Allogeneic hematopoietic stem cell transplantation (HSCT) remains the only curative therapy for sickle cell disease (SCD).1–4 Several barriers prevent its widespread application, including the lack of a suitable donor, risk of early and late onset of regimen-related toxicities, rejection and mortality. Despite these limitations, the number of transplants for hemoglobinopathies has been increasing in the last decade. The overall probability of survival (OS) for patients with SCD transplanted with a human lymphocyte antigen (HLA)- identical sibling graft ranges between 91 and 100% with an event-free survival (EFS) of 73-100%.3 A controversial issue is the ideal age to perform HSCT in SCD patients. In fact, whilst early age HSCT could prevent SCD-related organ damage, resulting in better patient outcomes, the emergence of new available SCD supportive care, promising curative therapies could justify not proceeding with HSCT in certain cases.2,5,6
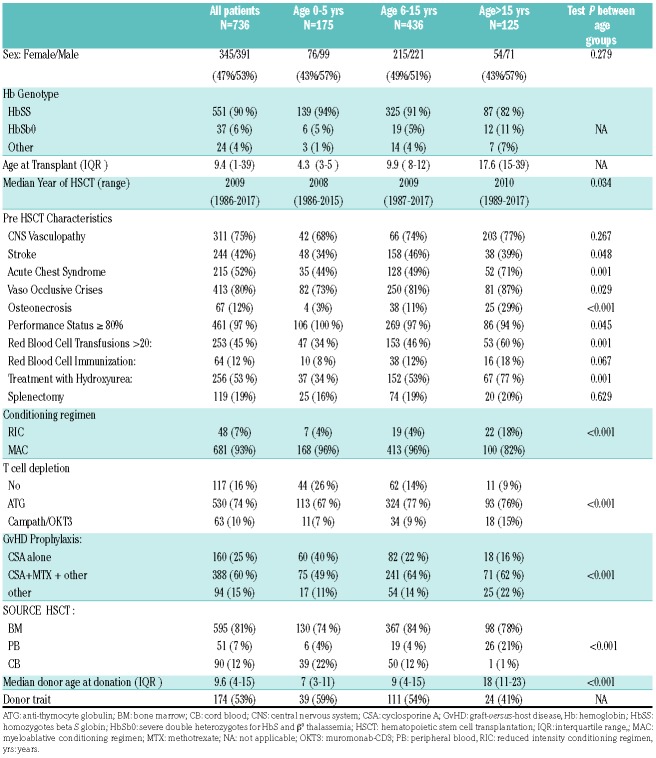
Herein, we report the outcomes of 736 SCD patients who underwent HSCT from an HLA-identical sibling between 1986 and 2017 and were reported to the European Blood Society for Blood and Marrow Transplantation (EBMT)/Eurocord registries. Based on previous reports suggesting improved HSCT outcomes in preschoolers1 and worst outcomes in adults, usually defined as patients older than 16 years in the SCD literature,7 three different groups were defined according to the age at transplant: 0-5 years: group 1 (n=175), 6-15 years: group 2 (n= 436) and more than 15 years: group 3 (n=125). Patients, donor and transplant characteristics are shown in Table 1. The cumulative incidence of neutrophil engraftment at day +60 was 96%, 98% and 95% in group 1, 2 and 3 respectively (P=0.017); platelet engraftment was 98%, 96% and 95% in the three groups respectively (P=0.033). In univariate analysis, in group 1, the cumulative incidence for neutrophil engraftment was higher for patients who received HSCT from a donor older than 9.6 years [98% (95% Comorbidity index (CI) 93-100%), P=0.031]; in group 2, it was also higher for patients who received HSCT from a donor older than 9.6 years [99% (95%CI 98-100%), P=0.037], who had an ABO blood group system compatibility or a minor incompatibility [99% (95%CI 97-100%), P=0.021] or who received anti-thymocyte globulin (ATG) [98% (95%CI 97-100%), P=0.003]; in group 3, none of the analyzed factors had a significant impact on neutrophil engraftment.
