ABSTRACT
Neutrophils in circulation experience significant shear forces due to blood flow when they tether to the vascular endothelium. Biochemical and biophysical responses of neutrophils to the physical force of flowing blood modulate their behavior and promote tissue recruitment under pro-inflammatory conditions. Neutrophil mechanotransduction responses occur through mechanisms that are not yet fully understood. In our recent work, we showed that GEF-H1, a RhoA specific guanine nucleotide exchange factor (GEF), is required to maintain neutrophil motility and migration in response to shear stress. GEF-H1 re-localizes to flottilin-rich uropods in neutrophils in response to fluid shear stress and promotes spreading and crawling on activated endothelial cells. GEF-H1 drives cellular contractility through myosin light chain (MLC) phosphorylation downstream of the Rho-ROCK signaling axis. We propose that GEF-H1-dependent cell spreading and crawling in shear stress-dependent neutrophil recruitment from the vasculature are due to the specific localization of Rho-induced contractility in the uropod.
KEYWORDS: GEF-H1, mechanotransduction, Neutrophil, RhoA, shear stress, small GTPase
Introduction
Neutrophils are important early responders of the innate immune system that are recruited to sites of infection from the circulation. To make their way to the site of infection they must recognize specific signals on the inflamed vascular endothelium, roll, adhere, crawl and migrate across the endothelial barrier. Furthermore, neutrophil responses on the endothelial surface are exquisitely sensitive to shear forces due to blood flow. In our recent work, we uncovered an important role of the guanine nucleotide exchange factor (GEF), GEF-H1, in neutrophil recruitment to the inflamed peritoneal cavity.1 Specifically, we demonstrated a role of GEF-H1 in neutrophil spreading and crawling in response to shear stress. Here we discuss our findings and review available evidence that provide insights in the regulation of mechanotransduction responses induced by shear forces.
Neutrophils, shear stress and mechanotransduction
Neutrophils are effector cells of the innate immune system that are critical for the response to inflammation. Their efficacy in providing protection against invading pathogens relies on their ability to rapidly get recruited from the blood to the site of infection. This recruitment is accomplished by adhesion to the activated vascular endothelium through a series of overlapping attachment processes that include selectin and integrin mediated capture. These events lead to neutrophil activation and their transmigration across the endothelial wall, which is followed by chemotactic migration through the interstitial space to the site of infection. At the site of infection neutrophils destroy invading pathogens through a variety of functions including phagocytosis, secretion of reactive oxygen species (ROS) and other cytotoxic molecules and secretion of neutrophil extracellular traps (NETs). Neutrophil activation and recruitment is a finely tuned process, as excessive neutrophil activity can result in significant tissue damage and increased morbidity.2
Mechanotransduction, the ability of cells to translate mechanical forces into biochemical responses, plays an essential role in many biologic processes, including embryonic3 and tissue development,4 cell fate determination,5 cell migration,6 cell morphology,7 cytokine activation,8 gene expression,5,9 and cancer cell invasion.10 Neutrophils and other circulating leukocytes have evolved in the context of shear forces due to blood flow, consequently, they have adapted mechanosensitive molecular switches that trigger appropriate biologic responses in a shear force sensitive manner.11-14 Leukocytes experience the strongest shear forces as they interact with and tether to the endothelial surface.
Evidence that appears to be in conflict suggests that mechanical and shear forces can serve to either activate or deactivate neutrophils.15 Shear forces have been shown to promote or limit neutrophil responses and recruitment to the endothelial surface depending on experimental context. In-vivo, in the normal healthy vasculature, in the absence of inflammation, shear was shown to induce pseudopod retraction,16 a response that depended on the presence of red blood cells.17 However, in a spontaneously hypersensitive rat (SPR) model fluid shear stress had the opposite effect.18 Centrifugation, which is known to stimulate neutrophils,19 and treatment with glucocoritcoids also reversed the shear stress response.20,21 Neutrophils plated under inflammatory conditions (i.e. activated neutrophils) had elevated crawling and extravasation,11 and increased neutrophil invagination into the apical endothelial interface,12 in response to shear. HL-60 cells differentiated into neutrophils, showed retraction in response to shear stress, however when the cells had been treated with f-Met-Leu-Phe (f MLP) they remained spread.22 In contrast to the observations above, one study using passively isolated human neutrophils, indicated that even in the absence of priming neutrophils have a cell spreading response within minutes of exposure to shear stress.23 Since shear stress helps to maintain the naïve phenotype of circulating neutrophils,16 it is possible that the lack of shear stress, after blood is removed from the vasculature, is sufficient to allow some neutrophil activation. Once these cells are re-exposed to shear stress they respond as activated cells. Alternatively, it is possible that some degree of activation occurred due to neutrophil interaction with glass coverslips or trace amounts of endotoxin.24 Overall the literature suggests that shear stress promotes the rounded-up phenotype of naïve neutrophils in the circulation, acting to limit neutrophil recruitment in the absence of inflammatory cues. However, neutrophils that become activated as they encounter pro-inflammatory cues at the endothelial surface, or isolated neutrophils, which are likely to experience some degree of activation during purification,24 exhibit the opposite response to shear stress, namely, increased spreading, crawling and extravasation.
