Abstract
There is an urgent need to identify novel potential therapeutic targets for diagnosis and treatment of glioma, a common primary tumor in brain, due to its high-level invasiveness. Long non-coding RNA (lncRNA) LINC00473 has been reported as potentially critical regulators in various types of cancer tumorigenesis. However, the effects of LINC00473 on glioma cells is unclear. The present study aimed to investigate the clinical significance, effects and mechanism of LINC00437 on glioma tumorigenesis. In the present study, LINC00473 was significantly increased in glioma tissues and in cell models, and predicted a poor prognosis in patients with glioma. Notably, LINC00473 knockdown not only suppressed cell proliferation, invasion and migration of glioma cells, but also blocked cell cycle progression and induced apoptosis. Subcutaneous xenotransplanted tumor model experiments revealed that reduced LINC00473 expression was able to suppress in vivo glioma growth. Mechanistically, LINC00473 functioned as a competing endogenous (ce)RNA to decrease microRNA (miR)-195-5p expression. Moreover, Yes-associated protein 1 (YAP1) and TEA domain family member 1 (TEAD1) were identified as downstream targets of miR-195-5p, whose expression levels were inhibited by miR-195-5p. LINC00473 knockdown suppressed glioma progression through the decrease of miR-195-5p and subsequent increase of YAP1 and TEAD1 expression levels. These results indicated LINC00473 might act as a ceRNA to sponge miR-195-5p, thus promoting YAP1 and TEAD1 expressions, and shedding light on the underlying mechanisms of LINC00473-induced glioma progression.
Keywords: long non-coding RNALINC00473, microRNA-195-5p, YAP1-TEAD1-Hippo signaling pathway, glioma progression
Introduction
Glioma is the most fatal and common primary malignant brain tumor of the adult central nervous system with a 3.3% mortality rate and a medical and neurologic morbidity rate of 31.7% (1,2); it is clinically classified into low-grade glioma (WHO grade I and II) and high-grade glioma (WHO grade III and IV), according to histopathological malignancy characteristics (3). Although combined therapeutic treatments have been improved tremendously in the last two decades, the 5-year survival rate is still <5% (4). Therefore, it is necessary to explore novel therapeutic targets and develop strategies for glioma treatment.
Long non-coding RNAs (lncRNAs) are key molecules in the progression of various types of human tumors (5,6), including glioma pathogenesis (7). lncRNA LINC00473 is an intergenic lncRNA located on the human 6q27 chromosome (8). Recently, LINC00473 expression has been found to be elevated in a variety of human cancers, including non-small cell lung cancer (9), Wilms tumor (10), hepatocellular carcinoma (11), cervical cancer (12), human mucoepidermoid carcinoma (13), fibrolamellar carcinoma (14) and gastric cancer (15). However, the clinical importance and biological functions of LINC00473 in glioma remains unclear.
lncRNAs can function as competing endogenous RNAs (ceRNAs) to sponge and inhibit the function of specific microRNA (miRNA) (16). As a ceRNA, LINC00473 regulates miRNA (miR)-15a-dependent Taxol resistance in colorectal cancer (17). Moreover, LINC00473 has been shown to antagonize the tumor suppressor miR-195 to mediate Wilms tumor (10), and miR-195 serves an antitumoral role in glioma (18-20). Therefore, we hypothesized that LINC00473 may function as a ceRNA to link miR-195 and post-transcriptional signaling in glioma cells.
The targeting of miR-195-5p to YAP1 (Yes-associated protein 1) has been reported in hepatocellular carcinoma (21), osteosarcoma (22) and colorectal cancer (23). The conserved Hippo signaling pathway mediates downstream transcriptional co-activators, such as YAP, which subsequently binds to and activates TEA domain family members (TEADs) that are responsible for cell proliferation and apoptosis (24). A previous study reported that YAP1 was upregulated in glioma cells and induced glioma growth (25). Ectopic expression of YAP binds to TEAD and controls the transcription of pro-proliferative and anti-apoptotic target genes in glioma (26). TEAD1 was also reported to be an important regulator of glioma cell migration (27). Therefore, LINC00473 might regulate glioma progression through YAP1-TEAD1-Hippo signaling pathway.
The present study hypothesized that LINC00473 may promote glioma development and, as such, investigated the effects of LINC00473 on tumor proliferation, migration and invasion in vivo and in vitro. In addition, the downstream targets of LINC00473 and the underlying mechanisms were also examined. The results of the present study may contribute to the application of LINC00473 in glioma therapy.
