowth. In a previously mentioned study, NT-3 was incorporated into poly(caprolactone-co-ethyl ethylene phosphate) nanofibers for use in a rat SCI model. Implants containing NT-3 exhibited the highest average neurite length compared to nanofiber-only groups [235].
Finally, magnetic nanoparticles functionalized with neurotrophic factors can enhance axonal regeneration through magnetic field-mediated spatiotemporal distribution of the nanoparticles near the regenerating axons and generating local force fields [264,265]. For instance, a study by Steketee et al. demonstrated that 50 nm superparamagnetic nanoparticles conjugated with TrkB agonist antibody (29D7) elongated neurite outgrowth and controlled growth cone motility in a magnetic force-dependent manner [266]. Activating TrkB signaling was key to the observations as control nanoparticles without 29D7 did not elicit similar responses. Similarly, NGF-bound magnetic nanoparticles promoted neuronal differentiation of PC-12 cells and oriented neuronal processes under magnetic field [267]. Magnetic nanoparticles hold great potential for clinical application as they can provide a non-invasive way of controlling axonal regeneration after SCI.
3.3.4. Gene delivery for axonal regeneration after SCI
Rather than directly introducing factors into the injury environment, some researchers have investigated delivery of genetic sequences to transduce endogenous cells to achieve sustained and localized production of neurotrophic factors in an injured spinal cord. For example, HEK-293T cell-derived lentiviruses encoding for either BDNF or NT-3 were incorporated into hydroxyapatite nanoparticles and loaded into poly(lactide-co-glycolide) multichannel bridges [268]. The bridges were then implanted into 4 mm T9-10 hemisections in rat spinal cord. Neurofilament staining in tissue rostral to the injury revealed that axon counts were significantly higher in both BDNF and NT-3 groups compared to empty channels. Axonal regeneration caudal to and in the center of lesions was not as substantial. More recently, delivery of nucleic acids for SCI was investigated [195]. In this study, MiR-222, which has been implicated in neuronal protein synthesis and has been found to enhance regrowth of injured neurons in vitro [269], was bound to micellar nanoparticles composed of poly(ε-caprolactone)-block-polyethylene glycol and poly(ε-caprolactone)-block-poly(2-aminoethyl ethylene phosphate). Nanoparticle-miRNA complexes were either added to poly(caprolactone-co-ethyl ethylene phosphate) solutions that were electrospun into aligned nanofibers with an average diameter of 820 nm or dispersed in collagen scaffolds. The aligned fibers were added to collagen in a cylindrical mold before gelation. Scaffolds were implanted into 1 mm C5 incisions in rat spinal cords. At 10 days post-implantation, neurofilament positive fibers were found growing into scaffolds that contained miR-222 more extensively than control scaffolds.
3.4. Emerging nanotechnologies for SCI repair
In addition to the studies reviewed thus far, there are several emerging technologies that hold great potential for revolutionizing SCI repair on their own or in combination with the nanosystems described earlier: optogenetics, immunotherapy, and genome editing through CRISPR/Cas9.
Optogenetics is a widely researched technique in neuroscience, primarily brain research, to control and study the behavior of excitable cells, such as neurons, with high spatiotemporal precision [270,271]. Optogenetics relies on genetically modifying target cells or animals that express light-sensitive transmembrane proteins, collectively called opsins, to enable optical stimulation [272]. Application of optogenetics for SCI repair in preclinical studies is on the rise, with studies showing functional improvements in respiration [273], functional synapse formation [274] and lower body function [275]. However, clinical translation of optogenetics for SCI faces several challenges, such as safe and effective delivery of vectors encoding opsins and limitations in transdermal illumination [270,276]. Nanomaterials can potentially provide solutions to overcome these limitations, by delivering vectors in nanocarriers that can localize into the lesion or utilizing nanomaterials that can convert near infrared-red light to wavelength-specific target light (a phenomenon called photon upconversion) [277], as demonstrated in a recent study where lanthanide-doped NaYF4:Yb/Tm upconversion nanoparticles were developed to improve deep-brain stimulation [278].
Recent successes in clinical trials of immunotherapy against cancer [279] can be benchmarked to develop novel therapeutic strategies for SCI. Preclinical studies suggest that vaccination of SCI patients can contribute to attenuating adaptive immune response and targeting myelin-derived inhibitors after SCI [280,281]. Currently, most immunotherapy strategies for SCI treatment target inflammatory response and myelin-derived inhibitors after the injury [282], however, one can envision similar approaches specifically to enhance axonal regeneration as well. Further, delivery of antibodies in nanomaterials may extend bioactivity of the injected vaccines and enhance therapeutic outcomes. However, clinical translation of this approach is premature at this stage, as safety concerns such as potential development of autoimmune diseases need to be thoroughly investigated before treating human patients is possible.
Finally, a recent development of a precise genome engineering tool, called clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system [283], holds potential to revolutionize SCI therapeutic and has already been applied to develop therapeutic strategies for cancer and many other genetic diseases [284]. Currently, no studies exist where CRISPR/Cas9 system was utilized to develop therapeutic strategies after SCI both in vitro and in vivo. Only a few studies exist in the neural space [285], with the applications including fluorescently labeling patient-derived neurons in vitro [286], editing genes in neural cells of the salamander [287] and C. elegans [288], and targeting multiple genes in the adult mouse brain in vivo [289]. CRISPR/Cas9 system is an exciting area of research not only for developing novel SCI repair strategies but also for creating nanocarrier delivery platforms. Incorporation of the CRISPR/Cas9 system in nanocarriers is in its nascent stages [290], as demonstrated in only a few delivery vehicles such as yarn-like DNA nanoparticles [291] or native Cas9:single guide RNA complexes that can be further complexed with supernegatively charged proteins [292], cationic arginine gold nanoparticles [293] or delivered in cationic lipofectamine transfection reagent [294]. Localized and sustained delivery of Cas9 and single guide RNA in nanomaterials will drastically improve SCI treatment strategies by targeting activated glial cells and other pro-inflammatory cells or promoting axonal regeneration.
3.5. Efforts and barriers to clinical translation
Despite the abundance of data that demonstrate the efficacy of nanomaterials for SCI repair in pre-clinical studies, nanotherapies are not utilized in treating SCI patients in the clinic. Currently, there are no clinical trials listed in the ClinicalTrials.gov website that involve any type of nanomaterials for SCI repair. In comparison, there are 49 clinical trials listed for nanotherapeutic strategies against cancer [48]. Possible reasons include lack of a universally standardized definition of nanoscale, complications with renal clearance and degradation products that remain in the body, the blood-spinal cord barrier, nanoparticles potentially having low payload, and low public awareness.
Currently, there exist many terminologies associated with nanoscale technologies (e.g., nanomedicine, nanoparticles, nanoscience, nanotherapeutic, nanodrugs) and yet no unanimous definition of “nanoscale” exists [1,66], One of the reasons behind this may stem from the fact that no universally established definition of nanoscale exists across various regulatory agencies that modulate clinical translation of nanotechnologies. Many researchers follow guidelines established by several entities such as the American Society for Testing and Materials International, who define nanoscale as 1 to 100 nm in size [1,3,295]. On the other hand, as mentioned in Section 1, the UK House of Lords Science and Technology Committee recommended that nanoscale be defined for up to 1000 nm in size [1]. Unanimous definition of nanoscale will facilitate regulatory pathway clearance towards clinical translation of various nanotechnologies developed, including the ones discussed in this review, and improve SCI treatment regimens.
Another critical challenge to clinical translation of nanomaterials is systemic clearance from the body. While several nanomaterials such as liposomes and certain polymeric/metallic/protein nanoparticles are approved by the US Food and Drug Administration (FDA) for clinical trials and/or use, the fate of these nanomaterials in the body is still widely unknown [296,297]. Molecules under 3 nanometers in size exhibit 100% urine/blood filterability, and once the hydrodynamic diameter reaches as low as 3.4 nm, clearance decreases to 80% [298]. Since most of the nanomaterials discussed here are over 3 nm in size, thorough investigations on systemic clearance of nanomaterials and the effects of degradation products on cell behavior in the body are much needed. In addition, individual carbon nanotubes can be cleared from the body, whereas aggregated carbon nanotubes can be accumulated in the glomerular capillaries and not filtered through the kidney [299]. When designing carbon nanotubes, several design parameters such as shape, dimensions, surface charges, and structural features need to be taken into consideration to not elicit any cytotoxic response from the patients.
The blood-brain barrier and the blood-spinal cord barrier strictly regulate diffusion transport of molecules to the spinal cord parenchyma, as they only allow molecules with MW < 400 Da to cross the endothelium [193]. As such, nanomaterials that can cross the blood-spinal cord barrier are limited. As discussed above, there exist certain nanomaterials, such as nanoparticles, dendrimers, liposomes, and micelles, which can selectively target glial cells or neurons in the spinal cord lesion after local administration. However, increased understanding of the ability of different nanomaterials to cross the blood-spinal cord barrier is crucial. Detailed in vivo studies will need to be carried out to assess not only the ability of these nanomaterials to cross the blood-spinal cord barrier but also the maintenance of nanoparticle specificity after systemic administration.
Nanoparticles in particular face challenges associated with release kinetics and payload. This is a significant issue as nanoparticles have a larger surface area-to-volume ratio, meaning most of the drugs encapsulated in nanoparticles can be at or near the surface of the particles and can be released faster [301]. In particular, hydrophilic polymer nanoparticles are subject to accelerated burst release because a large proportion of the encapsulated drug may have access to the external aqueous phase and rapid hydrolysis can occur as water penetrates the nanoparticles more quickly [302]. However, this can be addressed by modifying the surfaces of the nanoparticles to slow down nanoparticle hydrolysis and obtain a slow and sustained drug release [303,304]. Additionally, since more nanoparticles can be delivered without causing cytotoxicity or embolism, localized or systemic administration of a larger quantity of nanoparticles may compensate for payload compromise [66]. Furthermore, encapsulating drug-loaded nanoparticles in injectable hydrogels can provide an additional barrier and help control the rate of drug release [189,259].
Finally, increased awareness and positive acceptance of nanotechnology by the public is needed to successfully broaden the clinical application of nanomaterials. Although nanotechnology has not yet been met with public contempt as was the case for genetically modified crops [305], public awareness of nanotechnology is still very limited [306,307]. This gap in understanding of nanotechnology between experts and the general public is a global phenomenon, as reports from the European Commission and South Korea presented similar observations [308,309]. National and international organizations such as the US FDA, South Africa Nanotechnology Public Engagement Programme, International Organization for Standardization (ISO), and Organization for Economic Cooperation and Development (OECD) have launched public engagement programs to increase public awareness of nanotechnology. Positive perception of nanotechnology by the public will be crucial to achieving increased clinical use of nanomaterials, especially in the midst of environmental reports suggesting harmful effects of nanoparticles [310].
4. Conclusions and future directions
Over the past decade, significant advances have been reported in nanomaterials development for SCI repair. Pre-clinical successes of nanomaterials that present physical guidance cues for regenerating axons and/or are loaded with therapeutic drugs show great potential for revolutionizing SCI treatment. However, several hurdles still exist that hinder clinical translation of nanotechnologies. With a clear-cut definition of nanoscale, improved synthesis methods to facilitate complete clearance of nanomaterials and their toxic degradation products from the body, and increased public awareness and acceptance of nanomaterials, a broadened utilization of nanotherapeutic strategies for SCI repair may become a reality.
