Abstract
Circadian rhythms and exercise physiology are intimately linked, but the symbiosis of this relationship has yet to be fully unraveled. Exercise exerts numerous health benefits from the organelle to the organism. Proper circadian function is also emerging as a prerequisite for maintaining health. The positive effects of exercise on health may be partially mediated by an exercise-induced change in tissue molecular clocks and/or the outcomes of exercise may be modified depending on when exercise is performed. This review provides a brief overview of circadian biology and the influence of exercise on the molecular clock, with an emphasis on skeletal muscle. Additionally, we provide considerations for future investigations seeking to unravel the mechanistic interactions of exercise and the molecular clock.
Keywords: Circadian Rhythms, Exercise Physiology, Time-of-day
INTRODUCTION
The positive health effects of acute and chronic exercise on tissue-specific and organism-wide function have been a primary interest for nearly a century. Overall health improvements from exercise include reduced risk for cardiometabolic, neurodegenerative, and metastatic diseases, thereby reducing mortality risk [1]. The mechanisms through which exercise training confers health benefits are well-studied with selected tissues but incompletely understood at the system level [2,3]. To further address the relationship between exercise and health, the Molecular Transducers of Physical Activity Consortium (https://www.motrpac.org/ ) will provide data-rich resources to expand the breadth of existing knowledge in exercise physiology and health. While it is well-understood that the physiological responses to exercise are complex, the emergence of circadian rhythm biology as a parameter of human physiology has added another fundamental layer of complexity into understanding exercise responses.
The purpose of this review is to integrate the concepts of circadian biology and the molecular clock mechanism with growing research implementing different circadian concepts with exercise. We will briefly overview the molecular clock and its role in maintaining homeostasis. We will then highlight recent work integrating circadian concepts into exercise physiology two main themes. The first theme will review studies on how the timing of exercise impacts exercise outcomes and the second theme will review research that has contributed to our understanding of exercise as a time cue for skeletal muscle. Lastly, we will provide considerations for experimental design, and suggest intriguing future directions for the nascent field of exercise chronophysiology.
THE MAMMALIAN MOLECULAR CLOCK
Core Molecular Clock and the Clock-Controlled Transcriptional Program
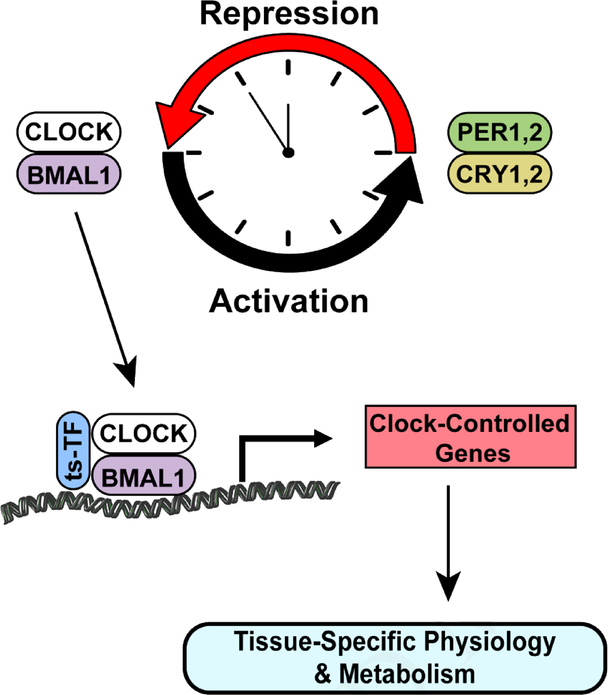
It is now well established that the primary mechanism underlying circadian rhythms is the molecular clock. The elucidation of this mechanism was recently recognized with a Nobel Prize in Physiology or Medicine in 2017. The molecular clock is a self-sustaining transcriptional-translational feedback loop (TTFL) that exists in virtually every cell of the body and functions to direct a daily program of gene transcription and a more in-depth review of the molecular clock can be found here [4]. The primary genes that comprise the molecular clock are ubiquitous in all cells and include Clock and Bmal1 which comprise the positive limb and induce the expression of Period1 (Per)1, Per2 and Cryptochrome (Cry)1, Cry2. The PERs and CRYs are part of the negative limb that function to repress the transcriptional activity of CLOCK and BMAL1, closing the approximate 24h loop. A simplified cartoon of the molecular clock is provided in Figure 1.