Although further work is necessary to elucidate the receptors that are responsible for mechanosensation, evidence implicates selectins,25 integrins,26-28 stretch activated calcium channels,29 and G-protein coupled receptors (GPCRs),30 including the f MLP receptor.31 The nucleus has also been proposed to have mechanosensory potential.32,33 The actin and microtubule (MT) cytoskeletons, polymeric structures that determine cell morphology, polarity and migrational capacity,34 are key downstream effectors of mechanotransducive signaling. While integrins are implicated as mechanosensory organelles in some contexts, they are also likely to be important downstream targets of the mechanosensory response. Shear stress induced strengthening of integrins by anchoring to cytoskeletal components was shown to be an important mechanism to enhance leukocyte adhesion in response to force.35
Rho-family small GTPases and cell motility
Successful extravasation of leukocytes to a site of infection requires highly orchestrated regulation of cell attachment, morphology, and polarity in response to extracellular cues. This is achieved through control of integrin based adhesions, cytoskeletal dynamics, and cell contractility. Maintaining traction for efficient cell migration depends on coordination between adhesion strengthening, protrusion at the leading edge, contraction of the cell body, and de-adhesion in the tail,36 and requires cyclical regulation of integrins and their association with the contractile F-actin cytoskeleton. Rho family small GTPases, such as RhoA, Rac1, and Cdc42, and their activators, the GEFs, are central regulators of these processes and control cell migration in fast moving innate immune cells37 and slow moving fibroblasts.38 Rho family small GTPases are important regulators of the cytoskeleton, and their roles in directed cell migration, polarity and the forces of adhesion, propulsion and retraction have been studied in various cell types.38,39 RhoA is an important regulator of the cellular contractile response. In its GTP bound form, RhoA activates its downstream effector ROCK, which in turn activates the myosin light chain (MLC) through direct phosphorylation,40 or by inhibiting myosin light chain phosphatase (MLCP).41 ROCK can also activate LIM kinase (LIMK) through direct phosphorylation. In its active state, LIMK phosphorylates cofilin, thus inactivating it and preventing cofilin dependent depolymerization of actin.42 Another RhoA effector, mDia, directly catalyzes F-actin polymerization.43 In fibroblasts and epithelial cells, the RhoA-induced contractile response, characterized by cell contraction, and the formation of actin stress fibers and focal adhesions, is induced by thrombin,44 lysophosphatidic acid (LPA),45 and disruption of the MT cytoskeleton.46 Interestingly, cell contraction itself drives stress fiber and focal adhesion formation in fibroblasts cells.47 Using transformed fibroblasts, RhoA dependent focal adhesion strengthening was demonstrated as a biochemical response to intracellular contractile tension, associated with the mechanosensory function of integrins.28 Rho family small GTPases have also been implicated in the shear stress response. There is evidence demonstrating that RhoA, Rac1 and Cdc42 have significant roles in the regulation of shear stress induced cytoskeletal dynamics in osteoblasts,48 chondrocytes49 and endothelial cells.50 In neutrophils exposed to shear forces, a reduction in active Rac and an increase in Rho activity have been shown to drive the cell rounding response.22
Soluble factors that promote neutrophil migration include chemokines, formylated peptides of bacterial origin, such as f MLP, and the complement fragment, C5a. These factors signal through a cognate GPCR, which activates PI3K and leads to enrichment of phophatidylinositol 3,4,5-trisphosphate (PIP3) at the leading edge. Enrichment of PIP3 at the leading edge and polarized activation of Rho GTPases are necessary for maintaining polarity in neutrophil chemotaxis.51 Rac1 is activated at the leading edge where it promotes F-actin based protrusion, while active RhoA is enriched at the sides and the back of the cell, where it drives myosin based contractile activity.52 The mutual exclusion of Rac1 to the front of the cell and RhoA to the back is thought to help establish and maintain self-organizing polarity during migration.52 RhoA dependent cell contraction is necessary for retraction of the trailing cell body in migrating neutrophils,53,54 and monocytes.55 Although RhoA dependent contractility is necessary for detachment of the tail, it is also known to strengthen integrin based adhesion in neutrophils,56 lymphocytes,57,58 and fibroblasts.59
GEF-H1 mediated mechanotransduction
GEF-H1 is a RhoA specific MT-associated GEF, and can be activated by MT-depolymerizing agents such as nocodazole and colchicine. In epithelial cells, GEF-H1 is necessary for RhoA-dependent contractility and stress fiber formation in response to MT depolymerization.60 Hence, GEF-H1 serves to promote F-actin and actomyosin based phenomena as a direct consequence of its release from MTs.
