Abstract
Purpose
In patients with advanced cancers, tumor genomic profiling (TGP) can reveal secondary germline findings (SGFs) with regard to inherited disease risks. This study examined the process by which patients with advanced cancers would decide about whether to learn these SGFs and their preferences about specific challenging decision scenarios, including whether patients should be required to receive SGFs and whether SGFs should be returned to the family after a patient’s death.
Patients and Methods
We conducted qualitative semistructured interviews with 40 patients with advanced breast, bladder, colorectal, or lung cancer who had undergone TGP. Data were collected on participants’ perspectives about the hypothetical decision to learn their SGFs, including their anticipated approach to the decision-making process, and their preferences about challenging decision scenarios. Data were evaluated by thematic content analysis.
Results
We identified themes with regard to participants’ preferred degree of decisional autonomy, perceived vital role of doctors, information needs, and anticipated process of deliberation. Although participants reported that this decision was ultimately their own, many wanted input from family and trusted others. Oncologists were expected to provide decision guidance and key clarifying information. Most participants stated that patients should be able to make a choice about receiving actionable SGFs, and a majority stated that SGFs should be available to family after a patient’s death.
Conclusion
These results provide insight into SGF decision-making processes of patients with advanced cancers, which can allow clinicians to provide patients with optimal decision support in this context. Patients with advanced cancers have specific information needs and decision-making preferences that educational and communication interventions should address to ensure that patients make informed choices about learning SGFs.
INTRODUCTION
Tumor genomic profiling (TGP) is revolutionizing cancer care. TGP involves the sequencing of somatic DNA to identify genetic variants indicative of tumor susceptibility to targeted therapeutics. TGP also can identify germline variants that indicate inherited disease risks detected either in the somatic DNA or through germline DNA directly sequenced for comparison with the somatic sequence. These germline variants are considered secondary findings when actively sought by researchers or clinicians (or incidental findings when not) because they arise outside the original purpose of TGP.1,2 Secondary germline findings (SGFs) that indicate risks for various health conditions are likely to be detected in a sizable minority of patients who receive TGP; for example, presumed pathogenic germline variants have been observed in 15.7% of patients who receive TGP at our institution.3
Current American College of Medical Genetics and Genomics recommendations state that individuals who undergo clinical genomic sequencing should be allowed to opt out of receiving SGFs.4 This recommendation plus the increasing adoption of TGP in clinical care ensure that many patients with cancer will be confronted with the decision about whether to learn their SGFs. This decision is likely to be challenging, particularly for patients with advanced cancers who are currently the primary users of TGP (because of its utility for identifying eligibility for clinical trials of novel therapeutics5,6). These individuals must choose whether to learn information about their future disease risks and potential shared familial risks while facing a poor prognosis and the psychosocial challenges of a terminal diagnosis.7 Although patients with varying stages of cancer have reported interest in receiving such information from TGP in real8 and hypothetical9-11 settings, how patients decide whether to learn SGFs is unclear. Understanding the decision-making processes of patients with advanced cancers would allow clinicians to anticipate patient informational and decision support needs in this context.
The current study describes processes by which patients with advanced cancers decide whether to learn SGFs that arise from TGP. We analyzed qualitative data collected through an investigation of attitudes about SGFs among patients who received TGP at our institution.12 These patients were informed about the possible incidental discovery of germline variants during TGP consent conducted by their primary medical oncologists; however, because our institution did not routinely conduct secondary analyses at the time of this study, none of the patients had made a definitive decision about learning their SGFs. We examined patients’ perspectives with regard to factors influential to their hypothetical decision about learning SGFs and preferences about their role in this decision-making process. We also assessed preferences with regard to specific challenging decision scenarios, including whether patients should be required to receive SGFs and whether SGFs should be returned to the family after a patient’s death.
PATIENTS AND METHODS
Study methods are described in detail elsewhere.12 In brief, we recruited 40 adults with advanced breast, bladder, colorectal, or lung cancer who had undergone TGP with an institutional somatic sequencing panel (MSK-IMPACT [Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Targets]13,14). The Memorial Sloan Kettering Cancer Center institutional review board approved this study.
Individual semistructured interviews15-18 were conducted with participants in person or by telephone on the basis of participant preference. All participants provided informed consent before the interview. Interviews lasted approximately 45 minutes and were audio recorded and transcribed. Demographic data were collected in the interview and abstracted from medical records. Participants received $25 for their contribution.