Interestingly, no preclinical studies exist on utilizing quantum dots as a drug delivery vehicle. As reviewed above, quantum dots and carbon nanodots have great potential for SCI repair, as their extremely small size can facilitate blood-spinal cord barrier extravasation and systemic clearance, maintain high neural cell viability after cellular uptake, and surface modification allows for enhanced biocompatibility and selective targeting via cell surface receptor interaction.
Finally, we envision widespread use of naturally-derived biomaterials as not only base materials for nanotherapeutics, as is the case in nanofibers, but also as injectable delivery vehicles of drug-loaded nanomaterials, as discussed in Section 3.5 (Figure 7). By combining therapeutic drugs in nanocarriers and injectable hydrogels with pro-regenerative extracellular matrix cues, researchers can drive synergistic effects in not only enhancing therapeutic outcomes but also overall tissue repair. For example, a recent publication from our lab demonstrated the therapeutic efficacy of injectable acellular peripheral nerve hydrogels not only on their own but also as a Schwann cell delivery vehicle [223,311]. One can envision delivering nanoscale therapeutics in naturally-derived injectable hydrogels to improve SCI repair strategies.
Figure 7.
Future directions include minimally invasive delivery of injectable hydrogels from naturally-derived biomaterials into the injured spinal cords. These hydrogels can serve as not only a delivery vehicle for therapeutic nanomaterials, but also a source of pro-regenerative ECM proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Bawa R, Audette GF, Rubinstein I, Handbook of Clinical Nanomedicine: Nanoparticles, Imaging, Therapy, and Clinical APplications, Pan Stanford Publishing, 2016. [Google Scholar]
- [2].Echave P, Conlon IJ, Lloyd AC, Cell size regulation in mammalian cells, Cell Cycle. 6 (2007) 218–224. doi: 10.4161/cc.6.2.3744. [DOI] [PubMed] [Google Scholar]
- [3].Wan ACA, Ying JY, Nanomaterials for in situ cell delivery and tissue regeneration, Adv. Drug Deliv. Rev 62 (2010) 731–740. doi: 10.1016/j.addr.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [4].Elliott Donaghue I, Tam R, Sefton MV, Shoichet MS, Cell and biomolecule delivery for tissue repair and regeneration in the central nervous system, J. Control. Release 190 (2014) 219–227. doi: 10.1016/j.jconrel.2014.05.040. [DOI] [PubMed] [Google Scholar]
- [5].Kabu S, Gao Y, Kwon BK, Labhasetwar V, Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury, J. Control. Release 219 (2015) 141–154. doi: 10.1016/j.jconrel.2015.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Assunção-Silva RC, Gomes ED, Sousa N, Silva NA, Salgado AJ, Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration, Stem Cells Int. 2015 (2015). doi: 10.1155/2015/948040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim H, Cooke MJ, Shoichet MS, Creating permissive microenvironments for stem cell transplantation into the central nervous system, Trends Biotechnol. 30 (2012) 55–63. doi: 10.1016/j.tibtech.2011.07.002. [DOI] [PubMed] [Google Scholar]
- [8].Eckert MJ, Martin MJ, Trauma: Spinal Cord Injury, Surg. Clin. North Am 97 (2017) 1031–1045. doi: 10.1016/j.suc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- [9].A.U. of A. at B. Birmingham, Facts and Figures at a Glance, 2017. https://www.nscisc.uab.edu/Public/Facts and Figures - 2017.pdf. [Google Scholar]
- [10].U.A.B. Medicine, NSCISC-Spinal Cord Injury (SCI) Facts and Figures at a Glance, (2016) 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheung V, Hoshide R, Bansal V, Kasper E, Chen CC, Methylprednisolone in the management of spinal cord injuries: Lessons from randomized, controlled trials., Surg. Neurol. Int 6 (2015) 142. doi: 10.4103/2152-7806.163452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al. , A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study, N Engl J Med. 322 (1990) 1405–1411. doi: 10.1056/nejm199005173222001. [DOI] [PubMed] [Google Scholar]
- [13].Bowers CA, Kundu B, Rosenbluth J, Hawryluk GWJ, Patients with spinal cord injuries favor administration of methylprednisolone, PLoS One. 11 (2016). doi: 10.1371/journal.pone.0145991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bydon M, Lin J, Macki M, Gokaslan ZL, Bydon A, The current role of steroids in acute spinal cord injury, World Neurosurg. 82 (2014) 848–854. doi: 10.1016/j.wneu.2013.02.062. [DOI] [PubMed] [Google Scholar]
- [15].Gerndt SJ, Rodriguez JL, Pawlik JW, Taheri PA, Wahl WL, Micheals AJ, Papadopoulos SM, Consequences of high-dose steroid therapy for acute spinal cord injury, J Trauma. 42 (1997) 279–284. [DOI] [PubMed] [Google Scholar]
- [16].Qian T, Guo X, Levi AD, Vanni S, Shebert RT, Sipski ML, High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients, Spinal Cord. 43 (2005) 199–203. doi: 10.1038/sj.sc.3101681. [DOI] [PubMed] [Google Scholar]
- [17].Tator CH, Update on the pathophysiology and pathology of acute spinal cord injury, Brain Pathol. 5 (1995) 407–413. [DOI] [PubMed] [Google Scholar]
- [18].Hayta E, Elden H, Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention, J Chem Neuroanat. 87 (2018) 25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [19].McDonald JW, Sadowsky C, Spinal-cord injury, Lancet. 359 (2002) 417–425. doi: 10.1016/s0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- [20].Morales D II Toscano-Tejeida A Ibarra, Non Pharmacological Strategies to Promote Spinal Cord Regeneration: A View on Some Individual or Combined Approaches, Curr Pharm Des. 22 (2016) 720–727. [DOI] [PubMed] [Google Scholar]
- [21].Tyler JY, Xu X-M, Cheng J-X, Nanomedicine for treating spinal cord injury, Nanoscale. 5 (2013) 8821. doi: 10.1039/c3nr00957b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Moreno-Flores MT, Avila J, The quest to repair the damaged spinal cord., Recent Pat. CNS Drug Discov 1 (2006) 55–63. doi: 10.2174/157488906775245264. [DOI] [PubMed] [Google Scholar]
- [23].Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG, Traumatic Spinal Cord Injury-Repair and Regeneration, Neurosurgery. 80 (2017) S9–s22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- [24].Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG, Modulating the immune response in spinal cord injury, Expert Rev Neurother. 16 (2016) 1127–1129. doi: 10.1080/14737175.2016.1207532. [DOI] [PubMed] [Google Scholar]
- [25].Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS, Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord, Exp Neurol. 184 (2003) 313–325. [DOI] [PubMed] [Google Scholar]
- [26].Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG, Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord, J. Neurosci 29 (2009) 13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Beattie MS, Inflammation and apoptosis: linked therapeutic targets in spinal cord injury, Trends Mol. Med 10 (2004) 580–583. [DOI] [PubMed] [Google Scholar]
- [28].Hulsebosch CE, Recent advances in pathophysiology and treatment of spinal cord injury, Adv Physiol Educ. 26 (2002) 238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- [29].Su Z, Yuan Y, Chen J, Zhu Y, Qiu Y, Zhu F, Huang A, He C, Reactive Astrocytes Inhibit the Survival and Differentiation of Oligodendrocyte Precursor Cells by Secreted TNF-α, J. Neurotrauma 28 (2011) 1089–1100. doi: 10.1089/neu.2010.1597. [DOI] [PubMed] [Google Scholar]
- [30].Silver J, Schwab ME, Popovich PG, Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia, Cold Spring Harb Perspect Biol. 7 (2014) a020602. doi: 10.1101/cshperspect.a020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Properzi F, Asher RA, Fawcett JW, Chondroitin sulphate proteoglycans in the central nervous system: changes and synthesis after injury, Biochem. Soc. Trans 31 (2003) 335–336. doi: 10.1042/bst0310335. [DOI] [PubMed] [Google Scholar]
- [32].Fawcett JW, Asher RA, The glial scar and central nervous system repair, Brain Res Bull. 49 (1999) 377–391. [DOI] [PubMed] [Google Scholar]
- [33].Bradbury EJ, Carter LM, Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury, Brain Res. Bull 84 (2011) 306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [34].Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM, Chondroitinase ABC Digestion of the Perineuronal Net Promotes Functional Collateral Sprouting in the Cuneate Nucleus after Cervical Spinal Cord Injury, J. Neurosci 26 (2006) 4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB, Chondroitinase ABC promotes functional recovery after spinal cord injury, Nature. 416 (2002) 636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- [36].Alilain WJ, Horn KP, Hu H, Dick TE, Silver J, Functional regeneration of respiratory pathways after spinal cord injury, Nature. 475 (2011) 196–200. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tester NJ, Plaas AH, Howland DR, Effect of Body Temperature on Chondroitinase ABC’s Ability To Cleave Chondroitin Sulfate Glycosaminoglycans, J. Neurosci. Res 85 (2007) 1110–1118. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- [38].Whetstone WD, Hsu JC, Eisenberg M, Werb Z, Linda J, Blood-Spinal Cord Barrier After Spinal Cord Injury: Relation to Revascularization and Wound Healing, 74 (2010) 227–239. doi: 10.1002/jnr.10759.Blood-Spinal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kumar P, Choonara YE, Modi G, Naidoo D, Pillay V, Nanoparticulate strategies for the five Rs of traumatic spinal cord injury intervention: Restriction, repair, regeneration, restoration and reorganization, Nanomedicine. 9 (2014) 331–348. doi: 10.2217/nnm.13.203. [DOI] [PubMed] [Google Scholar]
- [40].Upadhyay RK, Drug delivery systems, CNS protection, and the blood brain barrier, Biomed Res. Int 2014 (2014). doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hassanzadeh P, Atyabi F, Dinarvand R, Application of modelling and nanotechnologybased approaches: The emergence of breakthroughs in theranostics of central nervous system disorders, Life Sci. 182 (2017) 93–103. doi: 10.1016/j.lfs.2017.06.001. [DOI] [PubMed] [Google Scholar]
- [42].Wong PT, Choi SK, Mechanisms of Drug Release in Nanotherapeutic Delivery Systems, Chem. Rev 115 (2015) 3388–3432. doi: 10.1021/cr5004634. [DOI] [PubMed] [Google Scholar]
- [43].Provenzale JM, Silva GA, Uses of nanoparticles for central nervous system imaging and therapy, Am. J. Neuroradiol 30 (2009) 1293–1301. doi: 10.3174/ajnr.A1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pan W, Kastin AJ, Penetration of neurotrophins and cytokines across the blood-brain/blood-spinal cord barrier, Adv. Drug Deliv. Rev 36 (1999) 291–298. doi: 10.1016/S0169-409X(98)00086-6. [DOI] [PubMed] [Google Scholar]
- [45].Boisseau P, Loubaton B, Nanomedicine, nanotechnology in medicine, Comptes Rendus Phys. 12 (2011) 620–636. doi: 10.1016/j.crhy.2011.06.001. [DOI] [Google Scholar]
- [46].Spivey EC, Khaing ZZ, Shear JB, Schmidt CE, The fundamental role of subcellular topography in peripheral nerve repair therapies, Biomaterials. 33 (2012) 4264–4276. doi: 10.1016/j.biomaterials.2012.02.043. [DOI] [PubMed] [Google Scholar]
- [47].Giugliano M, Prato M, Ballerini L, Nanomaterial/neuronal hybrid system for functional recovery of the CNS, Drug Discov. Today Dis. Model 5 (2008) 37–43. doi: 10.1016/j.ddmod.2008.07.004. [DOI] [Google Scholar]
- [48].ClinicalTrials.gov, (2018).
