Short abstract
Background
Considering the slow-acting properties of traditional antidepressants, an important challenge in the field is the identification of early treatment response biomarkers. Reduced cortical thickness has been reported in neuroimaging studies of depression. However, little is known whether antidepressants reverse this abnormality. In this brief report, we investigated early cortical thickness changes following treatment with sertraline compared to placebo.
Methods
Participants (n = 215) with major depressive disorder were randomized to a selective serotonin reuptake inhibitor, sertraline, or to placebo. Structural magnetic resonance imaging scans were acquired at baseline and one week following treatment. Response was defined as at least 50% improvement in Hamilton rating scale for depression score at week 8. In a vertex-wise approach, we examined the effects of treatment, response, and treatment × response.
Results
Following correction for multiple comparisons, we found a significant effect of treatment, with widespread increase in cortical thickness following sertraline compared to placebo. Clusters with increased thickness were found in the left medial prefrontal cortex, right medial and lateral prefrontal cortex, and within the right parieto-temporal lobes. There were no sertraline-induced cortical thinning, and no significant response effects or treatment × response interactions.
Conclusion
Our findings suggest that cortical thickness abnormalities may be responsive to antidepressant treatment. However, a relationship between these early cortical changes and later treatment response was not demonstrated. Future studies would be needed to investigate whether those early effects are maintained at eight weeks and are associated with enhanced response.
Keywords: antidepressants, sertraline, structural abnormalities, cortical thickness, neuroimaging, major depressive disorder
Introduction
Major depressive disorder (MDD) is among the most prevalent psychiatric disorders, with one out of six individuals will be affected at least one time in their life.1 In addition to genetic and environmental factors that are important in the pathogenesis of this disorder,2 brain structural abnormalities mediating these effects have been suggested, particularly gray matter deficits such as cortical thinning.3 Currently, antidepressants and psychotherapies are recommended to treat patients with MDD.4 However, these treatments take weeks to months to achieve response. Hence, there is a need for biomarkers that might facilitate choosing the right initial treatment in an early stage which, in turn, may result in improved clinical outcome. In the current report, we used a vertex-wise approach with rigorous correction for multiple comparison to investigate whether the serotonin reuptake inhibitor sertraline exerts an effect on cortical thickness and whether these effects predate and predict treatment response in MDD patients.
Over the past two decades, accumulating preclinical evidence supported a synaptic model associating chronic stress and depression with gray matter atrophy due to stress-induced dendrites shrinkage and reduced synaptic density.5 These gray matter alterations are thought to be related to glutamate excitotoxicity and reduced brain-derived neurotrophic factor (BDNF). Monoaminergic antidepressants, such as sertraline, are believed to exert their therapeutic effects by increasing BDNF and reversing the stress-related gray matter deficits.6,7 In human, several vertex-based studies have found decreased cortical thickness in orbitofrontal, dorsolateral prefrontal, anterior cingulate, and rostral middle frontal cortices in MDD patients compared to healthy controls.8–12 Cortical thinning was also reported in the right rostral anterior cingulate cortex (rACC) of unmedicated MDD by a voxel-wise study examining structural differences between MDD patients and healthy controls.13 Region-of-interest studies also reported cortical thinning in the right rostral and dorsal anterior cingulate, medial and lateral orbitofrontal cortices, as well as right fusiform and right parahippocampal gyri.14–16
While a large number of previous studies documented reduced cortical thickness, some studies have reported thickness increases in MDD patients compared to healthy controls.14,17,18 Others have indicated both increases and decreases8 or found no differences in cortical thickness of MDD patients.19,20 In addition, there are few studies that examined the effect of treatment on structural brain changes in depression. One study investigating MDD patients using antidepressants reported thicker posterior cingulate cortex in remitters compared to non-remitters.9 Other studies reported increased cortical thickness in bilateral anterior cingulate cortex, superior temporal gyrus, temporal pole, parahipocampal gyrus, and bilateral insula following electroconvulsive therapy (ECT).21,22
To address a gap in the literature and complement available evidence, the current study investigated the effects of sertraline on cortical thickness vertex-wise, compared to placebo. We also investigated whether structural cortical changes would predict enhanced response at eight weeks. Consistent with the synaptic model of depression, we hypothesized that compared to placebo, sertraline will increase cortical thickness following treatment and this increase will predict remission at follow-up.
Methods
The data used in this study are part of the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care) trial and all details on randomization, patient characteristics and assessment measures can be found in Trivedi et al.23 Briefly, it is a double-blind controlled clinical trial which randomized participants to eight-week treatment with sertraline (200 mg daily) or placebo. All studies were approved by institutional review boards, and all participants signed informed consents.