Transcripts were analyzed through thematic content analysis, an inductive qualitative data analysis method that identifies recurring conceptual patterns directly from the data through intensive reading, coding, and interpretation.16,17,19-21 We used four coders to achieve analyst triangulation22 and iterative rounds of consensus analysis to ensure trustworthiness of the findings.23 ATLAS.ti was used to facilitate analysis.24 We selected illustrative participant quotes from the interviews to support our findings and computed descriptive statistics for demographic data.
RESULTS
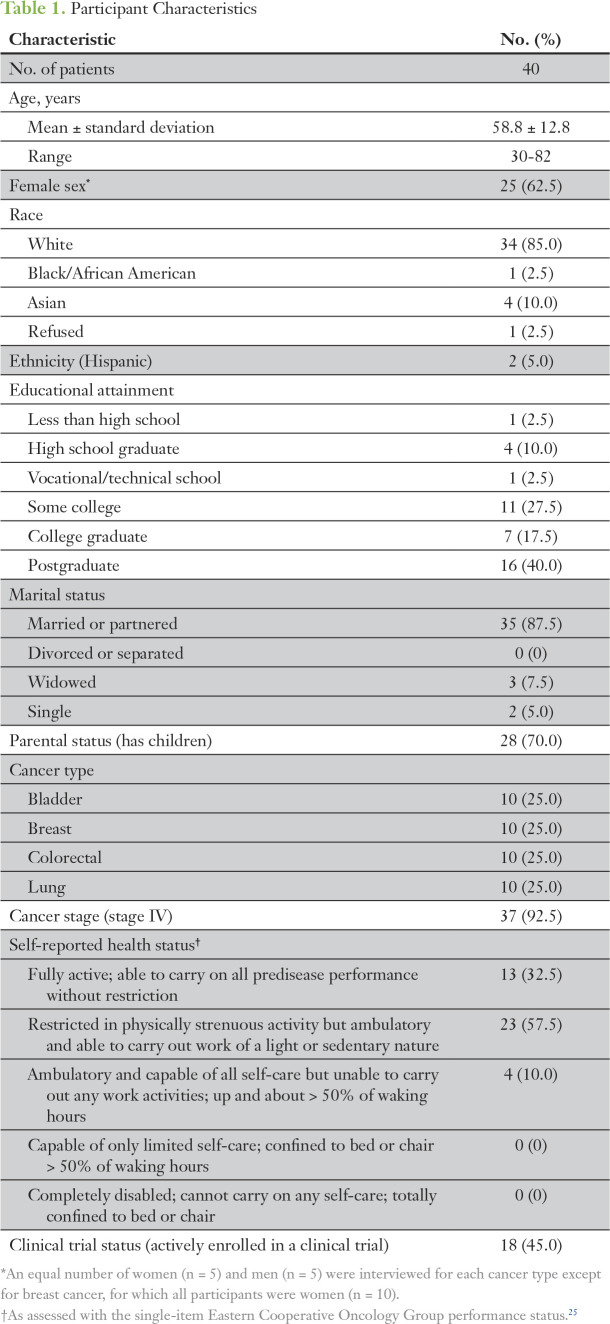
As listed in Table 1, study participants predominantly had stage IV cancer (92.5%); were white (85%), college graduates (57.5%), married/partnered (87.5%); and had at least one child (70%). Participants described how they would approach the decision if their doctor were to present the option of learning SGFs. We categorized participant responses into four key themes and relevant subthemes (indicated by italicized text); illustrative quotes appear in Table 2.
Table 1.