Work from our laboratory and others, has demonstrated that the association of GEF-H1 with the MT cytoskeleton depends on its interaction with the dynein light chain, Tctex-1.61 Phosphorylation of serine 885 in the C-terminus of GEF-H1 by PKA61 promotes binding to 14-3-3 proteins, which maintains GEF-H1 in an inactive state on the MTs. In addition to activation by MT-depolymerizing agents, signaling to GEF-H1 can be achieved through stimulation of several different kinds of cell surface receptors. GEF-H1 is activated downstream of LPA signaling in fibroblasts,61 thrombin stimulation of endothelial cells,62 TNF-α and epidermal growth factor (EGF) signaling in tubular epithelial cells,63 Wnt signaling in neuronal cells,64 NOD-like receptor stimulation in macrophages,65 and as a result of mechanosensory stimulation of integrins.66 ERK mediated phosphorylation of GEF-H1 on threonine 678 promotes its GEF activity.67 Recent evidence indicates that GEF-H1 and another GEF called LARG (leukemia-associated Rho GEF) are both necessary for RhoA-induced mechanical stiffening in response to force on integrins in fibroblasts.66 In this system LARG was activated downstream of the Src family tyrosine kinase Fyn, while GEF-H1 was activated by a FAK-Ras-ERK signaling axis. Moreover, TGF-β induced epithelial-to-mesenchymal transition (EMT) is mediated through enhanced proteosomal degradation of LARG and GEF-H1, which leads to stiffness attenuation and increased invasion capacity.68 Signaling induced phosphorylation or dephosphorylation of specific serine and threonine residues, release from the MT array, localized MT depolymerization, or some combination of these are all possible mechanisms of GEF-H1 activation that could potentially occur in different contexts. Elucidation of the relative contribution of these processes may be complicated by the fact that GEF-H1 itself stabilizes MTs.69 In the case of thrombin induced GEF-H1-dependent responses in endothelial cells, partial depolymerization of MTs has been observed.70 However, in the case of integrin induced mechanotransduction by GEF-H1, MT depolymerization is not necessary, since the MT stabilizing agent, taxol, did not block GEF-H1 activation.66 Differential association of GEF-H1 with specific binding partners regulates its intracellular localization and activity. In confluent epithelial cells, GEF-H1 is sequestered to the tight junctions through interaction with cingulin,71 and paracingulin.72 In neurons, MT-associated GEF-H1 is released in response to membrane depolarization, and binds to neurabin and spinophilin in dendritic spines, where it regulates dendritic spine morphology.73 GEF-H1 binds to ASAP1 (ArfGAP with SH3 domain, ankyrin repeat, and PH domain 1) in fibroblasts, where it negatively regulates podosomes.74 Recently, it was shown that tensional-mechanical forces induce RhoA activation through the FAK/p52(Shc) complex and the activation of p115-RhoGEF and GEF-H1 in endothelial cells.75
In our recent work, we have provided evidence that GEF-H1 is activated in response to shear stress and promotes neutrophil spreading, crawling and transmigration.1 Our results indicate that upon exposure to shear stress GEF-H1 becomes dephosphorylated at serine 885 (S885), and relocalizes to Flotillin-rich uropods. Previously, we demonstrated that dissociation of GEF-H1 from the dynein light chain protein, Tctex-1, and dephosphorylation at S885 was sufficient to induce GEF-H1 exchange activity toward Rho.76 Although it has not yet been determined whether Flotillin-associated GEF-H1 is dissociated from Tctex-1, our results suggest that localization of S885-dephosphorylated GEF-H1 to the uropod is sufficient to promote neutrophil spreading and crawling, likely through stimulation of the Rho-ROCK-pMLC (phospho-myosin light chain) signaling axis. In addition to being downstream of mechanical force, GEF-H1 induced cellular contractility, through the Rho pathway60 and produced intracellular tension,77 which could in theory generate feed forward amplification.
In addition to it's mechanosensory role in neutrophils, GEF-H1, p115-RhoGEF75 and LARG78 have all been implicated in mechanosensory mechanisms in endothelial cells. Mechanotransduction through GEF-H1 has been implicated in ventilator-induced vascular endothelial permeability in the lung.79 Interestingly, tractional forces generated by crawling leukocytes induce stiffening of underlying endothelial cells through a LARG-RhoA induced pathway, resulting in enhanced transendothelial migration.78 This illustrates the potential of GEF-dependent mechanosensory mechanisms to influence leukocyte recruitment by regulating endothelia.