Study Participants
Unmedicated (at least for three weeks) MDD participants (n = 215) aging from 18 to 65 years (135 females, mean age = 37.5, SD = 13.2) with successful pretreatment and one-week scans as well as eight-week depression scores were included in the current report. Of the 289 MDD participants with structural scans, 33 had only pretreatment scans (baseline) and 39 did not have depression scores at week 8. Of the remaining 217 participants, two were excluded because of poor segmentation. The clinical trial primary outcome was symptom severity which was rated on the 17-item Hamilton Rating Scale for Depression (HAMD) at baseline, weekly in the first month and every two other weeks in the second month. Eligible participants were required to have Quick Inventory of Depressive Symptomatology - self report (QIDS-SR) score of 14 or more and meet the DSM-IV criteria. Participants were allowed to take medication for general medical conditions or antidepressants side effects but no other type of medication or therapy for treating depression. Additional inclusion/exclusion criteria are available in Trivedi et al.23 Response was defined as at least 50% improvement in HAMD scores at week 8.
Neuroimaging Acquisition and Preprocessing
The high-resolution structural magnetic resonance imaging (MRI) scans were acquired using 3-T magnetic field scanners, with 1 × 1 × 1 mm3 resolution (for details, see Greenberg et al.24). All scans were processed using the Human Connectome Project implementation of FreeSurfer,25 as previously detailed.26 Briefly, the processing included the removal of non-brain tissue, intensity normalization, and segmentation of white matter/gray matter tissue. Following preprocessing, the quality of the cortical segmentations outputted from FreeSurfer was assessed by checking segmentation quality and visual inspection of cortex of each subject for possible inaccuracies. Thickness values were computed as the mean distance between vertices of a triangulated estimate of white matter and pial surfaces at each point across the cortex.
Statistical Analyses
Demographic and clinical characteristics were analyzed using the Statistical Package for Social Sciences software (SPSS, version 24; IBM). We used probability plots and standard deviation of mean to assess the normality assumption of the data. For all analyses, the level of significance was set at p < 0.05. A vertex-wise analysis of cortical thickness was performed to determine the effects of treatment (sertraline vs. placebo), response (responders vs. non-responders), and treatment × response interaction on cortical thickness changes from baseline (pretreatment) to week 1 (posttreatment) using general linear models (GLM) with age, gender, and site as covariates. Vertex-wise correction for multiple comparisons was conducted using false discovery rate (FDR) (q < 0.05).
Results
Demographics and clinical data are shown in Table 1. The two groups did not differ in terms of age, gender, and education.
Table 1.
Demographics and clinical characteristics.
| Characteristics | Sertraline (n = 103) | Placebo (n = 112) |
|---|---|---|
| Demographic | ||
| Age, mean (SD) | 38.6 (14.2) | 37.4 (12.7) |
| Male age, mean (SD) | 37.8 (13.8) | 38.2 (12.9) |
| Female age, mean (SD) | 39.4 (14.1) | 36.6 (12.5) |
| Male | 32% | 35.7% |
| Years of education, mean (SD) | 15 (3.1) | 15.4 (2.4) |
| Clinical features | ||
| Age of onset, mean (SD) | 13.9 (5.1) | 14.7 (5.3) |
| Chronic | 52% | 51% |
| Baseline HAMD, mean (SD) | 18.8 (4.5) | 18.5 (4.3) |
| W1 HAMD, mean (SD) | 16.1 (5.4) | 15.8 (4.9) |
| W8 HAMD, mean (SD) | 10.8 (6.5) | 11.9 (7.4) |
| W1 Response | 10% | 9% |
| W8 Response | 48% | 37%a |
W1: week 1; W8: week 8; HAMD: Hamilton Rating Scale for Depression; SD: standard deviation.
aThere were no significant (p > 0.1) differences between treatment groups, except for response rate at week 8 which showed a trend (chi-square = 2.65, p = 0.068).