- [49].Zhang C, Wangler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Kruger R, Huss R, Seliger C, Semmler W, Kiessling F, Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging, Langmuir. 23 (2007) 1427–1434. doi: 10.1021/la061879k. [DOI] [PubMed] [Google Scholar]
- [50].Sashiwa H, Yajima H, Aiba S, Synthesis of a chitosan-dendrimer hybrid and its biodegradation, Biomacromolecules. 4 (2003) 1244–1249. doi: 10.1021/bm030021w. [DOI] [PubMed] [Google Scholar]
- [51].Avgoustakis K, Beletsi A, Panagi Z, Klepetsanis P, Karydas AG, Ithakissios DS, PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vitro drug release and in vivo drug residence in blood properties, J Control Release. 79 (2002) 123–135. [DOI] [PubMed] [Google Scholar]
- [52].Patel T, Zhou J, Piepmeier JM, Saltzman WM, Polymeric Nanoparticles for Drug Delivery to the Central Nervous System, Adv Drug Deliv Rev. 64 (2012) 701–705. doi: 10.1016/j.addr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li M, Rouaud O, Poncelet D, Microencapsulation by solvent evaporation: State of the art for process engineering approaches, Int. J. Pharm 363 (2008) 26–39. doi: 10.1016/j.ijpharm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- [54].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V, PLGA-based nanoparticles: An overview of biomedical applications, J. Control. Release 161 (2012) 505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [55].Bhambere D, Shirivastava B, Sharma P, Bukane N, Gide P, Preparation and Optimization of Dry PLGA Nanoparticles by Spray Drying Technique, Part. Sci. Technol 31 (2013) 533–540. doi: 10.1080/02726351.2013.782932. [DOI] [Google Scholar]
- [56].Hans ML, Lowman AM, Biodegradable nanoparticles for drug delivery and targeting, Curr. Opin. Solid State Mater. Sci 6 (2002) 319–327. doi:https://doi.Org/10.1016/S1359-0286(02)00117-1. [Google Scholar]
- [57].Cho Y, Shi R, Borgens RB, Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury, J Exp Biol. 213 (2010) 1513–1520. doi: 10.1242/jeb.035162. [DOI] [PubMed] [Google Scholar]
- [58].Vila A, Sánchez A, Tobío M, Calvo P, Alonso MJ, Design of biodegradable particles for protein delivery, J. Control. Release 78 (2002) 15–24. doi: 10.1016/S0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- [59].Rampino A, Borgogna M, Blasi P, Bellich B, Cesáro A, Chitosan nanoparticles: Preparation, size evolution and stability, Int J Pharm. 455 (2013) 219–228. doi: 10.1016/j.ijpharm.2013.07.034. [DOI] [PubMed] [Google Scholar]
- [60].Cho Y, Shi R, Ivanisevic A, Ben Borgens R, Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury, J. Neurosci. Res 88 (2010) 1433–1444. doi: 10.1002/jnr.22309. [DOI] [PubMed] [Google Scholar]
- [61].White-Schenk D, Shi R, Leary JF, Nanomedicine strategies for treatment of secondary spinal cord injury, Int J Nanomedicine. 10 (2015) 923–938. doi: 10.2147/ijn.s75686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen B, Zuberi M, Ben Borgens R, Cho Y, Affinity for, and localization of, PEG-functionalized silica nanoparticles to sites of damage in an ex vivo spinal cord injury model, J. Biol. Eng 6 (2012). doi: 10.1186/1754-1611-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Stöber W, Fink A, Bohn E, Controlled growth of monodisperse silica spheres in the micron size range, J. Colloid Interface Sci 26 (1968) 62–69. doi: 10.1016/0021-9797(68)90272-5. [DOI] [Google Scholar]
- [64].Napierska D, Thomassen LC, Rabolli V, Lison D, Gonzalez L, Kirsch-Volders M, Martens JA, Hoet PH, Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells, Small. 5 (2009) 846–853. doi: 10.1002/smll.200800461. [DOI] [PubMed] [Google Scholar]
- [65].Ukmar T, Planinsek O, Ordered mesoporous silicates as matrices for controlled release of drugs, Acta Pharm. 60 (2010) 373–385. doi: 10.2478/v1007-010-0037-4. [DOI] [PubMed] [Google Scholar]
- [66].Suri S, Ruan G, Winter J, Schmidt CE, Microparticles and Nanoparticles, Third Edit, Elsevier, 2013. doi: 10.1016/B978-0-08-087780-8.00034-6. [DOI] [Google Scholar]
- [67].Hernandez DS, Ritschdorff ET, Seidlits SK, Schmidt CE, Shear JB, Functionalizing micro-3D-printed protein hydrogels for cell adhesion and patterning, J. Mater. Chem. B 4 (2016) 1818–1826. doi: 10.1039/c5tb02070k. [DOI] [PubMed] [Google Scholar]
- [68].Fozdar DY, Lee JY, Schmidt CE, Chen S, Hippocampal neurons respond uniquely to topographies of various sizes and shapes, Biofabrication. 2 (2010). doi: 10.1088/1758-5082/2/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fozdar DY, Lee JY, E Schmidt C, Chen S, Selective axonal growth of embryonic hippocampal neurons according to topographic features of various sizes and shapes, Int. J. Nanomedicine 6 (2011) 45–57. doi: 10.2147/IJN.S12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang X, Ding B, Li B, Biomimetic electrospun nanofibrous structures for tissue engineering, Mater. Today 16 (2013) 229–241. doi: 10.1016/j.mattod.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Rogina A, Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery, Appl. Surf. Sci 296 (2014) 221–230. doi: 10.1016/j.apsusc.2014.01.098. [DOI] [Google Scholar]
- [72].Franze K, Guck J, The biophysics of neuronal growth, Reports Prog. Phys 73 (2010). doi: 10.1088/0034-4885/73/9/094601. [DOI] [Google Scholar]
- [73].Yu LMY, Leipzig ND, Shoichet MS, Promoting neuron adhesion and growth, Mater. Today 11 (2008) 36–43. doi: 10.1016/S1369-7021(08)70088-9. [DOI] [Google Scholar]
- [74].Wittmer CR, Claudepierre T, Reber M, Wiedemann P, Garlick JA, Kaplan D, Egles C, Multifunctionalized electrospun silk fibers promote axon regeneration in the central nervous system, Adv. Funct. Mater 21 (2011) 4232–4242. doi: 10.1002/adfm.201100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu T, Houle JD, Xu J, Chan BP, Chew SY, Nanofibrous Collagen Nerve Conduits for Spinal Cord Repair, Tissue Eng. Part A 18 (2012) 1057–1066. doi: 10.1089/ten.tea.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Koutsopoulos S, Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications, J. Biomed. Mater. Res. - Part A 104 (2016) 1002–1016. doi: 10.1002/jbm.a.35638. [DOI] [PubMed] [Google Scholar]
- [77].Hamley IW, Self-assembly of amphiphilic peptides, Soft Matter. 7 (2011) 4122–4138. doi: 10.1039/c0sm01218a. [DOI] [Google Scholar]
- [78].Ravichandran R, Griffith M, Phopase J, Applications of self-assembling peptide scaffolds in regenerative medicine: the way to the clinic, J. Mater. Chem. B 2 (2014) 8466–8478. doi: 10.1039/C4TB01095G. [DOI] [PubMed] [Google Scholar]
- [79].Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA, Self-Assembling Nanofibers Inhibit Glial Scar Formation and Promote Axon Elongation after Spinal Cord Injury, J. Neurosci 28 (2008) 3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S, Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds, Proc. Natl. Acad. Sci 97 (2000) 6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Guo J, Su H, Zeng Y, Liang YX, Wong WM, Ellis-Behnke RG, So KF, Wu W, Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold, Nanomedicine Nanotechnology, Biol. Med 3 (2007) 311–321. doi: 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- [82].Cigognini D, Satta A, Colleoni B, Silva D, Donegá M, Antonini S, Gelain F, Evaluation of early and late effects into the acute spinal cord injury of an injectable functionalized self-assembling scaffold, PLoS One. 6 (2011). doi: 10.1371/journal.pone.0019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW, Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering, Biomaterials. 34 (2013) 2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- [84].Gelain F, Panseri S, Antonini S, Cunha C, Donega M, Lowery J, Taraballi F, Cerri G, Montagna ḰM, Baldissera ḰF, Vescovi A, Transplantation of Nanostructured Composite Scaffolds Results in the Spinal Cords, Acta Nano. 5 (2011) 227–236. doi: 10.1021/nn102461w. [DOI] [PubMed] [Google Scholar]
- [85].Sun Y, Li W, Wu X, Zhang N, Zhang Y, Ouyang S, Song X, Fang X, Seeram R, Xue W, He L, Wu W, Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration, ACS Appl. Mater. Interfaces 8 (2016) 2348–2359. doi: 10.1021/acsami.5b11473. [DOI] [PubMed] [Google Scholar]
- [86].Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI, Selective differentiation of neural progenitor cells by high-epitope density nanofibers, Science (80-.). 303 (2004) 1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- [87].Webber MJ, Kessler JA, Stupp SI, Emerging peptide nanomedicine to regenerate tissues and organs, in: J. Intern. Med, 2010: pp. 71–88. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].lijima S, Helical microtubules of graphitic carbon, Nature. 354 (1991) 56–58. doi: 10.1038/354056a0. [DOI] [Google Scholar]
- [89].Bekyarova E, Ni Y, Malarkey EB, Montana V, McWilliams JL, Haddon RC, Parpura V, Applications of Carbon Nanotubes in Biotechnology and Biomedicine, J. Biomed. Nanotechnol 1 (2005) 3–17. doi: 10.1166/jbn.2005.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Erol O, Uyan I, Hatip M, Yilmaz C, Tekinay AB, Guler MO, Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications, Nanomedicine Nanotechnology, Biol. Med (2016) 1–22. doi: 10.1016/j.nano.2017.03.021. [DOI] [PubMed] [Google Scholar]
- [91].Bronikowski MJ, Willis PA, Colbert DT, Smith KA, Smalley RE, Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study, J. Vac. Sci. Technol. A Vacuum, Surfaces, Film (2001). doi: 10.1116/1.1380721. [DOI] [Google Scholar]
- [92].Faccendini A, Vigani B, Rossi S, Sandri G, Bonferoni MC, Caramella CM, Ferrari F, Nanofiber scaffolds as drug delivery systems to bridge spinal cord injury, Pharmaceuticals. 10(2017) 1–30. doi: 10.3390/ph10030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].GhoshMitra S, Diercks DR, Mills NC, Hynds DAL, Ghosh S, Role of engineered nanocarriers for axon regeneration and guidance: Current status and future trends, Adv. Drug Deliv. Rev 64 (2012) 110–125. doi: 10.1016/j.addr.2011.12.013. [DOI] [PubMed] [Google Scholar]
- [94].Nick C, Yadav S, Joshi R, Thielemann C, Schneider JJ, Growth and structural discrimination of cortical neurons on randomly oriented and vertically aligned dense carbon nanotube networks, Beilstein J. Nanotechnol 5 (2014) 1575–1579. doi: 10.3762/bjnano.5.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Koppes AN, Keating KW, McGregor AL, Koppes RA, Kearns KR, Ziemba AM, McKay CA, Zuidema JM, Rivet CJ, Gilbert RJ, Thompson DM, Robust neurite extension following exogenous electrical stimulation within single walled carbon nanotube-composite hydrogels, Acta Biomater. 39 (2016) 34–43. doi: 10.1016/j.actbio.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rüegg UT, Hefti F, Growth of dissociated neurons in culture dishes coated with synthetic polymeric amines, Neurosci. Lett 49 (1984) 319–324. doi: 10.1016/0304-3940(84)90309-4. [DOI] [PubMed] [Google Scholar]
