ABSTRACT
Immunosurveillance plays an important role in breast cancer (BC) prognosis and progression, and can be geared by immunogenic chemotherapy. In a cohort of 1023 BC patients treated with neoadjuvant chemotherapy (NAC), 40% of the individuals took comedications mostly linked to aging and comorbidities. We systematically analyzed the off-target effects of 1178 concurrent comedications (classified according to the Anatomical Therapeutic Chemical (ATC) Classification System) on the density of tumor-infiltrating lymphocytes (TILs) and pathological complete responses (pCR). At level 1 of the ATC system, the main anatomical classes of drugs were those targeting the nervous system (class N, 39.1%), cardiovascular disorders (class C, 26.6%), alimentary and metabolism (class A, 16.9%), or hormonal preparations (class H, 6.5%). At level 2, the most frequent therapeutic classes were psycholeptics (N05), analgesics (N02), and psychoanaleptics (N06). Pre-NAC TIL density in triple-negative BC (TNBC) was influenced by medications from class H, N, and A, while TIL density in HER2+ BC was associated with the use of class C. Psycholeptics (N05) and agents acting on the renin-angiotensin system (C09) were independently associated with pCR in the whole population of BC or TNBC, and in HER2-positive BC, respectively. Importantly, level 3 hypnotics (N05C) alone were able to reduce tumor growth in BC bearing mice and increased the anti-cancer activity of cyclophosphamide in a T cell-dependent manner. These findings prompt for further exploration of drugs interactions in cancer, and for prospective drug-repositioning strategies to improve the efficacy of NAC in BC.
KEYWORDS: Breast cancer, immunomodulation, comedication, pCR, TILs
Introduction
Breast cancer (BC) incidence increases with age, as does the prevalence of many other chronic diseases, such as diabetes, hypertension, and cardiovascular disease. Molecular BC subtypes and the density of tumor-infiltrating immune cells are both considered as important predictive and prognostic factors for optimal risk stratification in BC patients. Denkert et al. first evidenced that the amount of stromal immune infiltration was positively associated with pathological complete response (pCR) after neoadjuvant chemotherapy (NAC).1,2 These results were recently confirmed on a pooled analysis of large cohort of 3771 patients receiving NAC from German Breast Group,3 showing that the relationships between TIL levels and pCR translates into improved disease-free survival in HER2-positive and triple-negative BC (TNBC).
The drivers of immunosurveillance have largely been studied in the past decade, and derive from both (i) tumor-intrinsic characteristics; and/or (ii) extrinsic factors related to the host or the environment.4-6 Among endogenous tumor characteristics, molecular features (BC subtype, proliferative patterns), expression of human leukocyte antigen (HLA)-class I, tumor mutational burden,4 activation of cellular pathways,5 or induction of autophagy7 have been found to be associated with immune infiltration. Extrinsic factors including host characteristics (gender,8 age,9 body mass index), environment (tobacco, alcohol), nutritional factors, diet, commensal microbiota, physical activity, hormonal exposure6,10 have been studied less extensively.
There is growing interest in chronically used medications that may influence the risk for and the progression of cancer.11,12 Some medications such as aspirin or non-steroidal anti-inflammatory drugs (NSAID) have been reported to decrease BC risk13 or BC recurrence (statins,14 NSAIDs,15 beta-blockers (BB)16 and metformin17,18). So far, the impact of chronic comedications on immune infiltration has not been investigated. A few studies suggest that drugs that do not fall into the class of antineoplastics may have an impact on immunosurveillance through various mechanisms. For instance, metformin may increase CD8+ TILs19 or potentiate PD-1 blockade through reduction of tumor hypoxia20 propranolol and etodolac modulate tumor infiltration21 zoledronic acid22 targets tumor-associated macrophages; proton pump inhibitors23-25 reverse T cell anergy; and tadalafil inhibits myeloid derived-suppressor cells26
In the current study, we hypothesized that some comedications might be associated with TIL levels in BC. We evaluated the interactions between comedications, immune infiltration at diagnosis and pCR rates in a cohort of 1023 non-metastatic BC patients treated with NAC. Here, we report on epidemiological associations between distinct classes of comedications, TIL density and pCR rates, as we exemplify the T lymphocyte-dependent anticancer effects of the psycholeptic zolpidem in preclinical mouse models. Altogether, the results of our hypothesis-generating study indicate that comedication may represent a confounding factor in BC clinical trials, and that the use of chronic drugs could modify the efficacy of BC therapies. These findings prompt for further validation studies, before their beneficial role could be prospectively assessed in the setting of drug-repositioning trials.
Results
Up to 40% BC patients at diagnosis take drugs affecting nervous, cardiovascular systems or alimentary tract
Overall, 1023 patients with different BC subtypes (luminal: 44.6% (n = 456); TNBC: 31.2% (n = 319), HER2-positive: 24.2% (n = 248)) were included in the analyses. Four hundred and eighty-two patients (47.1%) took at least one comedication (total number of comedications: n = 1178) and 421 (41.1%) had at least one comorbidity. The five main anatomical classes (level 1) were drugs for nervous system (Class N, n = 460, 39.1%), cardiovascular diseases (class C, n = 313, 26.6%), alimentary and metabolism (class A, n = 199, 16.9%), and hormonal preparations (class H, n = 76, 6.5%), whereas 130 comedications were grouped in the category “others” (11.0%) (Figure 1, Supplemental Table 1). At level 2, the most frequent therapeutic classes were psycholeptics (N05, n = 199), analgesics (N02, n = 118), and psychoanaleptics (N06, n = 114).
Figure 1.
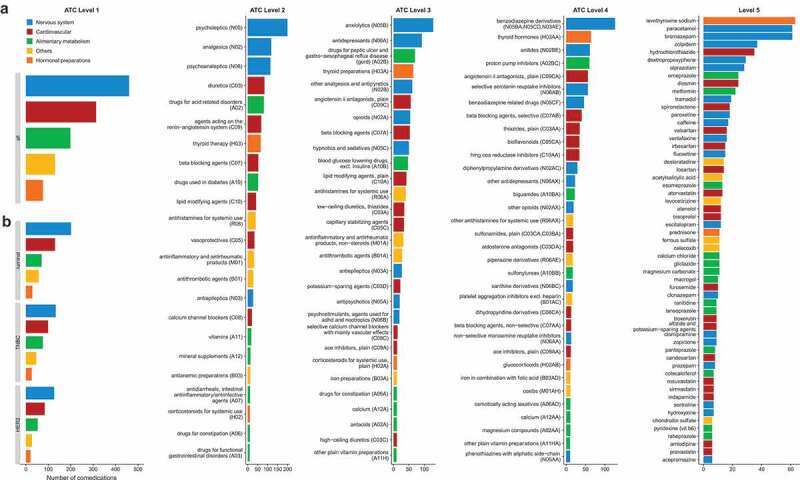
Distribution of the 1178 comedications according to the five levels of the ATC classification. Number of patients taking co-medications at ATC Level 1 in the whole population (a) and by BC subtype (b). Number of patients in the whole population taking co-medications at ATC Level 2 (c), at ATC Level 3 (d), at ATC Level 4 (e), at ATC Level 5 (f). Only classes with effectives ≥ 10 are reported.
The more frequent comorbidity was hypertension/heart disease (n = 177), followed by ulcer/gastritis (n = 109) (Supplemental Figure 1A). The number of comedications was strongly associated with the number of comorbidities (p < .001) (Supplemental Figure 1B). The majority of patients with a given comorbidity took at least one comedication from the corresponding class (57% of patients with depression/anxiety taking drugs for nervous system (N), 69% of patients with hypertension/heart disease taking cardiovascular drugs (C), 70% of patients with thyroid disorders taking drugs from class H mainly composed of thyroid therapy) (Supplemental Figure 1C). However, the class of the comedication was not always related to the very indication (Supplemental Figure 1D). Indeed, the use of compounds affecting the nervous system was frequently reported without any mention of an underlying psychiatric disease.
Patients with comedications were older, and/or more likely to be post-menopausal, and/or obese, and to have comorbidity than patients without comedication (Supplemental Table 2). Intrinsic tumor characteristics (tumor size, nodal status, grade, BC subtype, mitotic index) were not significantly associated with comedication use of any class (except for a lower tumor size in patients using a class A comedication, and a lower proportion of histologies of the nonspecific type (NST) in HER2-positive BC patients using class N drugs).
We conclude that a sizable proportion (approximately 40%) of BC patients took a chronic medication. However, as an aggregate, comedication is not associated with the disease presentation at diagnosis.
Some comedications are associated with pre-NAC TIL levels, mostly in TNBC
Information on pre-NAC TIL levels was available for 615 patients (60%). The TIL density was increased in BC patients taking drugs from class H (systemic hormonal preparations (H), Figure 2a). After stratification by BC subtype, TILs were higher in TNBC patients taking class N (nervous System), class A (alimentary tract) or class (H) drugs (Figure 2a–c) whereas in HER2-positive patients, TILs were higher in patients taking drugs from class C – cardiovascular system (Figure 2d). Conversely, TIL levels were not different according to any comorbidity (Supplemental Figure 2).
Figure 2.