Figure 1. Conceptual cartoon depicting links between the molecular clock and tissue-specific clock controlled gene expression.
Bmal1 and Clock direct the transcription of clock-controlled genes including Periods and Cryptochromes, which in turn form a heterodimer to repress activity of BMAL1 and CLOCK and maintain an approximate 24h rhythm of gene expression. In coordination with tissue-specific transcription factor(s) (ts-TF), BMAL1 and CLOCK direct the transcription of clock-controlled genes. This oscillating gene expression output from the molecular clock directs a daily program of transcription that impacts cellular physiology and metabolism.
In addition to its timekeeping function, the molecular clock regulates a daily transcriptional program. It is estimated that BMAL1 and CLOCK directly modulate expression of over 4000 genes and these genes are called clock-controlled genes (CCGs) [5]. In contrast to the core molecular clock factors, CCGs are expressed in a tissue-specific manner with very limited overlap in expression across all the tissues in the body [6]. Lineage-specific transcriptional regulators (e.g., MYOD1 in skeletal muscle) are likely key factors linking the ubiquitous clock with the tissue-specific CCG transcriptional profile [7,8]. The importance of this daily transcriptional program is highlighted by the recognition that CCGs include transcriptional regulators and rate-limiting enzymes important for cell-specific homeostasis [9,10]. Thus, the core molecular clock machinery through the tissue-specific CCG program play an important role in maintaining cell and tissue health.
Phase Setting of the Molecular Clock
As noted above the molecular clock within each cell is defined as a self-sustaining 24h TTFL that functions in the absence of signals from the environment. However, the phase of clock mechanism, as defined by the time of the peak, is sensitive to cues from the environment and can shift in response to the timing of the cues [11]. The most well-established environmental time cue is light. Light exposure during the dark phase modifies the phase of the molecular clock mechanism in the suprachiasmatic nuclei (SCN) in the brain, known as the central clock [12]. Historically, the phase of the molecular clocks across all the peripheral tissues were believed to be under direct control of the SCN through neurohumoral factors. However, studies in the last 10 years have demonstrated that time of feeding and time of physical activity/exercise are bone fide time cues that can modify the phase of the clock mechanisms in many peripheral tissues, and these changes occur independent of any phase change in the central clock [13–15]. These results are exciting as they have opened new concepts for using circadian or time of day principles when considering application of exercise interventions for the purpose of health or performance outcomes.
Circadian Rhythms in Rodents and Humans
One of the common questions within the circadian field is the translation of research findings from nocturnal mice to diurnal human beings. There has been significant work at the behavioral, physiological, and molecular levels to establish the commonality of the circadian system between these mammalian species [12,16,17]. Comparative analysis has demonstrated that many daily physiological rhythms in nocturnal species are at an opposite phase to humans [12,16,17]. For example, daily core body temperature, heart rate and blood pressure peak in the light or active phase and is lowest at dark or rest phase in humans. In contrast, these physiological variables peak in the dark or active phase and are lowest during the light or rest periods in rodents. The common principle here is that temperature, heart rate and blood pressure peak in the active phase of the mammal independent of lighting. This principle holds true with the molecular clock expression in rodents vs. humans [11,18]. Taking data from a time series of muscle biopsies from humans and comparing clock gene expression to muscle from rats and mice confirm that the core clock factors are commonly regulated across the active and inactive phases [11,18].