Participant Characteristics
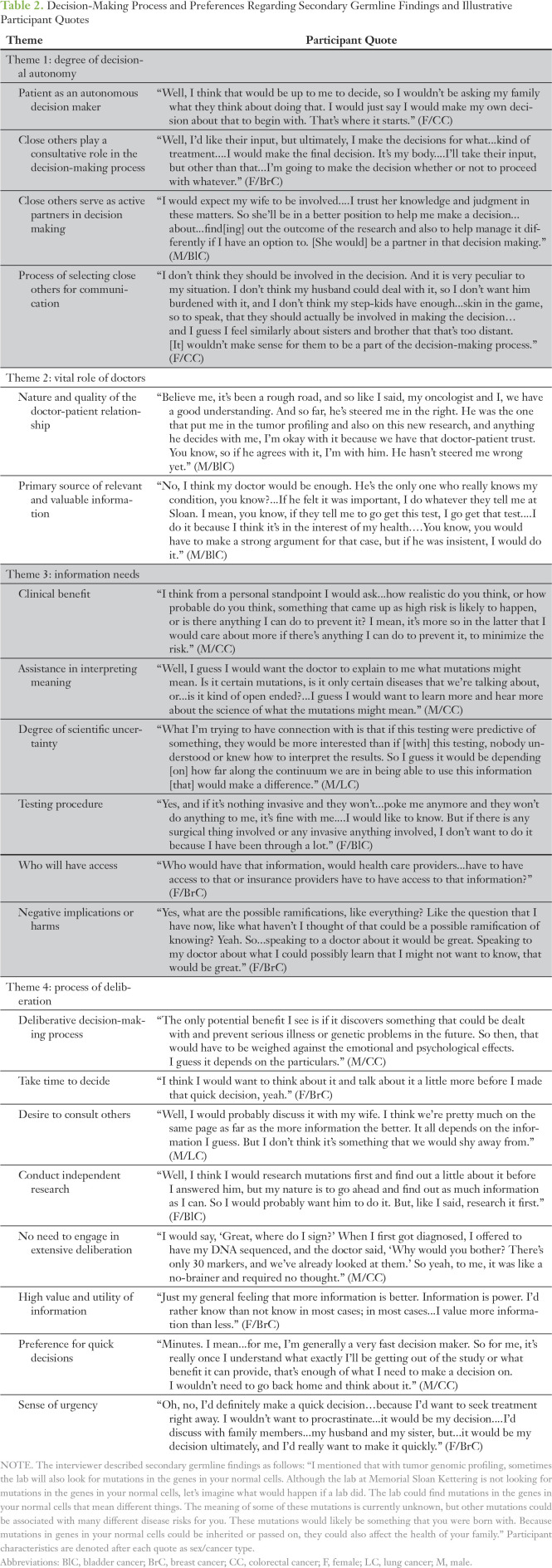
Table 2.
Decision-Making Process and Preferences Regarding Secondary Germline Findings and Illustrative Participant Quotes
Theme 1: Degree of Decisional Autonomy
As participants considered how they would decide whether to learn SGFs, a spectrum emerged with regard to participants’ preferred degree of decisional control and autonomy from close others. The close others that participants referred to primarily were significant others, close biologic family members (eg, siblings, children), and occasionally friends. One group of participants expressed a preference for the patient as an autonomous decision maker. These participants reported that they would prefer to make the SGFs decision on their own and neither needed nor desired input from others. Influential factors for this perspective included a view that the decision was “my choice” because it involved highly personal information fundamentally related to “my body” and a desire to avoid burdening others, particularly family, with potentially distressing information.
A second group of participants preferred that close others play a consultative role in the decision-making process. These participants anticipated communicating with close others about the option to learn SGFs and would consider their advice and opinions but would ultimately make the final decision on their own. Some in this group noted that their family’s views were highly valued but would not be determinants in their decision making.
Finally, a smaller group preferred that close others serve as active partners in decision making. These participants wanted their close others, particularly spouses/partners, to engage as full collaborators in the SGFs decision. Participants noted that as with other important life decisions, their spouses/partners naturally would be involved in this process. Others explained that their family members should be actively involved in this decision because SGFs may have direct health-related implications for them.
Participants who anticipated the involvement of others in their decision making also described their process of selecting close others for communication about the option of learning SGFs. Many participants would seek the perspectives of individuals (eg, siblings, children) who possess medical or scientific expertise. Participants also considered the intimacy of the relationship as well as the individual’s level of involvement in their overall health care. Finally, several participants deemed important the ability or appropriateness of the individual to participate in a discussion about this issue, which could depend on the individual’s age, cognitive ability, or capacity to cope emotionally with learning negative or upsetting information.
Theme 2: Vital Role of Doctors
Participants perceived their doctors (ie, oncologists) as a vital influence on their decision making. Several participants indicated that they would deeply value speaking with their doctor about the prospect of learning SGFs. The importance placed on this consultation and the doctor’s personal opinion was partly a result of the nature and quality of the doctor-patient relationship. For example, several participants reported great trust in their doctors on the basis of a foundation of past experiences and certainty that their doctors will act in their best interests. Their decision to learn SGFs was contextualized within an established, trusting relational dynamic; consequently, these participants indicated that they would be strongly inclined to learn SGFs if their doctor offered. Similarly, a few participants described how they generally feel comfortable with discussing important issues with their doctor. Doctors also were seen as experts who would serve as the primary source of relevant and valuable information necessary for the decision. Several participants anticipated that their doctors would possess expertise with regard to a range of issues relevant to SGFs and thus could help them to acquire all essential information.
