Adult T-cell leukemia/lymphoma (ATL) is an aggressive T-cell malignancy caused by human T lymphotropic virus type-1 (HTLV-1). Several patients with relapsed/refractory ATL treated with a monoclonal antibody that targets C-C chemokine receptor 4 (CCR4, www.clinicaltrials.gov NCT016266664)1 achieved durable remissions, including one patient who has remained in remission 64 months since trial entry. To define the molecular correlates of response to therapy, we quantified minimal residual disease (MRD) in four trial participants. Mogamulizumab reduced the HTLV-1 proviral load (PVL) and the abundance of the malignant clone by >2-logs (to <1% of peripheral blood mononulear cells (PBMC)) by day 56. In patients who achieved long-term progression-free survival, mogamulizumab reduced the abundance of the malignant clone to undetectable levels in the blood, and PVL was reduced to below the reported burden of patients at risk of ATL. Conversely, despite having a clinical response to therapy, the malignant clone remained highly abundant (9% to 55% of PBMC) in the patient with acute ATL who later progressed. We conclude that (1) anti-CCR4 should be further evaluated in clinical trials, particularly in the setting of ‘indolent’ ATL subtypes and (2) ATL-specific molecular diagnostic methods are critical for guiding therapeutic decisions with all agents.
CCR4 is highly expressed by HTLV-1 infected cells in asymptomatic carriers2 and by malignant cells in ATL,3 where increased surface expression of CCR4 is associated with cutaneous manifestations and poor overall survival (OS).4 Approximately 30% of ATL cases have gain-of function mutations (C-terminal truncations) in the CCR4 gene which inhibit receptor internalization after ligand binding.5,6 Mogamulizumab is a humanized, afucosylated monoclonal antibody that targets CCR4. In Japan, mogamulizumab was licensed for ATL following phase 1 and 2 clinical trials (NCT003554727 and NCT009207908) in the setting of relapsed/refractory ATL. Three out of six patients with the chronic unfavorable subtype showed progression-free survival (PFS) at 1 year in the phase 2 trial.9 Furthermore, the presence of gain-of-function mutations in CCR4 are associated with significantly longer PFS when treated with mogamuliuzmab.10
Quantitative analysis of HTLV-1 proviruses can provide clinically useful information about the response to therapy in ATL.11 During viral replication, the HTLV-1 provirus is inserted into the host DNA by integration at a semi-random location within the host cell genome.12 Each integrated provirus is inherited on cell division, thus, mapping and quantification of HTLV-1 integration sites can be used to measure the abundance of clonal populations of HTLV-1 infected cells. In 91% of ATL cases, malignant cells are clonal, with a single dominant proviral genomic integration site in the malignant tissue.13 Monoclonal ATL cells also share a unique T-cell receptor (TCR), and thus express a common TCRVbeta (TCRVβ) subunit.14 Together, HTLV-1 integration sites and TCR sequences can be utilized to monitor the kinetics of malignant cells and multidrug resistance (MRD) during treatment.
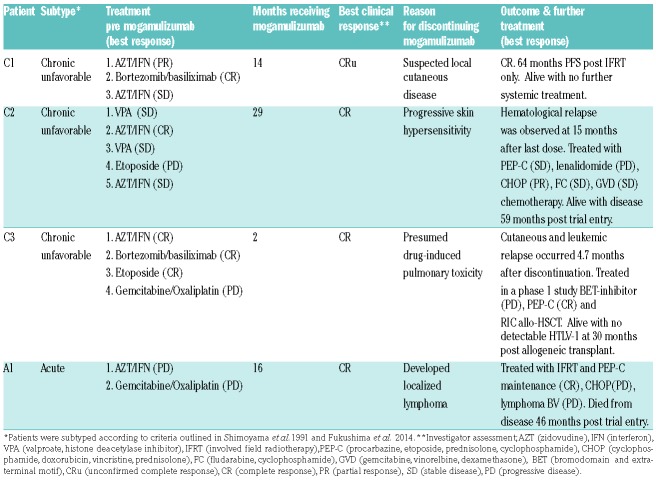
Outside of Japan, mogamulizumab has been investigated in the recently reported KW0761-009 study1 (intravenous mogamulizumab 1.0 mg/kg once weekly for 4 weeks and biweekly thereafter), where an 11% overall response rate with mogamulizumab was reported versus 0% with investigator’s choice chemotherapy. Here, we present HTLV PVL and MRD levels of the dominant clone in a cohort of patients treated as part of the KW0761-009 study in UK centers (Table 1). All patients had leukemia-type ATL (one acute and three chronic unfavorable), reached the first assessment point at the end of cycle 1 and were followed up for a median 60.1 months (range 46.1-63.7 months) from randomization (census date November 2018). Patients attended the National Center for Human Retrovirology (Imperial College Healthcare NHS Trust, St Mary’s Hospital, London) where written informed consent was obtained. Research was conducted under the governance of the Communicable Diseases Research Group Tissue Bank, approved by the UK National Research Ethics Service (09/H0606/106, 15/SC/0089).