- [97].Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V, Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth, J. Phys. Chem. B 109 (2005) 4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- [98].Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL, Single-Walled Carbon Nanotubes Chemically Functionalized with Polyethylene Glycol Promote Tissue Repair in a Rat Model of Spinal Cord Injury, J. Neurotrauma 28 (2011) 2349–2362. doi: 10.1089/neu.2010.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Fabbro A, Villari A, Laishram J, Scaini D, Toma FM, Turco A, Prato M, Ballerini L, Spinal cord explants use carbon nanotube interfaces to enhance neurite outgrowth and to fortify synaptic inputs, ACS Nano. 6 (2012) 2041–2055. doi: 10.1021/nn203519r. [DOI] [PubMed] [Google Scholar]
- [100].Sang L, Liu Y, Hua W, Xu K, Wang G, Zhong W, Wang L, Xu S, Xing MMQ, Qiu X, Thermally sensitive conductive hydrogel using amphiphilic crosslinker self-assembled carbon nanotube to enhance neurite outgrowth and promote spinal cord regeneration, RSC Adv. 6 (2016) 26341–26351. doi: 10.1039/C5RA20780K. [DOI] [Google Scholar]
- [101].Probst CE, Zrazhevskiy P, Bagalkot V, Gao X, Quantum dots as a platform for nanoparticle drug delivery vehicle design, Adv. Drug Deliv. Rev 65 (2013) 703–718. doi: 10.1016/j.addr.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Matea CT, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, lancu C, Mocan L, Quantum dots in imaging, drug delivery and sensor applications, Int. J. Nanomedicine 12 (2017) 5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pathak S, Cao E, Davidson MC, Jin S, Silva GA, Quantum Dot Applications to Neuroscience: New Tools for Probing Neurons and Glia, J. Neurosci 26 (2006) 1893–1895. doi: 10.1523/JNEUROSCI.3847-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S, Quantum dots: Synthesis, bioapplications, and toxicity, Nanoscale Res. Lett 7 (2012) 1. doi: 10.1186/1556-276X-7-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Winter JO, Liu TY, Korgel BA, Schmidt CE, Recognition Molecule Directed Interfacing Between Semiconductor Quantum Dots and Nerve Cells, Adv. Mater 13 (2001) 1673–1677. [Google Scholar]
- [106].Winter JO, Gomez N, Korgel BA, Schmidt CE, Quantum dots for electrical stimulation of neural cells, Proc. SPIE 5705 (2005) 235–246. doi: 10.1117/12.602363. [DOI] [Google Scholar]
- [107].Murray CB, Norris DJ, Bawendi MG, Synthesis and Characterization of Nearly Monodisperse CdE (E = S, Se, Te) Semiconductor Nanocrystallites, J. Am. Chem. Soc 115 (1993) 8706–8715. doi: 10.1021/ja00072a025. [DOI] [Google Scholar]
- [108].Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R, Jensen KF, Bawendi MG, (CdSe)ZnS Core–Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites, J. Phys. Chem. B 101 (1997) 9463–9475. doi: 10.1021/jp971091 y. [DOI] [Google Scholar]
- [109].Law WC, Yong KT, Roy I, Ding H, Hu R, Zhao W, Prasad PN, Aqueous-phase synthesis of highly luminescent CdTe/ZnTe core/shell quantum dots optimized for targeted bioimaging, Small. 5 (2009) 1302–1310. doi: 10.1002/smll.200801555. [DOI] [PubMed] [Google Scholar]
- [110].Kalasad MN, Rabinal MK, Mulimani BG, Ambient synthesis and characterization of high-quality CdSe quantum dots by an aqueous route, Langmuir. 25 (2009) 12729–12735. doi: 10.1021/la901798y. [DOI] [PubMed] [Google Scholar]
- [111].Chen HM, Huang XF, Xu L, Xu J, Chen KJ, Feng D, Self-assembly and photoluminescence of CdS-mercaptoacetic clusters with internal structures, Superlattices Microstruct. (2000). doi: 10.1006/spmi.1999.0819. [DOI] [Google Scholar]
- [112].Winter JO, Gomez N, Gatzert S, Schmidt CE, Korgel BA, Variation of cadmium sulfide nanoparticle size and photoluminescence intensity with altered aqueous synthesis conditions, Colloids Surfaces A Physicochem. Eng. Asp 254 (2005) 147–157. doi: 10.1016/j.colsurfa.2004.11.024. [DOI] [Google Scholar]
- [113].Peng ZA, Peng X, Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor, J. Am. Chem. Soc 123 (2001) 183–184. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- [114].He Y, Lu HT, Sai LM, Su YY, Hu M, Fan CH, Huang W, Wang LH, Microwave synthesis of water-dispersed CdTe/CdS/ZnS core-shell-shell quantum dots with excellent photostability and biocompatibility, Adv. Mater 20 (2008) 3416–3421. doi: 10.1002/adma.200701166. [DOI] [Google Scholar]
- [115].Xu B, Cai B, Liu M, Fan H, Ultraviolet radiation synthesis of water dispersed CdTe/CdS/ZnS core-shell-shell quantum dots with high fluorescence strength and biocompatibility, Nanotechnology. 24 (2013). doi: 10.1088/0957-4484/24/20/205601. [DOI] [PubMed] [Google Scholar]
- [116].Yang F, Xu Z, Wang J, Zan F, Dong C, Ren J, Microwave-assisted aqueous synthesis of new quaternary-alloyed CdSeTeS quantum dots; And their bioapplications in targeted imaging of cancer cells, Luminescence. 28 (2013) 392–400. doi: 10.1002/bio.2395. [DOI] [PubMed] [Google Scholar]
- [117].Zhou J, Yang Y, Zhang CY, Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application, Chem. Rev 115 (2015) 11669–11717. doi: 10.1021/acs.chemrev.5b00049. [DOI] [PubMed] [Google Scholar]
- [118].Su Y, Hu M, Fan C, He Y, Li Q, Li W, Wang LH, Shen P, Huang Q, The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties, Biomaterials. 31 (2010) 4829–4834. doi: 10.1016/j.biomaterials.2010.02.074. [DOI] [PubMed] [Google Scholar]
- [119].King-Heiden TC, Wiecinski PN, Mangham AN, Metz KM, Nesbit D, Pedersen JA, Hamers RJ, Heideman W, Peterson RE, Quantum Dot Nanotoxicity Assessment Using the Zebrafish Embryo, Environ. Sci. Technol 43 (2009) 1605–1611. doi: 10.1021/es801925c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lovrić J, Cho SJ, Winnik FM, Maysinger D, Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death, Chem. Biol 12 (2005) 1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [121].Derfus AM, Chan WCW, Bhatia SN, Probing the Cytotoxicity of Semiconductor Quantum Dots, Nano Lett. 4 (2004) 11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Baker SN, Baker GA, Luminescent carbon nanodots: Emergent nanolights, Angew. Chemie - Int. Ed 49 (2010) 6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- [123].Li H, Kang Z, Liu Y, Lee S-T, Carbon nanodots: synthesis, properties and applications, J. Mater. Chem 22 (2012) 24230. doi: 10.1039/c2jm34690g. [DOI] [Google Scholar]
- [124].Yang ST, Wang X, Wang H, Lu F, Luo PG, Cao L, Meziani MJ, Liu JH, Liu Y, Chen M, Huang Y, Sun YP, Carbon dots as nontoxic and high-performance fluorescence imaging agents, J. Phys. Chem. C 113 (2009) 18110–18114. doi: 10.1021/jp9085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Goryacheva IY, Sapelkin AV, Sukhorukov GB, Carbon nanodots: Mechanisms of photoluminescence and principles of application, TrAC - Trends Anal. Chem 90 (2017) 27–37. doi: 10.1016/j.trac.2017.02.012. [DOI] [Google Scholar]
- [126].Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie SY, Sun YP, Carbon dots for multiphoton bioimaging, J. Am. Chem. Soc 129 (2007) 11318–11319. doi: 10.1021/ja073527l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Y. Yang Shengtao; Cao Li; Luo Pengju G; Lu Fushen; Wang Xin; Wang Haifeng; Meziani Mohammed J.; Liu Yuanfang, Qi Gang; Sun, Carbon Dots for Optical Imaging in Vivo, J. Am. Chem. Soc 131 (2009) 11308–11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ajmal M, Yunus U, Matin A, Haq NU, Synthesis, characterization and in vitro evaluation of methotrexate conjugated fluorescent carbon nanoparticles as drug delivery system for human lung cancer targeting, J. Photochem. Photobiol. B Biol 153 (2015) 111–120. doi: 10.1016/j.jphotobiol.2015.09.006. [DOI] [PubMed] [Google Scholar]
- [129].Zeng Q, Shao D, He X, Ren Z, Ji W, Shan C, Qu S, Li J, Chen L, Li Q, Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo, J. Mater. Chem. B 4 (2016) 5119–5126. doi: 10.1039/C6TB01259K. [DOI] [PubMed] [Google Scholar]
- [130].Lai C-W, Hsiao Y-H, Peng Y-K, Chou P-T, Facile synthesis of highly emissive carbon dots from pyrolysis of glycerol; gram scale production of carbon dots/mSiO2 for cell imaging and drug release, J. Mater. Chem 22 (2012) 14403. doi: 10.1039/c2jm32206d. [DOI] [Google Scholar]
- [131].Majumdar S, Krishnatreya G, Gogoi N, Thakur D, Chowdhury D, Carbon-Dot-Coated Alginate Beads as a Smart Stimuli-Responsive Drug Delivery System, ACS Appl. Mater. Interfaces 8 (2016) 34179–34184. doi: 10.1021/acsami.6b10914. [DOI] [PubMed] [Google Scholar]
- [132].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Biotechnol 33 (2015) 941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C, Hirt UA, Walczak H, Falk W, Essig M, Edler L, Krammer PH, Martin-Villalba A, Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury, Nat. Med 10 (2004) 389–395. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- [134].Arnold SA, Hagg T, Anti-Inflammatory Treatments during the Chronic Phase of Spinal Cord Injury Improve Locomotor Function in Adult Mice, J. Neurotrauma 28 (2011) 1995–2002. doi: 10.1089/neu.2011.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Lima R, Monteiro S, Lopes JP, Barradas P, Vasconcelos NL, Gomes ED, Assunção-Silva RC, Teixeira FG, Morais M, Sousa N, Salgado AJ, Silva NA, Systemic interleukin-4 administration after spinal cord injury modulates inflammation and promotes neuroprotection, Pharmaceuticals. 10(2017) 1–16. doi: 10.3390/ph10040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Déry MA, Rousseau G, Benderdour M, Beaumont E, Atorvastatin prevents early apoptosis after thoracic spinal cord contusion injury and promotes locomotion recovery, Neurosci. Lett 453 (2009) 73–76. doi: 10.1016/j.neulet.2009.01.062. [DOI] [PubMed] [Google Scholar]
- [137].Jin W, Wang J, Zhu T, Yuan B, Ni H, Jiang J, Wang H, Liang W, Anti-inflammatory effects of curcumin in experimental spinal cord injury in rats, Inflamm. Res 63 (2014) 381–387. doi: 10.1007/s00011-014-0710-z. [DOI] [PubMed] [Google Scholar]
- [138].Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M, Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats, Nat. Med 4 (1998) 623–6. doi: 10.1038/nm0798-822. [DOI] [PubMed] [Google Scholar]