Theme 3: Information Needs
Participants described a typology of information that they would require to make an educated decision about learning SGFs, including an explanation of whether SGFs would provide a clinical benefit to the patient, his or her family, or other cancer patients and whether these benefits would outweigh possible harms; assistance in interpreting the meaning of SGFs, such as the degree of certainty of the results and meaning of specific mutations; degree of scientific uncertainty of SGFs and confidence in their applicability to health decisions; description of the testing procedure in terms of the invasiveness of sample acquisition; information about who will have access to the findings (eg, insurers, health care providers); and negative implications or harms of learning SGFs for the patient and family, including unanticipated consequences. Many participants stated that they would ask questions about these issues to feel adequately informed, yet a minority doubted that they would have any specific questions if presented with this decision primarily because of placing a high innate value on SGFs.
Theme 4: Process of Deliberation
Two preferences emerged among participants with regard to the necessity to engage in an extensive decision-making process. A majority anticipated a deliberative decision-making process characterized by weighing potential benefits against harms to determine their interest in learning SGFs. (A detailed description of these perceived benefits and harms is provided elsewhere.12) Participants described procedural aspects of their deliberation and expressed a preference to take time to decide, during which they would consider the option on their own and seek out information about the value of SGFs. These participants also expressed a desire to consult others for their perspectives, including family, friends, and health care providers. Furthermore, a few participants expressed a preference to conduct independent research to learn more about receiving SGFs and the meaning of potential mutations.
A minority of the sample articulated no need to engage in an extensive deliberation to determine their interest in SGFs. These participants reported that there was virtually no decision to make because they were already certain of their interest. Several factors informed this perspective. First, these individuals perceived a high value and utility of information, including knowledge in general and knowledge related to their present or future health. Second, many expressed a characteristic preference for quick decisions; thus, they would immediately respond to a doctor’s offer to learn SGFs without further contemplation. Finally, some described a sense of urgency about learning SGFs and stated the necessity to gain and act upon this information quickly to benefit their current health directly.
Preferences With Regard to Decision Scenarios
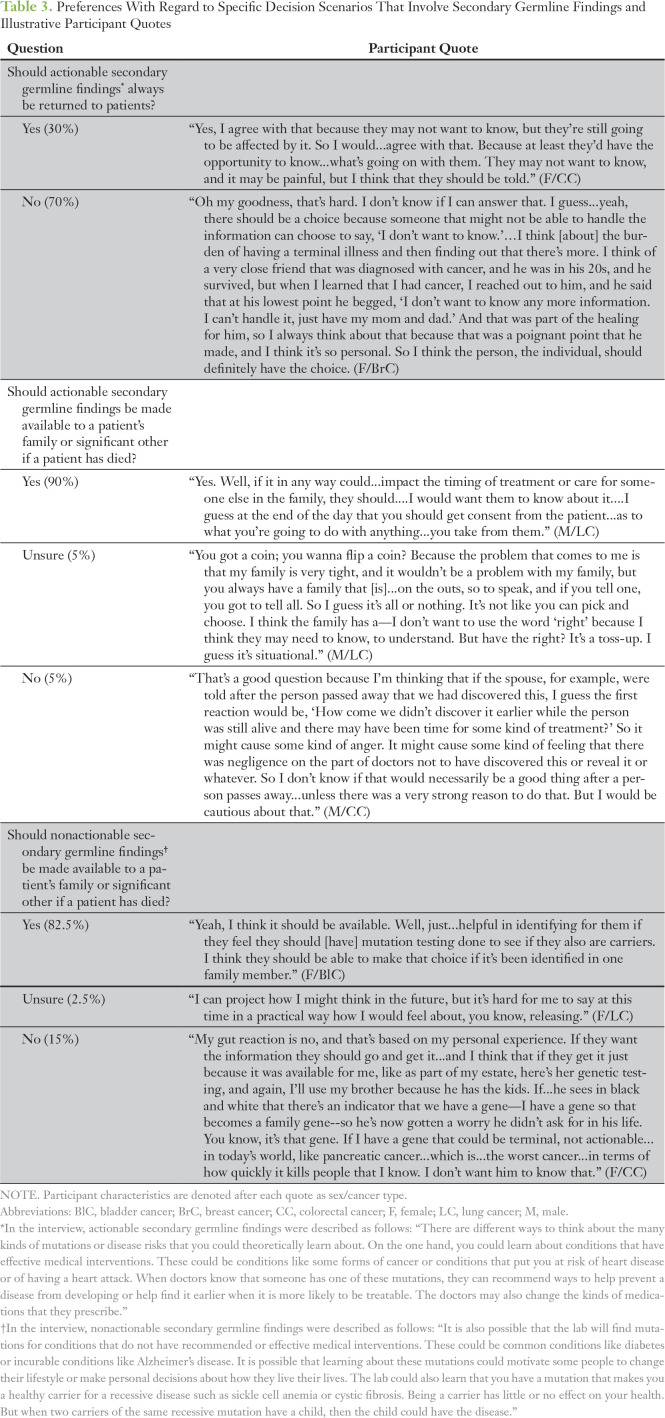
During the interview, participants were presented with challenging scenarios and asked to describe their preferences for how clinicians should handle these situations. Participants’ opinions were quantified and are listed with illustrative quotes in Table 3. First, in response to the debate about the disclosure of SGFs,26-29 we asked participants whether findings about diseases that have effective medical interventions or medication adverse effects (ie, actionable SGFs) should always be returned to patients. Most participants (28 [70%] of 40) stated that patients should be able to choose whether to receive this information, whereas a minority (12 [30%] of 40) stated that such information should always be disclosed to patients.
