ABSTRACT
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease; the identification of novel targets and development of effective treatment strategies are urgently needed to improve patient outcomes. Remodeling of the pancreatic stroma occurs during PDAC development, which drives disease progression and impairs responses to therapy. The actomyosin regulatory ROCK1 and ROCK2 kinases govern cell motility and contractility, and have been suggested to be potential targets for cancer therapy, particularly to reduce the metastatic spread of tumor cells. However, ROCK inhibitors are not currently used for cancer patient treatment, largely due to the overwhelming challenge faced in the development of anti-metastatic drugs, and a lack of clarity as to the cancer types most likely to benefit from ROCK inhibitor therapy. In 2 recent publications, we discovered that ROCK1 and ROCK2 expression were increased in PDAC, and that increased ROCK activity was associated with reduced survival and PDAC progression by enabling extracellular matrix (ECM) remodeling and invasive growth of pancreatic cancer cells. We also used intravital imaging to optimize ROCK inhibition using the pharmacological ROCK inhibitor fasudil (HA-1077), and demonstrated that short-term ROCK targeting, or ‘priming’, improved chemotherapy efficacy, disrupted cancer cell collective movement, and impaired metastasis. This body of work strongly indicates that the use of ROCK inhibitors in pancreatic cancer therapy as ‘priming’ agents warrants further consideration, and provides insights as to how transient mechanical manipulation, or fine-tuning the ECM, rather than chronic stromal ablation might be beneficial for improving chemotherapeutic efficacy in the treatment of this deadly disease.
KEYWORDS: cell contractility, cell invasion, intravital imaging, pancreatic cancer, Rho-kinase, tissue stiffness
Introduction
Despite there being several new therapeutics that have been developed for pancreatic cancer patient therapy, survival remains the lowest of all solid cancers, with 5-year survival rate being less than 7% and a median survival of 6 months.1 Despite pre-clinical efforts to develop new therapeutics,2 patient survival has not significantly improved over the last 4 decades, which highlights not only the need to identify new targets, but also to develop innovative treatment strategies to improve the outcomes of patients having this disease. In addition, development of diagnostic tools, for example based on detection of cancer-derived exosomes,3 to enable early detection of pancreatic cancer remains a critical challenge for this disease. Pancreatic ductal adenocarcinoma (PDAC) is characterized by extensive remodeling of the pancreatic stroma, with increased deposition and crosslinking of extracellular matrix (ECM) components and poor vascularization compared with normal pancreas.4,5 Alterations of the biochemical and mechanical properties of the ECM are known to influence cancer progression, invasion and responses to chemotherapy,6-9 however, recent studies assessing the efficacy of ECM-based pancreatic cancer therapies, for example via inhibition of Sonic Hedgehog signaling pathway, targeting of lysyl oxidase (LOX) activity or inhibition of hyaluronic acid (HA), have yielded conflicting results.4,10-16
Rho-associated protein kinases 1 and 2 (ROCK1 and ROCK2) are master regulators of the actomyosin cytoskeleton and govern force generation, cell invasion, proliferation and contractility.17-19 Numerous studies have established that ROCK inhibition disrupts tumor progression and metastasis in cell-based and in vivo models of various solid cancers.20-23 However, to date no compounds have progressed into the clinic for cancer therapy for several reasons. The development of anti-metastatic chemotherapeutics for clinical use is very challenging due to the need to detect a reduction in metastasis in patients over sustained periods (likely years) as a positive outcome,24 in contrast to chemotherapeutics that induce acute positive responses, such as tumor regression, which can be monitored in a clinical trial in a defined and relatively brief time period.24 Furthermore, the absence of correlations between defined genetic alterations, such as ROCK1 or ROCK2 mutations, with ROCK inhibitor sensitivity means that there is no simple genetic test for convenient patient stratification. As a result, ROCK inhibition has not been adopted as a cancer chemotherapy. In this commentary, we describe our recent findings25,26 demonstrating that ROCK activity promotes pancreatic cancer invasive growth via ECM remodeling. We also highlight how transient ROCK inhibition, or mechanical ‘priming’ with the pharmacological inhibitor fasudil affects tumor tissue tension, which in turn improves chemotherapy efficacy in primary and secondary tumor sites, while also disrupting collective movement of metastatic cancer cells.26 Lastly, we discuss potential translation of our findings into the clinic for pancreatic cancer therapy, where balancing cellular contractility via transient ROCK inhibition, rather than long-term ablation of the matrix, enables re-establishment of the normal mechanical features of the stroma.