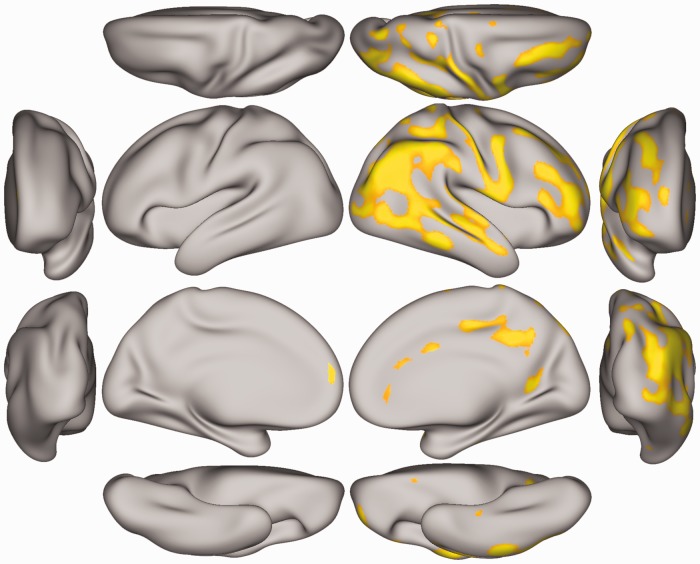
Following FDR correction for multiple comparisons, vertex-wise analysis showed a significant treatment effect, but no response and treatment × response interaction. The treatment effects were consistent with increased cortical thickness following sertraline treatment compared to placebo. There were no clusters of reduced cortical thickness. Brain regions exhibiting significantly increased cortical thickness are shown in Figure 1. These regions include parts of the superior frontal, caudal and rACC, posterior cingulate cortex, medial orbital frontal and parts of lingual region in medial right hemisphere and lateral occipital, inferior and superior parietal, supra marginal, middle and superior temporal, pars opercularis, pars tringularis, postcentral, rostral, and caudal middle frontal in lateral right hemisphere. We also observed a significant cortical thickness increase in the superior frontal region of medial left hemisphere in those who received sertraline compared to placebo.
Figure 1.
Vertex-based analysis of cortical thickness changes in MDD patients. The orange-yellow clusters mark the vertices with significantly increased cortical thickness following one-week treatment with sertraline compared to placebo. Shown here are left and right, lateral-medial, anterior-posterior, dorsal-ventral views.
Discussion
The study results demonstrated a significant effect of sertraline on cortical thickness abnormalities following a week of treatment. Areas of increased thickness after treatment with sertraline compared to placebo were primarily observed in the right hemisphere, especially in the prefrontal, parietal, and temporal lobes including rostral middle frontal, inferior parietal, and middle temporal as well as postcentral cortices. However, the results failed to demonstrate an association between cortical thickness and treatment response, suggesting that the cortical changes estimated by MRI are not directly related to the therapeutic benefit. Here, it is important to note that several clusters associated cortical changes with response; however, these did not survive correction for multiple comparisons.
An unpredicted finding is that despite the presence of significant antidepressant-induced increase in cortical thickness, these early changes did not predict response at week 8. Thus, while the pattern of normalization following sertraline is informative mechanistically, it is unlikely that early structural scans would have clinical utility in guiding treatment selection, a primary aim of the current study. Although the placebo response (37%) is comparable to clinical trials in the literature, it should be noted that response to sertraline (48%) was higher than placebo only at a trend level that did not reach statistical significance (p = 0.07). Therefore, it is possible that the lack of significant difference in antidepressant response between sertraline and placebo (see Table 1) may have contributed to a lower effect size preventing us from identifying significant treatment by response interaction. Another possibility is the use of conservative correction for multiple comparison, which might have reduced our ability to detect findings with small effect size. Recently, an elegant study conducted in a subgroup of the EMBARC sample, limited to select regions of interest, found an association between week-8 response and increased rostral anterior cingulate thickness at week 1 following sertraline treatment.27
Our laterality results are aligned with the findings of a recent study relating higher right hemisphere thickness to successful antidepressant treatment.28 We observed increased thickness in right caudal and rACC following treatment with sertraline. Anterior cingulate cortex is critical part of the limbic system, which is associated with sensory, motor, cognitive, and emotional processes.29 Furthermore, previous studies have reported thinning in anterior cingulate cortex of patients with MDD15,30 and bipolar disorder.31,32
Previous studies comparing cortical thickness between MDD patients and healthy controls indicated cortical thinning in rostral middle frontal cortex in patients.12,28 Our results showed increased cortical thickness in right rostral middle frontal in MDD participants given sertraline. This area is in Brodmann area 9 which is highly correlated with dorsolateral prefrontal cortex (dlPFC), the core region of the central executive cortex.12,33 Alterations in this region have been reported by previous studies using functional neuroimaging techniques.33–35 Similarly, Van Tol et al.36 found lower cortical thickness in the right dlPFC of MDD patients. Consistent with the MRI studies, human postmortem studies showed lower cortical thickness in dlPFC and rACC of individuals with MDD compared to healthy subjects.37,38 Therefore, our results may suggest that changes in cortical thickness are indicative of a normalization process induced by antidepressant treatment. Moreover, our findings in the superior temporal cortex are consistent with a previous study that showed increased thickness in this area after applying a course of ECT compared to baseline.21