Table 3.
Preferences With Regard to Specific Decision Scenarios That Involve Secondary Germline Findings and Illustrative Participant Quotes
Participants also were asked to decide whether they believed that if actionable SGFs were detected after a patient’s death, then these findings should be made available to a patient’s family or significant other. The majority (36 [90%] of 40) reported that such information should be made available after a patient’s death. This perspective was motivated predominantly by perceived family health benefits. A subset of participants (16 [69.5%] of 23) also expressed a belief that patients should be required to provide consent for this disclosure before their death, such as at the time of agreeing to TGP, whereas fewer (seven [30.5%] of 23) deemed patient consent unnecessary. Only a few participants were unsure about whether actionable SGFs should be available to a patient’s family after death (two [5%] of 40) or stated that such information should not be made available (two [5%] of 40). Preferences against disclosure were due to concerns about negative emotional implications of such information for families.
Participants were similarly asked to decide whether they believed that SGFs about diseases without effective medical interventions or that indicate one is a healthy carrier for recessive diseases (ie, nonactionable SGFs) should be made available to a patient’s family after a patient’s death. Again, a majority stated that such information should be made available (33 [82.5%] of 40) largely because of the potential for family health benefits. Most participants who provided an opinion regarding consent reported that patients should be required to consent to the disclosure of this information to their families (11 [92%] of 12). Fewer (six [15%] of 40) stated that nonactionable SGFs should not be made available to family after a patient’s death because of concerns about negative emotional reactions and the limited ability to intervene with such diseases. One participant (2.5%) was unsure. Finally, when comparing the preferences of participants with regard to the return of actionable versus nonactionable SGFs to family after a patient’s death, 22.5% (nine of 40) were discordant in their preferences across these scenarios.
DISCUSSION
This study clarifies the decision-making processes of patients with advanced cancer with regard to SGFs from TGP. Given the personal nature of genetic risk information, participants viewed the decision to learn SGFs as ultimately their own. However, consistent with other medical decision contexts,30-34 variability existed in participants’ preferences for involving others, including spouses/partners, children, and siblings, in their decision making. Consequently, when presenting the option of learning SGFs, clinicians must allow patients to solicit input from close others and help to navigate challenges inherent in decision making with multiple individuals.35 Additional research should investigate how such interpersonal influences shape, hinder, or support patients’ SGFs decision making.
Participants anticipated that their doctors (ie, oncologists) would be the primary source of guidance for this decision. They placed great trust in their oncologists and acknowledged the influence of their expertise and personal opinions on their decision making. Participants also anticipated that they would have extensive questions about the benefits, harms, interpretation, and process of obtaining SGFs and would expect their oncologists to provide answers. However, research has demonstrated that this may not be feasible because many oncologists have limited experience with germline testing and express concerns about their ability to address challenges presented by SGFs.8 Several approaches may help to bridge this gap between patient expectations and oncologist preparedness, including oncologist-targeted communication training, novel patient education materials, and referral to genetic counselors to address patient questions. Future research should evaluate which of these approaches are most effective at achieving the delicate balance between meeting patients’ information needs and practical challenges of cancer care delivery (eg, time demands, workforce limitations). Research should also examine how various models of patient education (eg, oncologist led, genetics professional led) influence patient SGFs decisions and how patients weigh the opinions of various care providers in this context.
Many participants anticipated a preference to undergo a thoughtful deliberation about the prospect of learning SGFs. Conversely, a minority believed that they would make an immediate decision guided by their personal values and beliefs. Research suggests that the adoption of a more intuitive decision-making approach can yield similar outcomes to deliberative decision making,36 although both approaches have benefits and drawbacks.37 Of note, some participants’ preferences for a quick decision were motivated by beliefs that SGFs would provide clinical utility or necessitate urgent action for them to reap health benefits. These expectations may be inaccurate for many patients with advanced cancers because the information revealed will not change their prognosis or clinical management. Accordingly, clinicians must ensure that all patients, including those immediately enthusiastic or accepting of SGFs, accurately understand the limitations of this risk information.