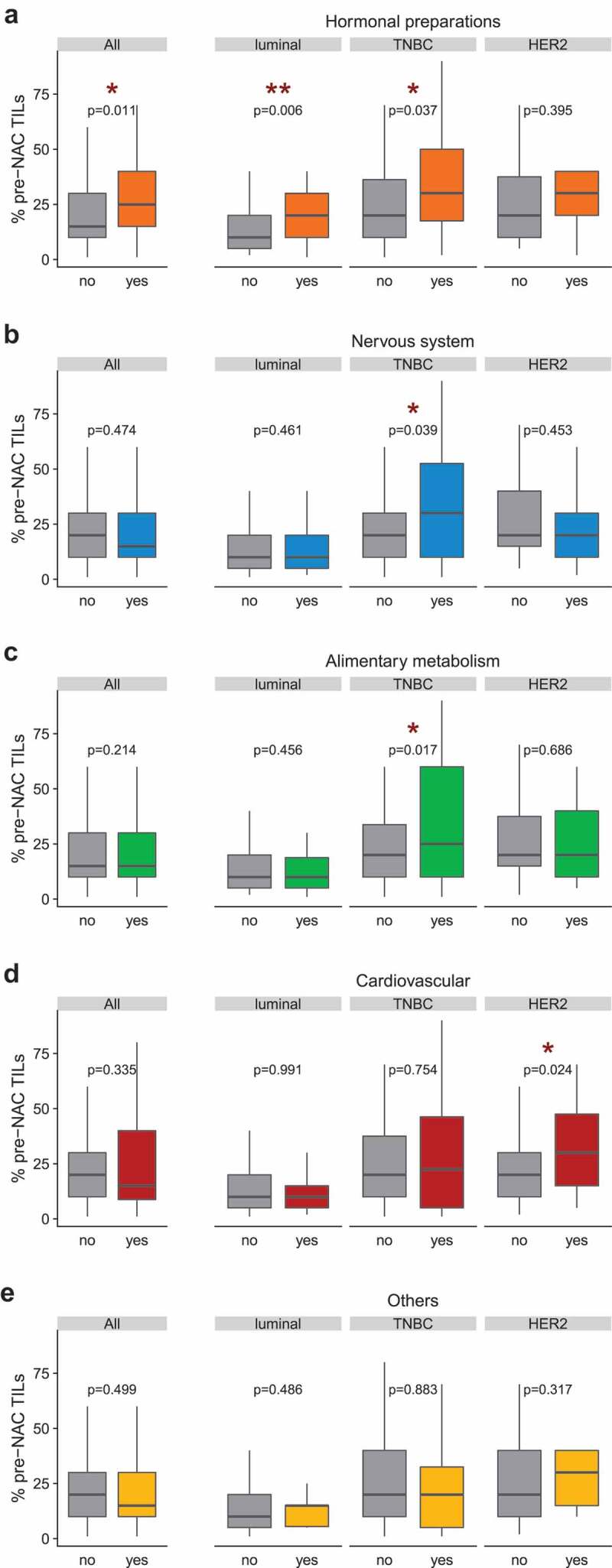
Pre-NAC TILs densities by comedication use (ATC level 1) in the whole population and by BC subtype. The TIL density (% pre-NAC TILs) was scored continuously as the average percentage of stromal area occupied by mononuclear cells as previously recommended.72 In the x-axis, patients were classified according to their use ("yes”) or absence of use ("no”) of a co-medication. (a) Systemic hormonal preparations (class H); (b) Nervous system (class N); (c) Alimentary and metabolism (class A); (d) Cardiovascular (class C); (e) Others. In boxplots, lower and upper bars represent the first and third quartile, respectively, the medium bar is the median, and whiskers extend to 1.5 times the inter-quartile range. TIL density was compared in Wilcoxon-Mann-Whitney tests (for groups including less than 30 patients) or with student t-test (n ≥30).
At the ATC level 2 (Supplemental Table 3), pre-NAC TILs were increased in patients with diuretics (C03) or thyroid therapy (H03) (Figure 3a,d). TIL levels were increased specifically in TNBC patients taking analgesics (N02) and drugs for [gastric] “acid-related disorders” (A02) (Figure 3b,c). This was not found in the other BC subtypes and the interactions tests were statistically significant (Pinteraction comedication/BC subtype = 0.019 and 0.027 for N02 and A02, respectively), meaning that the association of the comedication use and TIL levels differed by BC subtype. Conversely, in TNBC patients, TILs tended to be decreased (p = .175) in patients taking lipid-modifying agents (C10) and were significantly (p = .044) reduced in individuals consuming anti-inflammatory and anti-rheumatic products (M01) (Figure 3a,c).
Figure 3.
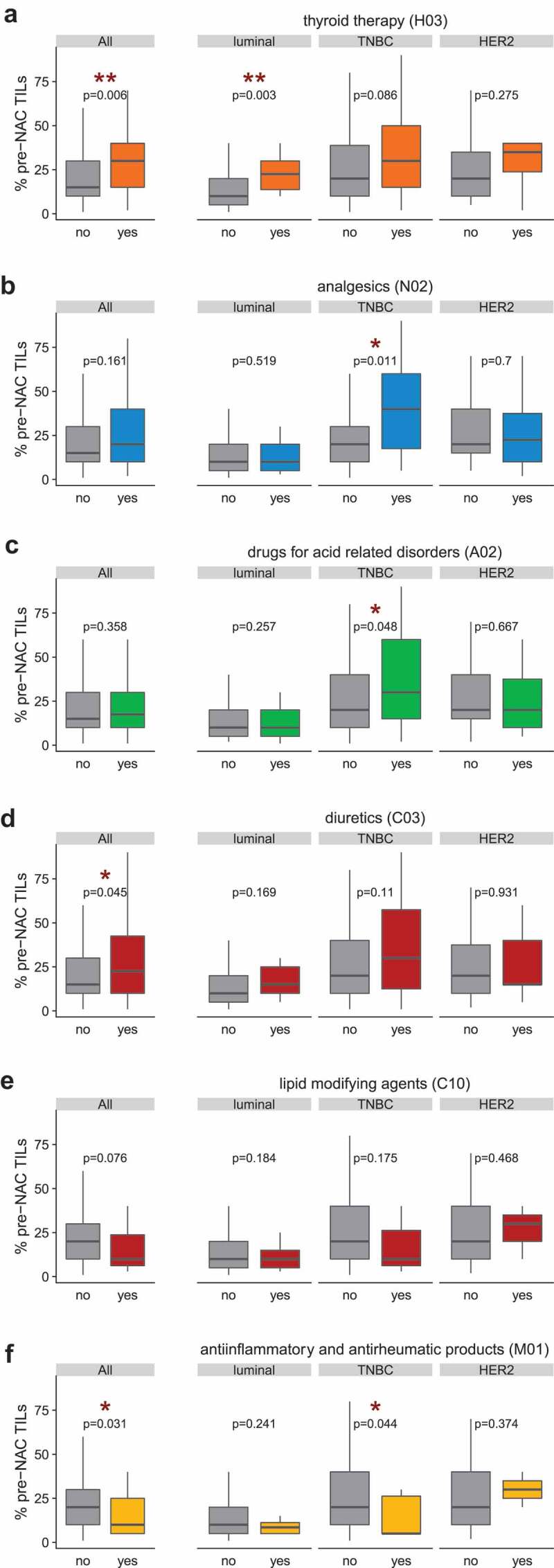
Pre-NAC TILs densities by comedication use (ATC level 2) in the whole population and by BC subtype. The TIL density (% pre-NAC TILs) was scored continuously as the average percentage of stromal area occupied by mononuclear cells as previously recommended.72 In the x-axis, patients were classified according to their use ("yes”) or absence of use ("no”) of a co-medication. (a) Thyroid therapy (class H03); (b) Analgesics (class N02); (c) Drugs for acid-related disorders (class A02); (d): Diuretics (C03); (e) Lipid-modifying agents (C10) ; (f) Anti-inflammatory and anti-rheumatic products (M01). In boxplots, lower and upper bars represent the first and third quartile, respectively, the medium bar is the median, and whiskers extend to 1.5 times the inter-quartile range. TIL density was compared in Wilcoxon-Mann-Whitney tests (for groups including less than 30 patients) or with student t-test (n ≥30).
We next analyzed gene expression profiles (GEPs) using RNA from baseline tumor samples in pre-NAC BC patients (n = 140). We focused on immune-related signatures that had been reported to correlate with clinical benefit in different clinical studies using immune checkpoint inhibitors for various cancer types.27,28 The T cell-inflamed GEP enriched in IFNγ–responsive genes related to antigen presentation, chemokine expression, cytotoxic activity, and adaptive immune resistance were found in about 40% specimen (Supplemental Figure 2). The level of the T cell-inflamed GEP or “IFN metagene” was significantly higher in patients taking hormonal preparations (whole population, luminal, HER2-positive, Figure 4a), and this association was different according to the molecular type among patients taking drugs from class A (lower in luminal, higher in TNBC patients) (Figure 4b,c).
Figure 4.