Table 1.
Patient, disease, donor and HSCT characteristics.*
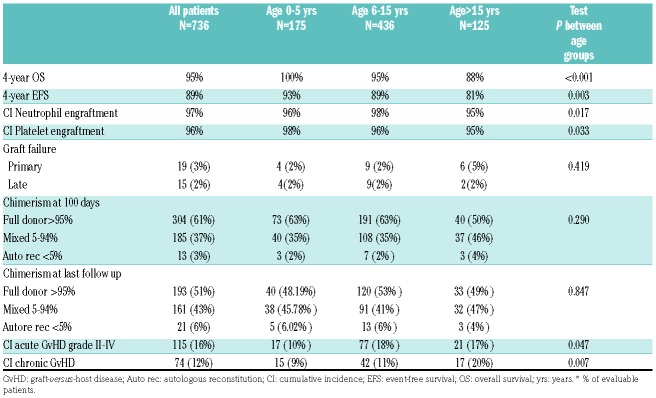
Primary graft failure was reported in 2% of the patients in group 1 (n=4) and in group 2 (n=9), and 5% in group 3 (n=6), whereas late graft failure occurred in 2% of the patients irrespective of the group (n=4, 9 and 2 in the three groups respectively).
At day +100, full chimerism was achieved in 63% of the patients in group 1 and 2 and in 50% of group 3; mixed chimerism in 35 % of patients in group 1 and 2 and 46% in group 3; autologous reconstitution was achieved in 2% of the patients of group 1 and 2 and in 4% of the patients in group 3.
The overall cumulative incidence of grade II-IV acute graft-versus-host disease (GvHD) was 16% (95%CI 13-18%) [group 1: 10% (95%CI 6-15%); group 2: 18% (95%CI 1-22%); group 3: 17% (95%CI 11-25%), P=0.047]. A univariate analysis of group 2 showed a higher incidence of acute GvHD in male patients [21% (95%CI 17-28%) versus 13% (95%CI 10-19%), P=0.038], in patients who received the grafts from donors older than 9.6 years [28% (95%CI 22-36%), P<0.001] or from a donor with positive cytomegalovirus (CMV) serology [22% (95%CI 17-27%) P=0.001].
The overall 4-year cumulative incidence of chronic GvHD was 12% (95%CI 10-15%) [group 1=9% (95% CI 6-15%), group 2=11% (95% CI 8-15%) and group 3=20% (95% CI 14-30%), P=0.007]. Chronic GvHD was extensive in four patients in group 1, in 21 patients in group 2 and in five patients in group 3.
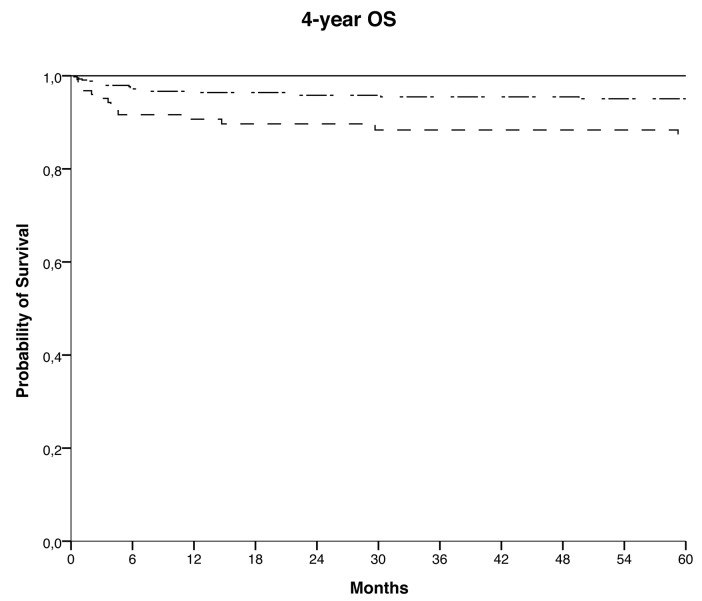
Four-year OS was 95 ± 1% for the whole cohort, being 100%, 95% and 88% for groups 1, 2 and 3, respectively (P<0.001) (Figure 1).