These results also provide insight into preferences of patients with advanced cancers with regard to challenging scenarios that involve the return of SGFs. Consistent with American College of Medical Genetics and Genomics recommendations4 and expert opinion,38 most participants stated that patients should choose whether they want to receive actionable SGFs from TGP. Participants acknowledged that some individuals may not want this information and that clinicians should honor such preferences. In addition, participants expressed diverse opinions about the management of SGFs after a patient’s death. Participants generally were more supportive of the return of actionable SGFs to family after a patient’s death than nonactionable SGFs; although, in both instances, a majority supported the sharing of this information with family largely because of perceived family health benefits. The observation that 22.5% of participants held discordant views about the appropriateness of sharing actionable versus nonactionable SGFs with family after a patient’s death highlights the importance of distinguishing the various categories of risk information that can be revealed through TGP39 when educating patients and eliciting their preferences.8,40 Participants’ general approval of obtaining patient consent at the time of TGP to ensure preference-concordant management of SGFs after death reinforces current ethical recommendations.41
This study has notable strengths. The qualitative design enabled an in-depth analysis of the decision-making preferences of a sample of patients with advanced cancers diverse in diagnosis, sex, and health status. However, the majority was well-educated (85% reporting at least some college); decision-making preferences and processes of these individuals may differ from those with less formal education. Additional limitations are that this sample was racially and ethnically homogenous, recruited from one institution, and assessed at a time when the decision about learning SGFs was hypothetical in nature; thus, the findings may not be generalizable to the broader population of patients with advanced cancers treated in other care settings who are navigating this decision in real time. Future work should examine decision-making processes of more-diverse patients and evaluate how various approaches to presenting patients with the option of learning SGFs (eg, education and consent led by oncologists v genetics professionals, presentation during a medical oncology visit v a separate visit) ultimately influence patient decision making.
In conclusion, this study provides important insight into how patients with advanced cancers approach the decision to learn SGFs and informs how developing precision oncology programs can manage the reporting of germline variants from TGP (Table 4). A paternalistic model of care in which patients lack a choice about receiving SGFs is inconsistent with patient preferences. Precision oncology programs instead should establish models that empower patients to make informed decisions about whether to learn SGFs. Patient preferences for involvement in this decision can be accommodated in both opt-in and opt-out models, although these models likely will differ in the resources necessary to support patient deliberation and in the number of patients who select to receive SGFs.42,43 Although most patients likely will want to retain decisional control in this context, some will desire time and space to include family and other influential figures in their decision making. Patients with advanced cancers likely have specific information needs, and possible misperceptions, about the implications and utility of SGFs. Oncologists will be the primary resource to which patients turn for clarity and guidance and must be prepared to meet these demands. Whereas the TGP decision may be time sensitive because of treatment implications, patients may benefit from efforts to ensure that the decision to receive SGFs can be pursued on a different temporal schedule that aligns with their preferences for information seeking and deliberation. Thus, educational and communication interventions targeted to patients, their families, and oncologists are needed to provide clear information that contextualizes the meaning of SGFs in the advanced cancer setting, assist the weighing of benefits and harms, and allow patients to explore and express their preferences about specific categories of SGFs and management of this information in the event of their death. Such interventions would enable the delivery of optimal decision support that matches patients’ needs and preferences in this era of precision cancer care.
Table 4.
Recommendations for Developing Precision Oncology Programs With Regard to How to Manage and Support Patient Decision Making About Secondary Germline Findings From Tumor Genomic Profiling
ACKNOWLEDGMENT
We are extremely grateful to all participating patients.
Footnotes
Supported by the Memorial Sloan Kettering Cancer Center Survivorship, Outcomes, and Risk Developmental Funds Award (J.G.H. and M.E.R.), National Cancer Institute Grant No. P30 CA008748, the Robert and Kate Niehaus Center for Inherited Cancer Genomics, and the Andrew Sabine Family Foundation. J.G.H. also was supported by a Mentored Research Scholar Grant in Applied and Clinical Research No. MRSG-16-020-01-CPPB from the American Cancer Society.
