Abstract
Objective:
We aimed to understand the role of stress eating (SE) and compulsive eating (CE) in metabolic health among adults with obesity, and whether mindful eating training may buffer these associations.
Method:
We used data from a trial in which we randomized 194 participants with obesity to a diet-exercise intervention with either mindful eating training plus mindfulness-based stress management (n=100) or active control components (n=94). We measured SE, CE, weight and fasting blood glucose (FBG) at baseline, 12 months, and 18 months. We tested SE and CE as both moderators and mediators of intervention effects on changes in metabolic health.
Results:
Participants higher (+1 SD) in CE at baseline randomized to mindful eating (vs. control) had greater improvements in FBG at 18 months (p=.05). Twelve-month reductions in CE mediated an association between intervention and changes in FBG and weight at 12 and 18 months post-baseline (p ≤. 05). Furthermore, those higher (+1 SD) in SE at baseline were nearly 2 BMI points higher than those lower (−1 SD) in stress eating (p < .01). Decreases in SE (F=3.42, p<.001, 95% CI: 2.55, 4.30) and CE (F=0.45, p<.001, 95% CI: 0.36, 0.54) in all participants at 6 months were associated with greater weight loss at 18 months.
Conclusions:
Those with greater compulsive eating may reduce risk for metabolic decline by receiving training in mindful eating training plus mindfulness-based stress management. Future obesity interventions should consider tailoring treatment towards trait-level characteristics, such as compulsive eating.
Keywords: Mindfulness, compulsive eating, metabolic outcomes, mindful eating, obesity management
INTRODUCTION
Current behavioral treatment options for obesity frequently fail to produce long-term, sustainable weight loss (Dombrowski, Knittle, Avenell, Araujo-Soares, & Sniehotta, 2014; Douketis, Macie, Thabane, & Williamson, 2005; Norris et al., 2016). Indeed, one of the greatest problems facing behavioral weight-loss interventions is the tendency towards weight regain following weight loss. This may be due to inadequate interventions targeting the underlying eating patterns to which individuals return after such interventions. Although weight loss can result in significant metabolic benefits, including decreased risk for heart disease and type 2 diabetes, most adults with obesity who lose weight in a diet and exercise intervention go on to regain lost weight within 3 to 5 years (Perri, 1998). High obesity rates highlight the extent to which the lack of more efficacious behavioral treatments poses a significant public health problem (Flegal, Carroll, Kuczmarski, & Johnson, 1998; Kuczmarski, Flegal, Campbell, & Johnson, 1994; Ogden, Carroll, Fryar, & Flegal, 2015; Ogden, Carroll, Kit, & Flegal, 2014), as obesity increases risk for metabolic dysfunction, morbidity, and mortality (Bray, 2003; Chen, Srinivasan, Elkasabany, & Berenson, 1999; Zimmet et al., 2007). Many factors determine obesity and subsequent metabolic dysfunction (Borecki et al., 1998; Kopelman, 2000). Some factors include genetic susceptibility, increased availability of highly palatable processed foods, decreased physical activity, and, though less commonly cited, psychological stress and compulsive eating.
Eating for psychological relief and compulsive eating are overlapping constructs in that they both often serve as ways to cope with negative emotions (Arnow, Kenardy, & Agras, 1992). Such patterns of eating beyond caloric needs can lead to maladaptive health outcomes (Hudson et al., 2010). Here, we define eating for psychological relief, or ‘stress eating,’ as eating in response to acute or chronic stress, or in response to negative emotional states (such as sadness and anger). We define ‘compulsive eating’ as eating beyond caloric needs in a way that involves both subjective and objective experiences of loss of control over eating (which is a key component of a DSM-5 diagnosis of binge eating disorder, or BED), including binge eating behaviors. Both stress eating and compulsive eating may be important behavioral targets in weight-loss interventions seeking to promote weight-loss maintenance (Lillis, Hayes, & Levin, 2011). Limited data speak to the comorbidity between stress eating and compulsive overeating, largely due to a lack of measures that adequately capture the concept of stress eating. However, emotional eating and compulsive eating are strongly correlated (r=.78, p<.001), and both of these eating behaviors are positively associated with BMI (Vainik, Neseliler, Konstabel, Fellows, & Dagher, 2015). By contrast, stress eating (defined by eating more in response to stress, as measured by the Salzberg Stress Eating Questionnaire), is not strongly correlated with emotional eating and BMI (Meule, Reichenberger, & Blechert, 2018), and is not universally maladaptive (Cummings, Mason, Puterman, & Tomiyama, 2018). To date, however, several studies have documented overlaps between high levels of perceived stress and high levels of emotional eating and/or compulsive eating (Groesz et al., 2012; Sims et al., 2008; Torres & Nowson, 2007). For example, adults with BED tend to report stress levels that are generally higher than representative norms (Pinaquy, Chabrol, Simon, Louvet, & Barbe, 2003).
Stress eating and compulsive eating, including binge eating, may independently increase metabolic health risk across several dimensions. Adults stress eat and compulsively eat (including binge eating) in both general and treatment-seeking populations are more likely to have obesity, hypertension, hypertriglyceridemia, low high-density lipoprotein (HDL), insulin resistance, and metabolic syndrome (Abraham, Massaro, Hoffmann, Yanovski, & Fox, 2014; Roehrig, Masheb, White, & Grilo, 2009). One explanation is that an objective binge, by definition, results in a large amount of food (calories) entering the digestive system at one time, which can overwhelm metabolic regulation. Binge eating is more common among adults with obesity than among individuals without obesity (Bruce & Agras, 1992; Striegel-Moore & Franko, 2008; Wonderlich, Gordon, Mitchell, Crosby, & Engel, 2009). Binge eating is strongly associated with severe obesity (BMI > 40 kg/m2), with up to 15% of individuals with a lifetime history of BED exhibiting severe obesity (Hudson, Hiripi, Pope, & Kessler, 2007). Up to 30% of those who seek treatment for obesity, and approximately 3–5% of individuals with obesity in the general population, meet diagnostic criteria for BED (de França, Gigante, & Olinto, 2014; Ivezaj, White, & Grilo, 2016; Kessler et al., 2013). Perhaps most concerning, stress eating and binge eating may predict the development of cardiovascular and endocrine problems (Hudson et al., 2010; Tanofsky-Kraff et al., 2012), including dyslipidemia, hypertension, and type-2 diabetes, even after accounting for weight status. These data support the potential importance of stress eating and compulsive eating as behavioral targets in the treatment of obesity.
To date, it is unclear whether interventions that promote decreases in stress and compulsive eating can improve metabolic health. Some data suggest that overweight individuals or individuals with obesity who also have a BED diagnosis are more likely to drop out of weight loss programs and tend to regain weight faster following weight loss interventions, compared to those without BED (Marcus, Wing, & Hopkins, 1988; Yanovski, Gormally, Leser, Gwirtsman, & Yanovski, 2012). This may be due to a failure to address the behavioral lynchpins that underlie the overweight or obesity: stress eating or compulsive overeating.
Mindfulness-based approaches that include mindful eating may be a promising treatment modality for adults with stress and compulsive eating. Mindfulness approaches aim to cultivate a non-judging awareness of experiences in the present-moment, and promote adaptive self-regulation (Brown, Ryan, & Creswell, 2007). Mindful eating interventions aim to cultivate awareness of internal body states related to eating, such as feelings of hunger and fullness, and attending to these interoceptive body cues (vs. non-homeostatic cues to eat, such as feelings of anxiety), in an effort to make more deliberate food choices (Kristeller & Wolever, 2011).
General mindfulness-based interventions may have a beneficial impact on lowering blood pressure (Carlson, Speca, Faris, & Patel, 2007) and improving stress reactivity (Hoge et al., 2013). Mindfulness-based approaches that specifically incorporate mindful eating techniques can lead to reductions in both the frequency and the severity of binge eating episodes in individuals with diagnosed BED (Kristeller & Hallett, 1999; Kristeller & Wolever, 2011; Kristeller, Wolever, & Sheets, 2014). However, randomized controlled trials (RCTs) of mindful eating interventions that follow individuals longer-term, and that measure changes in metabolic outcomes in addition to weight, are limited (Godfrey, Gallo, & Afari, 2015; Goyal et al., 2014).