Effects of nocodazole
Nocodazole is a MT depolymerizing agent that induces contractility and morphological effects in fibroblasts.80 In cultured fibroblasts, MT-depolymerization induces focal adhesion and actin stress fiber formation and reduced locomotion.81,82 However, in neutrophils nocodazole induces actomyosin contractility, polarization and spontaneous migration.83-85 We and others have found that GEF-H1 is an essential factor contributing to the cross-talk between the MT cytoskeleton and the actomyosin system.1,60 In neutrophils, we have shown that MT depolymerization with nocodazole stimulates GEF-H1 dependent activation of Rho-induced contractility. This stimulates small membrane blebs in the short term (5 minutes), which coincides with peak phosphorylation of MLC. After 30 minutes of stimulation with nocodazole a subset of wildtype neutrophils, but not GEF-H1−/− neutrophils, develop contractile uropods and exhibit random crawling. The most striking effects of nocodazole, which include neutrophil polarization, contractile morphological contortions and crawling, which we observed by live cell imaging, occur significantly after the peak in nocodazole induced pMLC. One possible explanation for this lag is that early contractile events are responsible for establishing neutrophil polarity, with GEF-H1-dependent membrane blebbing events promoting membrane re-configuration and enrichment of Flottilin-rich membrane rafts, eventually consolidating and forming stable uropods after 30 minutes. This explanation seams feasible when one considers that the membrane constitution of blebs is likely to be different from the parts of the membrane that don't take part in bleb formation. Furthermore, we consistently observed membrane blebbing of neutrophil differentiated HL60 cells immediately before shear stress induced spreading (unpublished result). It is of interest that only 20-30% of neutrophils develop uropods after treatment with nocodazole, which suggests the presence of neutrophil subsets in varied stages of differentiation or priming.
Similar to neutrophils, nocodazole induced contractility of fibroblasts depended on GEF-H1, and caused a reduction in spread area (unpublished results). This effect of GEF-H1 induced contractility in fibroblasts is opposite to the cell spreading and migration effect that we observed in neutrophils, although some studies have suggested that GEF-H1 promotes migration of fibroblasts through actions at the leading edge.86 One important factor that is likely to govern whether a cell contracts or spreads upon Rho-mediated contractility is the specific subcellular localization of the contractile events. Activated neutrophils possess an intrinsic polarity and chirality,87 and in this context restriction of GEF-H1-dependent contractility to the Flotillin-rich uropod is sufficient to drive spreading and crawling in response to shear stress. On the contrary, a non-activated/non-polarized neutrophil or fibroblast is likely to round-up/retract as a result of intracellular contractility that is not limited to a specific subcellular domain. It is likely that GEF-H1 induced contractility in the uropod produces intracellular tension that can induce cell spreading, consistent with the observation that mechanical deformation of neutrophils into narrow channels is sufficient to induce pseudopod formation.88 Use of new FRET-based techniques, which can measure localized intracellular force with piconewton sensitivity89 could be used to demonstrate subcellular localization of GEF-H1 induced contractility in neutrophils.
GEF-H1 translocation to the uropod
Bulk depolymerization of the MT cytoskeleton with nocodazole is a crude way of activating GEF-H1, and our results show that shear stress can accomplish GEF-H1 activation without MT-depolymerization, since the process was not inhibited by taxol. Furthermore, the kinetics of the neutrophil response to shear stress were much faster than the response to nocodazole. This suggests that mechanotransduction signaling produces neutrophil polarity and polar localization of activated GEF-H1 to the uropod more efficiently than release of GEF-H1 consequent to MT-depolymerization. This could be due to the presence of preformed membrane raft complexes and/or uropods due to f MLP or ICAM interactions in our experimental setup, or faster delivery of GEF-H1 to the uropod by unknown signaling mechanisms and chaperone factors. The rapid kinetics of shear stress induced GEF-H1 activation are consistent with signaling mediated activation of Rho downstream of receptor stimulation in leukocytes, which occurs within seconds.
GEF-H1-dependent contractility in the uropod, through the Rho-ROCK-pMLC signaling axis is sufficient to promote cell locomotion and spreading through a contractile flowing and squeezing mechanism, which is likely to be highly dependent on strong integrin attachments for anchoring. More work will be necessary to elucidate the mechanism by which localized GEF-H1 mediated contractility in the uropod induces neutrophil spreading. Neutrophils can exhibit amoeboid and mesenchymal modes of locomotion, and it is possible that GEF-H1 promotes a transition from an amoeboid (Rho-dependent) mode of migration to a more spread and flattened mesenchymal (Rac-dependent) mode in response to shear stress (Fig. 1). While the importance of integrins for shear-induced neutrophil spreading is obvious, further experiments will be necessary to determine if integrins are induced secondary to GEF-H1 effects or if load bearing integrins are the mechanosensory organelles that lie upstream of GEF-H1 activation.
Figure 1.
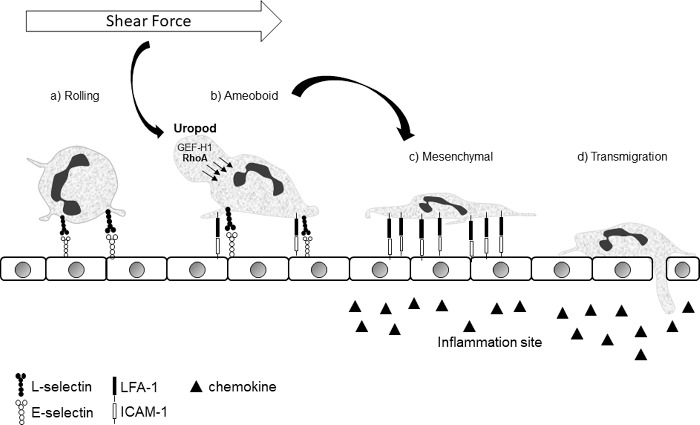
Crawling neutrophils can have an amoeboid or mesenchymal morphology. Neutrophils roll on the endothelial surface (a) until they encounter the right combination of pro-inflammatory signals to induce adhesion. Once adherent, they are able to crawl in a partly rounded up amoeboid (b) or a spread out mesenchymal (c) mode. In the amoeboid mode they are more exposed to shear forces, which results in GEF-H1 activation/dephosphorylation and relocalization to the uropod, where it promotes Rho-induced contractility. Contractile squeezing of the uropod to push the cytosol down and forward, in combination with strongly anchored integrins, will promote cell flattening. The impetus to promote the transition from (b) to (c) in the context of shear force is biologically coherent.