ROCK activity promotes PDAC progression
Genomic analyses have previously shown that the ROCK1 gene is amplified in 15% of pancreatic patient tumors,27 however the role of ROCK-mediated actomyosin contractility in PDAC had not been clearly established. To address this, we assessed ROCK expression in a patient tissue microarray (78 samples from patients with pancreatic cancers and 5 healthy human pancreas) and in human TCGA data sets, and determined that ROCK1 and ROCK2 expression increase with tumor stage and grade.25 In line with this, genomic alterations or mRNA amplification of ROCK1 and/or ROCK2 were found to be positively correlated with poorer survival, suggesting that ROCK signaling promotes pancreatic cancer progression.25
To further understand how ROCK influences the fate and behavior of pancreatic cancer cells, Cre-recombinase was expressed from the pancreatic epithelial selective Pdx1 promoter to induce pancreas-targeted recombination of LOX-STOP-LOX (LSL)-KrasG12D/+and LSL-Trp53R172H/+ (KPC) alleles in mice, which spontaneously develop PDAC that closely resembles human pancreatic cancer.28,29 In addition, KPC mice were crossed with LSL-ROCK2:ER mice30 to conditionally activate ROCK2 during PDAC progression. This model closely recapitulates the genomic features of human PDAC, where initiating mutations in Kras are found in almost 90% of patient tumors, while mutations in p53 are found in 50–75% of patient tumors.31 Consistent with the observed increased ROCK2 protein levels in advanced PDAC stages, as well as the correlation between increased ROCK1 and ROCK2 mRNA expression, along with a potentially activating truncation mutation (I383F-frameshift deletion; TCGA-HZ-8005–01), with poor survival from the TCGA human data set, conditional ROCK2 activation was associated with reduced PDAC mouse survival. Conditional ROCK2 activation in non metastatic PDAC cells isolated from genetically modified mice promoted pancreatic cancer cell invasion into 3D collagen matrices (see schematic representation of ROCK inhibition at the cellular level, Fig. 1A).25 Interestingly, analyses of cell-ECM interactions using Second Harmonic Generation (SHG) imaging, a label free imaging technique used to detect non-centrosymmetric entities such as crosslinked collagen fibers, or tannic acid-glutaraldehyde fixation of collagen fibers for transmission electron microscopy, revealed that ROCK activation induced extensive remodeling of the collagen matrix surrounding invading cancer cells.25
Figure 1.
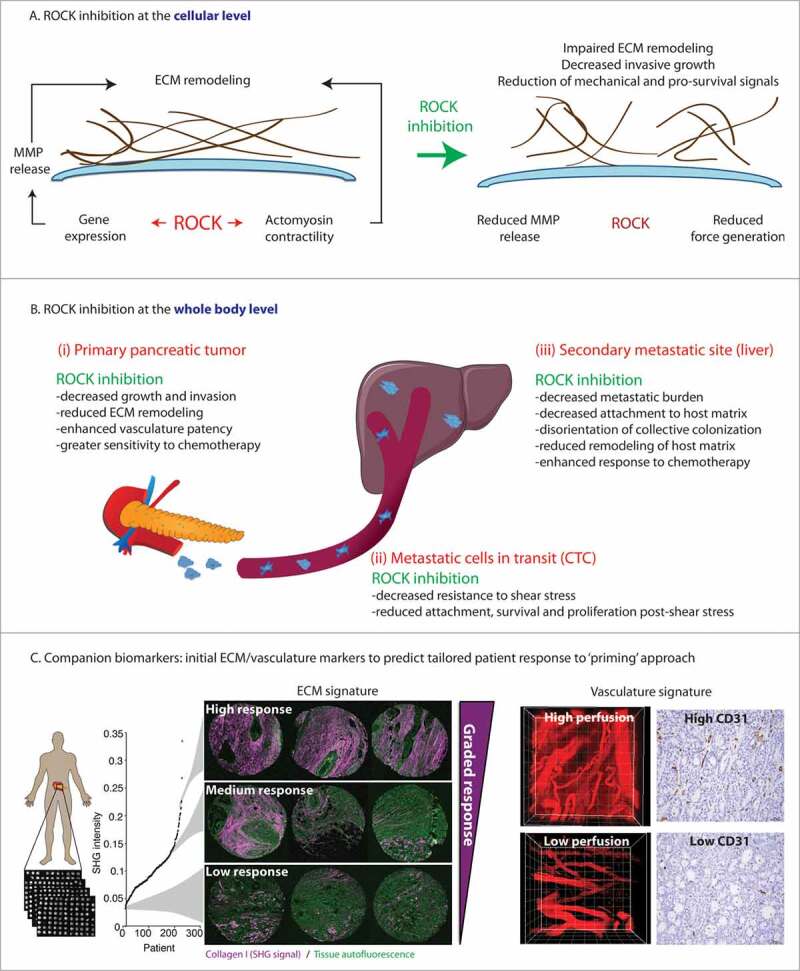
Schematic of the roles of ROCK activity and ROCK inhibition in pancreatic cancer: from cell-to-global effects to translation to patients. A. ROCK inhibition at the cellular level impairs ECM remodeling via decreased MMP release and impaired contractility. B. ROCK inhibition at the whole body, global level. Schematic representation of the effects of ROCK inhibition in primary tumor tissue (left hand panel), on circulating tumor cells (CTC, middle panel) and at secondary sites (right hand panel). Adapted from (Vennin et al., Science Translational Medicine 2017).26 Reprinted with permission from AAAS. C. Combination of ECM and vasculature markers as companion biomarkers for priming strategy. Left hand panel: Schematic representation of in-house automated Second Harmonic Generation (SHG) analysis of the ECM in the ICGC human TMA cohort, with examples of SHG signals in cores (triplicates) from patients with high, medium, or low SHG signal. Right hand panel: representative images of quantum dots and CD31 (cluster of differentiation 31) staining in tumors with high and low vascularity. Adapted from (Vennin etal., Science Translational Medicine 2017).26 Reprinted with permission from AAAS.