- [139].Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, Schmidt CE, High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury, J. Neural Eng 8 (2011). doi: 10.1088/1741-2560/8/4/046033. [DOI] [PubMed] [Google Scholar]
- [140].Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WEB, Baba H, Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury, J. Neurotrauma 29 (2012) 1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Knoller N, Auerbach G, Fulga V, Zelig G, Attias J, Bakimer R, Marder JB, Yoles E, Belkin M, Schwartz M, Hadani M, Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: Phase I study results, J. Neurosurg. Spine 3 (2005) 173–181. doi: 10.3171/spi.2005.3.3.0173. [DOI] [PubMed] [Google Scholar]
- [142].Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min B-H, Kim EY, Choi BH, Park H, Ha Y, Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial., Stem Cells. 25 (2007) 2066–73. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- [143].Hill CE, Moon LDF, Wood PM, Bunge MB, Labeled Schwann cell transplantation: Cell loss, host Schwann cell replacement, and strategies to enhance survival, Glia. 53 (2006) 338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- [144].Allison DJ, Thomas A, Beaudry K, Ditor DS, Targeting inflammation as a treatment modality for neuropathic pain in spinal cord injury: A randomized clinical trial, J. Neuroinflammation 13 (2016) 4–13. doi: 10.1186/s12974-016-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Allison DJ, Ditor DS, Targeting inflammation to influence mood following spinal cord injury: A randomized clinical trial, J. Neuroinflammation 12 (2015) 1–10. doi: 10.1186/s12974-015-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yang F, Murugan R, Wang S, Ramakrishna S, Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering, Biomaterials. 26 (2005) 2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- [147].Christopherson GT, Song H, Mao HQ, The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation, Biomaterials. 30 (2009) 556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- [148].Ebert E, Robert U, Johnson W, Gastrointestinal Involvement in Spinal Cord Injury: a Clinical Perspective, J Gastrointestin Liver Dis. 21 (2012) 75–82. [PubMed] [Google Scholar]
- [149].Chaw E, Shem K, Castillo K, Wong S, Chang J, Dysphagia and Associated Respiratory Considerations in Cervical Spinal Cord Injury, Top. Spinal Cord Inj. Rehabil 18 (2012) 291–299. doi: 10.1310/sci1804-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY, Methylprednisolone inhibition of TNF-alpha expression and NF-kB activation after spinal cord injury in rats., Brain Res. Mol. Brain Res 59 (1998) 135–142. doi: S0169328X98001429 [pii], [DOI] [PubMed] [Google Scholar]
- [151].Lu GB, Niu FW, Zhang YC, Du L, Liang ZY, Gao Y, Yan TZ, Nie ZK, Gao K, Methylprednisolone promotes recovery of neurological function after spinal cord injury: Association with Wnt/β-catenin signaling pathway activation, Neural Regen. Res 11 (2016) 1816–1823. doi: 10.4103/1673-5374.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Chvatal SA, Kim YT, Bratt-Leal AM, Lee H, Bellamkonda RV, Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord, Biomaterials. 29 (2008) 1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].tae Kim Y, Caldwell JM, Bellamkonda RV, Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury, Biomaterials. 30 (2009) 2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Cerqueira SR, Oliveira JM, Silva NA, Leite-Almeida H, Ribeiro-Samy S, Almeida A, Mano JF, Sousa N, Salgado AJ, Reis RL, Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury, Small. 9 (2013) 738–749. doi: 10.1002/smll.201201888. [DOI] [PubMed] [Google Scholar]
- [155].Gwak S-J, Koo H, Yun Y, Yhee JY, Lee HY, Yoon DH, Kim K, Ha Y, Multifunctional nanoparticles for gene delivery and spinal cord injury, J. Biomed. Mater. Res. Part A 103 (2015) 3474–3482. doi: 10.1002/jbm.a.35489. [DOI] [PubMed] [Google Scholar]
- [156].Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W, Minocycline Treatment Reduces Delayed Oligodendrocyte Death, Attenuates Axonal Dieback, and Improves Functional Outcome after Spinal Cord Injury, J. Neurosci 24 (2004) 2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH, Minocycline Reduces Cell Death and Improves Functional Recovery after Traumatic Spinal Cord Injury in the Rat, J. Neurotrauma 20 (2003) 1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- [158].Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM, Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury., Proc. Natl. Acad. Sci. U. S. A 101 (2004) 3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Papa S, Rossi F, Ferrari R, Mariani A, De Paola M, Caron I, Fiordaliso F, Bisighini C, Sammali E, Colombo C, Gobbi M, Canovi M, Lucchetti J, Peviani M, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P, Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury, ACS Nano. 7 (2013) 9881–9895. doi: 10.1021/nn4036014. [DOI] [PubMed] [Google Scholar]
- [160].Papa S, Caron I, Erba E, Panini N, De Paola M, Mariani A, Colombo C, Ferrari R, Pozzer D, Zanier ER, Pischiutta F, Lucchetti J, Bassi A, Valentini G, Simonutti G, Rossi F, Moscatelli D, Forloni G, Veglianese P, Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury, Biomaterials. 75 (2016) 13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- [161].Zhang Z, Wang Z, Nong J, Nix C, Ji H-F, Zhong Y, Metal ion-assisted self-assembly of complexes for controlled and sustained release of minocycline for biomedical applications, Biofabrication. 7 (2015) 15006. doi: 10.1088/1758-5090/7/1/015006.Metal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Wang Z, Nong J, Shultz RB, Zhang Z, Tom VJ, Ponnappan RK, Zhong Y, Local delivery of minocycline from metal ion-assisted self-assembled complexes promotes neuroprotection and functional recovery after spinal cord injury, Biomaterials. 112 (2017) 62–71. doi: 10.1016/j.biomaterials.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Saxena T, Loomis KH, Pai SB, Karumbaiah L, Gaupp E, Patil K, Patkar R, Bellamkonda RV, Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury, ACS Nano. 9 (2015) 1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- [164].Cox A, Varma A, Barry J, Vertegel A, Banik N, Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue, J. Neurotrauma 32 (2015) 1413–1421. doi: 10.1089/neu.2014.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bai F, Asojo OA, Cirillo P, Ciustea M, Ledizet M, Aristoff PA, Leng L, Koski RA, Powell TJ, Bucala R, Anthony KG, A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF), J. Biol. Chem 287 (2012) 30653–30663. doi: 10.1074/jbe.M112.385583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Maximov VD, Reukov VV, Barry JN, Cochrane C, Vertegel AA, Protein-nanoparticle conjugates as potential therapeutic agents for the treatment of hyperlipidemia, Nanotechnology. 21 (2010). doi: 10.1088/0957-4484/21/26/265103. [DOI] [PubMed] [Google Scholar]
- [167].Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, Van Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, Ma J, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC, Therapeutic Inflammatory Monocyte Modulation Using Immune-Modifying Microparticles, Sci. Transl. Med 6 (2014) 1–14. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Jeong SJ, Cooper JG, Ifergan I, McGuire TL, Xu D, Hunter Z, Sharma S, McCarthy D, Miller SD, Kessler JA, Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice, Neurobiol. Dis 108 (2017) 73–82. doi: 10.1016/j.nbd.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].David S, Aguayo AJ, Axonal Elongation into Peripheral Nervous System “Bridges” after Central Nervous System Injury in Adult Rats, Science (80-,). 214 (1981) 931–933. doi:0036-8075/81/1120. [DOI] [PubMed] [Google Scholar]
- [170].Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME, Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions, J Neurosci. 22 (2002) 3553–3567. doi:20026323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Ng WP, Cartel N, Li C, Roder J, Lozano A, Myelin from MAG-deficient mice is a strong inhibitor of neurite outgrowth, Neuroreport. 7 (1996) 861–864. doi: 10.1097/00001756-199603220-00005. [DOI] [PubMed] [Google Scholar]
- [172].Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B, Assessing Spinal Axon Regeneration and Sprouting in Nogo-, MAG-, and OMgp-Deficient Mice, Neuron. 66 (n.d.) 663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Fournier AE, Takizawa BT, Strittmatter SM, Rho kinase inhibition enhances axonal regeneration in the injured CNS., J. Neurosci 23 (2003) 1416–1423. doi:23/4/1416 [pii], [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Jones LL, Margolis RU, Tuszynski MH, The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury, Exp. Neurol 182 (2003) 399–411. [DOI] [PubMed] [Google Scholar]
- [175].Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, V Sofroniew Μ, Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury, J. Neurosci 24 (2004) 2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chen Z, Li Y, Yuan Q, Expression, purification and thermostability of MBP-chondroitinase ABC I from Proteus vulgaris, Int. J. Biol. Macromol 72 (2015) 6–10. doi: 10.1016/j.ijbiomac.2014.07.040. [DOI] [PubMed] [Google Scholar]
- [177].Crespo D, Asher RA, Lin R, Rhodes KE, Fawcett JW, How does chondroitinase promote functional recovery in the damaged CNS?, Exp. Neurol 206 (2007) 159–171. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [178].Lee H, Mckeon RJ, V Bellamkonda R, Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury, Proc. Natl. Acad. Sci 107 (2010) 3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wilems TS, Sakiyama-Elbert SE, Sustained dual drug delivery of anti-inhibitory molecules for treatment of spinal cord injury, J. Control. Release 213 (2015) 103–111. doi: 10.1016/j.jconrel.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wilems TS, Pardieck J, Iyer N, Sakiyama-Elbert SE, Combination therapy of stem cell derived neural progenitors and drug delivery of anti-inhibitory molecules for spinal cord injury, Acta Biomater. 28 (2015) 23–32. doi: 10.1016/j.actbio.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Xia T, Ni S, Li X, Yao J, Qi H, Fan X, Wang J, Sustained delivery of dbcAMP by poly(propylene carbonate) micron fibers promotes axonal regenerative sprouting and functional recovery after spinal cord hemisection, Brain Res. 1538 (2013) 41–50. doi: 10.1016/j.brainres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- [182].Rooney GE, Knight AM, Madigan NN, Gross L, Chen B, Giraldo CV, Seo S, Nesbitt JJ, Dadsetan M, Yaszemski MJ, Windebank AJ, Sustained Delivery of Dibutyryl Cyclic Adenosine Monophosphate to the Transected Spinal Cord Via Oligo [(Polyethylene Glycol) Fumarate] Hydrogels, Tissue Eng. Part A 17 (2011) 1287–1302. doi: 10.1089/ten.tea.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Li S, Strittmatter SM, Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury., J. Neurosci 23 (2003) 4219–27. doi:23/10/4219 [pii], [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Yusuke N, Atsushi N, Motokazu I, Masahito H, Kazuya M, Ryuichi F, Naoyuki S, Ichio A, Tsuneo S, Hong JL, Toshihiko W, Seung UK, Interferon-β Delivery via Human Neural Stem Cell Abates Glial Scar Formation in Spinal Cord Injury, Cell Transplant. 22 (2013) 2187–2201. doi: 10.3727/096368912X657882. [DOI] [PubMed] [Google Scholar]