Table 1.
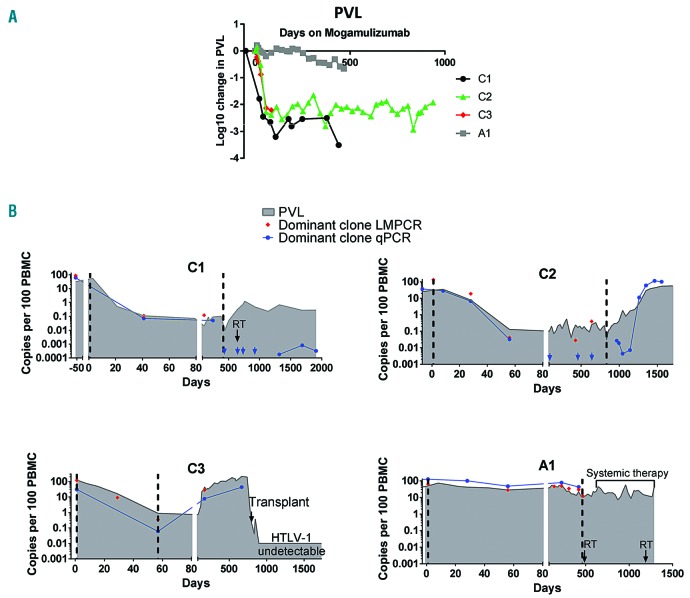
After two 28-day cycles of treatment with mogamulizumab, there was a significant reduction in the proportion PBMC infected with HTLV-1 in individuals with chronic unfavorable ATL (C1, C2 and C3, Figure 1A, B). By day 56 (Cycle 2, day 28), all three patients had experienced a 2-log reduction in PVL (proviruses per 100 PBMC) from premogamulizumab levels: C1 from 32% to 0.07% of PBMC; C2 from 25% to 0.13%; and C3 from 122% to 0.88%. At day 56, the patient with acute ATL, A1, experienced a modest reduction in PVL (~0.2-log from 44% to 28% of PBMC) with slower kinetics. Though A1 only experienced a modest reduction in PVL, he did experience a 1-log reduction in white cell count, consistent with hematological remission.
Figure 1.
Proviral load and minimal residual disease kinetics of four ATL patients. (A) Proviral load (PVL) during treatment with mogamulizumab. The proportion of peripheral blood monanuclear cells (PBMC) infected with HTLV-1 was estimated by quantitative PCR (qPCR) for HTLV-DNA and the beta globin gene using a method previously reported in Demontis et al.15 For each time point, log change was calculated using the formula: log10((time point value)/(premogamulizumab value)). (B) Kinetics of malignant (red and blue) and HTLV-1-infected cells (grey filled) in the study cohort. HTLV-1 integration sites were mapped using the ligation-meditated PCR (LM-PCR)/high throughput sequencing protocol described in detail in Gillet et al.12 Primers were designed to specifically amplify sequences unique to the malignant clone in each patient by pairing a primer located in the 3’LTR of HTLV-1 with a primer located in the human genome adjacent to the dominant HTLV-1 integration site.11 In the case of patient C2, the dominant integration site was located in a repetitive region of the human genome. Thus, specific primers were designed which amplified DNA of the rearranged T-cell receptor (TCR) in the malignant clone. The first (Cycle 1, day 1) and last mogamulizumab infusions are indicated by vertical dashed lines. RT indicates localized radiotherapy (black arrows). Copy numbers were estimated by quantitative PCR (blue) and linker-mediated PCR and next-generation sequencing (red), and expressed per 100 PBMC. Blue arrows indicate time points at which the malignant clone was undetectable by qPCR.
