Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by bone marrow fibrosis, cytopenias, splenomegaly, and elevated proinflammatory cytokine levels. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is the central pathway implicated in the pathogenesis of MF.1,2 One of the other pathways dysregulated in MF includes the phosphatidylinositol 3 kinase (PI3K)/Akt pathway.3 Ruxolitinib, a first-in-class JAK1/2 inhibitor (JAKi), is approved in the US and EU for treatment of MF and polycythemia vera after an inadequate response or intolerance to hydroxyurea. Buparlisib, an oral pan-PI3K inhibitor, showed a favorable tolerability/efficacy profile in patients with solid tumors.4
Targeting multiple signaling pathways might have a synergistic therapeutic effect on the underlying pathogenesis of MF.5 For patients who do not respond to JAKi or those who loose their response, a rational combination of other targeted agents is a promising strategy.6,7 Preclinical data suggest that inhibition of the PI3K/mTOR pathway has beneficial effects in MF.8–10 HARMONY, a phase 1b, 2-arm, open-label, multicenter, dose-finding study, investigated the safety and efficacy of the oral combination of ruxolitinib and buparlisib in adult patients with intermediate/high-risk primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF (Online Supplementary Figure 1). The maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D) was established at 15 mg twice daily (bid) for ruxolitinib and 60 mg once daily (qd) for buparlisib. The dose-limiting toxicity (DLT) and adverse event (AE) profile of the combination was similar to the safety profile of the individual drugs and resulted in an overall moderate clinical benefit compared with ruxolitinib monotherapy.
Patients without prior JAKi treatment (JAKi naïve) and with prior JAKi treatment including ruxolitinib (prior JAKi) were enrolled simultaneously into the two arms of the study.
The study consisted of two periods, namely the treatment period (cycle 1 day 1 to cycle 7 day 1 [C7D1]) and treatment extension period (C7D1 to cycle 12 day 28 [C12D28]). The treatment period comprised six cycles of 28 days per cycle. At the end of the treatment period, on C7D1, patients benefiting from the treatment as per the investigator’s discretion with no evidence of disease progression could enter the treatment extension period. Patients not meeting this criteria were discontinued from the study at the end of the treatment period. A follow-up visit was scheduled 30 days after the end-of-treatment visit (C7D1, C12D28, or premature discontinuation).
The study comprised dose-escalation and expansion phases. In the dose-escalation phase, successive cohorts of minimum three patients received increasing doses of ruxolitinib (5 mg bid to 40 mg bid) and buparlisib (40 mg qd to 100 mg qd) until the MTD/RP2D was reached. An additional six patients were enrolled to determine a dose level as the MTD/RP2D.
The primary objective was to establish the MTD/RP2D of the combination of ruxolitinib and buparlisib in each arm as assessed by the incidence rate of DLTs. The key secondary objective was to evaluate safety. Further details of the eligibility criteria, methods, other secondary objectives, and the statistical analysis used for this study are provided in the Online Supplementary Material.
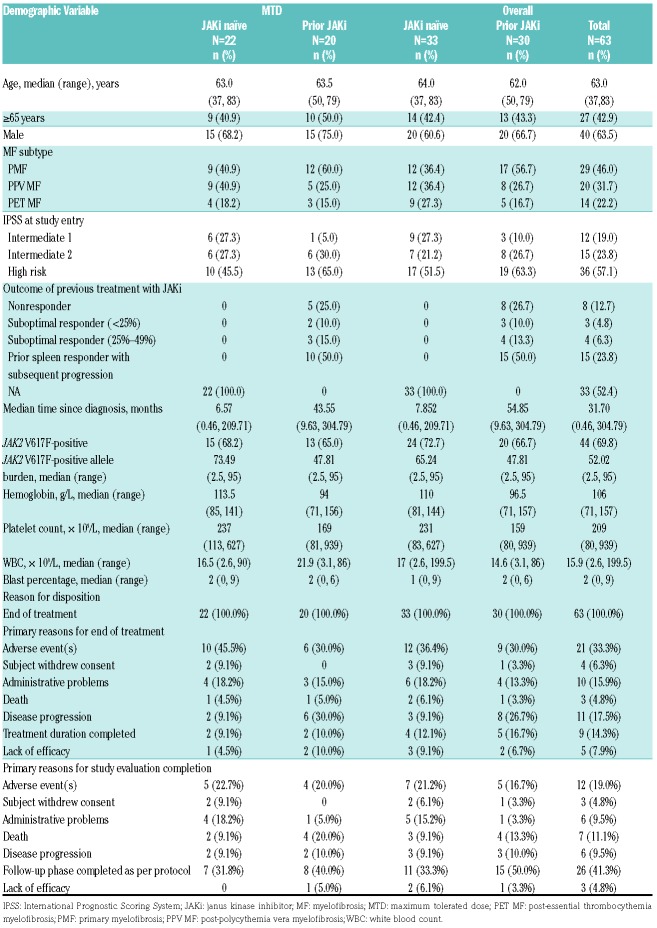
Sixty three patients (46% with primary MF, 31.7% with post-polycythemia vera MF, and 22.2% with post-essential thrombocythemia MF) were enrolled in the study (JAKi naïve, n=33; prior JAKi, n=30). The baseline characteristics and patient disposition are summarized in Table 1. At baseline, prior JAKi patients had lower hemoglobin and platelet counts and a higher white blood cell count compared with JAKi naïve patients. AE (33.3%) were the primary reason for the end of treatment. In the dose-escalation phase, dose levels included for the ruxolitinib bid/buparlisib qd combination were 10 mg/60 mg (dose level 1, n=15), 15 mg/60 mg (dose level 2, n=42), 15 mg/80 mg (dose level 3, n=3), and 20 mg/80 mg (dose level 4, n=3). The median duration of exposure to ruxolitinib at MTD was 79.5 weeks (12–167.6 weeks) and 54.6 weeks (8–151.3 weeks) in the JAKi naïve and prior JAKi arms, respectively, and to buparlisib was 79.4 weeks (2.4–167.4 weeks) and 54.5 weeks (7.1–151.1 weeks), respectively. The analysis set per dose level is presented in the Online Supplementary Table 1.