EXERCISE AND THE MOLECULAR CLOCK: OUTCOMES AND TIME CUE
Circadian Timing Affects Exercise Outcomes
Because exercise is a major physiological perturbation, the circadian oscillation of basal physiological rhythms has a direct effect on exercise responses. Several studies in humans and rodents have revealed that variables such as skeletal muscle strength and oxidative capacity demonstrate significant differences over time of day [19–22]. For example, studies have consistently demonstrated increased strength in the later afternoon versus morning [19] while oxidative capacity peaks in the late evening [22]. In addition, basal systemic hormone and metabolite concentrations oscillate over a 24h period, although the impact of these oscillation on exercise are unclear [20,22–25]. It is clear, however, that exercise at different times of day leads to different outcomes [19,25–27]. One recent example was provided by Dalbram and colleagues who reported that exercise in the late active phase of mice reduced the accumulation of body mass during high-fat diet compared to exercise in the early active phase [28]. The effect of circadian timing on an integrative outcome, such as weight gain, is exciting and will provide important considerations for future interventions. Additionally, future studies in both human and rodent interventions must take care to provide transparent reporting of circadian conditions (e.g., light/dark cycles, feeding status), as well as robust time of day sampling rates. Attention to these details are critical to help distinguish intrinsic circadian related changes vs. environmental/behavior effects [29]. We also suggest cautious assessments of experimental circadian controls prior to sweeping conclusions regarding the outcomes of an intervention.
Circadian timing has also been reported to affect exercise outcomes at the molecular level. The molecular responses of muscle to exercise are well-characterized. In particular, the mechanistic target of rapamycin complex 1 (mTORC1) and peroxisome proliferator activated receptor gamma coactivator 1 (PGC1) pathways are widely studied exercise-responsive pathways. Recently, these exercise-stimulated pathways were identified as being downstream of the molecular clock, providing a molecular mechanism through which circadian timing can influence exercise responses [30,31]. For instance, Wu and colleagues reported that PER2 lowers mTORC1 activity [32], and PER2 expression oscillates (peaking at the end of the inactive phase [33]), linking time-of-day to the exercise response. Additionally, morning resistance exercise, but not afternoon exercise in trained individuals lead to the activation of the mTORC1 signaling pathway, as assessed by p70S6K phosphorylation [34]. Despite the influence of circadian timing on hypertrophic signaling following acute resistance exercise, circadian timing of resistance exercise training does not influence skeletal muscle hypertrophy [27,34]. Endurance exercise increases PGC1α, which is a clock-controlled gene in skeletal muscle [35]. Thus, circadian timing may influence the endurance exercise response by modulating the activation PGC1 in skeletal muscle. To our knowledge, no investigations have assessed the impact of circadian timing as a modifier of endurance exercise training responses. However, it is unclear if any human investigation has performed exercise at the onset of the inactive phase (dark), which would closely mirror previous interventions using rodent models [15,36,37].
One approach to test the influence of the circadian clock on exercise outcomes is with genetic models of circadian disruption. In one model of circadian disruption (ClockΔ19), where mice have a 27–28h endogenous period length, mutant mice had a 49% reduction in treadmill exercise duration compared to wild type [38] suggesting that animals with internal clocks out of sync with environmental cues (i.e. misalignment) have reduced exercise capacity. Additionally, in a model of complete circadian disruption, activity levels in mice lacking Bmal1 were severely reduced (~2 fold) compared to wild type animals [39]. Together, these findings suggest that circadian disruption reduces exercise capacity. However, despite reduced exercise capacity in mice with circadian disruption, the animals retain plasticity. Specifically, exercise training restored the exercise capacity of the ClockΔ19 mice, although no studies have examined exercise training in Bmal1 knockout animals. Together, these findings have major therapeutic implications, as circadian disruption has been linked to numerous diseases [24,40,41]. Thus, exercise may reduce mortality through restoring the function of disrupted molecular clocks. Below we highlight the mechanisms through which exercise modulates the molecular clock.