In our recently completed randomized controlled trial, Supporting Health by Integrating Nutrition and Exercise (SHINE), 194 adults with obesity were randomized to a 5.5-month mindfulness-based weight loss intervention + diet-exercise program or a diet-exercise only program with identical non-restrictive diet-exercise guidelines. The mindfulness intervention included general mindfulness techniques drawn from mindfulness-based stress reduction, along with specific mindful eating training derived from mindfulness-based eating awareness training (MB-EAT;(Kristeller et al., 2014). The mindfulness intervention group lost an average of 1.9 kg more than the control group at 18 months compared to the active control intervention, but this difference was not statistically significant (95% CI: −4.5, 0.8 kg). However, participants in the mindfulness arm had greater improvements in fasting blood glucose and triglyceride/HDL levels one year after the end of the intervention (Daubenmier et al., 2016). Additionally, participants in the mindfulness arm experienced greater reductions in reward-based eating and these reductions mediated weight outcomes (Mason, Epel, Aschbacher, et al., 2016). Further, increases in mindful eating were associated with reduced fasting glucose and sweet food intake 6-months post-intervention (Mason, Epel, Kristeller, et al., 2016). Taken together, the SHINE trial provides preliminary support for certain effects of mindfulness and mindful eating training on metabolic health through changes in eating behavior. However, it is unknown if subgroups of participants who reported higher levels of stress eating or compulsive eating, specifically, derived greater health benefit from being randomized to the mindfulness arm.
In the current report, we hypothesized that (1) at baseline, participants with higher levels of stress and compulsive eating would have worse metabolic health (higher weight and FBG), and that (2) greater baseline stress and compulsive eating would predict worsening of these metabolic outcomes. Further, we anticipated that (3) decreases in stress and compulsive eating would predict improvements in weight and FBG, across interventions. Finally, we expected that (4) individuals higher in stress and compulsive eating, relative to those lower in stress and compulsive eating, would benefit the most from randomization to the mindfulness intervention, relative to the active control intervention, and that (5) decreases in stress and compulsive eating would mediate the effect of intervention on metabolic health. We selected FBG as a primary metabolic outcome measure (as opposed to the several other metabolic markers collected over the course of the trial) because the intervention had the greatest impact on this particular marker (Daubenmier et al., 2016) and previous data have implicated differences in FBG across individuals with and without overeating behavior. For example, Abraham and colleagues (2014) found that BMI accounted for associations between binge eating and several metabolic factors (e.g., HOMA-IR), but did not account for the association between binge eating and fasting glucose, suggesting fasting glucose to account for unique variance. FBG may also be a logical metabolic marker to examine in the context of a stress intervention, as stress responses stimulate cortisol and catecholamine release, which acutely impact blood glucose. Both these hormones serve as counter-regulatory hormones to insulin, and raise blood glucose, an effect that may be most clearly seen in the absence of food intake, in the fasting state. In contrast, glycated hemoglobin is influenced by both fasting glucose and by post-prandial glucose levels, and the latter may be less clearly influenced by physiological stress responses.
MATERIAL AND METHODS
We analyzed data from the SHINE trial (Clinicaltrials.gov registration: ), as described previously (Daubenmier et al., 2016). We pre-registered all hypotheses and corresponding analyses in the current study (https://aspredicted.org/nk4av.pdf; #8472).
Participants
Eligible participants were ≥18 years old, had a BMI between 30 and 45.9 kg/m2, and abdominal obesity. Exclusion criteria included a diagnosis of type 2 diabetes, participation in a weight-loss or mindfulness-based stress reduction program, medications for weight loss or those with known effects on endocrine functioning, an active diagnosis of bulimia nervosa, or women who were pregnant or breastfeeding. Participants with a diagnosis of BED were not excluded. We recruited participants from the San Francisco Bay Area. We advertised the study as a comparative weight loss intervention involving lifestyle changes in diet, exercise, and stress management. We obtained written informed consent from all study participants. The UCSF Institutional review board (IRB) approved all aspects of this study.
Study Design
Participants completed baseline assessment procedures, including a physical exam, fasting blood draw, measures of body composition, and self-report assessments of eating behaviors. We then randomized them to a diet-exercise intervention with (“mindful eating arm” or without (“active control arm”) a mindfulness component. We re-assessed participants on eating behavior and metabolic variables at 6, 12, and 18 months from the initiation of the intervention.
Interventions
As previously described (Daubenmier et al., 2016), both intervention arms included 12 weekly 2–2.5 hour group evening sessions, 3 bi-weekly sessions, 1 follow-up session 4 weeks later, and an all-day weekend session near the 8th week of the program, all over the course of a 5.5-month period. Both intervention arms received the same dietary and exercise guidelines (e.g., goal of reducing daily food intake of their choice by 500 calories, and increasing activity). We randomized participants in a 1:1 ratio to the mindful eating vs. active control arm via a computer-generated random allocation sequence using random block sizes of four to eight, programmed by a database manager (Daubenmier et al., 2016).
The mindfulness arm incorporated both specific mindful eating techniques as well as general mindfulness techniques. We adapted specific mindful eating elements from the Mindfulness-Based Eating Awareness Training program (MB-EAT;(Kristeller et al., 2014). These elements were designed to promote awareness and self-regulation of physical hunger, stomach fullness, taste satisfaction, food cravings, and other triggers for eating in the context of reduced caloric intake. In session, instructors guided participants in mindfulness exercises, with and without food. These exercises focused on attending to physical sensations of hunger, stomach fullness, taste satisfaction, and cravings for food. Instructors also guided participants to practice “mini-meditations” prior to meals and snacks at home, and at other times to help notice food cravings and triggers to eat when not physically hungry. Additionally, participants were encouraged to practice awareness and savoring of the taste and texture of foods, and to draw hedonic pleasure from smaller portions of their highly preferred foods. They were instructed to mindfully attend to ways to reduce food volume and caloric content that they expected would be both satisfying and sustainable.
We included general mindfulness techniques for stress management and emotion regulation, which were adapted from Mindfulness-based Stress Reduction programs (Kabat-Zinn, 1990). These techniques included body scan meditations, loving kindness and self-acceptance meditation, as well as sitting meditation and mindful yoga. Participants were taught a brief, extended exhalation breathing technique to promote physiological relaxation. They were instructed to practice these meditations at home for up to 30 minutes a day, 6 days per week, including mindful walking.
To ensure equivalence of time, attention, and engagement across intervention arms, the active control components included additional educational content, including information about nutrition and physical activity. It also included cognitive behavioral therapy tools and instruction in progressive muscle relaxation for stress management. See Daubenmier and colleagues (2016) for additional detail about intervention arms.
Measures
Physical examination and body composition.
All participants completed a medical history and physical examination to screen for significant medical problems. We measured body weight to the nearest 0.1 kg on a calibrated digital scale and height was measured to the nearest 0.1 cm; both were used to calculate BMI (kg/m2).
Fasting blood glucose.
We measured fasting blood glucose (FBG; mg/dl) using a fasting blood draw at Quest Laboratories using standard methods (i.e. immunoassay for glucose). We selected FBG as a primary metabolic outcome measure (as opposed to the several other metabolic markers collected over the course of the trial), as the mindfulness intervention had the greatest impact on this particular marker (Daubenmier et al., 2016), and previous data suggest that overeating is more strongly associated with FBG than other biomarkers of metabolic health (Abraham et al., 2014).
Stress and compulsive eating measures.
We assessed stress eating with the emotional eating subscale of the Dutch Eating Behavior Questionnaire (DEBQ-EE). This 13-item subscale assesses eating in response to emotional arousal states, including eating in response to diffuse and clearly labeled emotions. Item response choices range from 1 (never) to 5 (very often) and the total score of the emotional eating subscale is an average of the 13-items (range: 1 to 5). All of the subscales of the DEBQ (emotional eating, restrained eating, external eating) have demonstrated high internal consistency (Cronbach’s alpha=.94) and factorial validity (van Strien, Frijters, Bergers, & Defares, 1986). Among our study sample, emotional eating subscale internal consistency was high (α = 0.94).