Presented at the Society of Behavioral Medicine 37th Annual Meeting & Scientific Sessions, Washington, DC, March 30-April 2, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: Jada G. Hamilton, Jennifer L. Hay, Kenneth Offit, Mark E. Robson
Administrative support: Jada G. Hamilton
Collection and assembly of data: Jada G. Hamilton, Elyse Shuk
Data analysis and interpretation: Jada G. Hamilton, Elyse Shuk, Margaux Genoff Garzon, Vivian M. Rodríguez, Joy Westerman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Jada G. Hamilton
No relationship to disclose
Elyse Shuk
No relationship to disclose
Margaux Genoff Garzon
No relationship to disclose
Vivian M. Rodríguez
No relationship to disclose
Joy Westerman
No relationship to disclose
Jennifer L. Hay
No relationship to disclose
Kenneth Offit
No relationship to disclose
Mark E. Robson
Honoraria: AstraZeneca
Consulting or Advisory Role: McKesson, AstraZeneca
Research Funding: AstraZeneca (Inst), AbbVie (Inst), BioMarin (Inst), Medivation (Inst)
Travel, Accommodations, Expenses: AstraZeneca
REFERENCES
- 1.Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: Analysis and recommendations. J Law Med Ethics. 2008;36:219–248. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Presidential Commission for the Study of Bioethical Issues Anticipate and communicate: Ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts (December 2013 report of the Presidential Commission for the Study of Bioethical Issues) doi: 10.1093/aje/kwu217. https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf [DOI] [PubMed] [Google Scholar]
- 3.Schrader KA, Cheng DT, Joseph V, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–111. doi: 10.1001/jamaoncol.2015.5208. [Erratum: JAMA Oncol 2:279, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACMG Board of Directors ACMG policy statement: Updated recommendations regarding analysis and reporting of secondary findings in clinical genome-scale sequencing. Genet Med. 2015;17:68–69. doi: 10.1038/gim.2014.151. [DOI] [PubMed] [Google Scholar]
- 5.Tripathy D, Harnden K, Blackwell K, et al. Next generation sequencing and tumor mutation profiling: Are we ready for routine use in the oncology clinic? BMC Med. 2014;12:140. doi: 10.1186/s12916-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons DW, Roy A, Plon SE, et al. Clinical tumor sequencing: An incidental casualty of the American College of Medical Genetics and Genomics recommendations for reporting of incidental findings. J Clin Oncol. 2014;32:2203–2205. doi: 10.1200/JCO.2013.54.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor-Ford M. Clinical considerations for working with patients with advanced cancer. J Clin Psychol Med Settings. 2014;21:201–213. doi: 10.1007/s10880-014-9398-z. [DOI] [PubMed] [Google Scholar]
- 8.Gray SW, Park ER, Najita J, et al. Oncologists’ and cancer patients’ views on whole-exome sequencing and incidental findings: Results from the CanSeq Study. Genet Med. 2016;18:1011–1019. doi: 10.1038/gim.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray SW, Hicks-Courant K, Lathan CS, et al. Attitudes of patients with cancer about personalized medicine and somatic genetic testing. J Oncol Pract. 2012;8:329–335. doi: 10.1200/JOP.2012.000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yushak ML, Han G, Bouberhan S, et al. Patient preferences regarding incidental genomic findings discovered during tumor profiling. Cancer. 2016;122:1588–1597. doi: 10.1002/cncr.29951. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf RA, Rogith D, Hovick SR, et al. Attitudes toward molecular testing for personalized cancer therapy. Cancer. 2015;121:243–250. doi: 10.1002/cncr.28966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton JG, Shuk E, Genoff MC, et al. Interest and attitudes of patients with advanced cancer with regard to secondary germline findings from tumor genomic profiling. J Oncol Pract. 2017;13:e590–e601. doi: 10.1200/JOP.2016.020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won HH, Scott SN, Brannon AR, et al. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp. 2013;18:e50710. doi: 10.3791/50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkman S, Kvale S. InterViews: Learning the Craft of Qualitative Research Interviewing. (ed 3) Thousand Oaks, CA: Sage; 2015. [Google Scholar]
- 16.Green J, Thorogood N. Qualitative Methods for Health Research. (ed 3) London, UK: Sage; 2014. [Google Scholar]
- 17.Patton MQ. Qualitative Evaluation and Research Methods. (ed 3) Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 18.Rubin HJ, Rubin IS. Qualitative Interviewing: The Art of Hearing Data. (ed 3) Thousand Oaks, CA: Sage; 2012. [Google Scholar]