Materials and methods
Clinical tumor tissues
Paired glioma tissues and para-carcinoma tissues were obtained from 40 patients (17 male; 23 female; age, 55.8±4.7 years with range of 44-70 years) with primary gliomas between January 2014 and January 2017, patients with 10-year follow-up period underwent surgical treatment at Union Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). Patient glioma statuses were determined by imaging modalities (MRI scan) and verified though histological analysis. Tissue specimens were cut into pieces, flash frozen in liquid nitrogen and stored at −80°C for further experiments. The present study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (approval no. 2014-S034), and all the patients signed written informed consent.
Cell culture and transfection
Human glioma cell lines (U251, U118, LN229, U87 and SHG44) and a normal human astrocyte cell line (NHA) were obtained from Lonza Group AG and authenticated by short tandem repeat DNA profiling. Cells were cultured in DMEM supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) in a 37°C constant temperature incubator with 5% CO2.
Two short hairpin (sh)RNAs against LINC00473 (sh-LINC00473#1, 5′-AAC TGG ATC TTT GCA GAC AGG-3′; sh-LINC00473#2: 5′-AAG AAC CCA AGT CAT ATT CAT-3′) and a scramble negative control (sh-NC, 5′-CGG AUC AGC UCG CGC UAU CAU CGC A-3′) were inserted into a pLKO.1 expression vector (BioSettia, Inc.). A total of 40 nM sh-LINC00473#1, LINC00473#2 or shNC were co-transfected into 293 cells (4×105 cells/well) with psPAX2 and pMD2.G lentiviral vectors using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 48 h, according to the manufacturer’s protocols. Following incubation, lentiviral particles were harvested by precipitation in polyethylene glycol followed by centrifugation at 500 × g for 30 min at 4°C, and particles were transduced at 0.5 multiplicity of infection into U251 or U87 cells (1×106 cells) using ViraPower™ Packaging Mix (Thermo Fisher Scientific, Inc.) and 8 mg/ml polybrene at 37°C overnight. Cells were treated with 5 µg/ml puromycin (Sigma Aldrich; Merck KGaA) 1 week to establish stable cell lines.
Full-length of LINC00473 was amplified and cloned into pcDNA3.1 overexpression vector (Invitrogen; Thermo Fisher Scientific, Inc.). U251 or U87 cells (4×105 cells/well) were seeded in 12-well plates and transfected with 300 µg pcDNA3.1-LINC00473 or the empty vector control (pcDNA3.1) using Lipofectamine 2000 at 37°C for 48 h, according to the manufacturer’s protocols, and subsequently used for other experiments.
miR-195-5p mimics (5′-UAG CAG CAC AGA AAU AUU GGC-3′), miR-195 inhibitor (inh; 5′-GCC AAU AUU UCU GUG CUG CUA-3′), miR-NC (5′-UCA CAA CCU CCU AGA AAG AGU AGA-3′ and inh NC (5′-CAG UAC UUU UGU GUA GUA CAA-3′) were synthesized by Suzhou GenePharma Co., Ltd. U251 or U87 cells (1×106 cells) were transfected with miR-195-5p mimics, inhibitors or the respective controls (40 nM) using Lipofectamine 2000 at 37°C for 48 h, according to the manufacturer’s protocols, and subsequently used for other experiments. U251 and U87 cells were co-transfected with i) inh NC and sh-NC, ii) sh-LINC00473 and inh NC, iii) miR-195-5p inh and sh-NC, and iv) sh-LINC00473 and miR-195-5p inh to determine the functional roles of LINC00473/miR-195-5p/YAP1-TEAD1-Hippo axis in regulating glioma progression.
Cell viability assay
Transfected/transduced U251 or U87 cells (2×103 cells/well) were seeded in 96-well plates and incubated for 24, 48, 72 and 96 h. Subsequently, 20 µl Cell Counting Kit-8 (CCK8) solution (Dojindo Molecular Technologies, Inc.) was added into each well and incubated at 37°C for 2 h, according to the manufacturer’s protocols. The absorbance was measured at 450 nm using a Microplate Autoreader (Thermo Fisher Scientific, Inc.).