We used the Binge Eating Scale (BES) as a measure of compulsive eating (Gormally, Black, Daston, & Rardin, 1982). This 16-item scale assesses severity of binge eating behavior and attitudes, to discriminate between obese individuals with severe, moderate, or no binge eating problems (Gormally et al., 1982). Each item corresponds to a point value (0, 1, 2, or 3 points) and the total score is an average of the 16 items (range: 0 to 46). A score of ≤ 17 is considered “non-binging”; “moderate binging” is indicated by a score between 18 and 26; “severe binging” is indicated by a score ≥ 27 (Greeno, Marcus, & Wing, 1995). The BES has good test-retest reliability (r=.87) (Timmerman, 1999) and high internal consistency (Cronbach’s alpha=.85) (Gormally et al., 1982). However, the BES was not designed to differentiate clinically between individuals with DSM-diagnosable Binge Eating Disorder and those without.
Statistical Analysis
Data preparation.
We used SPSS (Version 24.0. Armonk, NY: IBM Corp.) for all variable preparation and statistical analysis. We computed summary statistics to evaluate the distributions of each study variable (DEBQ-EE, BES, weight, BMI, FBG) and assess potential outliers. We did not find any outliers with regard to our study variables (defined as > ± 3 standard deviations of the mean).
Stress eating, compulsive eating, and metabolic health.
To explore associations among baseline measures of stress or compulsive eating and weight and FBG, we assessed Pearson’s correlations between stress eating (DEBQ-EE) or compulsive eating (BES) measures with each metabolic health indicator at baseline (BMI, FBG). Additionally, for FBG, we used linear regression models with baseline stress or compulsive eating as an independent variable, FBG as a dependent variable, and baseline BMI as a covariate.
Baseline stress eating, compulsive eating, and change in metabolic health.
To test whether baseline stress or compulsive eating predicted changes in metabolic outcomes at 12-month and 18-month follow-up, we used linear regression models with baseline stress or compulsive eating as an independent variable (continuous) and 12-month and 18-month weight and FBG change as the dependent variables, across both intervention groups. We adjusted for corresponding changes in BMI in analyses with FBG as the dependent variable.
Changes in stress and compulsive eating, and change in metabolic health.
We used linear mixed models for repeated measures (using unstructured covariance) to examine the association between change in stress or compulsive eating from baseline to 6-month follow-up (immediately post-intervention) and change in weight and FBG across all follow-up timepoints. We conducted a series of separate linear regression models to examine the association between changes in stress or compulsive eating and changes in each metabolic outcome at each follow-up timepoint (6 months, 12 months, and 18 months).
Moderation analyses of interaction between stress or compulsive eating and intervention on change in metabolic health.
We conducted a series of linear regressions to explore whether baseline stress or compulsive eating at baseline (treated both as continuous and dichotomous [median split] variables) moderated the effect of the intervention on metabolic outcomes. We created an interaction term (between stress or compulsive eating and baseline X intervention) as our independent variable.
Assessing whether change in stress or compulsive eating mediates the effect of the intervention on changes in metabolic health.
We used PROCESS macro for SPSS (Model 4) to test change in stress or compulsive eating at 6-months as a mediator (ME; Δ DEBQ-EE; Δ BES) of the effect of intervention (X; mindful eating vs. active control) on change in each metabolic outcome at 12- and 18-months (Y; Δ weight, Δ BMI, Δ FBG) (Hayes, 2012). In exploratory analyses, we examined changes in stress and compulsive eating at 12 months as a mediator between intervention and 12 and 18-month changes in metabolic outcomes. We report regression coefficients for each model path, including Path A (association between X and ME), Path B (association between ME and Y), Path C (the total effect of X and ME on Y), Path C’ (the direct effect X on Y, without ME), and Path AB (the indirect effect of X on Y via ME). We determined the proportion of the effect of intervention on metabolic outcome by dividing the indirect effect (Path AB) by the total effect (C). PROCESS models in these analyses used 5,000 bootstrapped samples for the construction of 95% bias-corrected confidence intervals for indirect effects. In each mediation model, we flipped the predictor (X) and the mediator (M), to examine possible directions of any observed effects. In all analyses, we considered differences between groups significant when p values were ≤.05, using two-tailed tests. We conducted intention-to-treat analyses for all moderation and mediation analyses. We used linear mixed models where appropriate as our analysis strategy to address missing data, as these models are relatively robust to the effects of missing data (Singer, Willett, & Willett, 2003).
RESULTS
Participant Characteristics
At baseline, participants (n=194) had a mean BMI of 35.47 kg/m2 (range: 30–45.65 kg/m2) and mean FBG of approximately 86 mg/dl (range: 50–107 mg/dl). Thus, although all participants had obesity, the majority (95%) did not have prediabetes based on FBG (FBG <100 mg/dl). Participants’ mean stress eating (DEBQ-EE) score was 3.18 ± 0.90, which is significantly higher than a representative Dutch sample of 625 adults who were overweight (2.61 ± 0.9; t=7.71, p<.001; (van Strien, Herman, & Verheijden, 2009). Participants’ mean compulsive eating (BES) score was ~16 (range: 2–40), with most participants endorsing none (60%) to moderate levels (33%) of binge eating severity. When using a BES score of 27 as a cut-off for severe binge eating (Celio, Wilfley, Crow, Mitchell, & Walsh, 2004) approximately 6% (n=12) of participants met criteria (See Table 1 for demographics and health characteristics of study sample).
Table 1.
Baseline demographic and health characteristics of sample.
| Variable | Total Sample (n=194) | Active Control (n=94) | Mindfulness (n=100) |
|---|---|---|---|
| Demographics | |||
| Age (years)a | 46.98 ± 12.71 (18 – 69) | 46.76 ± 12.41 | 47.19 ± 13.03 |
| Sex (% female) | 80% | 81% | 79% |
| Race/Ethnicity (%) | |||
| White | 59.3% | 53.2% | 65.0% |
| Black | 12.9% | 12.8% | 13.0% |
| Latino | 11.9% | 17.0% | 7.0% |
| Asian/Pacific Islander | 9.8% | 11.7% | 8.0% |
| Native American | 1.0% | 2.1% | 0.0% |
| Other | 5.2% | 3.2% | 7.0% |
| Health Characteristics | |||
| DEBQ-EEa,b | 3.18 ± 0.90 (1.08 – 5.00) | 3.11 ± 0.89 | 3.23 ± 0.92 |
| BESa,c | 15.64 ±7.13 (2 – 40) | 15.79 ± 7.47 | 15.49 ± 6.83 |
| BES, 3 categoriesd (%) | 61% Non-binging 33% Moderate binging 6% Severe binging |
66% Non-binging 32% Moderate binging 2% Severe binging |
75% Non-binging 25% Moderate binging 0% Severe binging |
| Weight (kg)a | 97.22 ± 14.39 (70.5 – 138.9) | 96.68 ± 14.79 | 97.72 ± 14.05 |
| Body mass indexa (kg/m2) | 35.47 ± 3.62 (29.98 – 45.65) | 35.56 ± 3.75 | 35.38 ± 3.51 |
| FBGa,e (mg/dl) | 86.09 ± 8.20 (50 – 107) | 85.54 ± 7.67 | 86.61 ± 8.67 |
Note.
M ± SD (Range).
DEBQ-EE, Dutch Eating Behavioral Questionnaire, Emotional Eating Subscale.
BES, Binge Eating Scale (total score);
Categories according to Greeno, Marcus, & Wing (1995).
FBG, fasting blood glucose.
Stress eating, compulsive eating, and metabolic health
We found a strong correlation between baseline stress eating and baseline compulsive eating (r=.56, p<.001). Baseline stress eating was also associated with baseline BMI (r=.22, p=.002). However, stress eating was not significantly related to baseline FBG (r=.02, p=.78). Baseline compulsive eating tended to be positively associated with baseline BMI (r=.13, p=.08), but was not significantly related to baseline FBG (r=−.08, p=.25).
Baseline stress eating, compulsive eating, and change in metabolic health
Baseline stress eating did not predict changes in weight (ps>.23) or FBG (ps>.43) at either the 12 month or 18 month follow-up timepoint, across both intervention groups (Table 2, top). Although not statistically significant, the effects were generally negative, such that for each one unit increase in stress eating at baseline, the improvement in metabolic outcomes tended to decrease by a small margin (e.g., a decrease in weight by 0.54 to 0.91 kg). We observed a similar pattern in regard to compulsive eating at baseline. It did not predict changes in either weight or FBG at the 12 month or 18 month follow-up timepoints (ps>.31; Table 2, bottom).