Taken together, the study results showed sertraline-induced increased thickness in regions that are associated with cognitive dysfunction, emotion dysregulation, and self-reference in MDD.39 For example, rostral medial frontal cortex, a part of central executive network, is known to play an important role in emotional self-regulation whose deficit is one of the main characteristics of patients with MDD.39 Moreover, posterior cingulate cortex is a core node in the default mode network. Structural abnormalities in this brain region have not been frequently reported in MDD patients. However, several studies using functional images have frequently reported altered functional connectivity in this region.40–42 These abnormalities are thought to be related to dysregulation in rumination and self-referential processes.39,43
Finally, although some studies have previously reported left lateralization or no lateralization in patients with MDD, our results on right lateralization in cortical thickness changes after one week sertraline treatment is aligned with the hypothesis that the right side of the brain is primarily involved in processing of negative emotions.44
Limitations and Strengths
Among the limitations of the current study is that only one type of antidepressants was used, thus these findings may not necessarily generalize to other classes of antidepressants, especially drugs which are glutamate-based. In addition, findings of the current report may not generalize to MDD patients with higher severity as patients with psychotic features and suicidal risk were excluded. Another limitation is that the current study lacks cortical thickness measures at week 8, which would have revealed whether the cortical changes after full course of treatment are better associated with response.
The strengths of the study include a large sample with randomized double-blinded placebo-controlled design to account for non-specific effects using cortical thickness, a well-established structural measure, along with state-of-the-art preprocessing and rigorous FDR correction for multiple comparison using vertex-wise approach to facilitate the replication in future studies without being dependent on the selection of a priori region of interest.
Acknowledgements
The authors would like to thank the subjects who participated in these studies for their invaluable contribution. Data used in the preparation of this manuscript were obtained and analyzed from the controlled access data sets distributed from the National Institute of Mental Health (NIMH)-supported National Database for Clinical Trials (NDCT). NDCT is a collaborative informatics system created by the NIMH to provide a national resource to support and accelerate discovery related to clinical trial research in mental health. Data set identifier(s): STU 092010-151; Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIMH or of the submitters submitting original data to NDCT.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C. G. A. has served as a consultant, speaker and/or on advisory boards for Genentech, Janssen, Lundbeck, Psilocybin Labs, and FSV7, and editor of Chronic Stress for SAGE Publications, Inc.; filed a patent for using mTORC1 inhibitors to augment the effects of antidepressants (filed on Aug 20, 2018). S. N. declares no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support was provided by NIMH (K23MH101498) and the VA National Center for PTSD. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors, the Department of Veterans Affairs, NIH, or the U.S. Government.
ORCID iDs
Samaneh Nemati https://orcid.org/0000-0002-6610-5302
Chadi G. Abdallah https://orcid.org/0000-0001-5783-6181
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005; 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 2.Caspi A, Sugden K, Moffitt TEet al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003; 301: 386–389. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol Ther. 2018; 190: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath CL, Kelley ME, Holtzheimer PEet al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013; 70: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015; 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popoli M, Yan Z, McEwen BS, Sanacora GJ. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012; 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress (Thousand Oaks). 2017; 1: 2470547017692328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Eijndhoven P, van Wingen G, Katzenbauer Met al. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry. 2013; 170: 1477–1486. [DOI] [PubMed] [Google Scholar]
- 9.Järnum H, Eskildsen SF, Steffensen EGet al. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand. 2011; 124: 435–446. [DOI] [PubMed] [Google Scholar]
- 10.Na K-S, Chang HS, Won Eet al. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One. 2014; 9: e85425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Metzger CD, Li Wet al. Dissociation of glutamate and cortical thickness is restricted to regions subserving trait but not state markers in major depressive disorder. J Affect Disord. 2014; 169: 91–100. [DOI] [PubMed] [Google Scholar]
- 12.Tu P-C, Chen L-F, Hsieh J-C, Bai Y-M, Li C-T, Su T-P. Regional cortical thinning in patients with major depressive disorder: a surface-based morphometry study. Psychiatry Res. 2012; 202: 206–213. [DOI] [PubMed] [Google Scholar]
- 13.Lener MS, Kundu P, Wong Eet al. Cortical abnormalities and association with symptom dimensions across the depressive spectrum. J Affect Disord. 2016; 190: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papmeyer M, Giles S, Sussmann JEet al. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol Psychiatry. 2015; 78: 58–66. [DOI] [PubMed] [Google Scholar]