Table 1.
Baseline patient characteristics and patient disposition (full analysis set).
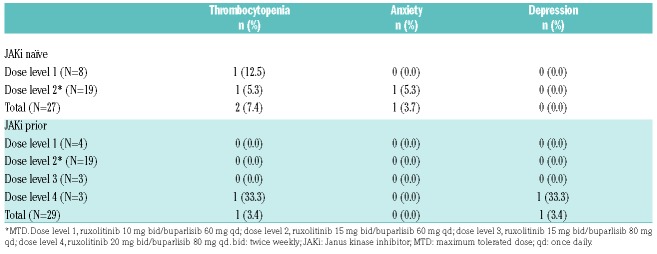
In the overall population, five patients (JAKi naïve, n=3; prior JAKi, n=2) experienced DLTs during the first 28 days; thrombocytopenia (JAKi naïve, n=2; prior JAKi, n=1), anxiety (JAKi naïve, n=1; prior JAKi, n=0), and depression (JAKi naïve, n=0; prior JAKi, n=1). MTD/RP2D was established at ruxolitinib 15 mg bid/buparlisib 60 mg qd (dose level 2) for both arms.11 The MTD/RP2D remained the same as confirmed by the results of the expansion cohort, based on the 56 patients in the dose-determining set (Table 2).
Table 2.
Dose-limiting toxicities occurring during cycle 1 (dose-determining set).
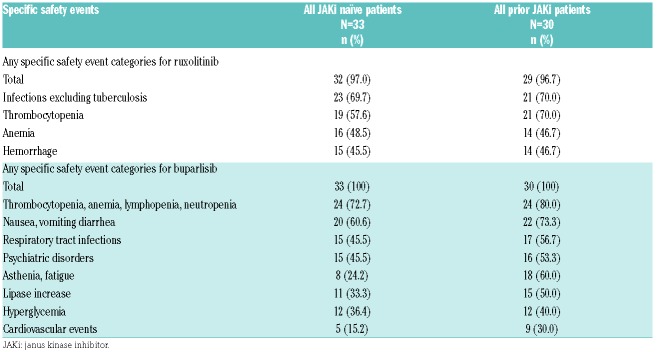
All patients included in the safety set (n=63) experienced ≥1 AE (Online Supplementary Table 2). In the MTD population, anxiety, depression, dizziness, dyspnea, and stomatitis were the most common nonhematologic AEs. The most frequent all-grade hematologic AE included thrombocytopenia (JAKi naïve, 63.6%; prior JAKi, 55.0%) and anemia (JAKi naïve, 50.0%; prior JAKi, 55.0%). Grade 3/4 thrombocytopenia was higher with prior JAKi (35.0%) compared with JAKi naïve (22.7%). Pneumonia (JAKi naïve, 9.1%; prior JAKi, 15%) and pyrexia (JAKi naïve, 4.5%; prior JAKi, 10%) were the most common serious AE in both arms. Progression to acute myeloid leukemia was also reported (JAKi naïve, 4.5%; prior JAKi, 10%).
Primary AE leading to study drug discontinuation in the MTD population included thrombocytopenia (n=3), anxiety (n=2), and depression (n=2) in the JAKi naïve arm and progression to acute myeloid leukemia (n=2) in the prior JAKi arm. Dose reduction/interruptions in the JAKi naïve arm and prior JAKi arm were due to thrombocytopenia (n=10; n=8, respectively) and anemia (n=2 in both arms).
The frequency of infections, thrombocytopenia, and psychiatric disorders were similar or slightly higher in the prior JAKi arm compared with the JAKi naïve arm (Table 3). Seven on-treatment deaths occurred, which were not related to the study treatment (Online Supplementary Table 3).
Table 3.
Adverse events of special interest (≥30% in either arm) for ruxolitinib and buparlisib, regardless of study drug relationship by arm (safety set).