Table 2.
Associations among baseline stress eating and compulsive eating and changes in study outcomes at multiple timepoints.
| B | SE(B) | β | t | P | 95% CI for B | ||
|---|---|---|---|---|---|---|---|
| LL | UL | ||||||
| DEBQ-EE (BL) | |||||||
| Δ Weight, 12m (n=149) | −0.54 | 0.64 | −0.07 | −0.84 | .40 | −1.81 | 0.73 |
| Δ Weight, 18m (n=147) | −0.91 | 0.77 | −0.10 | −1.18 | .24 | −2.44 | 0.62 |
| Δ FBG, 12ma (n=149) | −0.59 | 0.76 | −0.06 | −0.77 | .44 | −2.10 | 0.92 |
| Δ FBG, 18ma (n=147) | 0.28 | 0.72 | 0.03 | 0.38 | .70 | −1.15 | 1.71 |
| BES (BL) | |||||||
| Δ Weight, 12m (n=148) | 0.03 | 0.08 | 0.03 | 0.36 | .72 | −0.13 | 0.19 |
| Δ Weight, 18m (n=146) | −0.06 | 0.10 | −0.06 | −0.67 | .51 | −0.25 | 0.12 |
| Δ FBG, 12ma (n=148) | −0.10 | 0.10 | −0.08 | −0.99 | .32 | −0.29 | 0.09 |
| Δ FBG, 18ma (n=146) | 0.03 | 0.09 | 0.02 | 0.31 | .76 | −0.15 | 0.20 |
Note.
p ≤ .05,
p ≤ .01,
trend-level significance; Δ Weight and FBG computed as (later timepoint) – (baseline).
Adjusting for Δ weight. See Table 1 note.
Changes in stress and compulsive eating, and change in metabolic health
In a mixed effects model, decreases in stress eating at 6 months across intervention groups were associated with decreases in weight (p<.001) at all follow-up timepoints, but were not significantly related to changes in FBG, before and after adjusting for changes in weight (p=.82; Table 3, top; for individual time blocks, see Appendix A, top). Similarly, in a mixed effects model, decreases in compulsive eating at 6 months were associated with decreases in weight (p<.001) at all follow-up timepoints, but were unrelated to change in FBG, before and after adjusting for changes in weight (p=.99; Table 3, bottom; for individual time blocks, see Appendix A, bottom).
Table 3.
Changes in stress eating and compulsive eating at 6 months and changes as a predictor of study outcomes across subsequent timepoints (n=194).
| Estimate | SE | df | F | P | 95% CI for Estimate | ||
|---|---|---|---|---|---|---|---|
| LL | UL | ||||||
| Δ DEBQ-EE | |||||||
| Δ Weight | 3.42 | 0.45 | 1,522 | 59.21 | <.001** | 2.55 | 4.30 |
| Δ FBG | 0.10 | 0.45 | 1,561 | 0.05 | .82 | −0.78 | 0.99 |
| Δ BES | |||||||
| Δ Weight | 0.45 | 0.05 | 1,499 | 88.37 | <.001** | 0.36 | 0.54 |
| Δ FBG | <0.01 | 0.05 | 1,587 | <0.001 | .99 | −0.10 | 0.10 |
Note.
p ≤ .05,
p ≤ .01,
trend-level significance. Mixed effects linear model using unstructured covariance. See Table 1 note. Subsequent timepoints for outcomes included in the model were 12 and 18 months after study initiation.
Moderation analyses of interaction between stress or compulsive eating and intervention on change in metabolic health
Baseline stress eating did not moderate the effect of intervention on weight or FBG at 12 and 18 months (all ps>.28; Table 4, top). While these interaction terms were not significant, those high in stress eating tended to show greater weight loss and FBG at 12 months as well as greater decreases in FBG at 18 months when they were randomized to the mindful eating (vs. the active control) arm. By contrast, those lower in stress eating did not differ across intervention arms with regard to changes in outcomes (Table 4, top).
Table 4.
Effect of treatment arm (mindfulness vs. active control) on changes in study outcomes among those high vs. low in baseline stress eating (top) and compulsive eating (bottom).
| High DEBQ (>3.15) (n=73) | Low DEBQ (≤3.15) (n=75) | Treatment X DEBQ-EEa | |||||
|---|---|---|---|---|---|---|---|
| Active Control: M (SD) (95% CI) | Mindfulness: M (SD) (95% CI) | p-value | Active Control: M (SD) (95% CI) | Mindfulness: M (SD) (95% CI) | p-value | p-value of interaction term | |
| Δ Weight, 12m (kg) | −2.96 (6.27) (−5.23, −0.67) | −5.99 (7.37) (−8.23, −3.75) | .06† | −3.04 (6.75) (−5.35, −0.73) | −4.38 (6.79) (−6.46, −2.30) | .39 | .29 |
| Δ Weight, 18m (kg) | −3.82 (8.21) (−6.55, −1.10) | −4.86 (8.41) (−7.54, −2.17) | .59 | −2.33 (7.12) (−5.27, 0.60) | −5.04 (8.42) (−7.45, −2.63) | .16 | .74 |
| Δ FBG, 12m (mg/dl) | +2.08 (5.87) (−0.24, 4.41) | −0.97 (7.95) (−3.27, 1.32) | .07† | +4.50 (13.62) (1.01, 7.99) | +0.86 (6.22) (−2.28, 4.00) | .13 | .99 |
| Δ FBG, 18m (mg/dl) | +2.89 (8.83) (0.19, 5.59) | −0.87 (7.61) (−3.53, 1.79) | .05* | +3.38 (11.12) (0.06, 6.70) | −0.02 (7.17) (−2.75, 2.70) | .12 | .52 |
| High BES (>15) (n=74) | Low BES (< 15) (n=74) | Treatment X BESa | |||||
| Active Control: M (SD) (95% CI) | Mindfulness: M (SD) (95% CI) | p-value | Active Control: M (SD) (95% CI) | Mindfulness: M (SD) (95% CI) | p-value | p-value of interaction term | |
| Δ Weight, 12m (kg) | −2.27 (6.28) (−4.31, −0.22) | −5.16 (6.04) (−7.15, −3.16) | .05* | −3.77 (6.64) (−6.31, −1.24) | −5.28 (8.00) (−7.61, −2.94) | .39 | .42 |
| Δ Weight, 18m (kg) | −2.93 (8.10) (−5.94, 0.07) | −5.62 (9.36) (−8.50, −2.74) | .20 | −3.42 (7.44) (−6.07, −0.77) | −4.40 (7.57) (−6.68, −2.11) | .58 | .54 |
| Δ FBG, 12m (mg/dl) | +3.03 (5.46) (0.74, 5.32 | −1.08 (8.01) (−3.31, 1.15) | .01* | +3.50 (13.91) (−0.07, 7.07) | +0.95 (6.12) (−2.34, 4.24) | .30 | .50 |
| Δ FBG, 18m (mg/dl) | +3.91 (8.52) (1.15, 6.68) | −2.14 (7.67) (−4.79, 0.52) | .002** | +2.25 (11.12) (−0.88, 5.38) | +0.86 (6.80) (−1.84, 3.56) | .51 | .05* |
Note.
Interaction term created after mean centering stress eating variable (DEBQ-EE or BES).
p ≤ .05,
p ≤ .01,
trend-level significance. High vs. low stress and compulsive eating defined using median split.
See Table 1 note.