After the last infusion, patient C1 (who achieved a long remission) maintained a mean PVL of 0.4% post therapy (50 months follow up). In the two patients (C2 and C3) who relapsed after achieving a PVL of <1% during therapy, a PVL of <1% was maintained for 7 months (C2) and 2 months (C3) after the last infusion, rising gradually to the pre-mogamulizumab treatment levels at relapse (Figure 1B). The PVL of patient A1 returned to pre-mogamulizumab levels 16 months after the last infusion.
The genomic location of integrated HTLV-1 proviruses was mapped by high throughput sequencing. A single unique clonal integration site was dominant in pre-mogamulizumab samples from patients C1, C2 and A1. Patient C3 had five equally dominant integration sites which likely represent five HTLV-1 integrations in a single, TCRVβ3-expressing malignant clone.14 Integration-site mapping was performed at multiple time points in each individual (Figure 1B) and confirmed by quantitative PCR (qPCR) to detect the dominant clone.
A 2.7- to 3-log reduction in the abundance of the malignant clone was observed by day 56 in patients C1, C2 and C3 (Figure 1B). At later time points in C1 the malignant clone was either undetectable, or detectable at very low, stable levels off treatment (~5-log reduction from initial abundance). In patient C2, the abundance of the malignant clone was stable up to 11 months after the final infusion, while off all therapy, but increased by >3-log in abundance by 15 months after the final infusion. A relapse was confirmed at this point. The greatest reduction in the malignant clone abundance in patient A1 was 0.7-logs, achieved at the end of mogamulizumab treatment.
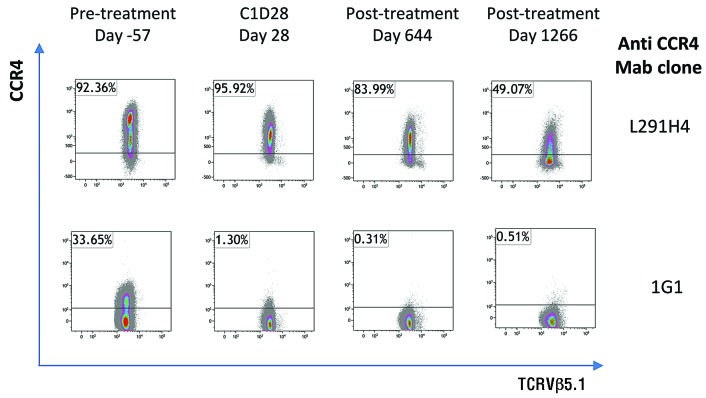
Given the different kinetics of response in patient A1, we analyzed the expression of CCR4 on the malignant clone (TCRVβ5.1+ cells) using two anti-CCR4 antibodies (clones 1G1 and L291H4), (Figure 2). Prior to treatment with mogamulizumab, expression of CCR4 on TCRVβ5.1+ cells was bimodal, with CCR4negative/medium by clone 1G1, CCR4medium/high by clone L291H4. After one cycle of mogamulizumab, the CCR4high TCRVβ5.1+ population was no longer detectable in PBMC. Six months after the final infusion, TCRVβ5.1+ cells were CCR4negative by 1G1 staining, and low or negative by L291H4 staining.
Figure 2.
Flow cytometric staining of CCR4 expression on malignant cells. PBMC from patient A1 were stained as described in Rowan et al.14: with a viability stain and antibodies specific for CD3, CD4, CD8, TCRVβ5.1 and with two different anti-CCR4 mAb clones. Cells were gated on live, single, CD3+CD8−TCRVβ5.1+ lymphocytes based on fluorescence minus one controls.