While ROCK is well known to induce force generation via its action on actomyosin structures,19 ROCK signaling also induces gene transcription.32 To identify ROCK induced gene expression changes, we performed RNA sequencing and identified 285 genes that were consistently and significantly found to be changed greater than twofold relative to control cells. Interestingly, conditional ROCK activation increased expression of metalloproteinases (MMP) Mmp10 and Mmp13, which was associated with increased release of these MMPs into the surrounding environment (see schematic representation of ROCK inhibition at the cellular level, Fig. 1A). These results indicated that ROCK mediates collagen remodeling by pancreatic cancer cells via transcription, synthesis and release of MMPs, in line with previous observations in melanoma cells,33 and in pancreatic cancer cells in which dasatinib-induced reduction of KPC cell migration was correlated with reduced production of MMP2 and MMP9.34 We also determined that ROCK-mediated remodeling of the surrounding matrix facilitated invasive growth of pancreatic cancer cells (see schematic representation of ROCK inhibition at the cellular and whole-body levels, Fig. 1A, B). These findings highlight the ability of cancer cells to adapt to the mechanical environment and to remodel the ECM to support their aberrant growth. These cell-based observations were further extended in KPC mice, where ROCK inhibition with fasudil significantly prolonged survival, and reduced collagen remodeling (see schematic representation of ROCK inhibition at the cellular and whole-body levels Fig. 1A, B).25 Together, these results shed light on novel roles of ROCK in driving pancreatic cancer progression, suggesting that targeting ROCK might be beneficial for the clinical management of the disease.
Transient ROCK inhibition with fasudil disrupts pancreatic cancer
Although ROCK-driven cell contractility and stromal remodeling are known to play crucial roles in cancer progression,7,19,35 ROCK inhibitors and ECM-based therapies have yet to be translated to the clinic. In our recent publication, we assessed the efficacy of fasudil to impair PDAC progression and to influence cell responses to chemotherapy.26 Fasudil is a ROCK inhibitor currently used clinically as a monotherapy for the treatment of cerebral vasospasm,36 and fasudil has also been shown to inhibit, in a less potent manner than for ROCK, other kinases such as PKA, PKC and MLCK.37 Meta-analysis of post-marketing surveillance data (> 3,000 patients) has demonstrated the safety of fasudil for clinical use in humans,38 which prompted us to assess the repurposing of fasudil for the treatment of pancreatic cancer. We combined mouse and stratified patient-derived models of pancreatic cancer with biosensor FLIM-FRET intravital imaging to monitor the effect of ROCK inhibition in real-time and in live tissues.39-42 Using an early, transient ‘priming’ regimen, where fasudil was administered for 3 days before chemotherapy, in line with its treatment regimen in patients with stable angina,43 we demonstrated that short-term ROCK inhibition with fasudil synchronized pancreatic cancer cell cycle progression, and rendered them more sensitive to subsequent treatment with anti-microtubule drugs and standard-of-care chemotherapy, both in primary tumors and metastatic sites (see schematic representation of ROCK inhibition at the whole-body level, Fig. 1B).26 We also observed that ‘priming’ with fasudil in the adjuvant setting disturbed coordinated cancer cell movement and impaired metastatic colonization in the liver (see schematic representation of ROCK inhibition at the whole-body level, Fig. 1B).