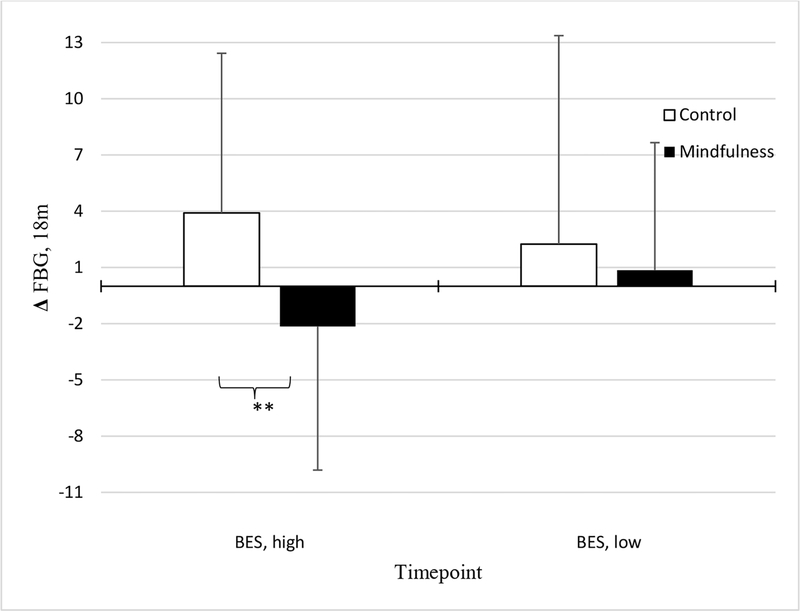
Baseline compulsive eating moderated the effect of intervention arm on FBG at 18 months, after adjusting for 18-month weight change (p=.05). It did not, however, moderate the effect of intervention arm on changes in weight at either timepoint (ps>.41; Table 4, bottom). Examining the simple slopes of this interaction term showed that the association between intervention group and FBG change was stronger at higher levels (+1 SD; B(SE)=−0.39 (0.20), p=.05; Figure 1) of compulsive eating, but not lower levels (−1 SD). Thus, participants with higher baseline compulsive eating appeared to have greater improvements in FBG at 18 months if randomized to the mindful eating (vs. active control) arm.
Figure 1.
Associations between treatment randomization and Δfasting blood glucose (FBG) at 18m, among those high (n=74) vs. low (n=74) in baseline compulsive eating (BES).
Note. **p<.01
Mediating role of changes in stress or compulsive eating on outcomes
Changes in stress eating at 6 months did not significantly mediate the effect of intervention arm on changes in weight or FBG at 12 and 18 month follow-up (Table 5). Further, we did not observe a direct effect of intervention on changes in weight at 12 or 18 months, but we did observe direct effects of intervention on changes in FBG at 12 and 18 months (ps≤ .06) as previously reported by Daubenmier and colleagues (2016). Similarly, changes in compulsive eating at 6 months did not mediate the association between intervention and changes in weight or FBG at 12 and 18 month follow-up (Table 5).
Table 5.
Conditional indirect effects of stress or compulsive eating on the effect of treatment randomization (mindfulness vs. active control) on change in study outcomes at multiple timepoints.
| Direct Effect of X on Y, B (SE) | 95% CI of Direct Effect | P | Point Estimate of Indirect Effect, B (SE) | 95% CI of Indirect Effect | |
|---|---|---|---|---|---|
| Δ Weight, 12m | |||||
| Mediator 1 (ME1): Δ DEBQ-EE, 6m | −1.69 (1.14) | (−3.94, 0.56) | .14 | −0.35 (.30) | (−1.20, 0.04) |
| Mediator 2 (ME2): Δ BES, 6m | −1.64 (1.13) | (−3.87, 0.59) | .15 | −0.37 (0.27) | (−1.07, 0.03) |
| Δ Weight, 18m | |||||
| Mediator 1 (ME1): Δ DEBQ-EE, 6m | −1.53 (1.40) | (−4.30, 1.23) | .27 | −0.24 (0.29) | (−1.11, 0.11) |
| Mediator 2 (ME2): Δ BES, 6m | −1.51 (1.40) | (−4.28, 1.26) | .28 | −0.23 (0.26) | (−0.98, 0.09) |
| Δ FBG, 12m | |||||
| Mediator 1 (ME1): Δ DEBQ-EE, 6m | −2.90 (1.50) | (−5.87, 0.08) | .06† | −0.28 (0.28) | (−1.15, 0.04) |
| Mediator 2 (ME2): Δ BES, 6m | −2.99 (1.52) | (−6.01, 0.02) | .05* | −0.22 (0.22) | (−0.92, 0.05) |
| Δ FBG, 18m | |||||
| Mediator 1 (ME1): Δ DEBQ-EE, 6m | −3.78 (1.45) | (−6.64, −0.91) | .01** | −0.23 (0.27) | (−1.05, 0.11) |
| Mediator 2 (ME2): Δ BES, 6m | −3.61 (1.43) | (−6.43, −0.79) | .01** | −0.32 (0.34) | (−1.31, 0.11) |
Note.
p ≤ .05,
p ≤ .01,
trend-level significance. All 95% Confidence Intervals (CIs) represent bootstrapped (5,000) and bias-corrected estimates (BCEs). SE=Standard Error. Effects are unstandardized coefficients from models testing each mediator (DEBQ-EE, BES) separately. X=mindfulness vs. control, Y=Δ each metabolic outcome at each timepoint, ME1= Δ stress eating at 6m; ME2 Δcompulsive eating at 6m.
See Table 1 note.
Effect of intervention on 12- and 18-month changes in stress and compulsive eating.
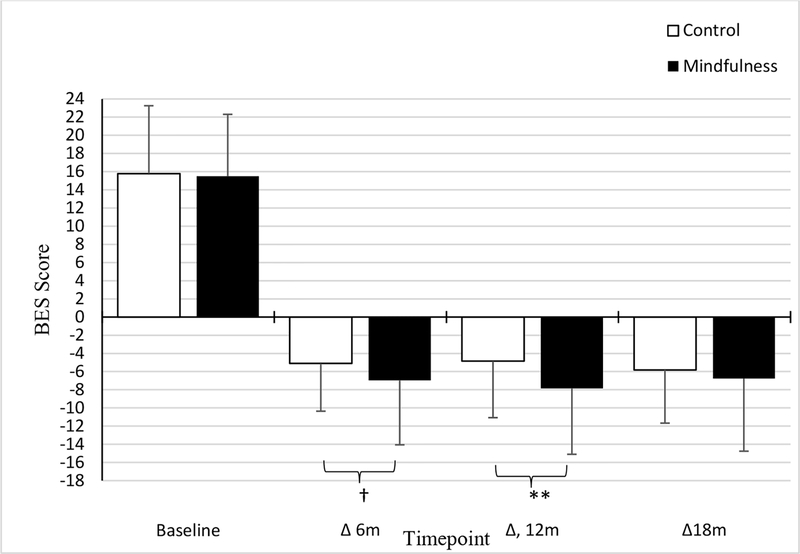
Intervention arm tended to influence change in stress eating at 12 months (p=.08), with a smaller effect at 6 months (p=.10; Appendix B, top; Figure 2). Similarly, we observed that the mindful eating arm showed greater decreases in compulsive eating at 12 months (p=.008), but this effect was marginally significant at 6 months (ps>.07) (Appendix B, bottom; Figure 3). Given this pattern of findings, we examined whether change in either eating measure at 12 months (vs. 6 months) mediated the association between intervention and metabolic outcomes at 12 and 18 months.
Figure 2.
Associations between treatment randomization and changes in stress eating.
Note. †trend-level significance
Figure 3.
Associations between treatment randomization and changes in compulsive eating.
Note. †trend-level significance, **p<.01, *p<.05
Exploratory: Changes in stress or compulsive eating in mediating intervention effects on metabolic health at 12 and 18 months.
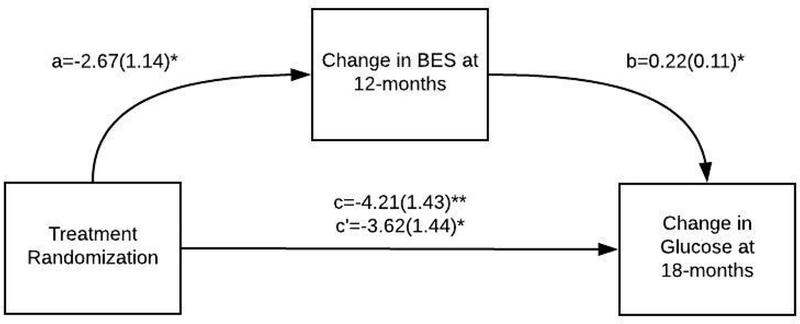
Changes in stress eating at 12 months did not mediate the effects of the intervention on changes in FBG or weight at 12 or 18 months (Appendix C). However, we found that changes in compulsive eating at 12 months mediated the intervention effects on changes in FBG at 18 months (indirect effect B(SE), CI= −0.58(0.41), CI: −1.73, −0.04; p=.04; Appendix C & Figure 4). The indirect effect through change in compulsive eating at 12 months explained 13.95% of the effect of intervention on changes in FBG at 18 months. However, changes in compulsive eating at 12 months was not a statistically significant mediator of the association between intervention and FBG at 12 months (Appendix C). Further, changes in compulsive eating at 12 months mediated the effects of the mindful eating intervention on changes in weight at 12 months (indirect effect B(SE), CI= −0.74(0.33), CI: −1.57, −0.21, p=.007); Figure 5; Appendix C). The indirect effect through change in compulsive eating at 12 months explained 32.54% of the total effect of intervention arm on changes in weight at 12 months. This mediation effect, however, was not statistically significant at 18 months (Appendix C).