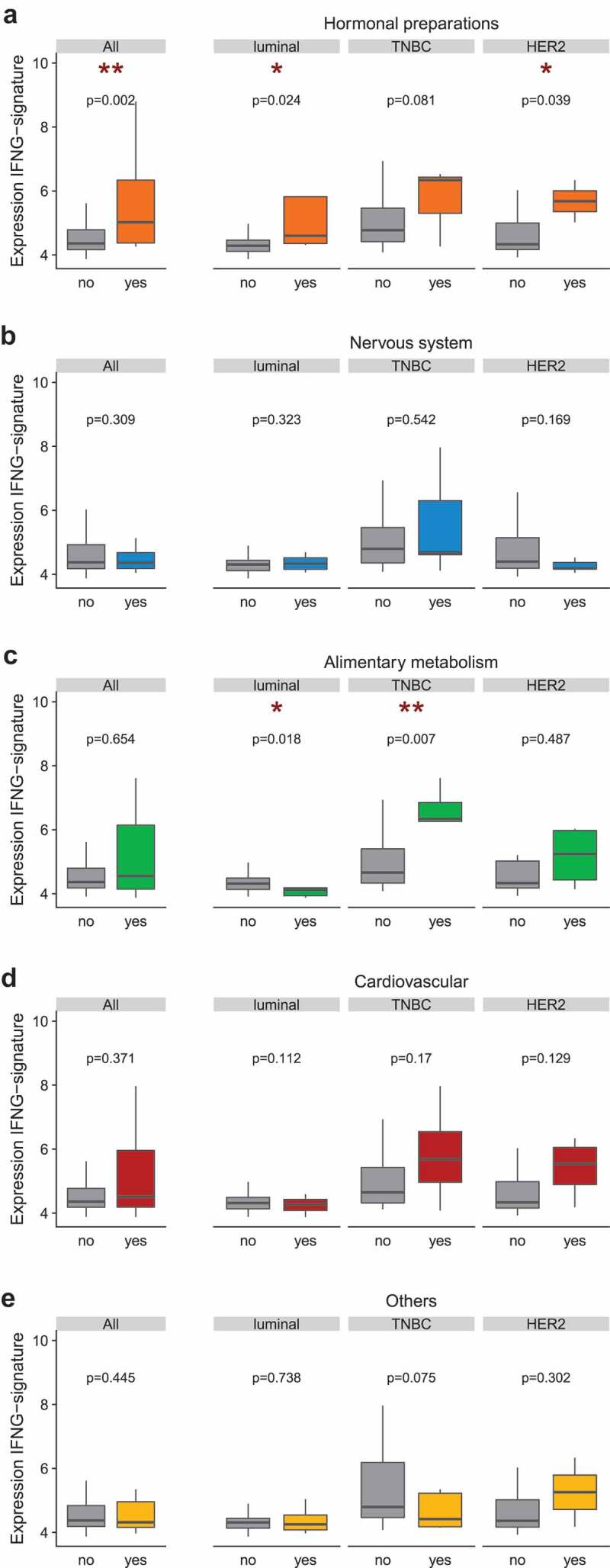
Levels of the T cell-inflamed gene expression profile or “IFN metagene” in the whole population and by BC subtype. Gene expression profiling on 140 pre-NAC tumor samples centered around the IFN-γ metagene described in Ayers et al.72 quantified according to the mean-normalized expression value for six genes (IFNG, IDO1, CXCL9, CXCL10, HLA-DRA, STAT1). In the x-axis, patients were classified according to their use ("yes”) or absence of use ("no”) of a co-medication. (a) Systemic hormonal preparations (class H); (b) Nervous system (class N); (c) Alimentary and metabolism (class A); (d) Cardiovascular (class C); (e) Others. In boxplots, lower and upper bars represent the first and third quartile, respectively, the medium bar is the median, and whiskers extend to 1.5 times the inter-quartile range. IFNG-metagene levels were compared in Wilcoxon-Mann-Whitney tests (for groups including less than 30 samples) or with student t-test (n≥30). Student t-test (n ≥30).
Altogether, hormonal preparations (mostly targeting thyroid disorders), nervous system-affecting drugs (such as analgesics), medications targeting cardiovascular diseases (such as diuretics) and compounds treating acid-related disorders were associated with increased lymphocytic infiltrates, and T cell inflamed GEP in all and/or triple-negative BC at diagnosis. In contrast, anti-inflammatory and anti-rheumatic products were negatively correlated with TIL density.
Comedication is associated with changes in pCR rates in BC
Bearing in mind the strong correlations between pre-NAC TIL density and pathological responses,1,2 we next undertook to analyze potential associations between comedications and rates of pathological complete responses (pCR) assessed by pathologists at surgery post-NAC. The use of drugs from the class N (Nervous system) was associated with higher pCR rates than no use (Supplemental Table 4) in the whole population (p = .035) and in TNBC patients (p = .026). At the level 2 (Supplemental Table 5), pCR rates were increased in patients taking psycholeptics (N05), agents acting on the renin-angiotensin system (C09), and TNBC patients taking psychoanaleptics (N06) (Figure 5a–c). Conversely, pCR rates tended to be decreased in TNBC patients taking vasoprotective drugs (C05) or anti-inflammatory and anti-rheumatic products (M01) (Figure 5d–e).
Figure 5.
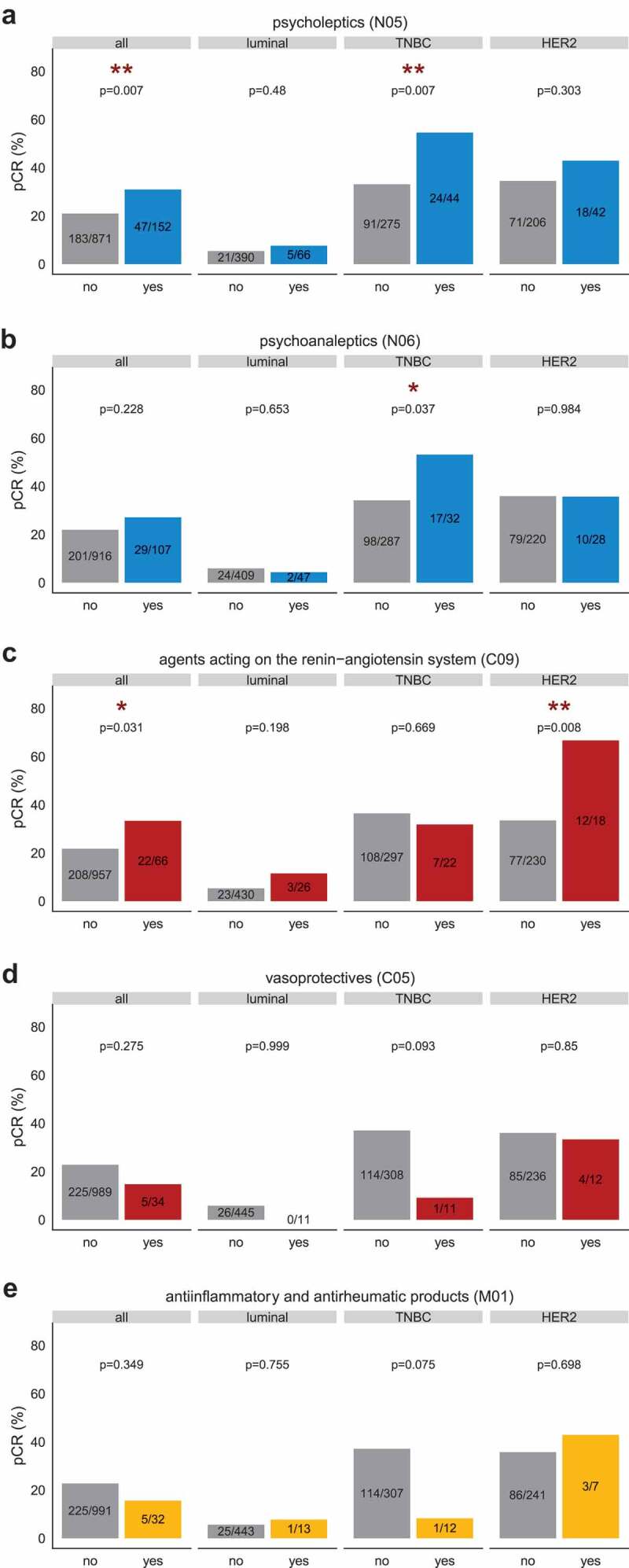
Pathological complete response (pCR) rates by comedication use (ATC level 2) in the whole population and by BC subtype. The pCR was assessed according to routine clinical guidelines.80 Effectives mentioned on the barplot represent the number of patients whose tumor reached pCR/total number of patients of the given category. In the x-axis, patients were classified according to their use ("yes”) or absence of use ("no”) of a co-medication. (a) Psycholeptics (N05); (b) Psychoanaleptics (N06); (c) agents acting on the renin-angiotensin system (class C09); (d) Vasoprotectives (class C05); (e) Anti-inflammatory and anti-rheumatic products (M01). The association between categorical variables was assessed with chi-square test or with the Fisher’s exact test if at least one category showed less than three patients.
After multivariate analysis, the association between psycholeptics (N05) and pCR remained statistically significant in the whole population (OR = 1.64, 95%CI [1.05–2.55], p = .027) and in TNBC patients (OR = 2.04, CI [1.06–3.97], p = .034). Accordingly, the association between pCR and agents acting on the renin-angiotensin system (C09) in HER2-positive BC withheld the multivariate Cox regression model (OR = 3.36, CI [1.2–10.33], p = .025) (Table 1). No comorbidity was significantly associated with pCR after multivariate analysis.
Table 1.
Study population characteristics.
| Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Whole population | OR | 95%CI | p | OR | 95%CI | p | |
| Age (y.) | 1 | [0.99–1.02] | 0.775 | ||||
| BMI | 1.01 | [0.98–1.04] | 0.628 | ||||
| Menopausal status | pre vs post menopausal | 0.78 | [0.58–1.06] | 0.113 | |||
| Tumor size (TNM) | T3 vs T1-T2 | 0.81 | [0.57–1.14] | 0.231 | |||
| clinical nodal status | N1-N2-N3 vs N0 | 0.98 | [0.73–1.32] | 0.889 | |||
| Histological type | other vs NST | 0.58 | [0.32–0.99] | 0.057 | |||
| Grade | Grade III vs I-II | 3.51 | [2.49–5.03] | <0.001 | 1.97 | [1.33–2.96] | 0.001 |
| Ki67 | ki67 ≥ 20 vs < 20 | 4.55 | [2.46–9.24] | <0.001 | |||
| BC subtype | TNBC vs luminal | 9.32 | [5.99–15] | <0.001 | 7.71 | [4.69–13.17] | <0.001 |
| HER2 vs luminal | 9.26 | [5.85–15.11] | <0.001 | 9.51 | [5.79–16.23] | <0.001 | |
| NAC regimen | Anthra taxanes vs anthra | 2.24 | [1.49–3.49] | <0.001 | |||
| Taxanes/others vs anthra | 1.69 | [0.9–3.13] | 0.097 | ||||
| Hypertension/H.D. | Yes vs no | 1.31 | [0.9–1.89] | 0.155 | |||
| Depression/Anxiety | Yes vs no | 1.11 | [0.65–1.82] | 0.699 | |||
| Dyslipidemia | Yes vs no | 1.26 | [0.71–2.13] | 0.411 | |||
| Diabete | Yes vs no | 1.62 | [0.78–3.2] | 0.175 | |||
| Ulcer/Gastritis | Yes vs no | 1.09 | [0.67–1.72] | 0.717 | |||
| Thyroid disorders | Yes vs no | 1.25 | [0.72–2.1] | 0.406 | |||
| Other comorbidity | Yes vs no | 1.05 | [0.62–1.73] | 0.84 | |||
| Psycholeptics (N05) | Yes vs no | 1.39 | [1.04–1.87] | 0.028 | 1.64 | [1.05–2.55] | 0.027 |
| Agents acting on the renin -angiotensin system (C09) |
Yes vs no |
1.8 |
[1.04–3.04] |
0.031 |
|
|
|
| Univariate analysis |
Multivariate analysis |
||||||
| TNBC |
OR |
95%CI |
p |
OR |
95%CI |
p |
|
| Age (y.) | 0.99 | [0.97–1.01] | 0.426 | ||||
| BMI | 0.97 | [0.92–1.02] | 0.298 | ||||
| Menopausal status | pre vs post menopausal | 1.08 | [0.68–1.75] | 0.739 | |||
| Tumor size (TNM) | T3 | 0.77 | [0.45–1.29] | 0.332 | |||
| clinical nodal status | N1-N2-N3 | 0.98 | [0.62–1.55] | 0.932 | |||
| Histological type | other | 0.92 | [0.38–2.1] | 0.852 | |||
| Grade | Grade III | 3.71 | [1.7–9.35] | 0.002 | 3.44 | [1.56–8.7] | 0.004 |
| Ki67 | ki67 ≥ 20 | 3.87 | [1.25–16.99] | 0.035 | |||
| NAC regimen | Anthra taxanes vs anthra | 1.79 | [0.97–3.44] | 0.069 | |||
| Taxanes/others vs anthra | 1.41 | [0.49–3.87] | 0.513 | ||||
| Hypertension/H.D. | Yes vs no | 0.98 | [0.53–1.78] | 0.954 | |||
| Depression/Anxiety | Yes vs no | 1.61 | [0.73–3.51] | 0.234 | |||
| Dyslipidemia | Yes vs no | 0.48 | [0.17–1.16] | 0.125 | |||
| Diabete | Yes vs no | 0.62 | [0.2–1.66] | 0.366 | |||
| Ulcer/Gastritis | Yes vs no | 0.44 | [0.17–1.01] | 0.067 | |||
| Thyroid disorders | Yes vs no | 1.16 | [0.51–2.55] | 0.709 | |||
| Other comorbidity | Yes vs no | 1 | [0.41–2.29] | 0.996 | |||
| Psycholeptics (N05) | yes vs no | 2.43 | [1.28–4.66] | 0.007 | 2.04 | [1.06–3.97] | 0.034 |
| Psychoanaleptics (N06) |
yes vs no |
2.19 |
[1.05–4.61] |
0.037 |
|
|
|
| Univariate analysis |
Multivariate analysis |
||||||
|
HER2 |
OR |
95%CI |
p |
OR |
95%CI |
p |
|
| Age (y.) | 1.03 | [1.01–1.06] | 0.016 | ||||
| BMI | 1.04 | [0.98–1.01] | 0.1493 | ||||
| Menopausal status | pre vs post menopausal | 0.47 | [0.28–0.8] | 0.006 | 0.55 | [0.31–0.98] | 0.043 |
| Tumor size (TNM) | T3 vs T1-T2 | 1.05 | [0.58–1.89] | 0.862 | |||
| clinical nodal status | N1-N2-N3 vs N0 | 0.8 | [0.48–1.36] | 0.415 | |||
| Histological type | other vs NST | 1.36 | [0.33–5.29] | 0.65 | |||
| Grade | Grade III vs I-II | 1.08 | [0.62–1.87] | 0.794 | |||
| Ki67 | ki67 ≥ 20 vs < 20 | 1.72 | [0.6–5.69] | 0.336 | |||
| ER status | positive versus negative | 0.42 | [0.24–0.71] | 0.001 | 0.39 | [0.22–0.68] | 0.001 |
| NAC regimen | Anthra taxanes vs anthra | 2.56 | [1.11–6.64] | 0.036 | 2.94 | [1.21–8.05] | 0.024 |
| Taxanes/others vs anthra | 2.2 | [0.74–6.93] | 0.163 | 2.17 | [0.68–7.33] | 0.197 | |
| Hypertension/H.D. | Yes vs no | 2.04 | [1.06–3.97] | 0.033 | |||
| Depression/Anxiety | Yes vs no | 0.73 | [0.23–2.04] | 0.565 | |||
| Dyslipidemia | Yes vs no | 2.3 | [0.98–5.48] | 0.054 | |||
| Diabete | Yes vs no | 1.52 | [0.43–5.18] | 0.501 | |||
| Ulcer/Gastritis | Yes vs no | 1.95 | [0.9–4.23] | 0.09 | |||
| Thyroid disorders | Yes vs no | 1.27 | [0.45–3.44] | 0.638 | |||
| Other comorbidity | Yes vs no | 1.31 | [0.54–3.07] | 0.535 | |||
| Agents acting on the renin -angiotensin system (C09) | Yes vs no | 3.97 | [1.48–11.79] | 0.008 | 3.13 | [1.1–9.71] | 0.037 |
Abbreviations: BMI: Body mass index (kg/m2). TNM: tumor node metastasis (AJCC staging). NAC: neoadjuvant chemotherapy
T cell-dependent antitumor effects of zolpidem in mouse breast cancer
We next analyzed cause–effect relationships between comedications taken by patients and natural or chemotherapy-induced cancer immunosurveillance in immunodeficient or immunocompetent mice bearing BC. First, we tested the combination of bromazepam with standard of care (anthracycline-based chemotherapy and taxanes) in the PDX model of TNBC HBCx-8 inoculated in immunosuppressed animals. HBCx-8 xenografts were treated with PBS, AC (adriamycin, 2 mg/kg, and cyclophosphamide (CTX), 100 mg/kg), or docetaxel (TXT), 20 mg/kg, given as single injection at day 1 by i.p. or i.v. injections, respectively, alone or combined with the benzodiazepine bromazepam (class N, ATC level 3, anxiolytics), given orally at 0.6 mg/kg, 5 days/week. Bromazepam alone did not reduce tumor growth. Both the AC or TXT regimens mediated marked antitumor effects, followed by tumor recurrence. The addition of bromazepam to AC and TXT did not delay the time until tumor recurrence (Figure 6a).
Figure 6.
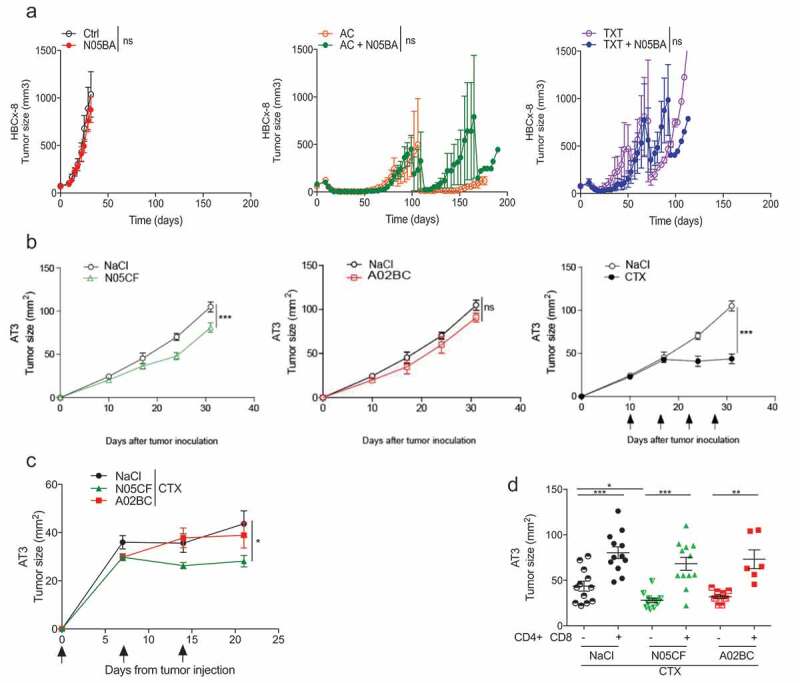
Immune effects of co-medication in mouse breast cancer models. (a) The PDX HBCx-8 xenograft established from a TNBC patient was transplanted into female 8-week-old Swiss nude mice and then, randomly assigned to the control or treatment groups (AC versus TXT alone or combined with bromazepam (N05BA)). Tumor growth kinetics with broma alone versus Ctrl, AC versus AC+ N05BA and TXT versus TXT+ N05BA are represented overtime, in six animals/group, in a representative experiment out of two yielding similar conclusions. Statistical analyses: *p < 0.05, ** p < 0.01, *** p < 0.001, ns=not significant. (b) and (c). Prophylactic and therapeutic i.p. administration of zolpidem (N05CF) or pantoprazole (A02BC) versus NaCl alone (b) or in combination with Cyclophosphamide (CTX) (c) in C57Bl/6 mice bearing the TNBC AT3. (d) Depletion of CD4+ or CD8+ lymphocytes with specific antibodies in the same setting as in (c). Tumor growth kinetics are depicted for a pool of two independent experiments comprising six mice/groups for (b) and (d). *p < 0.05, ** p < 0.01, *** p < 0.001, ns=not significant.