Assessment of the effect of ‘priming’ on key metastatic events revealed that ROCK inhibition rendered circulating tumor cells more sensitive to shear stress to which they are subjected in the blood circulation and in turn impaired their ability to extravasate and colonize host tissues (see schematic representation of ROCK inhibition at the whole-body level, Fig. 1B), consistent with previous studies.44,45 Additionally, analysis of collective cell movement, or streaming, upon ‘priming’ suggested that transient ROCK inhibition impaired coordinated cell migration and 3D cell movement of the metastatic emboli in the liver (see schematic representation of ROCK inhibition at the whole-body level, Fig. 1B),26 possibly due to disrupted durotaxis - where cell movement is directed by stiffness gradients - in the metastatic niche.46 The observed reduction of coordinated PDAC cell spread that we observed upon ROCK inhibition was also in line with previous work highlighting how the Rho-ROCK-LIMK pathway leads tumor cell invasion by driving path generation.47 ROCK inhibition was also found to reduce the ability of metastatic cells to remodel the host ECM and to create a favorable environment to support their growth in a distant site (see schematic representation of ROCK inhibition at the whole-body level Fig. 1B), as recently demonstrated in pancreatic cancer and melanoma.48-50 Assessment of the effects of ‘priming’ with fasudil on the stroma demonstrated that transient ROCK inhibition reduced ECM remodeling and tissue stiffness, thereby altering integrin signaling and depriving cancer cells of mechanical cues provided by the matrix.26 In addition, decompression of the tumor tissue upon ‘priming’ with fasudil was accompanied by relaxation and increased permeability of the tumor vasculature, as assessed by the imaging of quantum dots diffusing from blood vessels and into tumor tissue (see schematic representation of ROCK inhibition at the whole-body level Fig. 1B and Movie 1).26 This is in line with the current clinical use of fasudil for the treatment of cerebral vasospasm,36,43 and with recent work demonstrating that ROCK regulates vascular patency, or obstruction.51 Our findings therefore demonstrate that fasudil has a dual effect on both the ECM and the intratumoral vasculature, which together increased drug delivery and improved cancer cell responses to chemotherapy. This aligns with recent stromal-based strategies in metastatic colorectal cancer, where the combination of anti-VEGF therapy and anti-hyaluronic acid treatment significantly improved chemotherapy efficacy and prolonged survival compared with anti-VEGF therapy alone.52 Our work also indicates that rather than chronic treatment, which has a greater potential for adverse effects and toxicity,11,14 acute fasudil treatment to induce transient mechanical ‘priming’ was sufficient to re-equilibrate the pancreatic tumor stroma and to impair PDAC progression. Together, our findings demonstrate that ‘priming’ with fasudil might be beneficial both in the neo-adjuvant and adjuvant settings, which strongly suggests that further clinical assessment of fasudil in combination with standard-of-care chemotherapy, such as Gemcitabine and Abraxane, is warranted to improve PDAC patient outcomes.
Balancing cell contractility: A new approach to treat pancreatic cancer
While numerous studies have demonstrated that extensive transformation of the pancreatic stroma occurs during cancer development,5,53 previous work assessing ECM-based therapies have yielded conflicting data regarding the efficacy of stromal therapies in pancreatic cancer. As such, while pharmacological inhibition of the Hedgehog (Hh) signaling pathway,4 hyaluronic acid (HA) deposition13,15 or lysyl oxidase (LOX) activity12 resulted in impaired tumor growth and increased survival in mouse models of pancreatic cancer, genetic ablation of Hh signaling14 or myofibroblasts11 resulted in decreased survival. Importantly, ablation of fibrosis triggered adverse effects on the pancreatic stroma, such as profound alterations of the immune microenvironment, which in turn promoted cancer progression.11,14 Identification of new ECM targets and development of innovative therapeutic regimens to ‘fine-tune’ and manipulate the pancreatic stroma are therefore needed to improve pancreatic cancer patient outcomes. We believe that this balance is key to future development of stromal targeting strategies for this disease.