Flow cytometry
Transfected/transduced U251 or U87 cells (1×106 cells) were harvested using trypsin digestion. For cell cycle analysis, cells were stained with 5 µl propidium iodide (PI; 100 µg/ml) with 1 U/ml ribonuclease (Abcam) for 30 min at room temperature, according to the manufacturer’s protocols. For apoptosis analysis, cells were resuspended in 100 µl binding buffer (Nanjing KeyGen Biotech Co., Ltd.) containing 5 µl PI (100 µg/ml) with 1 U/ml ribonuclease in the dark for 30 min at room temperature, and then incubated with additional 5 µl of Annexin V-FITC for another 15 min in the dark at room temperature, according to the manufacturer’s protocols. Cells were subsequently analyzed by FACS using an Attune flow cytometer (Thermo Fisher Scientific, Inc.) with software FlowJo 1.1.0 (Tree Star, Inc.) for the determination of apoptotic rates, which is presented as a combination of early and late stage apoptosis.
Wound healing assay
Transfected/transduced U251 or U87 cells (5×105 cells/well) were seeded in six-well plates with complete medium. Cells ay 90-95% confluence were serum starved for 24 h. Cells with 95% confluence were used for the following experiment. A 200 µl sterile pipette tip was used to generate linear scratch wounds, and the plate was washed with PBS to remove debris and suspended cells. Plates were incubated at 37°C for 24 h post-wound generation, and images were captured under a light microscope (magnification, ×100) and the cell migration distance was measure compared with the 0 h time point.
Transwell invasion assay
Transfected/transduced U251 or U87 cells (2×104 cells/well) were suspended in 200 µl serum-free DMEM and plated in the upper Transwell chamber (Corning, Inc.) containing a pre-coated Matrigel-coated membrane (0.1 ml, 50 µg/ml; BD Biosciences). A total of 400 µl DMEM with 10% FBS was added to the lower chamber. Cells were incubated at 37°C for 24 h, and the invading cells at the bottom of chambers were stained with 1% crystal violet for 30 min. Cells were imaged and counted under a light microscope (magnification, ×100) (Olympus Corp).
Dual-luciferase reporter assay
Potential targets of miR-195-5p and LINC00743 were predicted using TargetScan release 7.1 (http://www.targetscan.org/vert_71) or starBase v2.0 (http://starbase.sysu.edu.cn), respectively. Sequences of wildtype (WT) or mutant (MUT) 3’-untranslated regions (UTRs) of LINC00473, YAP1 or TEAD1 were cloned into pmirGLO luciferase reporter vector (Promega Corporation). 293 cells (3×104 cells/well) were seeded in 24-well plates and co-transfected with either miR-195-5p mimics or NC mimics and pmirGLO-WT-LINC00473, pmirGLO-MUT-LINC00473, pmirGLO-WT-YAP1, pm i r GLO -MU T-YA P1, pm i r GLO -W T-TEA D1 or pmirGLO-MUT-TEAD1 using Lipofectamine 2000 at 37°C, according to the manufacturer’s protocols. At 48 h post-transfection, the luciferase activities were measured using a Lucifer Reporter Assay System (Promega Corporation), with firefly luciferase activity normalized to Renilla luciferase activity.
RNA immunoprecipitation (RIP)
A total of 1×106 trans-fected/transduced U251 or U87 cells were collected and lysed using Magna RIP Kit (EMD Millipore, Billerica, MA). Cell lysate was incubated with protein G Sepharose beads (GE Healthcare) coated with anti-argonaute 2 (Ago2) antibody (1:50; cat. no. ab186733; Abcam) at 4°C overnight. Anti-immunoglobulin (Ig)G antibody (1:50; cat. no. ab200699; Abcam) was used as the negative control, and anti-U1 small nucleoprotein 70 kDa (SNRNP70; 1:50; cat. no. ab83306; Abcam) was used as positive control. RNA was subsequently isolated for reverse transcription (RT)-qPCR, described below.
RT-qPCR
Total RNA was isolated from tissues (2 mg) or cell lines, including cells from RIP, (1×106 cells) using TRIzol® (Invitrogen; Thermo Fisher Scientific, Ind.), miRNAs were extracted using miRcute miRNA Isolation kit (Tiangen Biotech Co., Ltd.). A total of 2 µg RNA was reverse transcribed into cDNA using the PrimeScript RT Reagent kit (Takara Bio, Inc.). qPCR was conducted using SYBR Green Master mix (Roche Diagnostics GmbH) on a ViiA 7 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.), with the following thermocycling conditions: Initial denaturation at 95°C for 60 sec; followed by 40 cycles of extension at 95°C for 30 sec and annealing at 60°C for 40 sec. GAPDH or U6 was used as endogenous controls and for normalization of mRNA and miRNA expressions, respectively. Primer sequences are provided in Table I.
Table I.