Based on the findings that comedications correlated with the T cell inflamed GEP and TIL densities in tumor beds (Figures 3 and 4), and the assumption that an intact immune system is required for long-lasting anticancer protective effects induced by cytotoxicants, we challenged immunocompetent mice with the transplantable AT3 triple-negative mouse BC29 AT3 showed a significant albeit minimal response to zolpidem (N05CF) (10mg/kg/day, i.p. for 30 days), an imidazopyridine nonbenzodiazepine hypnotic drug (binding with high affinity to the α1 subunit of the gamma aminobutyric acid A receptor) (Figure 6b, left panel) but not to the proton pump inhibitor pantoprazole (A02BC) (100mg/kg/day), when the medication was initiated 14 days prior to tumor inoculation and pursued for >14 days (Figure 6b, middle panel). When combined to CTX (100 mg/kg weekly for 3 weeks) alone, Zolpidem (N05CF) (but not pantoprazole (A02BC)) ameliorated the anticancer effects (Figure 6c). The additive effects of the cytotoxicant CTX and hypnotic drugs were markedly abolished after the depletion of CD4+ and CD8+ T cells by means of suitable antibodies (Figure 6d), supporting the working hypothesis. Moreover, tissue immunofluorescence stainings revealed that AT3 TNBC were infiltrated with CD3+CD4− lymphocytes that were inversely correlated with tumor size across all experiments and animals (Figure 7a). Similarly, the T effector ratio over that of regulator T cells (Treg) negatively correlated with tumor size across all experiments (Figure 7b). Importantly, the density of such effectors was increased by concomitant therapy with Zolpidem (N05CF) alone as compared to untreated controls. Zolpidem combined with CTX also yielded a higher density of effector T cells compared to CTX alone (Figure 7c).
Figure 7.
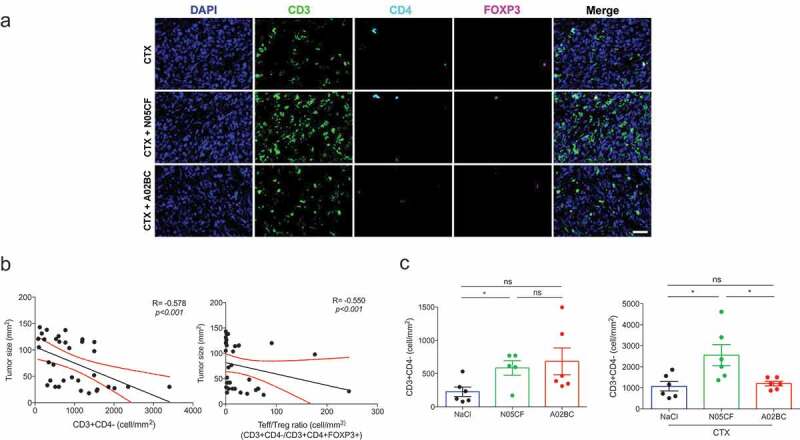
Comedications influence TIL densities in mouse TNBC. Representative micrograph pictures of co-immunofluorescence of CD3 (green), CD4 (cyan), FOXP3 (magenta), and DAPI stain (blue) in AT3 tumors at sacrifice in mice treated with CTX, zolpidem (N05CF) or pantoprazole (A02BC) alone or combined together. Scale bar: 20 µm (a). Spearman correlations between tumor sizes at sacrifice and CD3+ CD4− cell density (b, left) and the ratio of CD3+CD4− cells/ CD3+CD4+FOXP3+ cells across six experimental groups comprising 6 mice/group (b, right). Bar graphs showing CD3+CD4− cell density in AT3-bearing mice treated with NaCl, N05CF or A02BC, (c, left), CTX, CTX + N05CF or CTX + A02BC (c, right). Data are shown as means ± SEM. P values were obtained using ANOVA test.
These results illustrate that specific drugs modulating the nervous system can advantageously be combined with chemotherapy to increase tumor infiltration by T lymphocytes and to reduce tumor progression in immunocompetent (but not immunodeficient) TNBC bearing hosts.
Discussion
Comedication may represent an underestimated confounding factor in many clinical trials conducted in oncology. Off-target effects mediated by non-cytotoxic drugs with a satellite role in the oncological armamentarium may have direct or indirect anti-cancer effects through several mechanisms. These mechanisms include reduction of inflammation,21 decrease of invasion and metastasis,21 modulation of angiogenesis and vasculature,30 enhancement of apoptosis,31 inhibition of epithelia-mesenchymal transition,32 reversal of hypoxia20 and acidosis,33 decrease of proliferation,34 inhibition of critical growth or tumor suppressor pathways.35,36 These effects were evidenced either alone21,22 and in combination with other anti-cancer treatments (chemotherapy30,37 or radiotherapy38). The concomitant use of co-medication during NAC may also affect pharmacokinetic-related parameters, in as much as a wide range of medications interfere with cytochromes. For example, cimetidine has been described to modify the pharmacokinetic of epirubicin in advanced BC patients39 Similarly, the cytochrome P450 – (isoform 2C8 and 3A4) inhibitor amiodarone has been related to serious pharmacokinetics interactions when used with taxanes40 Comedications may also inhibit multi-drug resistance (MDR), involving efflux proteins of the ATP binding cassette transporter family translocating a substrate from the intracellular to the extracellular compartment. P-glycoproteins and breast cancer resistance protein can indeed be inhibited by atorvastatin,41 itraconazole,42 verapamil43 or PPI44
In this hypothesis-generating study performed in 1023 BC patients, we found that the use of several comedication classes was associated with either increased or decreased TIL levels, some of these associations translating into increased pCR rates. While independent from the intrinsic molecular and clinical characteristics of BC at disease presentation, the use of comedications, linked to age-related morbidities, was found to correlate with immune infiltration at diagnosis, knowing that a high TIL density is a prerequisite for optimal pathological response to NAC.1,2 Our preclinical data also support a mandatory role for T lymphocytes in the additive effects of hypnotic and cytotoxic compounds in immunocompetent tumor bearers while they failed to boost each other in PDX models established in immunodeficient hosts. These findings plead for immune-related effects mediated by comedications and their capacity to shape the tumor microenvironment to pave the way to the immunomodulatory role of chemotherapy45
While many retro- or prospective studies evaluated the links between aspirin or NSAID and reduced cancer occurrence,46,47 no such study has evaluated the potential association of daily administration of other types of self-medication or prescription by the general practitioner on immune functions and cancer immunosurveillance. In a randomized controlled trial in 38 BC patients, perioperative COX-2 and β-adrenergic blockade by propranolol and etodolac was associated with changes in immune profiles of surgical specimens with notably decreased tumor-infiltrating monocytes and increased tumor-infiltrating B cells21 Moreover, retrospective clinical data suggest that some anesthetic techniques can attenuate immunosuppression and minimize metastasis after cancer surgery.48,49 For example, in patients undergoing breast cancer surgery, propofol anesthesia with postoperative ketorolac analgesia reportedly has a favorable impact on NK cell cytotoxicity compared with sevoflurane anesthesia and postoperative fentanyl analgesia.50,51
Several mechanisms have been proposed to account for these off-target effects of distinct compounds, not necessarily annotated as “cytotoxic agents”. ER stress response inducers (i.e. thapsigargin52,53 or cardiac glycosides54,55,56) or autophagy inducers (such as aspirin, spermidine, hydroxycitrate57-59) could mediate a cellular stress of cancer cells associated with secretion of alarmins or cell surface expression of danger signals igniting the inflammasome and/or pattern recognition receptors45 These cell autonomous changes of cancerous cells preceding immunogenic cell death pave the way to synergistic anticancer activities when these compounds are combined with conventional chemotherapy, radiotherapy or targeted treatments. Other comedications can reprogram the tumor microenvironment by dampening myeloid suppressor cells. Thus, the anti-diabetic biguanide metformin may yield clinical benefit in ovarian cancer patients through improvement of antitumor T-cell immunity by dampening CD39/CD73-dependent MDSC immunosuppression60 Metformin may also act on the cognate arm of immunity i.e. CD8+ TILs and protect them from apoptosis and exhaustion,19 thereby potentiating the efficacy of PD-1 blockade. In addition, proton pump inhibitors could cause reversal of acidity-induced cancer immune escape23,24 and modulate myelopoiesis and the polarization of tumor-associated macrophages61 Compounds impacting the nervous system, more specifically hypnotic drugs are broadly prescribed. The hypnotic zolpidem was associated with higher TIL density in our retrospective clinical study and turned out to be immunogenic in combination with CTX in our preclinical AT3 model. These proinflammatory effects are consistent with correlative associations reported in a large population-based study of >59 000 individuals in the National Health Insurance Research Database (NHIRD) of Taiwan performed among patients with sleep disturbance taking zolpidem for at least 2 years (n = 14 000 patients). The authors found positive associations between the use of zolpidem and the risk of ischemic stroke,62 of Parkinson disease after 5 years of follow-up63 and of cancer occurrence64 (oral cancer (HR, 2.36; 95% CI, 1.57–3.56), as well as kidney cancer, esophageal cancer, and BC). Conversely, GABAergic modulation with classical benzodiazepines lorazepam and clonazepam, aside from exerting anxiolytic and antidepressant effects, may have therapeutic potential as neuroimmunomodulators during psychosocial stress. Lorazepam and clonazepam as well as the antidepressant imipramine blocked stress-induced accumulation of macrophages in the central nervous system, prevented neuroinflammatory signaling and reversed anxiety-like and depressive-like behavior in mice exposed to repeated social defeat.65,66 The use of beta-blocker, specifically the selective blockade of β2adrenergic receptors, correlated with better overall survival in metastatic melanoma patients and improved the efficacy of anti-PD1 and IL-2-based immunotherapies mobilizing T lymphocytic effectors in mice67 Conversely, in another experimental study where epinephrine-mobilized NK cells prevented tumor outgrowth following exercise, β-adrenergic signaling blunted training-dependent tumor inhibition and the trafficking of IL-6-dependent NK effectors into the tumor bed68 These apparent contradictions highlight the need for mechanistic exploration of the synergistic or antagonistic off-target bioactivity of these comedications.