Our 2 recent publications25,26 establish ROCK as a key regulator of matrix remodeling in pancreatic cancer, both via generation of contractile force, and regulation of MMP synthesis and release into the surrounding matrix (see schematic representation of ROCK inhibition at the cellular level, Fig. 1A). These findings align with recent work in pancreatic cancer demonstrating that the JAK/ROCK/STAT3 signaling pathway governs cancer cellular tension and promotes tumor progression via remodeling of the surrounding matrix in close proximity to the tumor.53 Our observations also highlight the intricate effects of ROCK-induced remodeling of the ECM. While prolonged exposure to fasudil significantly increased mechanical constraints and reduced tumor growth in the KPC model, potentially via reduced release of MMPs into the environment, transient ‘priming’ with fasudil led to reduced ECM crosslinking and relaxation of tumor tissue. This aligns with the emerging concept that the pancreatic stroma can both promote and restrain disease progression.8,16 Importantly, our work provides pre-clinical evidence that fine-tuning the ECM via transient ROCK inhibition using our ‘priming’ approach might provide new avenues for the treatment of pancreatic cancer. Potential hypotensive effects of ROCK inhibition with fasudil might be expected given its use for cerebral vasospasm, however the actions on the vasculature that we observe also have the potential beneficial effect of increasing drug delivery. Consistent with recently published work from the Weaver laboratory, we report no significant change in patient survival associated with bulk tumor stroma,26,53 however our study demonstrates a graded response to the ‘priming’ strategy in patient-derived xenografts that had been stratified based on their ECM signature.26 Where in tumors with high ECM content, ‘priming’ with fasudil greatly improved cancer cell responses to chemotherapy, delayed metastasis and approximately doubled survival compared with chemotherapy alone, this had a modest effect in tumors with low ECM content.26 This observation suggested that initial collagen content could be used as a surrogate biomarker alone, or because of the dual effects of fasudil ‘priming’ on the ECM and the intratumoral vasculature, in combination with tumor vasculature markers, such as CD31 (cluster of differentiation 31), to identify patients most likely to benefit from transient ROCK inhibition before chemotherapy (see schematic representation companion biomarker strategy, Fig. 1C). Additionally, non-invasive PET-reporters of fibrotic tissue are being developed for diagnosis of pulmonary fibrosis, which could be repurposed in this context.54 We propose that the repurposing of a low-cost, off-patent drug such as fasudil as a ‘priming’ agent might be beneficial for pancreatic cancer therapy. In addition, novel ROCK inhibitors such as AT13148, KD025 or CCT129254, currently in the clinical testing pipeline as anti-fibrotic agents, or in phase I clinical trial for the treatment of solid tumors (AT13148, NCT0158570155) could also have similar applications.56-59 Remodeling of the stroma has also been reported to occur in other solid cancers and to influence disease progression.7,48,60,61,62,63 Therefore, we envisage that fine-tuning the ECM via ROCK inhibition before standard-of-care therapies might lead to substantial therapeutic benefits in other forms of cancer.