Figure 1.
Overall Survival (OS), defined as the probability of survival, regardless of disease status, from the time of HSCT to the time of death or of the last follow-up, according to the age group; 4-year OS was 95 ± 1% : 100% in group 1 (solid line), 95% in group 2 (dash-dotted line), and 88% in group 3 (dashed line) (P<0.001).
Four-year event free survival (EFS) was, overall, 89 ± 2% (93% in group 1, 89% in group 2 and 81% in group 3, P=0.003). We did not observe any risk factor for OS and EFS in the univariate analysis. All children in group 1 were alive at the last follow-up; 36 patients died: 21 (5%) in group 2 and 15 (12%) in group 3. The most common causes of death were infections and GvHD. Outcomes according to the age group are shown in Table 2.
Table 2.
Outcomes according to age.*
Optimal timing for HSCT in patients with SCD with an available HLA-identical sibling donor is not well established. In our previous international report on patients transplanted from an HLA-identical sibling, we analyzed outcomes of 1000 patients transplanted before December 2015 in the EBMT and CIBMTR (Center for International Blood and Marrow Transplant Research) centers and showed that age was a prognostic factor for both EFS and OS 3. Recently, Arnold et al. suggested that a HSCT performed in patients before the age of 10 years is associated with better outcomes.8 Moreover, it is known that both the number and severity of SCD-related complications tend to increase with age.9 This was confirmed in our study where patients older than 15 years had more SCD related organ damage and a worse performance status at HSCT. In addition, because many adverse events may be exacerbated by HSCT, historically, patients aged >16 years were not considered eligible for HSCT due to SCD-related organ damage and inability to tolerate myeloablative conditioning.10 Nevertheless, the low percentage of primary graft failure was surprising in these heavily transfused patients. However, optimal pre-transplant methodology could not be determined due to the heterogeneity of the conditioning regimen, stem cell source and GvHD prophylaxis.
Despite improvements in immunosuppressive prophylaxis, GvHD remains one of the major causes of morbidity and mortality after HSCT. In our series, patients younger than 5 years had both less grade 2-4 acute GvHD and chronic GvHD, compared to older patients. This could be, partially, explained by the fact that the majority of peripheral blood stem cell (PBSC) transplantations were performed in patients from group 2 and 3. When analyzing bone marrow (BM) recipients only, group 1 patients had a total nucleated cells (TNC) count higher than the other two groups which may have accounted to better engraftment rates (median TNC=4.6×108, 3.3×108 and 2.7×108 for groups 1, 2 and 3, respectively). Of note, BM recipients who received stem cells from a donor older than 9.6 years received a higher number of TNC (median 3.56×108 versus 2.96×108 for donors younger than 9.6 years old); therefore we cannot discard the possibility that the higher incidence of acute GvHD was related to the cell dose rather than the age of the donor. In the setting of non-malignant disease, where graft-versus-leukemia is not needed, an efficient GvHD prophylaxis strategy is mandatory to lower the risk of GvHD and its deleterious effect on morbidity and survival.