Our findings suggest that the effect of comedications on TILs and pCR may vary by BC subtype. The multiple interactions and the high number of drugs to explore on a single cohort highlights the need for large-scale validation studies to address the immense complexity that likely underlies the interactions between comedication, immune infiltration, and chemotherapy outcome. Only very large patient cohorts would provide the sufficient statistical power for meaningful comparisons among tumor and drug subgroups.
We acknowledge several limitations of our work. First, we were not able to monitor whether the comedications retrieved in electronical medical records (EMR) truly reflected the chronic treatment. Patients may either over or underreport drugs to the physician, and measuring adherence to treatment remains a substantial challenge in medicine. In addition, we were not able to evaluate the respective roles of taxanes and anthracyclins in the drug interactions we evidenced, because most of BC patients received a sequential NAC regimen including anthracyclines first followed by taxanes, which represents the standard of care in the neoadjuvant setting. Second, no gold-standard method exists for measuring comorbidities in the context of cancer so far69 It is known that complexities exist in both measuring comorbidity in cancer patients and evaluating their impact on patients’ outcome.69,70 Plus, the effects of chronic diseases on outcomes vary by condition and by cancer type69 These pitfalls might limit the generalization of our findings to other cancer types. Third, we did not adjust our statistical analyses for multiple testing. While decreasing the risk of false-positive results, such conservative statistical procedures would have considerably increased the false-negative risk of this hypothesis-generating work, without overcoming the other limitations we previously described. Instead, we rather chose to validate our findings in an experimental manner, and we are launching a large-scale validation program in independent datasets of BC patients. Finally, as we based our results on real-world evidence, we cannot exclude that our findings might be hampered by inherent bias of retrospective studies. Thus, our study must clearly be considered as hypothesis generating, and further validation is highly needed.
The hypotheses we raised in the current work open several thrilling perspectives for drug repositioning. It paves the way to further explore the field of comedications as immunomodulators and chemotherapy sensitizers. As nearly half of the patients take one or more comedications, a considerable amount of untapped data is already available for exploitation in electronical health records of patients treated with NAC in cancer centers. We hypothesize that a variety of drugs that are not usually part of the oncological armamentarium may exert off-target effects against BC or other cancer types. Digging into such real-life data could help to identify drugs or lifestyle experiences that improve the response to antineoplastic treatments, followed by the design of clinical trials to quickly validate these hypotheses. In a context where the financial burden of innovative oncologic therapies jeopardizes health systems, repositioning routine prescriptions as anticancer treatments sensitizers could be an exciting strategy71
Material and methods
Patients, tumors and cancer treatments
We analyzed a cohort of 1023 T1-3NxM0 patients with invasive breast carcinoma (NEOREP Cohort, CNIL declaration number 1547270) treated with NAC at Institut Curie, Paris, between 2002 and 2012. We included only unilateral, non-recurrent, non-inflammatory, non-metastatic tumors, and excluded T4 tumors. All patients received NAC, followed by surgery and all but 21 patients received radiotherapy. The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules regarding research on tissue specimens and patients. Written informed consent from the patients was not required by French regulations. Analysis of human samples was performed in accordance with the French Bioethics Law 2004–800, the French National Institute of Cancer (INCa) Ethics Charter, and after approval by the Institut Curie review board and ethics committee (Comité de Pilotage du Groupe Sein) that waived the need for written informed consent from the participants. Women were informed of the research use of their tissues and did not declare any opposition for such research. Data were analyzed anonymously. Patients were treated according to national guidelines. NAC regimens changed over time (anthracycline-based regimen or sequential anthracycline-taxane regimen), with trastuzumab used in an adjuvant and/or neoadjuvant setting since the mid-2000s. Surgery was performed four to 6 weeks after the end of the chemotherapy. Endocrine therapy (tamoxifen, aromatase inhibitor, and/or GnRH agonists) was prescribed when indicated.
Tumor samples and pathology review
ER, PR and HER2 positivity determination is detailed in the supplemental material. BC subtypes were defined as follows: tumors positive for either ER or PR, and negative for HER2 were classified as luminal; tumors positive for HER2 were considered HER2-positive BC; tumors negative for ER, PR, and HER2 were considered as triple-negative BC (TNBC).
For a subset of patients (n = 615), central reading of tumor material was performed, and stromal TILs were retrospectively reviewed on pathological specimens of pre-NAC core needle biopsy (See supplemental methods). Pretreatment core needle biopsies were evaluated independently by two expert breast pathologists for the presence of a mononuclear cells infiltrate (including lymphocytes and plasma cells, excluding polymorphonuclear leukocytes) following the recommendations of the international TILs Working Group72 TILs were scored continuously on hematoxylin and eosin-stained sections without additional staining as the average percentage of stromal area occupied by mononuclear cells.
Comedications
Chronic concomitant therapies – designed throughout the manuscript as comedications – were extracted retrospectively from medical charts, as any chronic treatment for a chronic condition declared by the patient upon hospital admission at initial or anesthetics consultation, and reported in electronic medical record from BC diagnosis until the date of surgery. Intercurrent treatments lasting less than 1 week were excluded, as well as medications prescribed around chemotherapy (anti-vomiting drugs, granulocyte-colony stimulating factors, steroids), as they are systematically prescribed to all patients. As the information on drug dosing, schedule, or date of introduction was not constantly available, comedication use was coded as a binary variable (yes: at least one drug declaration; no: no comedication mentioned). They were classified according to the Anatomical Therapeutic Chemical (ATC) Classification System (ATC index 2018) controlled by the World Health Organization Collaboration (available at the URL https://www.whocc.no/atc_ddd_index/). ATC is used for the classification of active ingredients of drugs according to the organ or system on which they act and their therapeutic, pharmacological and chemical properties. The first level of the code indicates the anatomical main group (14 main groups) and consists of one letter; the second level of the code indicates the therapeutic subgroup; the third level and the fourth levels of the code indicate the chemical/therapeutic/pharmacological subgroup, and the fifth level indicates the chemical substance. The complete classification of metformin illustrates the structure of the code:
| A | Alimentary tract and metabolism (1st level, anatomical main group) |
| A10 | Drugs used in diabetes (2nd level, therapeutic subgroup) |
| A10B | Blood glucose lowering drugs, excl. insulins (3rd level, pharmacological subgroup) |
| A10BA | Biguanides (4th level, chemical subgroup) |
| A10BA02 | Metformin (5th level, chemical substance) |
Drugs from categories D (dermatologicals), P (antiparasitic products, insecticides, and repellents), L (antineoplastic and immunomodulating agents), S (sensory organs) were excluded from the analyses. Only chronic antivirals for systemic use (J05) and oral drugs for respiratory system (antihistamines for systemic use (R06)) were included in drugs from category J (anti-infectives for systemic use) and R (respiratory system) respectively. Anatomical classes with less than 50 comedications were grouped into the category “Others”.
Comorbidities
Comorbidities, defined as any chronic condition declared by the patient at initial or anesthetics consultation were extracted retrospectively from medical charts. Comorbidities were regrouped into six classes: hypertension/heart disease, depression/anxiety, dyslipidemia, diabetes, ulcer/gastritis, thyroid disorders, and the category “Others” regrouped the remaining chronic conditions.
Gene expression
Total RNA extraction from frozen pretreatment biopsies was previously performed for 140 patients who participated in the clinical trials REMAGUS0273,74 and REMAGUS0475 Human GeneChip U133 plus 2.0 microarray hybridization and quality controls have already been described in details elsewhere.75-78 For each dataset, batch effects were eliminated by the median centering of each probe set across arrays and the quantile normalization of all arrays separately for each set. The expression levels of six immune genes (IFNG, IDO1, CXCL9, CXCL10, HLA-DRA, STAT1) from the previously published Interferon-γ signature27 were extracted from the pooled gene expression matrix. We assessed Interferon-γ metagene expression by calculating the mean-normalized expression value for all the genes considered together and we generated a heatmap of the metagene expression profile using the gplot package.
Study endpoints
ypTN stage was defined according to the American Joint Committee on Cancer/Union for International Cancer Control staging79 A pathological complete response (pCR) was defined as an absence of invasive residual tumor in the breast, and of invasive disease in the axillary nodes (ypT0/is+ ypN0)80
Animal models
Immunodeficient xenograft model
The PDX HBCx-8 xenograft was established from a triple-negative negative breast cancer as previously described81 The in vivo efficacy study was conducted by transplanting HBCx-8 tumor fragments into female 8-week-old Swiss nude mice that were randomly assigned to the control or treated groups (six mice per group) when tumors reached a volume of 60 to 200 mm3. Adriamycin, 2 mg/kg (Doxorubicin, Teva Pharmaceuticals) and cyclophosphamide, 100 mg/kg (Endoxan, Baxter), or docetaxel, 20 mg/kg (Taxotere, Sanofi-Aventis) were given as single injection at day 1 by intraperitoneal (i.p.) and intravenous (i.v.) injections. Bromazepam was given orally at 0.6 mg/kg 5 days/week until ethical sacrifice. Tumor growth was evaluated by measurement of two perpendicular diameters of tumors with a caliper twice per week. Individual tumor volumes were calculated as V = axb 2/2, a being the largest diameter, b the smallest. Mice were ethically sacrificed when the tumor volume reached 1500 mm3.
Immunocompetent mice model
The C57BL/6 mice were injected intraperitoneally (i.p.) for 14 consecutive days with Zolpidem (5 mg/kg twice a day) or Pantoprazole (100 mg/kg once a day) or vehicle (NaCl). On day 14, 106 AT3 cells were inoculated and mice continued their treatment with Zolpidem or Pantoprazole or vehicle. When tumors reached 20 to 35 mm2 in size, mice received either NaCl or Cyclophosphamide (100 mg/kg of body weight) every 7 days x 3–4 injections. Tumor size was routinely monitored every 3 days by means of a caliper. In experiments using anti-CD4 mAb (clone GK1.5, 200μg per mouse) or anti-CD8 mAb (clone 53–6.72, 200μg per mouse) or their isotype controls (clone LTF-3 or clone 2A3), mAb were injected i.p. 2 days before Cyclophosphamide injection and then continued every 7 days starting from day 0 until the final Cyclophosphamide injection. All antibodies were purchased from BioXcell, NH, USA.