Supplementary Material
Funding
Funding was provided from Cancer Research UK to MFO (A18276) and to the Cancer Research UK Beatson Institute (A17196), NHMRC, Cancer Council NSW, Cancer Australia, Tour de Cure grants, Cancer Institute NSW, ARC Future, Lee Ainsworth and Philip Hemstritch Pancreatic Cancer Fellowships, Sydney Catalyst scholarship.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67:7-30; https://doi.org/ 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- [2].Spadi R, Brusa F, Ponzetti A, Chiappino I, Birocco N, Ciuffreda L, Satolli MA. Current therapeutic strategies for advanced pancreatic cancer: A review for clinicians. World J Clin Oncol 2016; 7:27-43; PMID:26862489; https://doi.org/ 10.5306/wjco.v7.i1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, Clardy S, Huang CH, Pille C, Ferrone S, et al.. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med 2017; 9:pii: eaal3226; https://doi.org/ 10.1126/scitranslmed.aal3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al.. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324:1457-61; PMID:19460966; https://doi.org/ 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neesse A, Michl P, Frese KK, Feig C, Cook N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al.. Stromal biology and therapy in pancreatic cancer. Gut 2011; 60:861-8; PMID:20966025; https://doi.org/ 10.1136/gut.2010.226092 [DOI] [PubMed] [Google Scholar]
- [6].Harris NL, Vennin C, Conway JR, Vine KL, Pinese M, Cowley MJ, Shearer RF, Lucas MC, Herrmann D, Allam AH, et al.. SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene 2017; 63:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 2014; 15:1243-53; PMID:25381661; https://doi.org/ 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rath N, Olson MF. Regulation of pancreatic cancer aggressiveness by stromal stiffening. Nat Med 2016; 22:462-3; PMID:27149218; https://doi.org/ 10.1038/nm.4099 [DOI] [PubMed] [Google Scholar]
- [9].Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J, Edward M, Campbell AD, McGarry LC, Evans TR, et al.. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res 2013; 73:4674-86; PMID:23749641; https://doi.org/ 10.1158/0008-5472.CAN-12-4545 [DOI] [PubMed] [Google Scholar]
- [10].Chang J, Lucas MC, Leonte LE, Garcia-Montolio M, Singh LB, Findlay AD, Deodhar M, Foot JS, Jarolimek W, Timpson P, et al.. Pre-clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget 2017; 8:26066-78; PMID:28199967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al.. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014; 25:719-34; PMID:24856586; https://doi.org/ 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, Timpson P, Leach J, McGarry L, Shanks E, et al.. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med 2015; 7:1063-76; PMID:26077591; https://doi.org/ 10.15252/emmm.201404827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al.. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013; 62:112-20; PMID:22466618; https://doi.org/ 10.1136/gutjnl-2012-302529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al.. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25:735-47; PMID:24856585; https://doi.org/ 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21:418-29; PMID:22439937; https://doi.org/ 10.1016/j.ccr.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cox T, Erler JT. Fibrosis and cancer: partner in crime or opposing forces? Trends in Cancer 2016; 2:279-82; https://doi.org/ 10.1016/j.trecan.2016.05.004 [DOI] [PubMed] [Google Scholar]
- [17].Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446-56; PMID:12778124; https://doi.org/ 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- [18].Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 2014; 5:e29846; PMID:25010901; https://doi.org/ 10.4161/sgtp.29846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rath N, Olson MF. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep 2012; 13:900-8; PMID:22964758; https://doi.org/ 10.1038/embor.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sadok A, McCarthy A, Caldwell J, Collins I, Garrett MD, Yeo M, Hooper S, Sahai E, Kuemper S, Mardakheh FK, et al.. Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Res 2015; 75:2272-84; PMID:25840982; https://doi.org/ 10.1158/0008-5472.CAN-14-2156 [DOI] [PubMed] [Google Scholar]
- [21].Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis 2009; 26:273-87; https://doi.org/ 10.1007/s10585-008-9174-2 [DOI] [PubMed] [Google Scholar]