Figure 4.
Change in compulsive eating (BES) as a mediator of the association between group (mindfulness vs. active control) and change in fasting blood glucose (FBG) at 18m.
Note. **p<.01, *p<.05. Path coefficients (a, b, c, c’), and corresponding standard errors in parentheses, for mediation analysis (PROCESS Model 4) are unstandardized regression coefficients testing the mediator (change in BES at 12 months). C=Total effect, C’=Direct effect. See Supplemental Table 4 for direct and indirect effects. SE=Standard error.
Figure 5.
Change in compulsive eating (BES) as a mediator of the association between group (mindfulness vs. active control) and change in weight at 12m.
Note. **p<.01, *p<.05. Path coefficients (a, b, c, c’) and corresponding standard errors in parentheses, for mediation analysis (PROCESS Model 4) are unstandardized regression coefficients testing the mediator (change in BES at 12-months). C=Total effect, C’=Direct effect. See Supplemental Table 4 for direct and indirect effects.
DISCUSSION
We assessed the role of stress eating and compulsive eating at baseline as moderators of the effects of a mindful eating intervention, as well as the role of changes in stress eating and compulsive eating during the intervention as mediators of the intervention effects on fasting blood glucose and weight. We found some support for cross-sectional associations between measures of stress and compulsive eating with BMI at baseline, prior to the intervention. Contrary to our hypothesis, we did not find strong evidence that baseline stress and compulsive eating moderated changes in these metabolic outcomes in most cases. The exception was that, as predicted, participants with high baseline BES appeared to derive greater benefit from the mindful eating group in regard to fasting blood glucose compared to those with lower baseline BES. Changes in stress and compulsive eating were associated with changes in metabolic outcomes, and we found some support for a mediating effect of compulsive eating on the impact of intervention condition on changes in metabolic outcomes. In particular, we found that changes in our compulsive eating measure from baseline to 12 months significantly mediated improvements in weight at 12 months and fasting glucose at 18 months.
Stress/Compulsive Eating Effects
Those above the median in stress eating at baseline had BMI levels approximately two points higher, compared to those below the median in stress eating. This finding is consistent with prior studies (Abraham et al., 2014; Roehrig et al., 2009). Contrary to our study hypotheses, and in contrast with several prior studies (Abraham et al., 2014; Raevuori et al., 2015; Webb, Applegate, & Grant, 2011), we found no significant association at baseline between compulsive or stress eating and FBG. Further, compulsive eating was associated with BMI at baseline, but this association did not reach statistical significance. Although most participants at baseline endorsed moderate to high levels of stress eating, the majority endorsed none (61%) or moderate levels of binge eating (33%). The fact that a much smaller proportion of participants endorsed significant compulsive and binge eating, compared to general stress-eating behavior, may explain discrepant findings across stress and compulsive eating in relation to BMI. Furthermore, decreases in stress and compulsive eating were associated with improvements in FBG and weight across intervention groups up to 18 months post-randomization. Given the prospective links between binge eating and worsening in metabolic health (Hudson et al., 2010), it would be logical to infer that improvements in these non-homeostatic eating behaviors would contribute to metabolic improvements. Indeed, in a study of youth with loss of control (LOC) eating (considered a pre-cursor to adult binge eating disorder) who were randomized to an interpersonal psychotherapy group or a health education control group, youth in both treatment arms whose LOC eating improved immediately following intervention evidenced decreases in FBG and lipids when compared to youth whose LOC persisted (Shank et al., 2018). Similarly, among adults with obesity, there is support for rapid reductions in binge eating frequency predicting greater weight loss, regardless of intervention assignment to orlistat + cognitive behavioral therapy, or placebo + cognitive behavioral therapy (Grilo & Masheb, 2007).
Intervention Effects
We found that the mindful eating intervention (vs. active control) led to greater decreases in compulsive eating and to trends in decreased stress eating at follow-up, similar to the work of Kristeller and colleagues (Kristeller et al., 2014). In that study, individuals with obesity (mean baseline BMI of 39.6 kg/m2) and diagnosed BED (per DSM criteria) evidenced lower BES scores at 4-months post-intervention (13.54) relative to at pre-intervention (28.98). Further, one RCT that compared a 3-month mindful eating program (MB-EAT) to a diabetes self-management education program among adults with type-2 diabetes found a significant reduction in glycemic load for both groups, indicating that a mindful eating program can be comparable to diabetes self-management with regard to metabolic improvement (Miller, Kristeller, Headings, Nagaraja, & Miser, 2012).
We found some support for a moderating effect of baseline compulsive eating; however, baseline stress eating did not significantly modify intervention effects. Few studies have examined the moderating role of trait level stress or compulsive eating on behavioral intervention effects on metabolic outcomes. A pilot intervention tailored towards adults with overweight who reported higher baseline levels of emotional eating, a 6-month acceptance-based behavioral intervention contributed to significant decreases in weight (an average weight loss of 12 kg) (Niemeier, Leahey, Palm Reed, Brown, & Wing, 2012). In particular, greater decreases in emotional eating over the course of the intervention were associated with even greater weight loss. While a significant limitation of this study was that it did not include an active control arm, the findings suggest that tailoring an intervention specifically towards those who endorse higher levels of non-homeostatic eating behaviors can contribute to greater weight loss than is typically observed in traditional behavioral weight loss interventions that target weight loss more generally. Surprisingly, limited data have considered the moderating role of trait-level compulsive and stress eating in the effect of intervention on metabolic outcomes.
We found preliminary support that changes in compulsive eating mediated mindfulness intervention effects on improvements in FBG. Specifically, the mindful eating intervention resulted in greater decreases in compulsive eating at 12 months post-randomization, which in turn contributed to greater decreases in FBG and weight. It should be noted that these specific analyses were not part of our pre-registered analytic plan, and are thus considered exploratory. We anticipated that mindful eating would contribute to immediate post-intervention (6 month) decreases in stress and compulsive eating. However, this effect did not occur until 12 months post-randomization, rather than immediately post-intervention, suggesting a delayed effect of mindfulness on these trait-eating patterns. Our findings, while tentative in nature, extend the work of Daubenmier and colleagues (2011) who found that women with obesity who were randomized to a mindfulness intervention for stress eating vs. a waitlist control showed greater decreases in external eating (as measured via the DEBQ) which in turn contributed to reductions in cortisol awakening response and weight maintenance. Similarly, Lillis and colleagues reported that changes in binge eating mediated the effects of acceptance and commitment therapy on change in weight at 3-month follow-up (Lillis et al., 2011). Most recently, Hanson and colleagues found that adding mindful eating strategies to a tier 3 obesity management service contributed to not only greater improvements in eating, but also greater weight loss vs. standard behavioral weight loss alone (Hanson et al., 2019).
Taken together, the current findings suggest that mindfulness-based interventions might be a better fit for adults with obesity who report experiencing higher levels compulsive eating, in comparison to standard behavioral weight loss (e.g., diet + exercise) interventions. Indeed, behavioral weight loss may be less effective for adults who struggle with non-homeostatic eating behaviors (Marcus et al., 1988; Yanovski et al., 2012). Mindfulness interventions adapted specifically towards aberrant eating behaviors may be able to more directly address the needs of adults with obesity who report higher levels of stress and/or compulsive eating (Godfrey et al., 2015; Goyal et al., 2014; Kristeller & Hallett, 1999; Kristeller & Wolever, 2011; Kristeller et al., 2014). In this regard, it is important to note that the intervention in this study was not a generic mindfulness intervention, but drew substantially on MB-EAT, which was developed in part for binge eating disorder. Our study findings add to research on the effects of mindfulness training on metabolic health (specifically FBG and weight) through changes in eating behavior (Daubenmier et al., 2011; Daubenmier et al., 2016; Mason, Epel, Aschbacher, et al., 2016; Mason, Epel, Kristeller, et al., 2016), extending these findings to subgroups of participants who report problems with stress and compulsive eating. Therefore, we recommend a greater focus on mindful eating training and tools, particularly for those who struggle with stress and/or compulsive eating, as a primary enhancement that can be made to existing mindfulness-based interventions. Data suggest that interventions with a specific mindful eating focus (as opposed to a general mindfulness focus) contribute to significant reductions in both cravings and weight (Mason, Jhaveri, Cohn, & Brewer, 2018).