Immunohistochemistry
Immunofluorescence staining, scanning, and analysis were performed for Foxp3, CD4 and CD3 expression in AT3 tumor from treated mice. For multiplexed staining, 3μm-thick sections of formalin-fixed, paraffin-embedded AT3 tumor from treated mouse were stained by means of an automated immunostainer (DISCOVERY ULTRA, Ventana, IGR). Heat-induced antigen retrieval in EDTA buffer (pH 8.0) for 48 min at 95°C was performed. The primary polyclonal Rabbit anti-human Foxp3 antibody (Thermo Fisher Scientific, #PA-1-46126, 1mg/mL) was applied on the slides for 1 h at 37°C, followed by detection using the biotin-free peroxidase system of detection, Discovery UltraMap anti-Rabbit HRP (Ventana, #760-4315). The Visualization of Foxp3 was accomplished using TSA fluorophore system, Discovery Rhodamine 6G kit (Ventana, #760-244). Heat-induced antigen retrieval in Citrate buffer (pH 6.0) for 10 min at 100°C was performed. Then, the slides were incubated on primary monoclonal Rabbit anti-human CD4 antibody (Abcam, EPR19514, 0.623mg/mL) for 1 h at 37°C, detected by Discovery UltraMap anti-rabbit HRP and visualized by Discovery Cy5 kit 360 (Ventana, #760-238). Heating step with Citrate Buffer was carried out, as described above. Next, the slides were incubated on primary polyclonal rabbit anti-human CD3 antibody (DAKO, #IS503, ready to use) for 32 min at RT, detected by Discovery UltraMap anti-rabbit HRP and visualized by Discovery FAM kit (Ventana, # 760–364 243). After the heating step with Citrate Buffer, nuclei were subsequently visualized with Spectral DAPI (Perkin Elmer, FP1490, 1:10). Images displayed in the figures were acquired as whole slide images (WSI) with a slide scanner Zeiss Axio Scan.Z1 (objective Plan-Apochromat 20x/0.8, 3CCD camera Hitachi HV-F202SCL) and exported from the Zeiss Zen 2 lite software as TIFF images. Image analysis of WSIs was performed using QuPath82 Regions of Interest were defined for tumor in each WSI by hand. Cells were detected based on the DAPI intensity. Next, CD3, CD4, and Foxp3 positive cells were determined by thresholds of each fluorescence intensity on QuPath.
Statistical analysis
Clinical cohort
The study population was described in terms of frequencies for qualitative variables, or medians and associated ranges for quantitative variables. All the analyses were performed on the whole population and after stratification by BC subtype. The association between TIL levels, qualitative variables, and comedications (ATC level 1,2,3) in classes were compared by student’s/ANOVA tests, or in Mann Whitney U/Kruskal–Wallis tests where indicated. Interactions tests were performed when a differential effect between TILs levels and comedication was suspected across BC subtypes. The relationships between pCR and comedications are reported according to the levels 1,2,3 of the ATC. Factors predictive of pCR (clinical, pathological variables, and comedication according to ATC level 1,2,3) were introduced into a univariate logistic regression model. The covariates selected for the multivariate analysis were the clinical, pathological variables, and comedications according to ATC level 2 classes with a likelihood ratio test p-value 0.05 or lower in univariate analysis. A multivariate logistic model was then implemented using a forward stepwise selection procedure. Analyses were performed with R software, version 3.1.2,83 with the ggplot2, dplyr, cowplot, tableone, and survival libraries.
Animal experiments
Data analyses were performed with the statistical environment Prism 6 (GraphPad, San Diego, CA, USA). Tumor size differences were calculated using ANOVA, Student’s t-test or dedicated software (https://kroemerlab.shinyapps.io/TumGrowth/). Briefly, tumor growth was subjected to a linear-mixed effect modeling applied to log pre-processed tumor surfaces. P-values were calculated by testing jointly whether both tumor growth slopes and intercepts (on a log scale) were different between treatment groups of interest. All reported tests are two-tailed and were considered significant at p < .05.
Study approval
All animal experiments were carried out in compliance with French and European laws and regulations. The local institutional animal ethics board and the French Ministry of Research approved all mouse experiments (permission numbers: 2014-071-1124, 2016-049-4646).
Author Contributions
Conceptualization, F.R. and L.Z; Experimentation and resources, S.Y., P.O., A.P., E. Marangoni, E.Montlaudon; Formal Analysis, A.-S. H., L. Derosa.; Data acquisition: M.P., J. G., D.d.C., M.L.; Data Curation; C.V., L.-S. T., L. Darrigues; Methodology, B.A., E.L.; Writing – Original Draft, A.-S. H., L. Derosa. F.R., and L.Z; Writing – Review & Editing, F.R., G.K. and L.Z; Supervision, F.R. and L.Z.; Funding Acquisition, F.R., and L.Z.
Acknowledgments
We are thankful to the animal facility team of Gustave Roussy and all the technicians form Center GF Leclerc. LZ and GK were supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation de France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Swiss Bridge Foundation; the Seerave and Carrefour Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Disclosure of Interest
LZ and GK are cofounders of EverImmune, a biotech company devoted to the use of commensal bacteria for the treatment of cancers.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(1):105–15. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 2.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 3.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 4.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N.. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res. 2016;22(6):1499–1509. doi: 10.1158/1078-0432.CCR-15-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azim HA, Vingiani A, Peccatori F, Viale G, Loi S, Pruneri G. Tumour infiltrating lymphocytes (TILs) in breast cancer during pregnancy. Breast Edinb Scotl. 2015;24(3):290–293. doi: 10.1016/j.breast.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Ladoire S, Enot D, Senovilla L, Ghiringhelli F, Poirier-Colame V, Chaba K, Semeraro M, Chaix M, Penault-Llorca F, Arnould L, et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy. 2016;12(5):864–875. doi: 10.1080/15548627.2016.1154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114(1):37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang TJ. Estrogen as an immunomodulator. Clin Immunol. 2004;113(3):224–230. doi: 10.1016/j.clim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109(3):573–579. doi: 10.1007/s10549-007-9683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin PJ, Stambolic V, Lemieux J, Chen BE, Parulekar WR, Gelmon KA, Hershman DL, Hobday TJ, Ligibel JA, Mayer IA, et al. Evaluation of metformin in early breast cancer: a modification of the traditional paradigm for clinical testing of anti-cancer agents. Breast Cancer Res Treat. 2011;126(1):215–220. doi: 10.1007/s10549-010-1224-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Zhu S, Li X, Wang F, Hu F, Li D, Zhang W, Li X. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117(1):141–150. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 14.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sørensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan ML, Habel LA, Slattery ML, Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control CCC. 2007;18(6):613–620. doi: 10.1007/s10552-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628–638. doi: 10.18632/oncotarget.101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29(19):2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 18.Haukka J, Niskanen L, Auvinen A. Risk of cause-specific death in individuals with cancer-modifying role diabetes, statins and metformin. Int J Cancer. August 25 2017. doi: 10.1002/ijc.31016. [DOI] [PubMed] [Google Scholar]
- 19.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112(6):1809. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharping NE, Menk AV, Whetstone RD, Zeng X, Delgoffe GM. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer Immunol Res. 2017;5(1):9–16. doi: 10.1158/2326-6066.CIR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-ii randomized trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2017. May 10;23:4651–4661. doi: 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junankar S, Shay G, Jurczyluk J, Ali N, Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, et al. Real-time intravital imaging establishes tumor-associated macrophages as the extraskeletal target of bisphosphonate action in cancer. Cancer Discov. 2015;5(1):35–42. doi: 10.1158/2159-8290.CD-14-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcinotto A, Filipazzi P, Grioni M, Iero M, Milito AD, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating t lymphocytes. Cancer Res. 2012;72(11):2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 24.Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. 2013;2(1):e22058. doi: 10.4161/onci.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellone M, Calcinotto A. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. 2013:3. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(1):30–38. doi: 10.1158/1078-0432.CCR-14-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribas A, Robert C, Hodi FS, Wolchok JD, Joshua AM, Hwu W-J, Weber JS, Zarour HM, Kefford R, Loboda A, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J Clin Oncol. 2015;33(15):3001. doi: 10.1200/jco.2015.33.15_suppl.3001. [DOI] [Google Scholar]
- 29.Liu J, Blake SJ, Yong MCR, Harjunpää H, Ngiow SF, Takeda K, Young A, O’Donnell JS, Allen S, Smyth MJ, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogribny IP, Tryndyak VP, Pogribna M, Shpyleva S, Surratt G, Gamboa Da Costa G, Beland FA. Modulation of intracellular iron metabolism by iron chelation affects chromatin remodeling proteins and corresponding epigenetic modifications in breast cancer cells and increases their sensitivity to chemotherapeutic agents. Int J Oncol. 2013;42(5):1822–1832. doi: 10.3892/ijo.2013.1855. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez-Martin A, Oliveras-Ferraros C, Cufí S, Del Barco S, Martin-Castillo B, Menendez JA. Metformin regulates breast cancer stem cell ontogeny by transcriptional regulation of the epithelial-mesenchymal transition (EMT) status. Cell Cycle Georget Tex. 2010;9:3807–3814. [PubMed] [Google Scholar]