Importantly, besides GvHD, other complications such as graft rejection, growth impairment, gonadal dysfunction, infertility, life threatening infections can occur after HSCT and need to be taken into account when proposing HSCT to very young children with a non-malignant disorder.11 The occurrence of these undesirable late effects must be balanced with the potential complications of SCD itself (cognitive deficits, iron overload, asplenism, and low quality of life). Although the high EFS observed in our cohort suggests a good quality of life after HSCT, studies comparing long term quality of life among patients with SCD undergoing HSCT in contrast with patients receiving standard treatment are needed to further illustrate the pros and cons of each therapeutic approach.12,13
Furthermore, because SCD is a chronic disease associated with costly complications and uncertainties that worsen throughout the patient’s life, a curative therapy such as HSCT may be also warranted to reduce healthcare costs. In fact, although HSCT seems to be an expensive alternative, it constitutes a more economic path over a short period of years. Given that the cost of treating SCD increases significantly with time, transplantation at a younger age can have significant economic implications.14 Nevertheless, the excellent OS and EFS we observed for patients younger than 5 years, strongly indicate that HLA-identical sibling HSCT should be proposed early in life, before complications occur. In the present study, only univariate analyses were performed; adjusted multivariate analyses were not performed due to the low number of events, especially in the youngest age group. Although this is a limitation of our study, as it makes controlling for potential confounders problematic, it also highlights the remarkable results observed for HSCT for SCD in this setting.
In conclusion, the comparison of these three age groups might help physicians to elaborate recommendations for transplant indications and to design conditioning protocols adapted to age, given the better OS and EFS and the lower incidence of acute GvHD and chronic GvHD in younger patients. These findings outline the importance of early referral to HSCT and the importance of adapting indications and protocols according to age. Decision making on time of transplant in 2019 is highly complex and we acknowledge the difficulties in providing advice to the families affected by SCD with respect to transplant, not least because of the progress in gene therapy/editing and new molecules currently in trial in SCD.
Acknowledgments
We thank the Pediatric Diseases Working Party of the EBMT and Arnaud Dalissier for selecting the data from the PROMISE database. We thank all the doctors and data managers who have collaborated on the study and updated the patient questionnaires.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Angelucci E, Matthes-Martin S, Baronciani D, et al. Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ware RE, de Montalembert M, Tshilolo L, et al. Sickle cell disease. Lancet. 2017;390(10091):311–323. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman E, Cappelli B, Bernaudin F, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernaudin F, Verlhac S, Peffault de Latour R, et al. DREPAGREFFE Trial Investigators Association of matched sibling donor hematopoietic stem cell transplantation with transcranial doppler velocities in children with sickle cell anemia. JAMA. 2019;321(3):266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tshilolo L, Tomlinson G, Williams TN, et al. Hydroxyurea for children with sickle cell anemia in subsaharian Africa. N Engl J Med. 2019;380(2):121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Montalembert M, Brousse V, Chakravorty S, et al. Are the risks of treatment to cure a child with severe sickle cell disease too high? BMJ. 2017;359:j5250. [DOI] [PubMed] [Google Scholar]
- 7.Gardner K, Douiri A, Drasar E, et al. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128(10):1436–1438. [DOI] [PubMed] [Google Scholar]
- 8.Arnold SD, Brazauskas R, He N, et al. Clinical risks and healthcare utilization of hematopoietic cell transplantation for sickle cell disease in the USA using merged databases. Haematologica. 2017; 102(11):1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra P, Caughey M, Robinson L, et al. Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica. 2017;102(4):626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King A, Shenoy S. Evidence-based focused review of the status of hematopoietic stem cell transplantation as treatment of sickle cell disease and thalassemia. Blood. 2014;123(20):3089–3094. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy S, Angelucci E, Arnold SD, et al. Current results and future research priorities in late effects after hematopoietic stem cell transplantation for children with sickle cell disease and thalassemia: a Consensus Statement from the second pediatric Blood and Marrow Transplant Consortium International Conference on late effects after pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23(4):552–561. [DOI] [PubMed] [Google Scholar]
- 12.Dampier C, LeBeau P, Rhee S, et al. Health-related quality of life in adults with sickle cell disease (SCD): a report from the comprehensive sickle cell centers clinical trial consortium. Am J Hematol. 2011; 86(2):203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBaun MR, Ghafuri DL, Rodeghier M, et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood. 2019;133(6):615–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saenz C, Tisdale JF. Assessing costs, benefits, and risks in chronic disease: taking the long view. Biol Blood Marrow Transplant. 2015; 21(7):1149–1150. [DOI] [PubMed] [Google Scholar]