The results presented here demonstrate that complete hematological responses can be achieved through mogamulizumab monotherapy: three heavily pretreated patients with aggressive leukemic ATL (presenting without significant nodal disease) have remained alive a mean of 61 months since trial entry (range 58-63 months).
Mogamulizumab induced a rapid response in patients with chronic unfavorable ATL, with a 2-log reduction in HTLV-1 proviral load within the first two cycles of therapy, and the malignant clone becoming undetectable in some patients. We previously reported a patient in whom oral zidovudine and subcutaneous interferon-a (AZT+IFN) induced durable treatment-free remission (5 years off therapy at the time of publication).11 The rapid reduction in the burden of malignant cells on treatment with mogamulizumab contrasts dramatically with the much slower rate at which malignant cells were cleared by AZT+IFN treatment: 4.5 years of AZT+IFN treatment were able to reduce the abundance of the malignant clone to 0.01% PBMC, versus ~3 months of mogamulizumab. Mogamulizumab also reduced the proviral load in the blood to levels (<1% of PBMC) below the expected viral burden of patients at risk of ATL.
Despite achieving a complete hematological response, the patient with the acute ATL maintained a high proviral load which was dominated by the malignant clone. Resistance to mogamulizumab therapy was attributed to a positive selection of a CCR4low/negative subpopulation of malignant cells which was present prior to treatment.
Being unable to detect MRD was associated with long remission after therapy in one patient (C1), whereas in two other patients (C2 and C3) MRD as low as 0.03% of PBMC was followed by a relapse with the same malignant clone. This was not attributable to the mutational status of CCR4: as whole exome sequencing detected a CCR4 mutation (Y331*) in patient C2 only. Despite the small number of patients in the present study, our data are consistent with published follow-up of trials conducted in Japan.9 In this report, a subgroup (25-31%) of patients with ATL who received mogamulizumab monotherapy survived >3 years.
Mogamulizumab clearly warrants further evaluation within clinical trials in chronic favorable and smoldering subtypes, before disease transformation. This and other studies indicate that individuals with high levels of MRD required further treatment and are likely to progress, whereas individuals with low/undetectable levels of MRD are more likely to achieve long-term treatment-free remission. Therefore, we recommend that real-time molecular analysis of MRD should form part of all future trials of anti-ATL agents.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Phillips AA, Fields PA, Hermine O, et al. Mogamulizumab versus investigator choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2019;104(5):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamano Y, Araya N, Sato T, et al. Abnormally high levels of virus- infected IFN-gamma+ CCR4+ CD4+ CD25+ T cells in a retrovirus-associated neuroinflammatory disorder. PLoS One. 2009;4(8):e6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–1511. [DOI] [PubMed] [Google Scholar]
- 4.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–3634. [PubMed] [Google Scholar]
- 5.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47(11):1304–1315. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M, Schmitz R, Xiao W, et al. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med. 2014; 211(13):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. [DOI] [PubMed] [Google Scholar]
- 8.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. [DOI] [PubMed] [Google Scholar]
- 9.Ishida T, Utsunomiya A, Jo T, et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108(10):2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Ishida T, Masaki A, et al. CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood. 2018;132(7):758–761. [DOI] [PubMed] [Google Scholar]
- 11.Cook LB, Rowan AG, Demontis MA, et al. Long-term clinical remission maintained after cessation of zidovudine and interferon-a therapy in chronic adult T-cell leukemia/lymphoma. Int J Hematol. 2018; 107(3):378–382. [DOI] [PubMed] [Google Scholar]
- 12.Gillet NA, Malani N, Melamed A, et al. The host genomic environment of the provirus determines the abundance of HTLV-1-infected T-cell clones. Blood. 2011;117(11):3113–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook LB, Melamed A, Niederer H, et al. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123(25):3925–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowan AG, Witkover A, Melamed A, et al. T Cell Receptor Vβ staining identifies the malignant clone in adult T cell leukemia and reveals killing of leukemia cells by autologous CD8+ T cells. PLOS Pathog. 2016;12(11):e1006030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demontis MA, Hilburn S, Taylor GP. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses. 2013; 29(2):359–364. [DOI] [PubMed] [Google Scholar]