Exercise as a Circadian Time Cue
Several studies have shown that exercise can impact circadian behavior (see [36,42]). For the purpose of this review we will focus on exercise and its role on the molecular clock mechanism. Data from the PER2::LUC [43] circadian reporter mouse, and in vitro assays [44] have revealed that exercise acts as a circadian time cue [15,37,45]. Specifically, four weeks of exercise on a treadmill or running wheel significantly shifted the phase of both the skeletal muscle and lung, but did not influence the phase of the central clock [15]. Exercise restored circadian patterns of activity, heart rate, and body temperature in mice with central circadian disruption [37]. Additionally, studies in humans have revealed that resistance [46,47], and endurance exercise [47] stimulate the expression of core clock genes. These data suggest that exercise serves as a circadian time cue, and changes the phase of the molecular clock, specifically in peripheral tissues. However, the specific mechanisms through which exercise serve as a circadian time cue have not been completely resolved. Below we highlight how the effects of exercise, or exercise-induced signaling on the molecular clock may serve as a circadian time cue.
In addition to core factors that directly repress the core molecular clock (e.g., Pers and Crys), factors external to the clock can regulate the stability, phase, or function of core molecular clock proteins. Emerging evidence suggests that exercise-responsive genes, including AMPK [31,48,49], HIF-1α [50], and PGC1α [51] influence the expression of core molecular clock genes, revealing a potential mechanism through which exercise serves as a circadian time cue. For instance, AMPK activity increases in response to exercise and increased AMPK activity alters the stability of PER and CRY proteins to affects the expression of core molecular clock genes [31,52]. Specifically, AMPK activation results in reduced stability of CRY1, allowing derepression of Bmal1:Clock targets [52]. Additionally, CRY1/2 proteins reduce exercise capacity through PPARδ repression [53], suggesting that AMPK activity directly influences the molecular clock, revealing a mechanism through which exercise acts as a circadian time cue. Exercise also induces HIF-1α expression, which influences the output of the molecular clock through direct binding to core clock gene promoters [50]. Thus, HIF-1α may partially mediate the effects of exercise as a circadian time cue. Another potential mechanism through which exercise may act as a time cue is through mechanical input, as cellular compression has been reported to influence molecular clock oscillation in mammary cells [54]. Considering the mechanical effect of exercise on skeletal muscle [55], mechanical changes may contribute to the effect of exercise on the molecular clock, although the exercise-related regulation of CCG expression is likely multifactorial.
CONCLUDING REMARKS & FUTURE DIRECTIONS
Exercise imparts numerous health benefits, and the mechanisms through which exercise improves health continue to be revealed [1,2]. With this review, we suggest two new considerations: 1) Applying circadian or time of day principles to exercise interventions hold promise for improving the outcomes for exercise for healthy subjects, patient populations as well as elite athletes. 2) The molecular clock with its impact through clock controlled genes maybe one of the mechanisms through which exercise improves cell/tissue health. This is still a new field and there is much to be learned about fundamental molecular clock function across all cell types as well as how these clocks in peripheral tissues sense and respond to exercise. Further, it is our sincere hope that future investigations aimed to examine the interplay between exercise physiology and circadian biology implement rigorous study designs [29] to allow for an in-depth understanding of the interaction of these two exciting fields. While our review highlights the unknowns of circadian exercise biology from the perspective of the skeletal muscle, the intricate system-wide effects of exercise and the global physiological enhancements on health that are associated with exercise timing and training remain unexplored.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL, Kaminsky LA, Cardiorespiratory Fitness and Mortality in Healthy Men and Women., J. Am. Coll. Cardiol 72 (2018) 2283–2292. doi: 10.1016/j.jacc.2018.08.2166. [DOI] [PubMed] [Google Scholar]
- [2].Cartee GD, Hepple RT, Bamman MM, Zierath JR, Exercise Promotes Healthy Aging of Skeletal Muscle., Cell Metab. 23 (2016) 1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gabriel BM, Zierath JR, The Limits of Exercise Physiology: From Performance to Health, Cell Metab. 25 (2017) 1000–1011. doi: 10.1016/j.cmet.2017.04.018. [DOI] [PubMed] [Google Scholar]
- [4].Takahashi JS, Transcriptional architecture of the mammalian circadian clock., Nat. Rev. Genet 18 (2017) 164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS, Transcriptional architecture and chromatin landscape of the core circadian clock in mammals, Science (80−. ). (2012). doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB, A circadian gene expression atlas in mammals: Implications for biology and medicine, Proc. Natl. Acad. Sci 111 (2014) 16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hodge BA, Zhang X, Gutierrez-Monreal MA, Cao Y, Hammers DW, Yao Z, Wolff CA, Du P, Kemler D, Judge AR, Esser KA, MYOD1 functions as a clock amplifier as well as a critical cofactor for downstream circadian gene expression in muscle., Elife. 8 (2019). doi: 10.7554/eLife.43017.* This paper provides a framework to emphasize that there are likely tissue-specific transcriptional complexes that contribute to the expression of clock-controlled genes. Further, clock controlled gene expression is dependent upon when/where Bmal1 binds to the DNA.