Primer sequences for reverse transcription-qPCR.
| Gene | Sequence (5′→3′) |
|---|---|
| GAPDH | F: ACCACAGTCCATGCCATCAC |
| R: TCCACCACCCTGTTGCTGTA | |
| LINC00473 | F: TCATTTCCCTACCTGCTCCT |
| R: CAGTGTCTGCACATCGCTAAT | |
| microRNA-195-5p | F: GGGGTAGCAGCACAGAAAT |
| R: TCCAGTGCGTGTCGTGGA | |
| YAP1 | F: CGCTCTTCAACGCCGTCA |
| R: AGTACTGGCCTGTCGGGAGT | |
| TEAD1 | F: AACATGGAAAGGATGAGCGACT |
| R: TCAGTCCTTCACAAGCCTGTAGA | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
F, forward; R, reverse; TEAD1, TEA domain family member 1; YAP1, Yes-associated protein 1.
Western blotting
Total protein was extracted from glioma and para-cancerous tissues (100 mg) and glioma cell lines (1×107 cells) using RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Protein concentrations were quantified by BCA and a total of 30 µg protein lysate was separated by 10% SDS-PAGE and subsequently electrotransferred onto PVDF membranes. After blocking with 5% BSA (Sigma-Aldrich; Merck KGaA) at 37°C for 1 h, the membrane was incubated overnight with primary antibodies against YAP1 (1:1,500; cat. no. ab56701; Abcam), TEAD1 (1:1,500; cat. no. ab109080, Abcam), CTGF (1:2,500; cat. no. ab6992; Abcam) and GAPDH (1:3,000; cat. no. ab9485; Abcam) at 4°C. Following incubation with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:5,000; cat. no. ab205718; Abcam) or goat anti-mouse (1:5,000; cat. no. ab205719; Abcam) secondary antibody at 4°C for 2 h, the immunoreactivities were detected by Enhanced Chemiluminescence (Nanjing KeyGen Biotech Co., Ltd.). Densitometric analysis was performed using ImageJ v2.4.1 (National Institutes of Health) with protein expression levels normalized to GAPDH.
Mouse xenograft assay
All studies involving animals were approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Male BALB/c nude mice (n=12; age, 5 weeks; weight, 20-25 g; Shanghai Experimental Animal Centre) were randomly separated into two groups, sh-NC and shRNA-LINC00473#2, (n=6 mice/group), and used for tumor formation assay. A total of 1×108 LINC00473#2- or sh-NC-transfected U251 cells in 100 µl PBS were subcutaneously injected in the right flank of nude mice. Tumors were measured with digital calipers every 7 days; the largest tumor diameter was 4.83 mm, and tumor volume was calculated. At 5 weeks post-injection, the mice were anaesthetized with 40 mg/kg sodium pentobarbital (i.p.) and then sacrificed by 10% formalin perfusion fixation of central nervous system; death was confirmed by completely stopping of the heartbeat and breathing, as well as disappearance of the foot withdrawal reflex. The tumor tissues were isolated and weighed, and RNAs and proteins were extracted for analysis following the aforementioned protocols.
Hematoxylin and Eosin (H&E) staining
Glioma tissues from mice were fixed in 4% paraformaldehyde at 4°C for 24 h, embedded in paraffin and cut into 7 µm sections with an RM2245 microtome (Leica Microsystems GmbH). The sections were deparaffinized in xylene, rehydrated in serially diluted ethanol and stained with H&E (Sigma-Aldrich; Merck KGaA) at 4°C for 10 min. Representative photomicrographs were captured using a light microscope (Olympus Corporation), magnification, ×200.
Immunohistochemical (IHC) staining
Mouse glioma tissues were fixed with 4% paraformaldehyde at 4°C for 24 h, embedded in paraffin and sectioned (4 µm). After dewaxing with xylene and rehydration through an ethanol series, sections were incubated in 3% H2O2 to inhibit endogenous peroxidases. For antigen retrieval, sections were immersed in Tris-EDTA buffer containing 0.05% Tween 20 (pH 9.0), in a water bath at 95°C for 30 min. After washing with PBS, the sections were incubated in 4% dry milk with 0.3% goat serum (Sigma-Aldrich; Merck KGaA) in PBS solution for 20 min to block non-specific binding, and then incubated overnight with anti- Ki67 (1:100; cat. no. ab15580; Abcam) antibody in the presence of 10% rabbit serum (Sigma-Aldrich; Merck KGaA). After washing with PBS, the sections were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (1:100; cat. no. ab6721; Abcam). Slides were counterstained with hematoxylin to stain cell nuclei, dehydrated in an ethanol series and examined under a light microscope (Olympus Corporation); magnification, ×200).