- [185].Ito S, Natsume A, Shimato S, Ohno M, Kato T, Chansakul P, Wakabayashi T, Kim SU, Human neural stem cells transduced with IFN-beta and cytosine deaminase genes intensify bystander effect in experimental glioma., Cancer Gene Ther. 17 (2010) 299–306. doi: 10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- [186].Zuidema JM, Gilbert RJ, Osterhout DJ, Nanoparticle Technologies in the Spinal Cord, Cells Tissues Organs. 202 (2016) 102–115. doi: 10.1159/000446647. [DOI] [PubMed] [Google Scholar]
- [187].Varma AK, Das A, Wallace IV G, Barry J, Vertegel A. a., Ray SK, Banik NL, Spinal cord injury: A review of current therapy, future treatments, and basic science frontiers, Neurochem. Res 38 (2013) 895–905. doi: 10.1007/s11064-013-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Baumann MD, Kang CE, Tator CH, Shoichet MS, Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury, Biomaterials. 31 (2010) 7631–7639. doi: 10.1016/j.biomaterials.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [189].Stanwick JC, Baumann MD, Shoichet MS, In vitro sustained release of bioactive anti-NogoA, a molecule in clinical development for treatment of spinal cord injury, Int. J. Pharm 426 (2012) 284–290. doi: 10.1016/j.ijpharm.2012.01.035. [DOI] [PubMed] [Google Scholar]
- [190].Wu J, Stoica BA, Dinizo M, Pajoohesh-Ganji A, Piao C, Faden AI, Delayed cell cycle pathway modulation facilitates recovery after spinal cord injury, Cell Cycle. 11 (2012) 1782–1795. doi: 10.4161/cc.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Ren H, Han M, Zhou J, Zheng Z-F, Lu P, Wang J-J, Wang J-Q, Mao Q-J, Gao J-Q, Ouyang HW, Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles, Biomaterials. 35 (2014) 6585–6594. doi: https://doi.Org/10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- [192].Papa S, Ferrari R, De Paola M, Rossi F, Mariani A, Caron I, Sammali E, Peviani M, Dell’Oro V, Colombo C, Morbidelli M, Forloni G, Perale G, Moscatelli D, Veglianese P, Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury, J. Control. Release 174 (2014) 15–26. doi: 10.1016/j.jconrel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- [193].Liu T, Xu J, Chan BP, Chew SY, Sustained release of neurotrophin-3 and chondroitinase ABC from electrospun collagen nanofiber scaffold for spinal cord injury repair, J. Biomed. Mater. Res. Part A 100A (2012) 236–242. doi: 10.1002/jbm.a.33271. [DOI] [PubMed] [Google Scholar]
- [194].Chew SY, Mi R, Hoke A, Leong KW, The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation, Biomaterials. 29 (2008) 653–661. doi: https://doi.Org/10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Nguyen LH, Gao M, Lin J, Wu W, Wang J, Chew SY, Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment, Sci. Rep 7 (2017) 42212. doi: 10.1038/srep42212https://www.nature.com/articles/srep42212#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA, Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination, Neuron. 65 (2010) 597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Zawko S, Schmidt CE, Giving Hydrogels Backbone: Incorporating Physical Architecture into Soft Biomaterials, SurFACTS Biomater. 17 (2012) 13–14. [Google Scholar]
- [198].Santhosh KT, Alizadeh A, Karimi-Abdolrezaee S, Design and optimization of PLGA microparticles for controlled and local delivery of Neuregulin-1 in traumatic spinal cord injury, J. Control. Release 261 (2017) 147–162. doi: 10.1016/j.jconrel.2017.06.030. [DOI] [PubMed] [Google Scholar]
- [199].Khaing ZZ, Agrawal NK, Park JH, Xin S, Plumton GC, Lee KH, Huang Y-J, Niemerski AL, Schmidt CE, Grau JW, Localized and sustained release of brain-derived neurotrophic factor from injectable hydrogel/microparticle composites fosters spinal learning after spinal cord injury, J. Mater. Chem. B 4 (2016) 7560–7571. doi: 10.1039/C6TB01602B. [DOI] [PubMed] [Google Scholar]
- [200].Kaplan DR, Miller FD, Neurotrophin signal transduction in the nervous system., Curr. Opin. Neurobiol 10(2000) 381–91. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- [201].Gao Y, Nikulina E, Mellado W, Filbin MT, Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase, J. Neurosci 23 (2003) 11770–11777. doi:23/37/11770 [pii], [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Minichiello L, TrkB signalling pathways in LTP and learning, Nat. Rev. Neurosci 10 (2009) 850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- [203].Ansorena E, De Berdt P, Ucakar B, Simón-Yarza T, Jacobs D, Schakman O, Jankovski A, Deumens R, Blanco-Prieto MJ, Préat V, Des Rieux A, Injectable alginate hydrogel loaded with GDNF promotes functional recovery in a hemisection model of spinal cord injury, Int. J. Pharm 455 (2013) 148–158. doi: 10.1016/j.ijpharm.2013.07.045. [DOI] [PubMed] [Google Scholar]
- [204].Seidlits SK, Schmidt CE, Shear JB, High-resolution patterning of hydrogels in three dimensions using direct-write photofabrication for cell guidance, Adv. Funct. Mater 19 (2009) 3543–3551. doi: 10.1002/adfm.200901115. [DOI] [Google Scholar]
- [205].Hardy JG, Hernandez DS, Cummings DM, Edwards FA, Shear JB, Schmidt CE, Multiphoton microfabrication of conducting polymer-based biomaterials, J. Mater. Chem. B 3 (2015) 5001–5004. doi: 10.1039/c5tb00104h. [DOI] [PubMed] [Google Scholar]
- [206].Spivey EC, Ritschdorff ET, Connell JL, McLennon CA, Schmidt CE, Shear JB, Multiphoton lithography of unconstrained three-dimensional protein microstructures, Adv. Funct. Mater 23 (2013) 333–339. doi: 10.1002/adfm.201201465. [DOI] [Google Scholar]
- [207].Zawko SA, Schmidt CE, Crystal templating dendritic pore networks and fibrillar microstructure into hydrogels, Acta Biomater. 6 (2010) 2415–2421. doi: 10.1016/j.actbio.2010.02.021. [DOI] [PubMed] [Google Scholar]
- [208].Hardy J, Cornelison R, Sukhavasi R, Saballos R, Vu P, Kaplan D, Schmidt C, Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering, Bioengineering. 2 (2015) 15–34. doi: 10.3390/bioengineering2010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Thomas RC, Vu P, Modi SP, Chung PE, Landis RC, Khaing ZZ, Hardy JG, Schmidt CE, Sacrificial Crystal Templated Hyaluronic Acid Hydrogels As Biomimetic 3D Tissue Scaffolds for Nerve Tissue Regeneration, ACS Biomater. Sci. Eng 3 (2017) 1451–1459. doi: 10.1021/acsbiomaterials.7b00002. [DOI] [PubMed] [Google Scholar]
- [210].Gomez N, Lee JY, Nickels JD, Schmidt CE, Micropatterned polypyrrole: A combination of electrical and topographical characteristics for the stimulation of cells, Adv. Funct. Mater 17 (2007) 1645–1653. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Thomas AM, Kubilius MB, Holland SJ, Seidlits SK, Boehler RM, Anderson AJ, Cummings BJ, Shea LD, Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury, Biomaterials. 34 (2013) 2213–2220. doi: 10.1016/j.biomaterials.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, McDonald JW, Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers, Biomaterials. 32 (2011) 6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Thomas AM, Shea LD, Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury, J. Control. Release 170 (2013) 421–429. doi: 10.1016/j.jconrel.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Yoshii S, Ito S, Shima M, Taniguchi A, Akagi M, Functional restoration of rabbit spinal cord using collagen-filament scaffold, (2009) 19–25. doi: 10.1002/term. [DOI] [PubMed] [Google Scholar]
- [215].Tsintou M, Dalamagkas K, Seifalian AM, Advances in regenerative therapies for spinal cord injury: A biomaterials approach, Neural Regen. Res 10 (2015) 726–742. doi: 10.4103/1673-5374.156966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Muheremu A, Peng J, Ao Q, Stem cell therapies for spinal cord injury, Nat Rev Neurol. 6 (2010) 363–372. doi:nrneurol.2010.73 [pii]\r 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Schaal SM, Kitay BM, Cho KS, Lo TP, Barakat DJ, Marcillo AE, Sanchez AR, Andrade CM, Pearse DD, Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion, Cell Transplant. 16 (2007) 207–228. doi: 10.3727/000000007783464768. [DOI] [PubMed] [Google Scholar]
- [218].Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, Ozawa H, Itoi E, Pearse DD, Bunge MB, Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury., J. Neurosci 34 (2014) 1838–55. doi: 10.1523/JNEUROSCI.2661-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Bunge MB, Efficacy of Schwann cell transplantation for spinal cord repair is improved with combinatorial strategies., J. Physiol 594 (2016) 3533–8. doi: 10.1113/JP271531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Gros T, Sakamoto JS, Blesch A, Havton LA, Tuszynski MH, Regeneration of longtract axons through sites of spinal cord injury using templated agarose scaffolds, Biomaterials. 31 (2010) 6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- [221].Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, Tuszynski MH, Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection, Biomaterials. 34 (2013) 1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Patel V, Joseph G, Patel A, Patel S, Bustin D, Mawson D, Tuesta LM, Puentes R, Ghosh M, Pearse DD, Suspension matrices for improved Schwann-cell survival after implantation into the injured rat spinal cord., J. Neurotrauma 27 (2010) 789–801. doi: 10.1089/neu.2008.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Cerqueira SR, Lee Y, Cornelison RC, Mertz MW, Wachs RA, Schmidt CE, Bunge MB, Decellularized nerve matrix supports Schwann cell transplants and axon growth following spinal cord injury, Biomaterials. (2018). doi: 10.1016/j.biomaterials.2018.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Johnson PJ, Tatara A, McCreedy DA, Shiu A, Sakiyama-Elbert SE, Tissueengineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI, Soft Matter. 6 (2010) 5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Seidlits SK, Lee JY, Schmidt CE, Nanostructured scaffolds for neural applications, Nanomedicine. 3(2008) 183–199. doi: 10.2217/17435889.3.2.183. [DOI] [PubMed] [Google Scholar]