- [22].Rodriguez-Hernandez I, Cantelli G, Bruce F, Sanz-Moreno V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Res 2016; 5:674; PMID:27158457; https://doi.org/ 10.12688/f1000research.7909.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prudnikova TY, Rawat SJ, Chernoff J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res 2015; 21:24-9; PMID:25336694; https://doi.org/ 10.1158/1078-0432.CCR-14-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Steeg PS. Targeting metastasis. Nat Rev Cancer 2016; 16:201-18; PMID:27009393; https://doi.org/ 10.1038/nrc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rath N, Morton JP, Julian L, Helbig L, Kadir S, McGhee EJ, Anderson KI, Kalna G, Mullin M, Pinho AV, et al.. ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol Med 2017; 9:198-218; PMID:28031255; https://doi.org/ 10.15252/emmm.201606743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vennin C, Chin VT, Warren SC, Lucas MC, Herrmann D, Magenau A, Melenec P, Walters SN, Del Monte-Nieto G, Conway JR, et al.. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med 2017; 9:pii: eaai8504; PMID:28381539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, et al.. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015; 518:495-501; PMID:25719666; https://doi.org/ 10.1038/nature14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005; 7:469-83; PMID:15894267; https://doi.org/ 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]
- [29].Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al.. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4:437-50; PMID:14706336; https://doi.org/ 10.1016/S1535-6108(03)00309-X [DOI] [PubMed] [Google Scholar]
- [30].Samuel MS, Rath N, Masre SF, Boyle ST, Greenhalgh DA, Kochetkova M, Bryson S, Stevenson D, Olson MF. Tissue-selective expression of a conditionally-active ROCK2-estrogen receptor fusion protein. Genesis 2016; 54:636-46; PMID:27775859; https://doi.org/ 10.1002/dvg.22988 [DOI] [PubMed] [Google Scholar]
- [31].Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002; 2:897-909; PMID:12459728; https://doi.org/ 10.1038/nrc949 [DOI] [PubMed] [Google Scholar]
- [32].Rajakyla EK, Vartiainen MK. Rho, nuclear actin, and actin-binding proteins in the regulation of transcription and gene expression. Small GTPases 2014; 5:e27539; PMID:24603113; https://doi.org/ 10.4161/sgtp.27539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Orgaz JL, Pandya P, Dalmeida R, Karagiannis P, Sanchez-Laorden B, Viros A, Albrengues J, Nestle FO, Ridley AJ, Gaggioli C, et al.. Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat Commun 2014; 5:4255; PMID:24963846; https://doi.org/ 10.1038/ncomms5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, Doyle B, McKay C, Heung MY, Oien KA, et al.. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 2010; 139:292-303; PMID:20303350; https://doi.org/ 10.1053/j.gastro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- [35].Pajic M, Herrmann D, Vennin C, Conway JR, Chin VT, Johnsson AK, Welch HC, Timpson P. The dynamics of Rho GTPase signaling and implications for targeting cancer and the tumor microenvironment. Small GTPases 2015; 6:123-33; PMID:26103062; https://doi.org/ 10.4161/21541248.2014.973749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chin VT, Nagrial AM, Chou A, Biankin AV, Gill AJ, Timpson P, Pajic M. Rho-associated kinase signalling and the cancer microenvironment: novel biological implications and therapeutic opportunities. Expert Rev Mol Med 2015; 17:e17; PMID:26507949; https://doi.org/ 10.1017/erm.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J, Hidaka H. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta 2005; 1754:245-52; PMID:16213195; https://doi.org/ 10.1016/j.bbapap.2005.06.015 [DOI] [PubMed] [Google Scholar]
- [38].Liu GJ WZ, Wang YF, Xu LL, Wang XL, Liu Y, Luo GJ, He GH, Zeng YJ. Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid haemorrhage. Eur J Clin Pharmacol 2012; 68:131-9; PMID:21837395; https://doi.org/ 10.1007/s00228-011-1100-x [DOI] [PubMed] [Google Scholar]
- [39].Conway JRW, Warren SC, Timpson P. Context-dependent intravital imaging of therapeutic response using intramolecular FRET biosensors. Methods 2017. (in press); PMID:28435000; https://doi.org/ 10.1016/j.ymeth.2017.04.014 [DOI] [PubMed] [Google Scholar]
- [40].Conway JR, Carragher NO, Timpson P. Developments in preclinical cancer imaging: innovating the discovery of therapeutics. Nat Rev Cancer 2014; 14:314-28; PMID:24739578; https://doi.org/ 10.1038/nrc3724 [DOI] [PubMed] [Google Scholar]
- [41].Vennin C, Herrmann D, Lucas MC, Timpson P. Intravital imaging reveals new ancillary mechanisms co-opted by cancer cells to drive tumor progression. F1000Res 2016; 5:892; https://doi.org/ 10.12688/f1000research.8090.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nobis M, Carragher NO, McGhee EJ, Morton JP, Sansom OJ, Anderson KI, Timpson P. Advanced intravital subcellular imaging reveals vital three-dimensional signalling events driving cancer cell behaviour and drug responses in live tissue. FEBS J 2013; 280:5177-97; PMID:23678945; https://doi.org/ 10.1111/febs.12348 [DOI] [PubMed] [Google Scholar]
- [43].Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U, et al.. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol 2005; 46:1803-11; PMID:16286163; https://doi.org/ 10.1016/j.jacc.2005.07.047 [DOI] [PubMed] [Google Scholar]