Statistical analysis
Experiments were repeated three times and data were expressed as mean ± SEM. GraphPad Prism software v5.0 (GraphPad Prism Software, Inc.) was used for statistical analysis by one-way ANOVA and Tamhane’s T2 post hoc test. Kaplan-Meier method and log-rank test were used to analyze differences between patients with high or low levels of LINC00473 expression and overall survival (OS). χ2 was used to analyze the data in Table II. Paired Student’s t-test was used to analyze differences between paired tumor and para-cancerous tissues. Pearson’s correlation test was used for correlation analysis. Multivariate and univariate analysis of prognostic parameters in patients with glioma by Cox regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Table II.
Associations between LINC00473 expression and clinicopathological features of patients with glioma were determined by χ2 analysis.
| Clinicopathological feature | LINC00473 expression
|
|||
|---|---|---|---|---|
| Total (n=40) | Low (n=23) | High (n=17) | P-value | |
| Age (years) | ||||
| <50 | 15 | 7 | 8 | 0.283 |
| ≥50 | 25 | 16 | 9 | |
| Sex | ||||
| Male | 17 | 7 | 10 | 0.073 |
| Female | 23 | 16 | 7 | |
| Histopathology | ||||
| Conventional | 22 | 12 | 10 | 0.676 |
| Chordioid | 18 | 11 | 7 | |
| Tumor recurrence | ||||
| Yes | 23 | 17 | 6 | 0.015 |
| No | 17 | 6 | 11 | |
| Tumor grade | ||||
| I-II | 11 | 11 | 0 | 0.001 |
| III | 14 | 9 | 5 | |
| IV | 15 | 3 | 12 | |
Results
lncRNA LINC00473 is highly expressed in both glioma tissues and cell lines
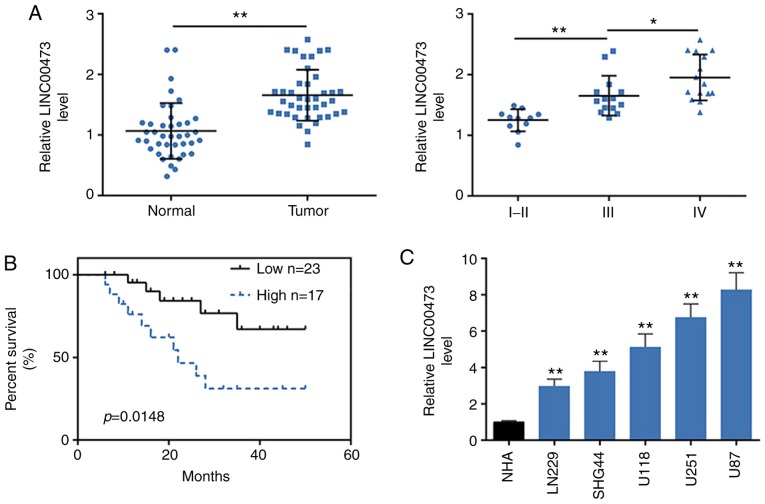
To determine the association between LINC00473 and glioma tumorigenesis, the expression levels of LINC00473 were first determined in glioma and para-cancerous tissues from 40 patients. The results demonstrated that LINC00473 was significantly increased in tumoral tissues compared with the para-carcinoma tissues (P<0.01; Fig. 1A). Moreover, LINC00473 levels were positively related to the WHO grades of glioma (Fig. 1A), as there was higher expression level in WHO grade III and IV compared with WHO grade I and II, which indicated that LINC00473 may be associated with malignancy of glioma. Median expression level of LINC00473 in patients was considered as a cut-off, patients were then divided into two groups: High LINC00473 expression group (fold change ≥2.5; n=17) and low LINC00473 expression group (n=23). Further association analysis between LINC00473 expression and clinicopathological characteristics of patients with glioma demonstrated that among the 40 patients, high LINC00473 expression level was significantly associated with tumor recurrence (P=0.015) and tumor grade (P<0.001) (Table II). No significant associations were determined between LINC00473 expression and the other clinicopathological features, such as the age (P=0.283), histopathology (P=0.676) and sex (P=0.073) (Table II). The association between LINC00473 expression and prognosis of patients with glioma was determined using Kaplan-Meier survival analysis (Fig. 1B). Patients with high LINC00473 level exhibited shorter OS compared with patients with low LINC00473 expression (P=0.0148). Cox multivariate survival analysis indicated tumor grade (P=0.039) and LINC00473 expression (P=0.030) were independent risk prognostic factors of glioma (Table III). However, univariate analyses identified no clinicopathological features that were significantly associated with glioma prognosis (Table III). The univariate and multivariate analysis results suggested that there may be complex interactions between the independent variables.