Future
While we have demonstrated a role for GEF-H1 in neutrophil spreading and crawling in response to shear stress, other roles of GEF-H1 in mechanotransduction are possible. Future work will be necessary to determine if GEF-H1 is involved in the rounding up of naïve neutrophils in response to shear. Furthermore, since neutrophils experience mechanical stress in the 3-dimensional tissue matrix, it will be of interest to determine whether GEF-H1 plays a role in migration in this context. Finally, since other leukocyte subsets, including monocytes and lymphocytes, which also cross the endothelial barrier under shear forces, also form uropods during extravasation,90 it will be of interest to determine if GEF-H1 plays a similar role in these cell types.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Fine N, Dimitriou D, Rullo J, Sandi M, Petri B, Haitsma J, Ibrahim H, La Rose J, Glogauer M, Kubes P, et al.. GEF-H1 is necessary for neutrophil shear stress-induced migration during inflammation. J Cell Biol. 2016;215:107-19. doi: 10.1083/jcb.201603109. PMID:27738004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159-75. doi: 10.1038/nri3399. PMID:23435331 [DOI] [PubMed] [Google Scholar]
- [3].Patwari P, Lee RT. Mechanical control of tissue morphogenesis. Circ Res. 2008;103:234-43. doi: 10.1161/CIRCRESAHA.108.175331. PMID:18669930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dorland YL, Huveneers S. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci. 2017;74:279-92. doi: 10.1007/s00018-016-2325-8. PMID:27506620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Le HQ, Ghatak S, Yeung CY, Tellkamp F, Gunschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, et al.. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18:864-75. doi: 10.1038/ncb3387. PMID:27398909 [DOI] [PubMed] [Google Scholar]
- [6].Mascharak S, Benitez PL, Proctor AC, Madl CM, Hu KH, Dewi RE, Butte MJ, Heilshorn SC. YAP-dependent mechanotransduction is required for proliferation and migration on native-like substrate topography. Biomaterials. 2016;115:155-66. doi: 10.1016/j.biomaterials.2016.11.019. PMID:27889666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf) 2017;219:382-408. doi: 10.1111/apha.12725. PMID:27246807 [DOI] [PubMed] [Google Scholar]
- [8].Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311-23. doi: 10.1083/jcb.200704042. PMID:18086923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Missirlis YF. Mechanoepigenetics. Front Cell Dev Biol. 2016;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shah AD, Bouchard MJ, Shieh AC. Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling. PLoS One. 2015;10:e0142337. doi: 10.1371/journal.pone.0142337. PMID:26560447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kitayama J, Hidemura A, Saito H, Nagawa H. Shear stress affects migration behavior of polymorphonuclear cells arrested on endothelium. Cell Immunol. 2000;203:39-46. doi: 10.1006/cimm.2000.1671. PMID:10915560 [DOI] [PubMed] [Google Scholar]
- [12].Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol (Baltimore, Md: 1950). 2004;173:7282-91. doi: 10.4049/jimmunol.173.12.7282. PMID:15585851 [DOI] [PubMed] [Google Scholar]
- [13].Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17-27. doi: 10.1016/j.immuni.2007.01.002. PMID:17241958 [DOI] [PubMed] [Google Scholar]
- [14].Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol. 2008;20:525-32. doi: 10.1016/j.ceb.2008.04.003. PMID:18499427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ekpenyong AE, Toepfner N, Chilvers ER, Guck J. Mechanotransduction in neutrophil activation and deactivation. Biochim Biophys Acta. 2015;1853:3105-16. doi: 10.1016/j.bbamcr.2015.07.015. PMID:26211453 [DOI] [PubMed] [Google Scholar]
- [16].Moazzam F, DeLano FA, Zweifach BW, Schmid-Schonbein GW. The leukocyte response to fluid stress. Proc Natl Acad Sci U S A. 1997;94:5338-43. doi: 10.1073/pnas.94.10.5338. PMID:9144238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Komai Y, Schmid-Schonbein GW. De-activation of neutrophils in suspension by fluid shear stress: a requirement for erythrocytes. Ann Biomed Eng. 2005;33:1375-86. doi: 10.1007/s10439-005-6768-6. PMID:16240086 [DOI] [PubMed] [Google Scholar]
- [18].Fukuda S, Yasu T, Kobayashi N, Ikeda N, Schmid-Schonbein GW. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ Res. 2004;95:100-8. doi: 10.1161/01.RES.0000133677.77465.38. PMID:15166092 [DOI] [PubMed] [Google Scholar]
- [19].Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105-11. PMID:1907873 [PubMed] [Google Scholar]
- [20].Fukuda S, Schmid-Schonbein GW. Centrifugation attenuates the fluid shear response of circulating leukocytes. J Leukoc Biol. 2002;72:133-9. PMID:12101272 [PubMed] [Google Scholar]