Future investigations might consider targeting weight loss interventions towards an individual’s more salient presenting issues. While adults with obesity who do not report significant levels of stress or compulsive eating may benefit from standard diet and exercise interventions, adults who report moderate to high levels of these behaviors may be better suited for a mindful eating intervention. Furthermore, understanding the mechanisms that explain the role of changes in stress and compulsive eating in improving metabolic health will be important in future clinical trials among adults with obesity. The current study was limited by a sample size that may have been too small to detect modest interaction effects of intervention arm with stress or compulsive eating at baseline. Thus, despite promising trends, we were likely under-powered to detect some potentially relevant moderating or mediating effects of stress eating and compulsive eating. Further, our measures of stress eating (DEBQ) and compulsive eating (BES) may not fully reflect non-homeostatic eating behavior. A semi-structured interview measure of eating pathology, fully assessing the presence of clinical BED, would have strengthened our ability to draw conclusions about the influence of non-homeostatic eating behaviors on metabolic outcomes. Finally, we did not have a high proportion of participants who reported symptoms of BED using the BES, and most were in the healthy FBG range, making our findings even more broadly relevant to community, in addition to clinical samples of people with obesity.
CONCLUSIONS
One of the greatest problems in the field of obesity management is the tendency towards weight regain following most behavioral weight loss interventions. One potential reason for this finding is that the underlying cause of overweight for some is psychological stress, emotionally-triggered eating, and/or compulsive overeating, which most behavioral weight loss programs do not thoroughly assess. Programs such as mindfulness-based eating interventions specifically target these issues. Thus, tailoring treatment options towards individuals based on mechanisms for overeating and obesity may be a crucial factor in improving metabolic outcomes. This RCT suggests that those with a tendency toward greater compulsive eating may derive the greatest benefit in regard to metabolic outcomes such as reducing fasting blood glucose if they receive an intervention similar to our mindful eating training (as opposed to behavioral weight loss alone). Future obesity interventions should consider tailoring treatment options towards baseline trait-level characteristics, such as stress or compulsive overeating.
Supplementary Material
Acknowledgements:
We gratefully acknowledge our participants for donating their time to this study. We presented portions of this manuscript at the Society for Behavioral Medicine annual meeting in Washington, DC in March of 2019.
Funding Sources: Funding for this study was provided by the National Center for Complementary and Integrative Health (NCCIH; formerly NCCAM) F32 AT009649 (to RMR), P01AT005013 (to FH), K24AT007827 (to FH), K01AT004199 (to JD); the National Heart, Lung, and Blood Institute (NHLBI) K23 HL133442 (to AEM), as well as the National Center for Advancing Translational Sciences, UCSF-CTSI Grant Number UL1 TR000004. NCCIH and NHLBI had no role in the study design, collection, analysis, or interpretation of data, writing the manuscript, or the decision to submit the paper for publication. FMH was on the Scientific Advisory Board for Virta during preparation of this manuscript; he is no longer. All remaining authors declare they have no conflicts of interest.
Footnotes
Trial Registration: ClinicalTrials.gov identifier . Preregistration with AsPredicted (https://aspredicted.org/nk4av.pdf; #8472)
REFERENCES
- Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, & Fox CS (2014). Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring), 22(11), 2441–2449. doi: 10.1002/oby.20867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow B, Kenardy J, & Agras WS (1992). Binge eating among the obese: a descriptive study. J Behav Med, 15(2), 155–170. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1583679 [DOI] [PubMed] [Google Scholar]
- Borecki IB, Higgins M, Schreiner PJ, Arnett DK, Mayer-Davis E, Hunt SC, & Province MA (1998). Evidence for multiple determinants of the body mass index: the National Heart, Lung, and Blood Institute Family Heart Study. Obes Res, 6(2), 107–114. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9545016 [DOI] [PubMed] [Google Scholar]
- Bray GA (2003). Risks of obesity. Endocrinol Metab Clin North Am, 32(4), 787–804, viii Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14711062 [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM, & Creswell JD (2007). Mindfulness: Theoretical Foundations and Evidence for its Salutary Effects. Psychological Inquiry, 18(4), 211–237. doi: 10.1080/10478400701598298 [DOI] [Google Scholar]
- Bruce B, & Agras WS (1992). Binge eating in females: A population based investigation. International Journal of Eating Disorders, 12(4), 365–373. [Google Scholar]
- Carlson LE, Speca M, Faris P, & Patel KD (2007). One year pre–post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, behavior, and immunity, 21(8), 1038–1049. [DOI] [PubMed] [Google Scholar]
- Celio AA, Wilfley DE, Crow SJ, Mitchell J, & Walsh BT (2004). A Comparison of the Binge Eating Scale, Questionnaire for Eating and Weight Patterns-Revised, and Eating Disorder Examination Questionnaire with Instructions with the Eating Disorder Examination in the Assessment of Binge Eating Disorder and its Symptoms. International Journal of Eating Disorders, 36(4), 434–444. doi: 10.1002/eat.20057 [DOI] [PubMed] [Google Scholar]
- Chen W, Srinivasan SR, Elkasabany A, & Berenson GS (1999). Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol, 150(7), 667–674. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10512420 [DOI] [PubMed] [Google Scholar]
- Cummings JR, Mason AE, Puterman E, & Tomiyama AJ (2018). Comfort Eating and All-Cause Mortality in the US Health and Retirement Study. International Journal of Behavioral Medicine, 25(4), 473–478. doi: 10.1007/s12529-017-9706-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, … Epel E (2011). Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J Obes, 2011, 651936. doi: 10.1155/2011/651936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, … Hecht FM (2016). Effects of a mindfulness-based weight loss intervention in adults with obesity: A randomized clinical trial. Obesity (Silver Spring), 24(4), 794–804. doi: 10.1002/oby.21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de França GVA, Gigante DP, & Olinto MTA (2014). Binge eating in adults: prevalence and association with obesity, poor self-rated health status and body dissatisfaction. Public health nutrition, 17(04), 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, & Sniehotta FF (2014). Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ, 348, g2646. doi: 10.1136/bmj.g2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douketis JD, Macie C, Thabane L, & Williamson DF (2005). Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond), 29(10), 1153–1167. doi: 10.1038/sj.ijo.0802982 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kuczmarski RJ, & Johnson CL (1998). Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord, 22(1), 39–47. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9481598 [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Gallo LC, & Afari N (2015). Mindfulness-based interventions for binge eating: a systematic review and meta-analysis. J Behav Med, 38(2), 348–362. doi: 10.1007/s10865-014-9610-5 [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, & Rardin D (1982). The assessment of binge eating severity among obese persons. Addict Behav, 7(1), 47–55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7080884 [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, … Haythornthwaite JA (2014). Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med, 174(3), 357–368. doi: 10.1001/jamainternmed.2013.13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeno CG, Marcus MD, & Wing RR (1995). Diagnosis of binge eating disorder: Discrepancies between a questionnaire and clinical interview. International Journal of Eating Disorders, 17(2), 153–160. doi:doi: [DOI] [PubMed] [Google Scholar]
- Grilo CM, & Masheb RM (2007). Rapid response predicts binge eating and weight loss in binge eating disorder: findings from a controlled trial of orlistat with guided self-help cognitive behavioral therapy. Behav Res Ther, 45(11), 2537–2550. doi: 10.1016/j.brat.2007.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, … Epel E (2012). What is eating you? Stress and the drive to eat. Appetite, 58(2), 717–721. doi: 10.1016/j.appet.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P, Shuttlewood E, Halder L, Shah N, Lam FT, Menon V, & Barber TM (2019). Application of Mindfulness in a Tier 3 Obesity Service Improves Eating Behavior and Facilitates Successful Weight Loss. J Clin Endocrinol Metab, 104(3), 793–800. doi: 10.1210/jc.2018-00578 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper]. Retrieved from Retrieved from http://www.afhayes.com/public/process2012.pdf