- [226].Islam M, Atmaramani R, Mukherjee S, Ghosh S, Iqbal SM, Enhanced proliferation of PC12 neural cells on untreated, nanotextured glass coverslips, Nanotechnology. 27 (2016). doi: 10.1088/0957-4484/27/41/415501. [DOI] [PubMed] [Google Scholar]
- [227].Baranes K, Chejanovsky N, Alon N, Sharoni A, Shefi O, Topographic cues of nanoscale height direct neuronal growth pattern, Biotechnol. Bioeng 109 (2012) 1791–1797. doi: 10.1002/bit.24444. [DOI] [PubMed] [Google Scholar]
- [228].Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim DH, Suh KY, Nanotopography-guided tissue engineering and regenerative medicine, Adv. Drug Deliv. Rev 65 (2013) 536–558. doi: 10.1016/j.addr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Hoffman-Kim D, Mitchel JA, Bellamkonda RV, Topography, Cell Response, and Nerve Regeneration, Annu. Rev. Biomed. Eng 12 (2010) 203–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Gomez N, Chen S, Schmidt CE, Polarization of hippocampal neurons with competitive surface stimuli: Contact guidance cues are preferred over chemical ligands, J. R. Soc. Interface 4 (2007) 223–233. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Wang HB, Mullins ME, Cregg JM, McCarthy CW, Gilbert RJ, Varying the diameter of aligned electrospun fibers alters neurite outgrowth and Schwann cell migration, Acta Biomater. 6 (2010) 2970–2978. doi: 10.1016/j.actbio.2010.02.020. [DOI] [PubMed] [Google Scholar]
- [232].Gnavi S, Fornasari BE, Tonda-Turo C, Ciardelli G, Zanetti M, Geuna S, Perroteau I, The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth, Mater. Sci. Eng. C 48 (2015) 620–631. doi: 10.1016/j.msec.2014.12.055. [DOI] [PubMed] [Google Scholar]
- [233].Zhu W, Masood F, O’Brien J, Zhang LG, Highly aligned nanocomposite scaffolds by electrospinning and electrospraying for neural tissue regeneration, Nanomedicine Nanotechnology, Biol. Med 11 (2015) 693–704. doi: 10.1016/j.nano.2014.12.001. [DOI] [PubMed] [Google Scholar]
- [234].Lee JY, Bashur CA, Goldstein AS, Schmidt CE, Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications, Biomaterials. 30 (2009) 4325–4335. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Milbreta U, Nguyen LH, Diao H, Lin J, Wu W, Sun CY, Wang J, Chew SY, Three-Dimensional Nanofiber Hybrid Scaffold Directs and Enhances Axonal Regeneration after Spinal Cord Injury, ACS Biomater. Sci. Eng 2 (2016) 1319–1329. doi: 10.1021/acsbiomaterials.6b00248. [DOI] [PubMed] [Google Scholar]
- [236].Zamani F, Amani-Tehran M, Latifi M, Shokrgozar MA, Zaminy A, Promotion of spinal cord axon regeneration by 3D nanofibrous core-sheath scaffolds, J. Biomed. Mater. Res. - Part A 102 (2014) 506–513. doi: 10.1002/jbm.a.34703. [DOI] [PubMed] [Google Scholar]
- [237].Jang MJ, Namgung S, Hong S, Nam Y, Directional neurite growth using carbon nanotube patterned substrates as a biomimetic cue, Nanotechnology. 21 (2010). doi: 10.1088/0957-4484/21/23/235102. [DOI] [PubMed] [Google Scholar]
- [238].Gupta P, Sharan S, Roy P, Lahiri D, Aligned carbon nanotube reinforced polymeric scaffolds with electrical cues for neural tissue regeneration, Carbon N. Y 95 (2015) 715–724. doi: 10.1016/j.carbon.2015.08.107. [DOI] [Google Scholar]
- [239].Jin G, Kim M, Shin US, Kim H, Effect of carbon nanotube coating of aligned nanofibrous polymer scaffolds on the neurite outgrowth of PC-12 cells, Cell Biol. Int 35 (2011) 741–745. doi: 10.1042/CBI20100705. [DOI] [PubMed] [Google Scholar]
- [240].Hu H, Ni Y, Montana V, Haddon RC, Parpura V, Chemically functionalized carbon nanotubes as substrates for neuronal growth, Nano Lett. 4 (2004) 507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Lee SJ, Zhu W, Nowicki M, Lee G, Heo DN, Kim J, Zuo YY, Zhang LG, 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration, J. Neural Eng 15 (2018). doi: 10.1088/1741-2552/aa95a5. [DOI] [PubMed] [Google Scholar]
- [242].Hwang JY, Shin US, Jang WC, Hyun JK, Wall IB, Kim HW, Biofunctionalized carbon nanotubes in neural regeneration: A mini-review, Nanoscale. 5 (2013) 487–497. doi: 10.1039/c2nr31581e. [DOI] [PubMed] [Google Scholar]
- [243].Sorkin R, Greenbaum A, David-Pur M, Anava S, Ayali A, Ben-Jacob E, Hanein Y, Process entanglement as a neuronal anchorage mechanism to rough surfaces, Nanotechnology. 20 (2009). doi: 10.1088/0957-4484/20/1/015101. [DOI] [PubMed] [Google Scholar]
- [244].Usmani S, Aurand ER, Medelin M, Fabbro A, Scaini D, Laishram J, Rosselli FB, Ansuini A, Zoccolan D, Scarselli M, De Crescenzi M, Bosi S, Prato M, Ballerini L, 3D meshes of carbon nanotubes guide functional reconnection of segregated spinal explants, Sci. Adv 2 (2016) 1–11. doi: 10.1126/sciadv.1600087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ruoslahti E, RGD AND OTHER RECOGNITION SEQUENCES FOR INTEGRINS, Annu. Rev. Cell Dev. Biol (1996). doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- [246].Ellis-Behnke RG, Liang Y-X, You S-W, Tay DKC, Zhang S, So K-F, Schneider GE, Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision, Proc. Natl. Acad. Sci 103 (2006) 5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Gelain F, Cigognini D, Caprini A, Silva D, Colleoni B, Donegá M, Antonini S, Cohen BE, Vescovi A, New bioactive motifs and their use in functionalized self-assembling peptides for NSC differentiation and neural tissue engineering, Nanoscale. 4 (2012) 2946–2957. doi: 10.1039/c2nr30220a. [DOI] [PubMed] [Google Scholar]
- [248].Sun J, Zheng Q, Experimental study on self-assembly of KLD-12 peptide hydrogel and 3-D culture of MSC encapsulated within hydrogel in vitro, J. Huazhong Univ. Sci. Technol. - Med. Sci 29 (2009) 512–516. doi: 10.1007/s11596-009-0424-6. [DOI] [PubMed] [Google Scholar]
- [249].Sun J, Zheng Q, Wu Y, Liu Y, Guo X, Wu W, Biocompatibility of KLD-12 peptide hydrogel as a scaffold in tissue engineering of intervertebral discs in rabbits, J. Huazhong Univ. Sci. Technol. - Med. Sci 30 (2010) 173–177. doi: 10.1007/s11596-010-0208-z. [DOI] [PubMed] [Google Scholar]
- [250].Raspa A, Marchini A, Pugliese R, Mauri M, Maleki M, Vasita R, Gelain F, A biocompatibility study of new nanofibrous scaffolds for nervous system regeneration, Nanoscale. 8 (2016) 253–265. doi: 10.1039/C5NR03698D. [DOI] [PubMed] [Google Scholar]
- [251].Tysseling VM, Sahni V, Pashuck ET, Birch D, Hebert A, Czeisler C, Stupp S.l., Kessler JA, Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury, J. Neurosci. Res 88 (2010) 3161–3170. doi: 10.1002/jnr.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD, Self-assembly of multidomain peptides: Balancing molecular frustration controls conformation and nanostructure, J. Am. Chem. Soc 129 (2007) 12468–12472. doi: 10.1021/ja072536r. [DOI] [PubMed] [Google Scholar]
- [253].Liu Y, Ye H, Satkunendrarajah K, Yao GS, Bayon Y, Fehlings MG, A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury, Acta Biomater. 9 (2013) 8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- [254].ming Wan J, le Liu L, fang Zhang J, wei Lu J, Li Q, Promotion of neuronal regeneration by using self-polymerized dendritic polypeptide scaffold for spinal cord tissue engineering, J. Mater. Sci. Mater. Med 29 (2018) 2–8. doi: 10.1007/s10856-017-6010-8. [DOI] [PubMed] [Google Scholar]
- [255].Huang EJ, Reichardt LF, Neurotrophins: Roles in Neuronal Development and Function, Annu. Rev. Neurosci 24 (2001) 677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Ramer MS, Priestley JV, McMahon SB, Functional regeneration of sensory axons into the adult spinal cord, Nature. 403 (2000) 312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- [257].Harvey AR, Lovett SJ, Majda BT, Yoon JH, Wheeler LPG, Hodgetts SI, Neurotrophic factors for spinal cord repair: Which, where, how and when to apply, and for what period of time?, Brain Res. 1619 (2015) 36–71. doi: 10.1016/j.brainres.2014.10.049. [DOI] [PubMed] [Google Scholar]
- [258].Wang YC, Wu YT, Huang HY, Lin H.l., Lo LW, Tzeng SF, Yang CS, Sustained intraspinal delivery of neurotrophic factor encapsulated in biodegradable nanoparticles following contusive spinal cord injury, Biomaterials. 29 (2008) 4546–4553. doi: 10.1016/j.biomaterials.2008.07.050. [DOI] [PubMed] [Google Scholar]
- [259].Stanwick JC, Baumann MD, Shoichet MS, Enhanced neurotrophin-3 bioactivity and release from a nanoparticle-loaded composite hydrogel, J. Control. Release 160 (2012) 666–675. doi: 10.1016/j.jconrel.2012.03.024. [DOI] [PubMed] [Google Scholar]
- [260].Elliott Donaghue I, Tator CH, Shoichet MS, Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord, Biomater. Sci 3 (2015) 65–72. doi: 10.1039/c4bm00311j. [DOI] [PubMed] [Google Scholar]
- [261].Kang CE, Baumann MD, Tator CH, Shoichet MS, Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury, Cells Tissues Organs. 197 (2012) 55–63. doi: 10.1159/000339589. [DOI] [PubMed] [Google Scholar]
- [262].Valmikinathan CM, Defroda S, Yu X, Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor, Biomacromolecules. 10 (2009) 1084–1089. doi: 10.1021/bm8012499. [DOI] [PubMed] [Google Scholar]
- [263].Li X, Su Y, Liu S, Tan L, Mo X, Ramakrishna S, Encapsulation of proteins in poly(llactide-co-caprolactone) fibers by emulsion electrospinning, Colloids Surfaces B Biointerfaces. 75 (2010) 418–424. doi: 10.1016/j.colsurfb.2009.09.014. [DOI] [PubMed] [Google Scholar]
- [264].Pita-Thomas W, Magnetic nanotechnology to study and promote axon growth, Neural Regen. Res 10(2015) 1037–1039. doi: 10.4103/1673-5374.160067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Gahl TJ, Kunze A, Force-mediating magnetic nanoparticles to engineer neuronal cell function, Front. Neurosci 12 (2018) 1–16. doi: 10.3389/fnins.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Steketee MB, Moysidis SN, Jin X-L, Weinstein JE, Pita-Thomas W, Raju HB, Iqbal S, Goldberg JL, Nanoparticle-mediated signaling endosome localization regulates growth cone motility and neurite growth, Proc. Natl. Acad. Sci 108 (2011) 19042–19047. doi: 10.1073/pnas.1019624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Riggio C, Calatayud MP, Giannaccini M, Sanz B, Torres TE, Fernández-Pacheco R, Ripoli A, Ibarra MR, Dente L, Cuschieri A, Goya GF, Raffa V, The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 1549–1558. doi: 10.1016/j.nano.2013.12.008. [DOI] [PubMed] [Google Scholar]