- [21].Fukuda S, Mitsuoka H, Schmid-Schonbein GW. Leukocyte fluid shear response in the presence of glucocorticoid. J Leukoc Biol. 2004;75:664-70. doi: 10.1189/jlb.1003464. PMID:14726499 [DOI] [PubMed] [Google Scholar]
- [22].Makino A, Glogauer M, Bokoch GM, Chien S, Schmid-Schonbein GW. Control of neutrophil pseudopods by fluid shear: role of Rho family GTPases. Am J Physiol Cell Physiol. 2005;288:C863-71. doi: 10.1152/ajpcell.00358.2004. PMID:15561759 [DOI] [PubMed] [Google Scholar]
- [23].Coughlin MF, Schmid-Schonbein GW. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys J. 2004;87:2035-42. doi: 10.1529/biophysj.104.042192. PMID:15345579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101-10. PMID:2984939 [PMC free article] [PubMed] [Google Scholar]
- [25].Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein lig and 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol (Baltimore, Md: 1950). 2004;172:7780-90. doi: 10.4049/jimmunol.172.12.7780. PMID:15187162 [DOI] [PubMed] [Google Scholar]
- [26].Li Z, Lee H, Zhu C. Molecular mechanisms of mechanotransduction in integrin-mediated cell-matrix adhesion. Exp Cell Res. 2016;349(1):85-94. doi: 10.1016/j.yexcr.2016.10.001. PMID:27720950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dixit N, Yamayoshi I, Nazarian A, Simon SI. Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J Immunol (Baltimore, Md: 1950). 2011;187:472-81. doi: 10.4049/jimmunol.1004197. PMID:21632714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175-86. doi: 10.1083/jcb.153.6.1175. PMID:11402062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466-76. doi: 10.1152/ajpheart.00854.2009. PMID:19966050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633-9. doi: 10.1152/ajpcell.00576.2005. PMID:16436471 [DOI] [PubMed] [Google Scholar]
- [31].Chen AY, DeLano FA, Valdez SR, Ha JN, Shin HY, Schmid-Schonbein GW. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am J Physiol Cell Physiol. 2010;299:C1441-9. doi: 10.1152/ajpcell.00157.2010. PMID:20861466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Enyedi B, Jelcic M, Niethammer P. The cell nucleus serves as a Mechanotransducer of tissue damage-induced inflammation. Cell. 2016;165:1160-70. doi: 10.1016/j.cell.2016.04.016. PMID:27203112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849-54. doi: 10.1073/pnas.94.3.849. PMID:9023345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fine N, Khaliq S, Hassanpour S, Glogauer M. Role of the cytoskeleton in myeloid cell function. Microbiol Spectr. 2016;4:1-15. doi: 10.1128/microbiolspec.MCHD-0029-2016. PMID:27726772 [DOI] [PubMed] [Google Scholar]
- [35].Alon R, Feigelson SW, Manevich E, Rose DM, Schmitz J, Overby DR, Winter E, Grabovsky V, Shinder V, Matthews BD, et al.. Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain. J Cell Biol. 2005;171:1073-84. doi: 10.1083/jcb.200503155. PMID:16365170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schwartz MA, Horwitz AR. Integrating adhesion, protrusion, and contraction during cell migration. Cell. 2006;125:1223-5. doi: 10.1016/j.cell.2006.06.015. PMID:16814706 [DOI] [PubMed] [Google Scholar]
- [37].Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163-71. doi: 10.1016/j.tcb.2005.01.002. PMID:15752980 [DOI] [PubMed] [Google Scholar]
- [38].Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235-44. doi: 10.1083/jcb.144.6.1235. PMID:10087266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhelev DV, Alteraifi A. Signaling in the motility responses of the human neutrophil. Ann Biomed Eng. 2002;30:356-70. doi: 10.1114/1.1477446. PMID:12051620 [DOI] [PubMed] [Google Scholar]
- [40].Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al.. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996;271:20246-9. doi: 10.1074/jbc.271.34.20246. PMID:8702756 [DOI] [PubMed] [Google Scholar]
- [41].Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al.. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Sci (New York, NY). 1996;273:245-8. doi: 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- [42].Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S, et al.. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Sci (New York, NY). 1999;285:895-8. doi: 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- [43].Zigmond SH. Formin-induced nucleation of actin filaments. Curr Opinion Cell Biol. 2004;16:99-105. doi: 10.1016/j.ceb.2003.10.019. PMID:15037312 [DOI] [PubMed] [Google Scholar]
- [44].Ruiz-Loredo AY, Lopez E, Lopez-Colome AM. Thrombin promotes actin stress fiber formation in RPE through Rho/ROCK-mediated MLC phosphorylation. J Cell Physiol. 2011;226:414-23. doi: 10.1002/jcp.22347. PMID:20672289 [DOI] [PubMed] [Google Scholar]