- [8].Beytebiere JR, Trott AJ, Greenwell BJ, Osborne CA, Vitet H, Spence J, Yoo S-H, Chen Z, Takahashi JS, Ghaffari N, Menet JS, Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer-enhancer interactions., Genes Dev. 33 (2019) 294–309. doi: 10.1101/gad.322198.118.** The authors present a robust dataset revealing unique transcriptional regulatory elements of the molecular clock. The tissue-specific binding of Bmal1 across the genome provides an exciting launching point for circadian biology, organismal development, and potentially reveals a targetable therapeutic approach.
- [9].Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H, Regulation of clock-controlled genes in mammals, PLoS One. (2009). doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Korenčič A, Košir R, Bordyugov G, Lehmann R, Rozman D, Herzel H, Timing of circadian genes in mammalian tissues, Sci. Rep (2014). doi: 10.1038/srep05782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dibner C, Schibler U, Circadian timing of metabolism in animal models and humans, J. Intern. Med (2015). doi: 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- [12].Challet E, Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals, Endocrinology. (2007). doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- [13].Tahara Y, Shibata S, Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition, Free Radic. Biol. Med 119 (2018) 129–138. doi: 10.1016/j.freeradbiomed.2017.12.026. [DOI] [PubMed] [Google Scholar]
- [14].Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Tahara Y, Shibata S, Forced rather than voluntary exercise entrains peripheral clocks via a corticosterone/noradrenaline increase in PER2::LUC mice, Sci. Rep 6 (2016) 1–15. doi: 10.1038/srep27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wolff G, Esser KA, Scheduled exercise phase shifts the circadian clock in skeletal muscle., Med. Sci. Sports Exerc 44 (2012) 1663–70. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Refinett R, Rhythms of body temperature and temperature selection are out of phase in a diurnal rodent, Octodon degus, Physiol. Behav (1996). doi: 10.1016/S0031-9384(96)00147-3. [DOI] [PubMed] [Google Scholar]
- [17].Kalsbeek A, Verhagen LAW, Schalij I, Foppen E, Saboureau M, Bothorel B, Buijs RM, Pévet P, Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species, Eur. J. Neurosci (2008). doi: 10.1111/j.1460-9568.2008.06057.x. [DOI] [PubMed] [Google Scholar]
- [18].Mohawk JA, Green CB, Takahashi JS, Central and Peripheral Circadian Clocks in Mammals, Annu. Rev. Neurosci 35 (2012) 445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Atkinson G, Reilly T, Circadian variation in sports performance, Sport. Med (1996). doi: 10.2165/00007256-199621040-00005. [DOI] [PubMed] [Google Scholar]
- [20].De Goede P, Wefers J, Brombacher EC, Schrauwen P, Kalsbeek A, Circadian rhythms in mitochondrial respiration, J. Mol. Endocrinol (2018). doi: 10.1530/JME-17-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wens I, Hansen D, Muscle Strength, But Not Muscle Oxidative Capacity, Varies Between the Morning and the Afternoon in Patients with Multiple Sclerosis, Am. J. Phys. Med. Rehabil 96 (2017) 828–830. doi: 10.1097/PHM.0000000000000703. [DOI] [PubMed] [Google Scholar]