- 19.Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. (ed 5) Thousand Oaks, CA: Sage; 2009. [Google Scholar]
- 20.Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. Thousand Oaks, CA: Sage; 2014. [Google Scholar]
- 21.Saldana J. The Coding Manual for Qualitative Researchers. (ed 2) London, UK: Sage; 2013. [Google Scholar]
- 22.Denzin NK. The Research Act: A Theoretical Introduction to Sociological Methods. (ed 5) New Brunswick, NJ: Aldine Transaction; 2009. [Google Scholar]
- 23.Morse JM, Barrett M, Mayan M, et al. Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Methods. 2002;1:1–19. [Google Scholar]
- 24.Friese S. Qualitative Data Analysis With ATLAS.ti. (ed 2) London, UK: Sage; 2014. [Google Scholar]
- 25.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 26.Burke W, Antommaria AH, Bennett R, et al. Recommendations for returning genomic incidental findings? We need to talk! Genet Med. 2013;15:854–859. doi: 10.1038/gim.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross LF, Rothstein MA, Clayton EW. Mandatory extended searches in all genome sequencing: “Incidental findings,” patient autonomy, and shared decision making. JAMA. 2013;310:367–368. doi: 10.1001/jama.2013.41700. [DOI] [PubMed] [Google Scholar]
- 28.Green RC, Lupski JR, Biesecker LG. Reporting genomic sequencing results to ordering clinicians: Incidental, but not exceptional. JAMA. 2013;310:365–366. doi: 10.1001/jama.2013.41703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klitzman R, Appelbaum PS, Chung W. Return of secondary genomic findings vs patient autonomy: Implications for medical care. JAMA. 2013;310:369–370. doi: 10.1001/jama.2013.41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini C, Jandorf L, Goldsmith RE, et al. Interpersonal influences on patients’ surgical decision making: The role of close others. J Behav Med. 2011;34:396–407. doi: 10.1007/s10865-011-9323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coyne JC, Anderson KK. Marital status, marital satisfaction, and support processes among women at high risk for breast cancer. J Fam Psychol. 1999;13:629–641. [Google Scholar]
- 32.Davison BJ, Oliffe JL, Pickles T, et al. Factors influencing men undertaking active surveillance for the management of low-risk prostate cancer. Oncol Nurs Forum. 2009;36:89–96. doi: 10.1188/09.ONF.89-96. [DOI] [PubMed] [Google Scholar]
- 33.Hallowell N, Ardern-Jones A, Eeles R, et al. Men’s decision-making about predictive BRCA1/2 testing: The role of family. J Genet Couns. 2005;14:207–217. doi: 10.1007/s10897-005-0384-3. [DOI] [PubMed] [Google Scholar]
- 34.Stiggelbout AM, Jansen SJ, Otten W, et al. How important is the opinion of significant others to cancer patients’ adjuvant chemotherapy decision-making? Support Care Cancer. 2007;15:319–325. doi: 10.1007/s00520-006-0149-z. [DOI] [PubMed] [Google Scholar]
- 35.Laidsaar-Powell RC, Butow PN, Bu S, et al. Physician-patient-companion communication and decision-making: A systematic review of triadic medical consultations. Patient Educ Couns. 2013;91:3–13. doi: 10.1016/j.pec.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Kruglanski AW, Gigerenzer G. Intuitive and deliberate judgments are based on common principles. Psychol Rev. 2011;118:97–109. doi: 10.1037/a0020762. [DOI] [PubMed] [Google Scholar]
- 37.de Vries M, Fagerlin A, Witteman HO, et al. Combining deliberation and intuition in patient decision support. Patient Educ Couns. 2013;91:154–160. doi: 10.1016/j.pec.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Scheuner MT, Peredo J, Benkendorf J, et al. Reporting genomic secondary findings: ACMG members weigh in. Genet Med. 2015;17:27–35. doi: 10.1038/gim.2014.165. [DOI] [PubMed] [Google Scholar]
- 39.Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: Meeting the challenge one bin at a time. Genet Med. 2011;13:499–504. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- 40.Kaphingst KA, Ivanovich J, Biesecker BB, et al. Preferences for return of incidental findings from genome sequencing among women diagnosed with breast cancer at a young age. Clin Genet. 2016;89:378–384. doi: 10.1111/cge.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf SM, Branum R, Koenig BA, et al. Returning a research participant’s genomic results to relatives: Analysis and recommendations. J Law Med Ethics. 2015;43:440–463. doi: 10.1111/jlme.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357:1340–1344. doi: 10.1056/NEJMsb071595. [DOI] [PubMed] [Google Scholar]
- 43.Ojerholm E, Halpern SD, Bekelman JE. Default options: Opportunities to improve quality and value in oncology. J Clin Oncol. 2016;34:1844–1847. doi: 10.1200/JCO.2015.64.8741. [DOI] [PubMed] [Google Scholar]