- [45].Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science (New York, NY). 1997;275:1308-11. doi: 10.1126/science.275.5304.1308 [DOI] [PubMed] [Google Scholar]
- [46].Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, et al.. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55-70. doi: 10.1002/jcp.20055. PMID:15281089 [DOI] [PubMed] [Google Scholar]
- [47].Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797-806. doi: 10.1083/jcb.150.4.797. PMID:10953004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wan Q, Cho E, Yokota H, Na S. Rac1 and Cdc42 GTPases regulate shear stress-driven beta-catenin signaling in osteoblasts. Biochem Biophys Res Commun. 2013;433:502-7. doi: 10.1016/j.bbrc.2013.03.020. PMID:23524265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wan Q, Kim SJ, Yokota H, Na S. Differential activation and inhibition of RhoA by fluid flow induced shear stress in chondrocytes. Cell Biol Int. 2013;37:568-76. doi: 10.1002/cbin.10072. PMID:23408748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 2006; 98:176-85. doi: 10.1161/01.RES.0000200162.94463.d7. PMID:16456110 [DOI] [PubMed] [Google Scholar]
- [51].Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437-45. doi: 10.1083/jcb.200604113. PMID:16864657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814-20. doi: 10.1182/blood-2006-01-010363. PMID:16809619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Eddy RJ, Pierini LM, Matsumura F, Maxfield FR. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 2000;113(Pt 7):1287-98. PMID:10704379 [DOI] [PubMed] [Google Scholar]
- [54].Yoshinaga-Ohara N, Takahashi A, Uchiyama T, Sasada M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp Cell Res. 2002;278:112-22. doi: 10.1006/excr.2002.5571. PMID:12126963 [DOI] [PubMed] [Google Scholar]
- [55].Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154:147-60. doi: 10.1083/jcb.200103048. PMID:11448997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981-3. doi: 10.1126/science.271.5251.981. PMID:8584934 [DOI] [PubMed] [Google Scholar]
- [57].Soede RD, Zeelenberg IS, Wijnands YM, Kamp M, Roos E. Stromal cell-derived factor-1-induced LFA-1 activation during in vivo migration of T cell hybridoma cells requires Gq/11, RhoA, and myosin, as well as Gi and Cdc42. J Immunol (Baltimore, Md: 1950). 2001;166:4293-301. doi: 10.4049/jimmunol.166.7.4293. PMID:11254681 [DOI] [PubMed] [Google Scholar]
- [58].Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, Laudanna C. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20:25-35. doi: 10.1016/S1074-7613(03)00350-9. PMID:14738762 [DOI] [PubMed] [Google Scholar]
- [59].Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403-15. doi: 10.1083/jcb.133.6.1403. PMID:8682874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294-301. doi: 10.1038/ncb773. PMID:11912491 [DOI] [PubMed] [Google Scholar]
- [61].Meiri D, Greeve MA, Brunet A, Finan D, Wells CD, LaRose J, Rottapel R. Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation. Mol Cell Biol. 2009;29:5963-73. doi: 10.1128/MCB.01268-08. PMID:19667072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AD. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L540-8. doi: 10.1152/ajplung.00259.2005 [DOI] [PubMed] [Google Scholar]
- [63].Kakiashvili E, Dan Q, Vandermeer M, Zhang Y, Waheed F, Pham M, Szászi K. The epidermal growth factor receptor mediates tumor necrosis factor-alpha-induced activation of the ERK/GEF-H1/RhoA pathway in tubular epithelium. J Biol Chem. 2011;286:9268-79. doi: 10.1074/jbc.M110.179903. PMID:21212278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tsuji T, Ohta Y, Kanno Y, Hirose K, Ohashi K, Mizuno K. Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells. Mol Biol Cell. 2010;21:3590-600. doi: 10.1091/mbc.E10-02-0095. PMID:20810787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhao Y, Alonso C, Ballester I, Song JH, Chang SY, Guleng B, Arihiro S, Murray PJ, Xavier R, Kobayashi KS, et al.. Control of NOD2 and Rip2-dependent innate immune activation by GEF-H1. Inflammatory Bowel Dis. 2012;18:603-12. doi: 10.1002/ibd.21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722-7. doi: 10.1038/ncb2254. PMID:21572419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fujishiro SH, Tanimura S, Mure S, Kashimoto Y, Watanabe K, Kohno M. ERK1/2 phosphorylate GEF-H1 to enhance its guanine nucleotide exchange activity toward RhoA. Biochem Biophys Res Commun. 2008;368:162-7. doi: 10.1016/j.bbrc.2008.01.066. PMID:18211802 [DOI] [PubMed] [Google Scholar]