- 33.Wang B-Y, Zhang J, Wang J-L, Sun S, Wang Z-H, Wang L-P, Zhang Q-L, Lv -F-F, Cao E-Y, Shao Z-M, et al. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J Exp Clin Cancer Res CR. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Uppal H, Demaria M, Desprez P-Y, Campisi J, Kapahi P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep. 2015;(5). doi: 10.1038/srep17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alalem M, Ray A, Ray BK. Metformin induces degradation of mTOR protein in breast cancer cells. Cancer Med. 2016;5(11):3194–3204. doi: 10.1002/cam4.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujihara S, Morishita A, Ogawa K, Tadokoro T, Chiyo T, Kato K, Kobara H, Mori H, Iwama H, Masaki T. The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKa/mTOR pathway in vitro and in vivo. Oncotarget. 2016;8(5):8536–8549. doi: 10.18632/oncotarget.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polydorou C, Mpekris F, Papageorgis P, Voutouri C, Stylianopoulos T. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget. 2017;8(15):24506–24517. doi: 10.18632/oncotarget.15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cong J, Wang Y, Zhang X, Zhang N, Liu L, Soukup K, Michelakos T, Hong T, DeLeo A, Cai L, et al. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett. 2017. August 30;409:9–19. doi: 10.1016/j.canlet.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Murray LS, Jodrell DI, Morrison JG, Cook A, Kerr DJ, Whiting B, Kaye SB, Cassidy J. The effect of cimetidine on the pharmacokinetics of epirubicin in patients with advanced breast cancer: preliminary evidence of a potentially common drug interaction. Clin Oncol. 1998;10(1):35–38. doi: 10.1016/S0936-6555(98)80109-X. [DOI] [PubMed] [Google Scholar]
- 40.Hammann F, Gotta V, Conen K, Medinger M, Cesana P, Rochlitz C, Taegtmeyer AB. Pharmacokinetic interaction between taxanes and amiodarone leading to severe toxicity. Br J Clin Pharmacol. 2017;83(4):927–930. doi: 10.1111/bcp.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues AC, Curi R, Britto LRG, Rebbechi IMM, Hirata MH, Bertolami MC, Bernik MMS, Dorea EL, Hirata RDC. Down-regulation of ABCB1 transporter by atorvastatin in a human hepatoma cell line and in human peripheral blood mononuclear cells. Biochim Biophys Acta. 2006;1760(12):1866–1873. doi: 10.1016/j.bbagen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Wang E, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother. 2002;46(1):160–165. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vdovin AS, Maximchik PV, Kulikov AV, Zhivotovsky BD, Gogvadze VG. Inhibition of P-glycoprotein stimulates cell death under Hypoxia-mimicking conditions. Dokl Biochem Biophys. 2017;472(1):27–30. doi: 10.1134/S1607672917010100. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, Sawada Y, Kohda Y. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67(1):44–49. doi: 10.1111/j.1365-2125.2008.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Trabert B, Ness RB, Lo-Ciganic W-H, Murphy MA, Goode EL, Poole EM, Brinton LA, Webb PM, Nagle CM, Jordan SJ, et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the ovarian cancer association consortium. J Natl Cancer Inst. 2014;106(2):djt431. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM. Australian national endometrial cancer study group. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case-control study, systematic review and meta-analysis. Int J Cancer. 2013;132(5):1146–1155. doi: 10.1002/ijc.27717. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, Lu P, Chen L, Yang S, Shen H-Y, Yu D, Zhang X, Zhong S, Zhao J, Tang J. Perioperative propofol-paravertebral anesthesia decreases the metastasis and progression of breast cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2015;36(11):8259–8266. doi: 10.1007/s13277-015-4027-5. [DOI] [PubMed] [Google Scholar]
- 49.Anderson SL, Duke-Novakovski T, Singh B. The immune response to anesthesia: part 2 sedatives, opioids, and injectable anesthetic agents. Vet Anaesth Analg. 2014;41(6):553–566. doi: 10.1111/vaa.12191. [DOI] [PubMed] [Google Scholar]
- 50.Cho JS, Lee M-H, Kim SI, Park S, Park HS, Oh E, Lee JH, Koo B-N. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study. Int J Med Sci. 2017;14(10):970–976. doi: 10.7150/ijms.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(1):i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 52.Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 53.Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene. 2011;30(10):1147–1158. doi: 10.1038/onc.2010.500. [DOI] [PubMed] [Google Scholar]
- 54.Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4(143):143ra99. doi: 10.1126/scitranslmed.3003807. [DOI] [PubMed] [Google Scholar]
- 55.Martins I, Kepp O, Menger L, Michaud M, Adjemian S, Sukkurwala AQ, Vacchelli E, Galluzzi L, Kroemer G. Fluorescent biosensors for the detection of HMGB1 release. Methods Mol Biol Clifton NJ. 2013;1004:43–56. doi: 10.1007/978-1-62703-383-1_4. [DOI] [PubMed] [Google Scholar]
- 56.Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology. 2013;2(2):e23082. doi: 10.4161/onci.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359:6374. doi: 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- 58.Pietrocola F, Bravo-San Pedro JM, Galluzzi L, Kroemer G. Autophagy in natural and therapy-driven anticancer immunosurveillance. Autophagy. 2017;13(12):2163–2170. doi: 10.1080/15548627.2017.1310356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, Levesque S, Castoldi F, Jacquelot N, Yamazaki T, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. 2016;30(1):147–160. doi: 10.1016/j.ccell.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, Zhang C, Yue D, Qin G, Zhang T, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. 2018;78(7):1779–1791. doi: 10.1158/0008-5472.CAN-17-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vishvakarma NK, Singh SM. Augmentation of myelopoiesis in a murine host bearing a T cell lymphoma following in vivo administration of proton pump inhibitor pantoprazole. Biochimie. 2011;93(10):1786–1796. doi: 10.1016/j.biochi.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 62.Huang W-S, Tsai C-H, Lin -C-C, Muo C-H, Sung F-C, Chang Y-J, Kao C-H. Relationship between zolpidem use and stroke risk: a Taiwanese population-based case-control study. J Clin Psychiatry. 2013;74(5):e433–438. doi: 10.4088/JCP.12m08181. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y-W, Hsieh T-F, Yu C-H, Huang Y-S, Lee -C-C, Tsai T-H. Zolpidem and the risk of Parkinson’s disease: a nationwide population-based study. J Psychiatr Res. 2014;58:84–88. doi: 10.1016/j.jpsychires.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Kao C-H, Sun L-M, Liang J-A, Chang S-N, Sung F-C, Muo C-H. Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc. 2012;87(5):430–436. doi: 10.1016/j.mayocp.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramirez K, Sheridan JF. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav Immun. 2016;57:293–303. doi: 10.1016/j.bbi.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, Schell TD. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7(3):e1405205. doi: 10.1080/2162402X.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Renzi C, Kaushal A, Emery J, Hamilton W, Neal RD, Rachet B, Rubin G, Singh H, Walter FM, de, et al. Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat Rev Clin Oncol. 2019. July 26. doi: 10.1038/s41571-019-0249-6. [DOI] [PubMed] [Google Scholar]
- 70.Geraci JM, Escalante CP, Freeman JL, Goodwin JS. Comorbid disease and cancer: the need for more relevant conceptual models in health services research. J Clin Oncol. September 21 2016. doi: 10.1200/JCO.2004.00.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The Repurposing Drugs in Oncology (ReDO) project. Ecancermedicalscience. 2014;8:442. doi: 10.3332/ecancer.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van Den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierga J-Y, Delaloge S, Espié M, Brain E, Sigal-Zafrani B, Mathieu M-C, Bertheau P, Guinebretière JM, Spielmann M, Savignoni A, et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122(2):429–437. doi: 10.1007/s10549-010-0939-3. [DOI] [PubMed] [Google Scholar]
- 74.Giacchetti S, Hamy A-S, Delaloge S, Brain E, Berger F, Sigal-Zafrani B, Mathieu M-C, Bertheau P, Guinebretière JM, Saghatchian M, et al. Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status. Eur J Cancer. 2017;75(SupplementC):323–332. doi: 10.1016/j.ejca.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Pierga J. Whole expression genome array can be used in daily practice for selecting neo-adjuvant treatment. Ann Oncol Abstr ESMO. 2012;23(9):Abstract 245O. [Google Scholar]
- 76.de Cremoux P, Valet F, Gentien D, Lehmann-Che J, Scott V, Tran-Perennou C, Barbaroux C, Servant N, Vacher S, Sigal-Zafrani B, et al. Importance of pre-analytical steps for transcriptome and RT-qPCR analyses in the context of the phase II randomised multicentre trial REMAGUS02 of neoadjuvant chemotherapy in breast cancer patients. BMC Cancer. 2011;11:215. doi: 10.1186/1471-2407-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valet F, de Cremoux P, Spyratos F, Servant N, Dujaric ME, Gentien D, Lehmann-Che J, Scott V, Sigal-Zafrani B, Mathieu MC, et al. Challenging single- and multi-probesets gene expression signatures of pathological complete response to neoadjuvant chemotherapy in breast cancer: experience of the REMAGUS 02 phase II trial. Breast Edinb Scotl. 2013;22(6):1052–1059. doi: 10.1016/j.breast.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Hamy AS, Bieche I, Lehmann-Che J, Scott V, Bertheau P, Guinebretière JM, Matthieu MC, Sigal-Zafrani B, Tembo O, Marty M, et al. BIRC5 (survivin): a pejorative prognostic marker in stage II/III breast cancer with no response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2016. September 3;159:499–511. doi: 10.1007/s10549-016-3961-2. [DOI] [PubMed] [Google Scholar]
- 79.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 80.Provenzano E, Bossuyt V, Viale G, Cameron D, Badve S, Denkert C, MacGrogan G, Penault-Llorca F, Boughey J, Curigliano G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol Off J US Can Acad Pathol Inc. 2015;28(9):1185–1201. doi: 10.1038/modpathol.2015.74. [DOI] [PubMed] [Google Scholar]
- 81.Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, de Plater L, Guyader C, De Pinieux G, Judde J-G, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13(13):3989–3998. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 82.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.R Development Core Team . R: a language and environment for statistical computing. R Found Stat Computing, Vienna, Austria. 2011. http://www.R-project.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