Figure 1.
LncRNA LINC00473 is highly expressed in glioma tissues and cell lines. (A) RT-qPCR was used to determine lncRNA LINC00473 expression levels in glioma tissues (Tumor) and para-carcinoma tissues (Normal), and in high-grade glioma (WHO grade III and IV) than low-grade glioma (WHO grade I and II) tissues. n=40; *P<0.05; **P<0.01. (B) Kaplan-Meier OS analysis of patients with glioma with highly and lowly expression of LINC00473. (C) LINC00473 expression levels in human glioma cell lines (U251, U118, LN229, U87 and SHG44) and normal human astrocytes cell line (NHA) were detected by RT-qPCR. **P<0.01 vs. NHA. OS, overall survival; RT-qPCR, reverse transcription-qPCR.
Table III.
Multivariate and univariate analysis of prognostic parameters in patients with glioma by Cox regression analysis.
| Clinicopathological feature | Multivariate P-value | Univariate P-value |
|---|---|---|
| Age (years) | ||
| <50 years | 0.288 | 0.181 |
| ≥50 years | ||
| Sex | ||
| Male | 0.11 | 0.275 |
| Female | ||
| Histopathology | ||
| Conventional | 0.233 | 0.733 |
| Chordioid | ||
| Invasion condition | ||
| Yes | 0.056 | 0.165 |
| No | ||
| Tumor grade | ||
| I-II | 0.039 | 0.244 |
| III | ||
| IV | ||
| LINC00473 expression | ||
| Low | 0.030 | 0.291 |
| High |
LINC00473 expression levels were also increased in human glioma cell lines (U251, U118, LN229, U87 and SHG44) compared with NHA cells (Fig. 1C). As U251 and U87 cells exhibited the highest expression levels of LINC00473, they were chosen for the subsequent functional assays.
LINC00473 knockdown suppresses glioma cell proliferation, migration and invasion, and induces apoptosis
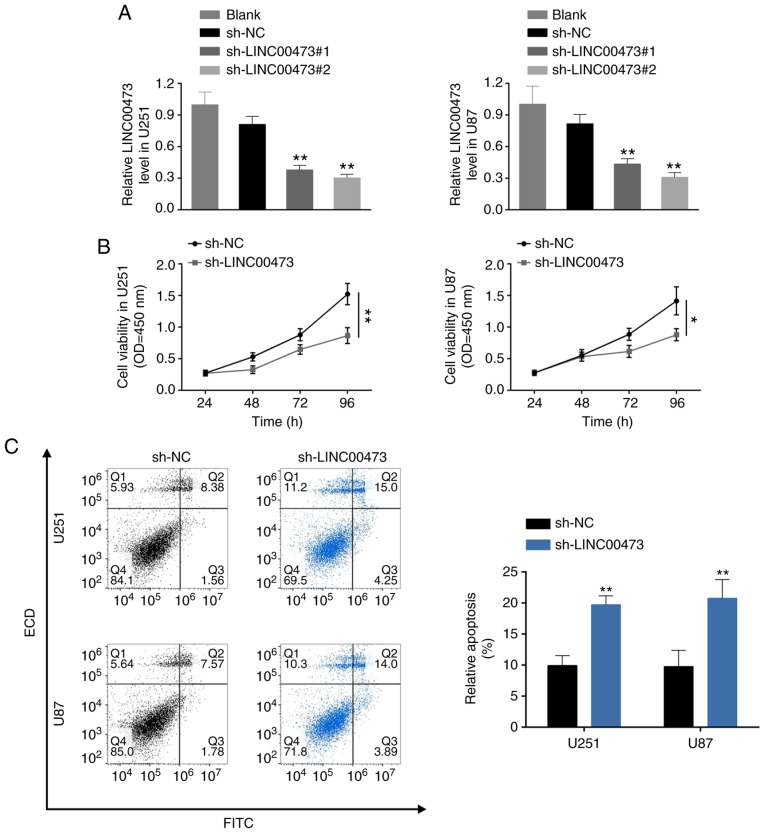
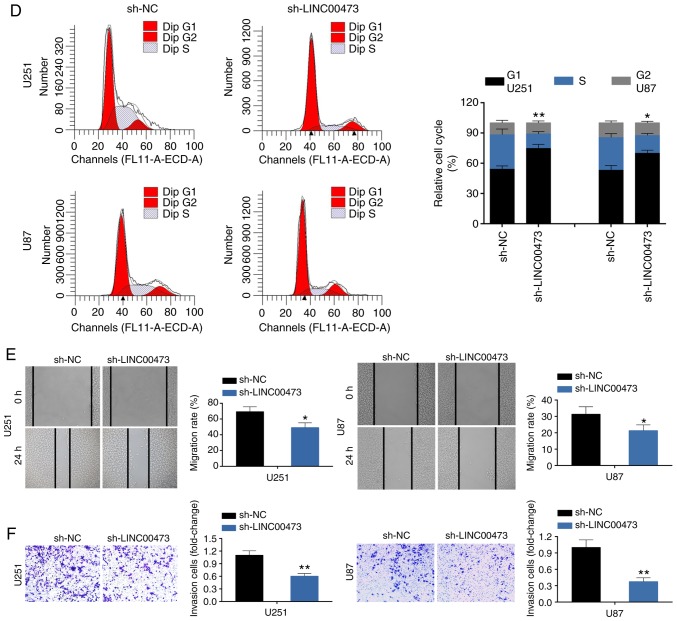
To further investigate the biological role of LINC00473 in glioma cells, U251 and U87 cells were transfected with sh-LINC00473#1 and sh-LINC00473#2. RT-qPCR analysis confirmed the knockdown efficiency of LINC00473 in U251 and U87 cells (Fig. 2A); sh-LINC00473#2 was used for the subsequent assays, and will be referred to as sh-LINC00473. Loss-of-function assays demonstrated that LINC00473 knockdown inhibited cell viability (Fig. 2B), induced apoptosis (Fig. 2C) and blocked cell cycle progression at the G0/G1 phase (Fig. 2D). Moreover, down-regulation of LINC00473 also substantially inhibited cell migration (Fig. 2E) and invasion (Fig. 2F). In general, LINC00473 knockdown suppressed glioma cell proliferation, migration and invasion, and induced apoptosis.