- [68].Osborne LD, Li GZ, How T, O'Brien ET, Blobe GC, Superfine R, Mythreye K. TGF-beta regulates LARG and GEF-H1 during EMT to affect stiffening response to force and cell invasion. Mol Biol Cell. 2014;25:3528-40. doi: 10.1091/mbc.E14-05-1015. PMID:25143398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yoshimura Y, Miki H. Dynamic regulation of GEF-H1 localization at microtubules by Par1b/MARK2. Biochem Biophys Res Commun. 2011;408:322-8. doi: 10.1016/j.bbrc.2011.04.032. PMID:21513698 [DOI] [PubMed] [Google Scholar]
- [70].Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004;18:1879-90. doi: 10.1096/fj.04-2328com. PMID:15576491 [DOI] [PubMed] [Google Scholar]
- [71].Aijaz S, D'Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777-86. doi: 10.1016/j.devcel.2005.03.003. PMID:15866167 [DOI] [PubMed] [Google Scholar]
- [72].Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell. 2008;19:4442-53. doi: 10.1091/mbc.E08-06-0558. PMID:18653465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ryan XP, Alldritt J, Svenningsson P, Allen PB, Wu GY, Nairn AC, Greengard P. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron. 2005;47:85-100. doi: 10.1016/j.neuron.2005.05.013. PMID:15996550 [DOI] [PubMed] [Google Scholar]
- [74].Shiba Y, Randazzo PA. GEFH1 binds ASAP1 and regulates podosome formation. Biochem Biophys Res Commun. 2011;406:574-9. doi: 10.1016/j.bbrc.2011.02.093. PMID:21352810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wu RF, Liao C, Fu G, Hayenga HN, Yang K, Ma Z, Liu Z, Terada LS. p66Shc couples mechanical signals to RhoA through FAK-dependent recruitment of p115-RhoGEF and GEF-H1. Mol Cell Biol. 2016;36:2824-37. doi: 10.1128/MCB.00194-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Meiri D, Marshall CB, Greeve MA, Kim B, Balan M, Suarez F, Bakal C, Wu C, Larose J, Fine N, et al.. Mechanistic insight into the microtubule and actin cytoskeleton coupling through dynein-dependent RhoGEF inhibition. Mol Cell. 2012;45:642-55. doi: 10.1016/j.molcel.2012.01.027. PMID:22405273 [DOI] [PubMed] [Google Scholar]
- [77].Wang HL, Yang CH, Lee HH, Kuo JC, Hur SS, Chien S, Lee OK, Hung SC, Chang ZF. Dexamethasone-induced cellular tension requires a SGK1-stimulated Sec5-GEF-H1 interaction. J Cell Sci. 2015;128:3757-68. doi: 10.1242/jcs.169961. PMID:26359301 [DOI] [PubMed] [Google Scholar]
- [78].Lessey-Morillon EC, Osborne LD, Monaghan-Benson E, Guilluy C, O'Brien ET, Superfine R, Burridge K. The RhoA guanine nucleotide exchange factor, LARG, mediates ICAM-1-dependent mechanotransduction in endothelial cells to stimulate transendothelial migration. J Immunol (Baltimore, Md: 1950). 2014;192:3390-8. doi: 10.4049/jimmunol.1302525. PMID:24585879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837-48. doi: 10.1152/ajplung.00263.2009. PMID:20348280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93(Pt 2):255-66. PMID:2482296 [DOI] [PubMed] [Google Scholar]
- [81].Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317-26. doi: 10.1247/csf.21.317. PMID:9118237 [DOI] [PubMed] [Google Scholar]
- [82].Grigoriev IS, Chernobelskaya AA, Vorobjev IA. Nocodazole, vinblastine and taxol at low concentrations affect fibroblast locomotion and saltatory movements of organelles. Membr Cell Biol. 1999;13:23-48. PMID:10661468 [PubMed] [Google Scholar]
- [83].Kolodney MS, Elson EL. Contraction due to microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proc Natl Acad Sci U S A. 1995;92:10252-6. doi: 10.1073/pnas.92.22.10252. PMID:7479762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813-22. doi: 10.1242/jcs.00306. PMID:12571279 [DOI] [PubMed] [Google Scholar]
- [85].Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619-38. doi: 10.1016/S1357-2725(03)00144-4. PMID:12962702 [DOI] [PubMed] [Google Scholar]
- [86].Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell. 2009;20:4070-82. doi: 10.1091/mbc.E09-01-0041. PMID:19625450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A. 2007;104:9296-300. doi: 10.1073/pnas.0703153104. PMID:17517645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yap B, Kamm RD. Mechanical deformation of neutrophils into narrow channels induces pseudopod projection and changes in biomechanical properties. J Appl Physiol (1985). 2005;98:1930-9 [DOI] [PubMed] [Google Scholar]
- [89].Freikamp A, Cost AL, Grashoff C. The piconewton force awakens: quantifying mechanics in cells. Trends Cell Biol. 2016;26:838-47. doi: 10.1016/j.tcb.2016.07.005. PMID:27544876 [DOI] [PubMed] [Google Scholar]
- [90].Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209:1349-62. doi: 10.1084/jem.20111426. PMID:22711877 [DOI] [PMC free article] [PubMed] [Google Scholar]