- [44].Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol 2003; 161:429-39; PMID:12719476; https://doi.org/ 10.1083/jcb.200210135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barnes JM, Nauseef JT, Henry MD. Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS One 2012; 7:e50973; PMID:23226552; https://doi.org/ 10.1371/journal.pone.0050973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sunyer R, Conte V, Escribano J, Elosegui-Artola A, Labernadie A, Valon L, Navajas D, García-Aznar JM, Muñoz JJ, Roca-Cusachs P, et al.. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 2016; 353:1157-61; PMID:27609894; https://doi.org/ 10.1126/science.aaf7119 [DOI] [PubMed] [Google Scholar]
- [47].Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, et al.. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol 2010; 191:169-85; PMID:20876278; https://doi.org/ 10.1083/jcb.201002041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell 2015; 27:574-88; PMID:25873177; https://doi.org/ 10.1016/j.ccell.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al.. Tumour exosome integrins determine organotropic metastasis. Nature 2015; 527:329-35; PMID:26524530; https://doi.org/ 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al.. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015; 17:816-26; PMID:25985394; https://doi.org/ 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Johansson-Percival A, Li ZJ, Lakhiani DD, He B, Wang X, Hamzah J, Ganss R. Intratumoral LIGHT Restores Pericyte Contractile Properties and Vessel Integrity. Cell Rep 2015; 13:2687-98; PMID:26711337; https://doi.org/ 10.1016/j.celrep.2015.12.004 [DOI] [PubMed] [Google Scholar]
- [52].Rahbari NN, Kedrin D, Incio J, Liu H, Ho WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, et al.. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med 2016; 8:360ra135; PMID:27733559; https://doi.org/ 10.1126/scitranslmed.aaf5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, Hill RC, Lakins JN, Schlaepfer DD, Mouw JK, et al.. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 2016; 22:497-505; PMID:27089513; https://doi.org/ 10.1038/nm.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Desogere P, Tapias LF, Hariri LP, Rotile NJ, Rietz TA, Probst CK, Blasi F, Day H, Mino-Kenudson M, Weinreb P, et al.. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med 2017; 9:pii: eaaf4696; PMID:28381537; https://doi.org/ 10.1126/scitranslmed.aaf4696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kumar R, Mateo J, Smith AD, Khan KH, Ruddle R, Swales KE, et al.. First-in-human, first-in-class phase 1 study of a novel oral multi-AGC kinase inhibitor AT13148 in patients (pts) with advanced solid tumors. J Clin Oncol 2014; 15:2554 [Google Scholar]
- [56].Flynn R, Paz K, Du J, Reichenbach DK, Taylor PA, Panoskaltsis-Mortari A, Vulic A, Luznik L, MacDonald KK, Hill GR, et al.. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood 2016; 127:2144-54; PMID:26983850; https://doi.org/ 10.1182/blood-2015-10-678706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sadok A, Marshall CJ. Rho GTPases: masters of cell migration. Small GTPases 2014; 5:e29710; PMID:24978113; https://doi.org/ 10.4161/sgtp.29710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zanin-Zhorov A, Weiss JM, Trzeciak A, Chen W, Zhang J, Nyuydzefe MS, Arencibia C, Polimera S, Schueller O, Fuentes-Duculan J, et al.. Cutting edge: Selective oral ROCK2 inhibitor reduces clinical scores in patients with psoriasis vulgaris and normalizes skin pathology via concurrent regulation of IL-17 and IL-10. J Immunol 2017; 198(10):3809-14 (in press); https://doi.org/ 10.4049/jimmunol.1602142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bertolini F, Sukhatme VP, Bouche G. Drug repurposing in oncology–patient and health systems opportunities. Nat Rev Clin Oncol 2015; 12:732-42; PMID:26483297; https://doi.org/ 10.1038/nrclinonc.2015.169 [DOI] [PubMed] [Google Scholar]
- [60].Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, et al.. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011; 19:776-91; PMID:21665151; https://doi.org/ 10.1016/j.ccr.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al.. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009; 139:891-906; PMID:19931152; https://doi.org/ 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Madsen CD, Pedersen JT, Venning FA, Singh LB, Moeendarbary E, Charras G, Cox TR, Sahai E, Erler JT. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep 2015; 16:1394-408; PMID:26323721; https://doi.org/ 10.15252/embr.201540107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kular J, Scheer KG, Pyne NT, Allam AH, Pollard AN, Magenau A, Wright RL, Kolesnikoff N, Moretti PA, Wullkopf L, et al.. A negative regulatory mechanism involving 14-3-3zeta limits signaling downstream of ROCK to regulate tissue stiffness in epidermal homeostasis. Dev Cell 2015; 35:759-74; PMID:26702834; https://doi.org/ 10.1016/j.devcel.2015.11.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