Figure 2.
LINC00473 knockdown suppresses glioma cell proliferation, migration and invasion, and induces apoptosis. (A) Transfection efficiency of sh-LINC00473#1 or sh-LINC00473#2 in U251 and U87 cells was detected by reverse transcription-qPCR. **P<0.01 vs. sh-NC (P<0.01). (B) The influence of sh-LINC00473 on cell viability was detect by Cell Counting Kit-8. *P<0.05; **P<0.01. (C) The influence of sh-LINC00473 on apoptosis was determined by flow cytometry. **P<0.01. LINC00473 knockdown suppresses glioma cell proliferation, migration and invasion, and induces apoptosis. (D) The influence of sh-LINC00473 on cell cycle progression was detected by flow cytometry *P<0.05 and **P<0.01 vs. sh-NC G1. (E) The influence of sh-LINC00473 on cell migration was determined by wound healing assay. *P<0.01 vs sh-NC. (F) The influence of sh-LINC00473 on cell invasive ability was detect by Transwell assay. **P<0.01 vs. sh-NC. NC, negative control; sh, short hairpin RNA.
LINC00473 knockdown inhibits glioma growth in vivo
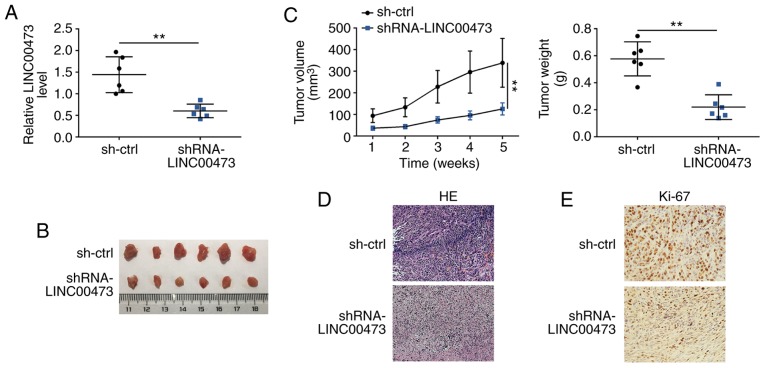
The effects of LINC00473 on glioma growth were examined in vivo. At 5 weeks post-transplantation, subcutaneous tumors were harvested. The knockdown efficiency of sh-LINC00473 in subcutaneous xenotransplanted tumors was confirmed by RT-qPCR (Fig. 3A). Mice in the sh-LINC00473 group exhibited smaller tumor sizes and volumes compared with those of the sh-NC group (Fig. 3B and C, respectively). Tumor weight was also significantly decreased in sh-LINC00473 group compared with the control (Fig. 3C). Tumor sections were stained with H&E, which revealed that LINC00473 knockdown decreased degrees of malignancy, as tissues in sh-NC group exhibited dissimilar cells with bigger nucleus (Fig. 3D), and IHC staining demonstrated that Ki67 expression levels were also notably reduced in the xenografted tumor tissues of mice injected with sh-LINC00473-trans-fected cells (Fig. 3E).