At C7D1 and C12D28, the proportion of MTD patients achieving a ≥50% reduction in the spleen length was 12 of 16 (75.0%) and 13 of 15 (86.7%), respectively, in the JAKi naïve arm and 6 of 17 (35.3%) and 4 of 11 (36.4%), respectively, in the prior JAKi arm. At C7D1, the proportion of MTD patients in the expansion phase achieving a ≥35% reduction in the spleen volume was 5 of 9 (55.6%) in the JAKi naïve arm and 3 of 7 (42.9%) in the prior JAKi arm (Online Supplementary Figure 2A and Online Supplementary Figure 2B). The best response in the spleen volume reduction in MTD patients is presented in the Online Supplementary Figure 2C.
There was a remarkable improvement in the global health status/quality of life, which was more prominent in the JAKi naïve arm than the prior JAKi arm at both C7D1 and C12D28. Improvement in the 7-day Myelofibrosis Symptom Assessment Form (MFSAF) from baseline to week 24 in both the arms is shown in the Online Supplementary Table 4. A modest effect was observed on allele burden in both arms. Among MTD patients, improvement in bone marrow fibrosis (n=1 in each arm), stabilization (JAKi naïve, n=2; prior JAKi, n=4), and worsening by the end of treatment (JAKi naïve, n=1) were observed. Data for the overall population are presented in the Online Supplementary Table 5. Buparlisib did not impact the pharmacokinetics of ruxolitinib (Online Supplementary Table 6).
To the best of our knowledge, HARMONY is the first study presenting safety and efficacy data on ruxolitinib and buparlisib combination in patients with MF. MTD for the combination was determined to be 15 mg bid for ruxolitinib and 60 mg qd for buparlisib in both JAKi naïve and prior JAKi arms.11 Individual MTD were 25 mg bid for ruxolitinib and 100 mg qd for buparlisib.12,13 In our study, MTD for the combination constituted lower doses for both the drugs when compared with the single agents. Additionally, no unexpected DLT were observed with the combination. Thrombocytopenia, anxiety, and depression were the DLT observed with the combination in our study, which are consistent with the known profile of these two drugs.12,13 The combination was well tolerated with a manageable safety profile and provided clinically relevant efficacy. No new safety signals were observed.
Approximately, 40% of the patients had a spleen volume reduction of ≥35% with the combination in the expansion phase. With ruxolitinib alone, 41.9% of patients at week 24 in COMFORT-I and 28% of patients at week 48 in COMFORT-II achieved a spleen volume reduction of ≥35%.14,15 In our study, the anticipated synergistic effect of the combination was not observed on the spleen response. The combination demonstrated a modest effect in the JAK allele burden in both arms, and only a few patients had improvement or stabilization in bone marrow fibrosis. However, two factors have to be taken into account before interpreting this data; the small sample size and short duration of the study.
Based on the modest, overall benefit-risk profile and efficacy, the ruxolitinib and buparlisib combination will not be progressed at this time.
Footnotes
Funding: the Novartis Pharmaceuticals Corporation
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Keohane C, Radia DH, Harrison CN. Treatment and management of myelofibrosis in the era of JAK inhibitors. Biologics. 2013;7:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014; 123(22):e123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartalucci N, Guglielmelli P, Vannucchi AM. Rationale for targeting the PI3K/Akt/mTOR pathway in myeloproliferative neoplasms. Clin Lymphoma Myeloma Leuk. 2013;13 Suppl 2:S307–S309. [DOI] [PubMed] [Google Scholar]
- 4.Ragon BK, Kantarjian H, Jabbour E. Buparlisib, a PI3K inhibitor, demonstrates acceptable tolerability and preliminary activity in a phase I trial of patients with advanced leukemias. Am J Hematol. 2017;92(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogani C, Bartalucci N, Martinelli S, et al. Associazione Italiana per la Ricerca sul Cancro AGIMM Gruppo Italiano Malattie Mieloproliferative. mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms. PLoS One. 2013;8(1):e54826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose P, Verstovsek S. Myelofibrosis: an update on drug therapy in 2016. Expert Opin Pharmacother. 2016;17(18):2375–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison CN, Kiladjian J, Passamonti F, et al. A phase 1b dose-finding study of ruxolitinib plus panobinostat in patients with primary myelofibrosis, post-polycythemia vera myelofibrosis, or post-essential thrombocythemia myelofibrosis [abstract 0364]. Haematologica. 2012;97 Suppl 1:146. [Google Scholar]
- 8.Bartalucci N, Calabresi L, Balliu M, et al. Inhibitors of the PI3K/mTOR pathway prevent STAT5 phosphorylation in JAK2V617F mutated cells through PP2A/CIP2A axis. Oncotarget. 2017;8(57):96710–96724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartalucci N, Tozzi L, Bogani C, et al. Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J Cell Mol Med. 2013;17(11):1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choong ML, Pecquet C, Pendharkar V, et al. Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med. 2013;17(11):1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrant S, Nagler A, Vannucchi AM, et al. An open-label, multicenter, 2-arm, dose-finding, phase 1b study of the combination of ruxolitinib and buparlisib (BKM120) in patients with myelofibrosis: results from HARMONY study. Blood. 2015;126:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30(3):282–290. [DOI] [PubMed] [Google Scholar]
- 13.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 15.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]