- 15.Meier TB, Drevets WC, Wurfel BEet al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. 2016; 53: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won E, Choi S, Kang J, Lee M-S, Ham B-J. Regional cortical thinning of the orbitofrontal cortex in medication-naïve female patients with major depressive disorder is not associated with MAOA-uVNTR polymorphism. Ann Gen Psychiatry. 2016; 15: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu L, Lui S, Kuang Wet al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Translat Psychiatry. 2014; 4: e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds S, Carrey N, Jaworska N, Langevin LM, Yang X-R, MacMaster FP. Cortical thickness in youth with major depressive disorder. BMC Psychiatry. 2014; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han K-M, Choi S, Jung Jet al. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. 2014; 155: 42–48. [DOI] [PubMed] [Google Scholar]
- 20.Koolschijn PCM, van Haren NE, Schnack HG, Janssen J, Pol HEH, Kahn RS. Cortical thickness and voxel-based morphometry in depressed elderly. Eur Neuropsychopharmacol. 2010; 20: 398–404. [DOI] [PubMed] [Google Scholar]
- 21.Pirnia T, Joshi S, Leaver Aet al. Electroconvulsive therapy and structural neuroplasticity in neocortical, limbic and paralimbic cortex. Translat Psychiatry. 2016; 6: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartorius A, Demirakca T, Böhringer Aet al. Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol. 2016; 26: 506–517. [DOI] [PubMed] [Google Scholar]
- 23.Trivedi MH, McGrath PJ, Fava Met al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): rationale and design. J Psychiatr Res. 2016; 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg T, Chase HW, Almeida JRet al. Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. Am J Psychiatry. 2015; 172: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glasser MF, Sotiropoulos SN, Wilson JAet al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013; 80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdallah CG, Dutta A, Averill CLet al. Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress (Thousand Oaks). 2018; 2: 2470547018796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett EA, DeLorenzo C, Sharma Pet al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology. 2018; 1: 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saricicek Aydogan A, Oztekin E, Esen MEet al. Cortical thickening in remitters compared to non‐remitters with major depressive disorder following 8‐week antidepressant treatment. Acta Psychiatr Scand. 2019; 140: 217–226. [DOI] [PubMed] [Google Scholar]
- 29.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000; 4: 215–222. [DOI] [PubMed] [Google Scholar]
- 30.Li R, Ma Z, Yu J, He Y, Li J. Altered local activity and functional connectivity of the anterior cingulate cortex in elderly individuals with subthreshold depression. Psychiatry Res. 2014; 222: 29–36. [DOI] [PubMed] [Google Scholar]
- 31.Brooks JO, III, Foland-Ross LC, Thompson PM, Altshuler LL. Preliminary evidence of within-subject changes in gray matter density associated with remission of bipolar depression. Psychiatry Res. 2011; 193: 53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oertel-Knöchel V, Reuter J, Reinke Bet al. Association between age of disease-onset, cognitive performance and cortical thickness in bipolar disorders. J Affect Disord. 2015; 174: 627–635. [DOI] [PubMed] [Google Scholar]
- 33.Dannlowski U, Ohrmann P, Konrad Cet al. Reduced amygdala–prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009; 12: 11–22. [DOI] [PubMed] [Google Scholar]
- 34.Oda K, Okubo Y, Ishida Ret al. Regional cerebral blood flow in depressed patients with white matter magnetic resonance hyperintensity. Biol Psychiatry. 2003; 53: 150–156. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence NS, Williams AM, Surguladze Set al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004; 55: 578–587. [DOI] [PubMed] [Google Scholar]
- 36.Van Tol M-J, Li M, Metzger Cet al. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med. 2014; 44: 2053–2065. [DOI] [PubMed] [Google Scholar]
- 37.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002; 12: 386–394. [DOI] [PubMed] [Google Scholar]
- 38.Rajkowska G, Miguel-Hidalgo JJ, Wei Jet al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999; 45: 1085–1098. [DOI] [PubMed] [Google Scholar]
- 39.Li BJ, Friston K, Mody M, Wang HN, Lu HB, Hu DW. A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci Therapeut. 2018; 24: 1004–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdallah CG, Averill LA, Collins KAet al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology. 2017; 42: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012; 139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greicius MD, Flores BH, Menon Vet al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007; 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berman MG, Misic B, Buschkuehl Met al. Does resting-state connectivity reflect depressive rumination? A tale of two analyses. Neuroimage. 2014; 103: 267–279. [DOI] [PubMed] [Google Scholar]
- 44.Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res. 2010; 68: 77–87. [DOI] [PubMed] [Google Scholar]