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, … Simon NM (2013). Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry, 74(8), 786–792. doi: 10.4088/JCP.12m08083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, Hiripi E, Pope HG, & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry, 61(3), 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, … Pope HGJ (2010). Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge eating disorder. Am J Clin Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivezaj V, White MA, & Grilo CM (2016). Examining binge eating disorder and food addiction in adults with overweight and obesity. Obesity, 24(10), 2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York, N.Y.: Dell Publishing [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, … Benjet C (2013). The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biological psychiatry, 73(9), 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman PG (2000). Obesity as a medical problem. Nature, 404, 635. doi: 10.1038/35007508 [DOI] [PubMed] [Google Scholar]
- Kristeller J, & Hallett CB (1999). An exploratory study of a meditation-based intervention for binge eating disorder. Journal of health psychology, 4(3), 357–363. [DOI] [PubMed] [Google Scholar]
- Kristeller J, & Wolever RQ (2011). Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord, 19(1), 49–61. doi: 10.1080/10640266.2011.533605 [DOI] [PubMed] [Google Scholar]
- Kristeller J, Wolever RQ, & Sheets V (2014). Mindfulness-Based Eating Awareness Training (MB EAT) for Binge Eating: A Randomized Clinical Trial. J Mindfulness, 5(3), 282–297. doi: 10.1007/s12671-012-0179-1 [DOI] [Google Scholar]
- Kuczmarski RJ, Flegal KM, Campbell SM, & Johnson CL (1994). Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA, 272(3), 205–211. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8022039 [DOI] [PubMed] [Google Scholar]
- Lillis J, Hayes SC, & Levin ME (2011). Binge eating and weight control: the role of experiential avoidance. Behav Modif, 35(3), 252–264. doi: 10.1177/0145445510397178 [DOI] [PubMed] [Google Scholar]
- Marcus MD, Wing RR, & Hopkins J (1988). Obese binge eaters: affect, cognitions, and response to behavioural weight control. J Consult Clin Psychol, 56(3), 433–439. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3397436 [DOI] [PubMed] [Google Scholar]
- Mason AE, Epel ES, Aschbacher K, Lustig RH, Acree M, Kristeller J, … Daubenmier J (2016). Reduced reward-driven eating accounts for the impact of a mindfulness-based diet and exercise intervention on weight loss: Data from the SHINE randomized controlled trial. Appetite, 100, 86–93. doi: 10.1016/j.appet.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Epel ES, Kristeller J, Moran PJ, Dallman M, Lustig RH, … Daubenmier J (2016). Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: data from the SHINE randomized controlled trial. J Behav Med, 39(2), 201–213. doi: 10.1007/s10865-015-9692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Jhaveri K, Cohn M, & Brewer JA (2018). Testing a mobile mindful eating intervention targeting craving-related eating: feasibility and proof of concept. Journal of Behavioral Medicine, 41(2), 160–173. doi: 10.1007/s10865-017-9884-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Reichenberger J, & Blechert J (2018). Development and preliminary validation of the Salzburg Stress Eating Scale. Appetite, 120, 442–448. doi: 10.1016/j.appet.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Miller CK, Kristeller JL, Headings A, Nagaraja H, & Miser WF (2012). Comparative Effectiveness of a Mindful Eating Intervention to a Diabetes Self-Management Intervention among Adults with Type 2 Diabetes: A Pilot Study. Journal of the Academy of Nutrition and Dietetics, 112(11), 1835–1842. doi: 10.1016/j.jand.2012.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier HM, Leahey T, Palm Reed K, Brown RA, & Wing RR (2012). An acceptance-based behavioral intervention for weight loss: a pilot study. Behav Ther, 43(2), 427–435. doi: 10.1016/j.beth.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SL, Zhang X, Avenell A, Gregg E, Brown T, Schmid CH, & Lau J (2016). Long-term non-pharmacological weight loss interventions for adults with type 2 diabetes mellitus. Sao Paulo Med J, 134(2), 184. doi: 10.1590/1516-3180.20161342T2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, & Flegal KM (2015). Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS Data Brief(219), 1–8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26633046 [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, & Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA, 311(8), 806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri MG (1998). The Maintenance of Treatment Effects in the Long-Term Management of besity. 5(4), 526–543. doi:doi: 10.1111/j.1468-2850.1998.tb00172.x [DOI] [Google Scholar]
- Pinaquy S, Chabrol H, Simon C, Louvet J-P, & Barbe P (2003). Emotional Eating, Alexithymia, and Binge-Eating Disorder in Obese Women. Obesity Research, 11(2), 195–201. doi: 10.1038/oby.2003.31 [DOI] [PubMed] [Google Scholar]
- Raevuori A, Suokas J, Haukka J, Gissler M, Linna M, Grainger M, & Suvisaari J (2015). Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. Int J Eat Disord, 48(6), 555–562. doi: 10.1002/eat.22334 [DOI] [PubMed] [Google Scholar]
- Roehrig M, Masheb RM, White MA, & Grilo CM (2009). The metabolic syndrome and behavioral correlates in obese patients with binge eating disorder. Obesity (Silver Spring), 17(3), 481–486. doi: 10.1038/oby.2008.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank LM, Tanofsky-Kraff M, Radin RM, Shomaker LB, Wilfley DE, Young JF, … Yanovski JA (2018). Remission of loss of control eating and changes in components of the metabolic syndrome. Int J Eat Disord, 51(6), 565–573. doi: 10.1002/eat.22866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims R, Gordon S, Garcia W, Clark E, Monye D, Callender C, & Campbell A (2008). Perceived stress and eating behaviors in a community-based sample of African Americans. Eat Behav, 9(2), 137–142. doi: 10.1016/j.eatbeh.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB, & Willett JB (2003). Applied longitudinal data analysis: Modeling change and event occurrence: Oxford university press. [Google Scholar]
- Striegel-Moore RH, & Franko DL (2008). Should binge eating disorder be included in the DSM-V? A critical review of the state of the evidence. Annu Rev Clin Psychol, 4, 305–324. doi: 10.1146/annurev.clinpsy.4.022007.141149 [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Shomaker LB, Stern EA, Miller R, Sebring N, Dellavalle D, … Yanovski JA (2012). Children’s binge eating and development of metabolic syndrome. Int J Obes (Lond), 36(7), 956–962. doi: 10.1038/ijo.2011.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman GM (1999). Binge Eating Scale: Further Assessment of Validity and Reliability1. Journal of Applied Biobehavioral Research, 4(1), 1–12. doi:doi: 10.1111/j.1751-9861.1999.tb00051.x [DOI] [Google Scholar]
- Torres SJ, & Nowson CA (2007). Relationship between stress, eating behavior, and obesity. Nutrition, 23(11–12), 887–894. doi: 10.1016/j.nut.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Vainik U, Neseliler S, Konstabel K, Fellows LK, & Dagher A (2015). Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite, 90, 229–239. doi: 10.1016/j.appet.2015.03.004 [DOI] [PubMed] [Google Scholar]
- van Strien T, Frijters JER, Bergers GPA, & Defares PB (1986). The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders, 5(2), 295–315. doi:doi: [DOI] [Google Scholar]
- van Strien T, Herman CP, & Verheijden MW (2009). Eating style, overeating, and overweight in a representative Dutch sample. Does external eating play a role? Appetite, 52(2), 380–387. doi: 10.1016/j.appet.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Webb JB, Applegate KL, & Grant JP (2011). A comparative analysis of Type 2 diabetes and binge eating disorder in a bariatric sample. Eat Behav, 12(3), 175–181. doi: 10.1016/j.eatbeh.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, & Engel SG (2009). The validity and clinical utility of binge eating disorder. Int J Eat Disord, 42(8), 687–705. doi: 10.1002/eat.20719 [DOI] [PubMed] [Google Scholar]
- Yanovski SZ, Gormally JF, Leser MS, Gwirtsman HE, & Yanovski JA (2012). Binge Eating Disorder Affects Outcome of Comprehensive Very-Low Calorie Diet Treatment. Obesity research, 2(3), 205–212. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, … Caprio S (2007). The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes, 8(5), 299–306. doi:PDI271 [pii] 10.1111/j.1399-5448.2007.00271.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.