- [22].van Moorsel D, Hansen J, Havekes B, Scheer FAJL, Jörgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MKC, Staels B, Schrauwen P, Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity., Mol. Metab 5 (2016) 635–645. doi: 10.1016/j.molmet.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dyar KA, Hubert MJ, Mir AA, Ciciliot S, Lutter D, Greulich F, Quagliarini F, Kleinert M, Fischer K, Eichmann TO, Wright LE, Peña Paz MI, Casarin A, Pertegato V, Romanello V, Albiero M, Mazzucco S, Rizzuto R, Salviati L, Biolo G, Blaauw B, Schiaffino S, Uhlenhaut NH, Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock., PLoS Biol. 16 (2018) e2005886. doi: 10.1371/journal.pbio.2005886.** This rich dataset provides an important baseline dataset for muscle with and without Bmal1. Specifically, the authors provide multi-omics data within a 24h period in mice with or without intact skeletal muscle Bmal1. These baseline data can be used to infer how exercise at different times of day may influence transcription and metabolomic responses.
- [24].Dyar KA, Eckel-Mahan KL, Circadian metabolomics in time and space, Front. Neurosci 11 (2017) 1–10. doi: 10.3389/fnins.2017.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Machado FSM, Fóscolo DRC, Poletini MO, Coimbra CC, Influence of Time-of-Day on Maximal Exercise Capacity Is Related to Daily Thermal Balance but Not to Induced Neuronal Activity in Rats, Front. Physiol 7 (2016) 464. doi: 10.3389/fphys.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boukelia B, Gomes EC, Florida-James GD, Diurnal Variation in Physiological and Immune Responses to Endurance Sport in Highly Trained Runners in a Hot and Humid Environment, Oxid. Med. Cell. Longev 2018 (2018) 1–9. doi: 10.1155/2018/3402143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kuusmaa M, Schumann M, Sedliak M, Kraemer WJ, Newton RU, Malinen JP, Nyman K, Hakkinen A, Hakkinen K, Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations, Appl Physiol Nutr Metab. 41 (2016) 1285–1294. doi: 10.1139/apnm-2016-0271. [DOI] [PubMed] [Google Scholar]
- [28].Dalbram E, Basse AL, Zierath JR, Treebak JT, Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action, J Appl Physiol. (2019). doi: 10.1152/japplphysiol.00737.2018. [DOI] [PubMed] [Google Scholar]
- [29].Hughes ME, Abruzzi KC, Allada R, Anafi R, Arpat AB, Asher G, Baldi P, de Bekker C, Bell-Pedersen D, Blau J, Brown S, Ceriani MF, Chen Z, Chiu JC, Cox J, Crowell AM, DeBruyne JP, Dijk D-J, DiTacchio L, Doyle FJ, Duffield GE, Dunlap JC, Eckel-Mahan K, Esser KA, FitzGerald GA, Forger DB, Francey LJ, Fu Y-H, Gachon F, Gatfield D, de Goede P, Golden SS, Green C, Harer J, Harmer S, Haspel J, Hastings MH, Herzel H, Herzog ED, Hoffmann C, Hong C, Hughey JJ, Hurley JM, de la Iglesia HO, Johnson C, Kay SA, Koike N, Kornacker K, Kramer A, Lamia K, Leise T, Lewis SA, Li J, Li X, Liu AC, Loros JJ, Martino TA, Menet JS, Merrow M, Millar AJ, Mockler T, Naef F, Nagoshi E, Nitabach MN, Olmedo M, Nusinow DA, Ptáček LJ, Rand D, Reddy AB, Robles MS, Roenneberg T, Rosbash M, Ruben MD, Rund SSC, Sancar A, Sassone-Corsi P, Sehgal A, Sherrill-Mix S, Skene DJ, Storch K-F, Takahashi JS, Ueda HR, Wang H, Weitz C, Westermark PO, Wijnen H, Xu Y, Wu G, Yoo S-H, Young M, Zhang EE, Zielinski T, Hogenesch JB, Guidelines for Genome-Scale Analysis of Biological Rhythms., J. Biol. Rhythms 32 (2017) 380–393. doi: 10.1177/0748730417728663.* This resource provides a thorough overview of considerations in experimental designs for circadian outcomes. We suggest investigators new to the concept of circadian biology review these guidelines to help develop rigorous experiments.