- [268].Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, Ko SY, Margul DJ, Bartels AK, Boehler RM, Cummings BJ, Anderson AJ, Shea LD, Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury, Biomaterials. 33 (2012) 1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Zhou S, Shen D, Wang Y, Gong L, Tang X, Yu B, Gu X, Ding F, microRNA-222 Targeting PTEN Promotes Neurite Outgrowth from Adult Dorsal Root Ganglion Neurons following Sciatic Nerve Transection, PLoS One. 7 (2012) 1–11. doi: 10.1371/journal.pone.0044768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL, Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system, Sci. Transl. Med 8 (2016). doi: 10.1126/scitranslmed.aad7577. [DOI] [PubMed] [Google Scholar]
- [271].Erofeev A, Zakharova O, Terekhin S, Plotnikova P, Bezprozvanny I, Vlasova O, Future of Optogenetics: Potential Clinical Applications?, Opera Med Physiol. 2 (2016) 117–121. doi: 10.20388/OMP2016.002.0032. [DOI] [Google Scholar]
- [272].Fenno L, Yizhar O, Deisseroth K, The Development and Application of Optogenetics, Annu. Rev. Neurosci (2011). doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J, Light-Induced Rescue of Breathing after Spinal Cord Injury, J. Neurosci (2008). doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Jayaprakash N, Wang Z, Hoeynck B, Krueger N, Kramer A, Balle E, Wheeler DS, Wheeler RA, Blackmore MG, Optogenetic Interrogation of Functional Synapse Formation by Corticospinal Tract Axons in the Injured Spinal Cord, J. Neurosci 36 (2016) 5877–5890. doi: 10.1523/JNEUROSCI.4203-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Awad BI, Gutierrez DV, Alilain W, Steinmetz MP, Optogenetic Photostimulation to Control Bladder Function After Experimental Spinal Cord Injury, Spine J. 13 (2013) S12. doi: 10.1016/j.spinee.2013.07.058. [DOI] [Google Scholar]
- [276].Mallory GW, Grahn PJ, Hachmann JT, Lujan JL, Lee KH, Optical stimulation for restoration of motor function after spinal cord injury, Mayo Clin. Proc 90 (2015) 300–307. doi: 10.1016/j.mayocp.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Huang K, Dou Q, Loh XJ, Nanomaterial mediated optogenetics: Opportunities and challenges, RSC Adv. 6 (2016) 60896–60906. doi: 10.1039/c6ra11289g. [DOI] [Google Scholar]
- [278].Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, Huang AJY, Hashimotodani Y, Kano M, Iwasaki H, Parajuli LK, Okabe S, Loong Teh DB, All AH, Tsutsui-Kimura I, Tanaka KF, Liu X, McHugh TJ, Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics, Science (80-,). (2018). doi: 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]
- [279].Banchereau J, Palucka K, Immunotherapy: Cancer vaccines on the move, Nat. Rev. Clin. Oncol (2017). doi: 10.1038/nrclinonc.2017.149. [DOI] [PubMed] [Google Scholar]
- [280].Wang Y-T, Lu X-M, Chen K-T, Shu Y-H, Giu C-H, Immunotherapy strategies for spinal cord injury., Curr. Pharm. Biotechnol 16 (2015) 492–505. doi: 10.2174/138920101606150407112646. [DOI] [PubMed] [Google Scholar]
- [281].Putatunda R, Bethea JR, Hu W-H, Potential immunotherapies for traumatic brain and spinal cord injury, Chinese J. Traumatol (2018). doi: 10.1016/J.CJTEE.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Lu X-M, Wei J-X, Xiao L, Shu Y-H, Wang Y-T, Experimental and Clinical Advances in Immunotherapy Strategies for Spinal Cord Injury Target on MAIs and Their Receptors., Curr. Pharm. Des 22 (2016) 728–737. doi: 10.2174/1381612822666151204000855. [DOI] [PubMed] [Google Scholar]
- [283].Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F, Multiplex genome engineering using CRISPR/Cas systems, Science (80-.). 339 (2013) 819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Ahmed Khan F, Pandupuspitasari NS, Chun-Jie H, Ao Z, Jamal M, Zohaib A, Ahmed Khan F, Raihana Hakim M, ShuJun Z, CRISPR/Cas9 therapeutics: a cure for cancer and other genetic diseases, Oncotarget. 7 (2016) 52541–52552. doi: 10.18632/oncotarget.9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Pardieck J, Sakiyama-Elbert S, Genome engineering for CNS injury and disease, Curr. Opin. Biotechnol 52 (2018) 89–94. doi: 10.1016/j.copbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Liu J, Gao C, Chen W, Ma W, Li X, Shi Y, Zhang H, Zhang L, Long Y, Xu H, Guo X, Deng S, Yan X, Yu D, Pan G, Chen Y, Lai L, Liao W, Li Z, CRISPR/Cas9 facilitates investigation of neural circuit disease using human iPSCs: Mechanism of epilepsy caused by an SCN1A loss-of-function mutation, Transl. Psychiatry 6 (2016). doi: 10.1038/tp.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Fei JF, Schuez M, Tazaki A, Taniguchi Y, Roensch K, Tanaka EM, CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration, Stem Cell Reports. 3 (2014) 444–459. doi: 10.1016/j.stemcr.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Shen Z, Zhang X, Chai Y, Zhu Z, Yi P, Feng G, Li W, Ou G, Conditional knockouts generated by engineered CRISPR-Cas9 endonuclease reveal the roles of coronin in c.elegans neural development, Dev. Cell 30 (2014) 625–636. doi: 10.1016/j.devcel.2014.07.017. [DOI] [PubMed] [Google Scholar]
- [289].Swiech L, Heidenreich M, Banerjee A, Habib N, Li Y, Trombetta J, Sur M, Zhang F, In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9, Nat. Biotechnol 33 (2015) 102–106. doi: 10.1038/nbt.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Liu C, Zhang L, Liu H, Cheng K, Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications, J. Control. Release 266 (2017) 17–26. doi: 10.1016/j.jconrel.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Sun W, Ji W, Hall JM, Hu Q, Wang C, Beisel CL, Gu Z, Self-Assembled DNA Nanoclews for the Efficient Delivery of CRISPR-Cas9 for Genome Editing, Angew. Chemie - Int. Ed 54 (2015) 12029–12033. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR, Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo, Nat. Biotechnol 33 (2015) 73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K, Rotello VM, Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing, ACS Nano. 11 (2017) 2452–2458. doi: 10.1021/acsnano.6b07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Liang X, Potter J, Kumar S, Zou Y, Quintanilla R, Sridharan M, Carte J, Chen W, Roark N, Ranganathan S, Ravinder N, Chesnut JD, Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection, J. Biotechnol 208 (2015) 44–53. doi: 10.1016/j.jbiotec.2015.04.024. [DOI] [PubMed] [Google Scholar]
- [295].Zhang L, Webster TJ, Nanotechnology and nanomaterials: Promises for improved tissue regeneration, Nano Today. 4 (2009) 66–80. doi: 10.1016/j.nantod.2008.10.014. [DOI] [Google Scholar]
- [296].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date, Pharm. Res 33 (2016) 2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- [297].Bourquin J, Milosevic A, Hauser D, Lehner R, Blank F, Petri-Fink A, Rothen-Rutishauser B, Biodistribution, Clearance, and Long-Term Fate of Clinically Relevant Nanomaterials, Adv. Mater 30 (2018) 1–31. doi: 10.1002/adma.201704307. [DOI] [PubMed] [Google Scholar]
- [298].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV, Renal clearance of quantum dots, Nat. Biotechnol 25 (2007) 1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Lacerda L, Herrero MA, Venner K, Bianco A, Prato M, Kostarelos K, Carbonnanotube shape and individualization critical for renal excretion, Small. 4 (2008) 1130–1132. doi: 10.1002/smll.200800323. [DOI] [PubMed] [Google Scholar]
- [300].Juillerat-Jeanneret L, The targeted delivery of cancer drugs across the blood-brain barrier: chemical modifications of drugs or drug-nanoparticles?, Drug Discov. Today 13 (2008) 1099–1106. doi: 10.1016/j.drudis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [301].Singh Lillard, Nanoparticle-based targeted drug delivery, Exp. Mol. Pathol 86 (2009) 215–223. doi: 10.1016/j.yexmp.2008.12.004.Nanoparticle-based. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Kohane DS, Microparticles and Nanoparticles for Drug Delivery, Biotechnol. Bioeng 96 (2007) 203–209. doi: 10.1002/bit.21301. [DOI] [PubMed] [Google Scholar]
- [303].Fan X, Wang X, Cao M, Wang C, Hu Z, Wu YL, Li Z, Loh XJ, “y”-shape armed amphiphilic star-like copolymers: Design, synthesis and dual-responsive unimolecular micelle formation for controlled drug delivery, Polym. Chem 8 (2017) 5611–5620. doi: 10.1039/c7py00999b. [DOI] [Google Scholar]
- [304].Li Z, Yuan D, Jin G, Tan BH, He C, Facile Layer-by-Layer Self-Assembly toward Enantiomeric Poly(lactide) Stereocomplex Coated Magnetite Nanocarrier for Highly Tunable Drug Deliveries, ACS Appl. Mater. Interfaces 8 (2016) 1842–1853. doi: 10.1021/acsami.5b09822. [DOI] [PubMed] [Google Scholar]
- [305].Resnik DB, Tinkle SS, Ethical issues in clinical trials involving nanomedicine, Contemp. Clin. Trials 28 (2007) 433–441. doi: 10.1016/j.cct.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Cobb MD, Macoubrie J, Public perceptions about nanotechnology: Risks, bene.ts and trust, J. Nano. Res 0 (2004) 1–11. [Google Scholar]
- [307].Besley J, Current research on public perceptions of nanotechnology., Emerg. Health Threats J 3 (2010) e8. doi: 10.3134/ehtj.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].European Comission, Eurobarometer 55.2 Europeans, Science and Technology - 2001, (2001). [Google Scholar]
- [309].Kim YR, Lee EJ, Park SH, Kwon HJ, An SSA, Son SW, Seo YR, Pie JE, Yoon M, Kim JH, Kim MK, Comparative analysis of nanotechnology awareness in consumers and experts in South Korea, Int. J. Nanomedicine. 9 (2014) 21–27. doi: 10.2147/IJN.S57921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Wilson N, Nanoparticles: Environmental Problems or Problem Solvers?, Bioscience. 68 (2018) 241–246. doi: 10.1093/biosci/biy015. [DOI] [Google Scholar]
- [311].Cornelison RC, Gonzalez-Rothi E, Porvasnik SL, Wellman SM, Park JH, Fuller DD, Schmidt CE, Injectable hydrogels of optimized acellular nerve for injection in the injured spinal cord, Biomed. Mater (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Weishaupt N, Blesch A, Fouad K, BDNF: The career of a multifaceted neurotrophin in spinal cord injury, Exp. Neurol 238 (2012) 254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]