- [30].Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, Cao R, Liu AC, mTOR signaling regulates central and peripheral circadian clock function, PLoS Genet. 14 (2018) e1007369. doi: 10.1371/journal.pgen.1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Um JH, Pendergast JS, Springer DA, Foretz M, Viollet B, Brown A, Kim MK, Yamazaki S, Chung JH, AMPK regulates circadian rhythms in a tissue- and isoform-specific manner, PLoS One. 6 (2011) e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wu R, Dang F, Li P, Wang P, Xu Q, Liu Z, Li Y, Wu Y, Chen Y, Liu Y, The Circadian Protein Period2 Suppresses mTORC1 Activity via Recruiting Tsc1 to mTORC1 Complex, Cell Metab. 29 (2019) 653–667.e6. doi: 10.1016/j.cmet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- [33].Pizarro A, Hayer K, Lahens NF, Hogenesch JB, CircaDB: a database of mammalian circadian gene expression profiles, Nucleic Acids Res. 41 (2013) D1009–13. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sedliak M, Zeman M, Buzgó G, Cvecka J, Hamar D, Laczo E, Okuliarova M, Vanderka M, Kampmiller T, Häkkinen K, Ahtiainen JP, Hulmi JJ, Nilsen TS, Wiig H, Raastad T, Morphological, molecular and hormonal adaptations to early morning versus afternoon resistance training, Chronobiol Int. 35 (2018) 450–464. doi: 10.1080/07420528.2017.1411360. [DOI] [PubMed] [Google Scholar]
- [35].Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, Campbell KS, Arbogast S, Reid MB, Walker JR, Hogenesch JB, Takahashi JS, Esser KA, CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function., Proc. Natl. Acad. Sci. U. S. A 107 (2010) 19090–5. doi: 10.1073/pnas.1014523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Edgar DM, Dement WC, Regularly scheduled voluntary exercise synchronizes the mouse circadian clock., Am. J. Physiol (1991). doi: 10.1152/ajpregu.1991.261.4.R928. [DOI] [PubMed] [Google Scholar]
- [37].Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS, Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice., J. Physiol 590 (2012) 6213–26. doi: 10.1113/jphysiol.2012.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pastore S, Hood DA, Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice, J Appl Physiol 114 (2013) 1076–1084. doi: 10.1152/japplphysiol.01505.2012. [DOI] [PubMed] [Google Scholar]
- [39].Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA, Mop3 is an essential component of the master circadian pacemaker in mammals., Cell. 103 (2000) 1009–17. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang XS, Armstrong MEG, Cairns BJ, Key TJ, Travis RC, Shift work and chronic disease: The epidemiological evidence, Occup. Med. (Chic. Ill). 61 (2011) 78–89. doi: 10.1093/occmed/kqr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gabriel BM, Zierath JR, Circadian rhythms and exercise - re-setting the clock in metabolic disease, Nat Rev Endocrinol. (2019). doi: 10.1038/s41574-018-0150-x. [DOI] [PubMed] [Google Scholar]
- [42].Hughes ATL, Piggins HD, Feedback actions of locomotor activity to the circadian clock., Prog. Brain Res 199 (2012) 305–36. doi: 10.1016/B978-0-444-59427-3.00018-6. [DOI] [PubMed] [Google Scholar]
- [43].Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS, PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues, Proc Natl Acad Sci U S A. 101 (2004) 5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saracino PG, Rossetti ML, Steiner JL, Gordon BS, Hormonal regulation of core clock gene expression in skeletal muscle following acute aerobic exercise, Biochem Biophys Res Commun. 508 (2019) 871–876. doi: 10.1016/j.bbrc.2018.12.034. [DOI] [PubMed] [Google Scholar]
- [45].Sasaki H, Hattori Y, Ikeda Y, Kamagata M, Iwami S, Yasuda S, Shibata S, Phase shifts in circadian peripheral clocks caused by exercise are dependent on the feeding schedule in PER2::LUC mice, Chronobiol Int. 33 (2016) 849–862. doi: 10.3109/07420528.2016.1171775. [DOI] [PubMed] [Google Scholar]
- [46].Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR, Time- and exercise-dependent gene regulation in human skeletal muscle., Genome Biol. 4 (2003) R61. doi: 10.1186/gb-2003-4-10-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dickinson JM, D’Lugos AC, Naymik MA, Siniard AL, Wolfe AJ, Curtis DP, Huentelman MJ, Carroll CC, Transcriptome response of human skeletal muscle to divergent exercise stimuli, J. Appl. Physiol (2018) japplphysiol.00014.2018. doi: 10.1152/japplphysiol.00014.2018. [DOI] [PubMed] [Google Scholar]
- [48].Lassiter DG, Sjögren RJO, Gabriel BM, Krook A, Zierath JR, AMPK activation negatively regulates GDAP1, which influences metabolic processes and circadian gene expression in skeletal muscle, Mol Metab. 16 (2018) 12–23. doi: 10.1016/j.molmet.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schmitt K, Grimm A, Dallmann R, Oettinghaus B, Restelli LM, Witzig M, Ishihara N, Mihara K, Ripperger JA, Albrecht U, Frank S, Brown SA, Eckert A, Circadian Control of DRP1 Activity Regulates Mitochondrial Dynamics and Bioenergetics, Cell Metab. (2018). doi: 10.1016/j.cmet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- [50].Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, Bonar NA, McNulty MR, Ramsey KM, Bass J, Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle, Cell Metab. 25 (2017) 86–92. doi: 10.1016/j.cmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu C, Li S, Liu T, Borjigin J, Lin JD, Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism, Nature. (2007). doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- [52].Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM, AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation, Science (80−. ). 326 (2009) 437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jordan SD, Kriebs A, Vaughan M, Duglan D, Fan W, Henriksson E, Huber A-L, Papp SJ, Nguyen M, Afetian M, Downes M, Yu RT, Kralli A, Evans RM, Lamia KA, CRY1/2 Selectively Repress PPARδ and Limit Exercise Capacity., Cell Metab. 26 (2017) 243–255.e6. doi: 10.1016/j.cmet.2017.06.002.** The authors reveal an important role of both AMPK and the Cry1/2 proteins in skeletal muscle circadian biology. Cry1/2 modulate PPARs in an AMPK-dependent manner, revealing a mechanism through which the core molecular clock can alter exercise capacity. The paper begins to examine how exercise can influence the activity of the molecular clock in skeletal muscle.
- [54].Yang N, Williams J, Pekovic-Vaughan V, Wang P, Olabi S, McConnell J, Gossan N, Hughes A, Cheung J, Streuli CH, Meng QJ, Cellular mechano-environment regulates the mammary circadian clock, Nat. Commun (2017). doi: 10.1038/ncomms14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hornberger TA, Esser KA, Mechanotransduction and the regulation of protein synthesis in skeletal muscle., Proc. Nutr. Soc 63 (2004) 331–5. doi: 10.1079/PNS2004357. [DOI] [PubMed] [Google Scholar]