Figure 3.
LINC00473 knockdown inhibits glioma growth in vivo. (A) Stable knockdown of LINC00473 by sh-LINC00473 in the subcutaneous xenotrans-planted tumors was confirmed by reverse transcription-qPCR. **P<0.01. (B) sh-LINC00473 inhibited glioma growth in xenografted mice. (C) sh-LINC00473 inhibited glioma tumor volume and weight. **P<0.01. (D) Tumor sections were stained with H&E, which demonstrated that sh-LINC00473 decreased degrees of glioma malignancy. (E) Immunohistochemical staining indicated that the expression of Ki67 was reduced in the xenograft tumor tissues of mice injected with U251 cells transfected with sh-LINC00473. **P<0.01. H&E, hematoxylin and eosin; sh, short hairpin RNA.
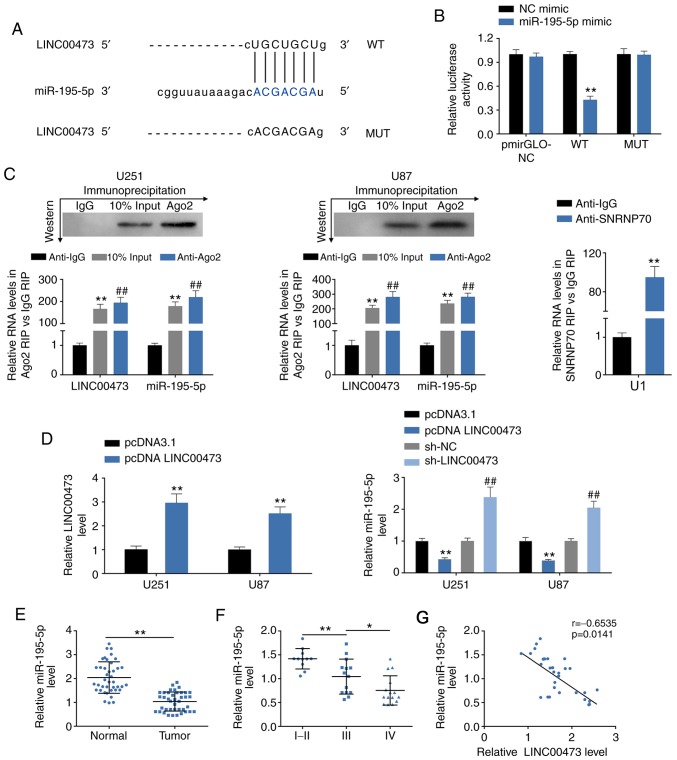
LINC00473 directly binds to and decreases miR-195-5p expression
StarBase was used to determine if LINC00473 contained potential target sites for miR-195-5p (Fig. 4A). Dual luciferase reporter assays confirmed that miR-195-5p significantly decreased the luciferase activity of pmirGLO-wt-LINC00473, but did not have an effect on pmirGLO-mut-LINC00473 (Fig. 4B), which suggested a potential binding ability between LINC00473 and miR-195-5p. Results from the RIP assay revealed that LINC00473 and miR-195-5p were both significantly enriched in Ago2-containing beads compared with the IgG-containing beads, the same as that of the SNRNP70 positive control (Fig. 4C). Taken together, these results indicated that there may be an interaction between LINC00473 and miR-195-5p.
Figure 4.
LINC00473 directly binds to and decreases miR-195-5p expression. (A) Predicted target sites of miR-195-5p in LINC00473. (B) Effect of miR-195-5p mimics on luciferase activity of reporter gene with WT or MUT LINC00473 target regions. **P<0.01. (C) RIP assays were performed using the Ago2 or IgG antibodies, and RT-qPCR was used to detect the expression levels of miR-195-5p and LINC00473 in U251 and U87 cells. **P<0.01 and ##P<0.01 vs. anti-IgG. (D) Transfection efficiency of pcDNA3.1-LINC00473 overexpression (left) and the effects of pcDNA3.1-LINC00473 and sh-LINC00473 on miR-195-5p expression in U251 and U87 cells was detected by RT-qPCR. **P<0.01 vs. pcDNA3.1; ##P<0.01 vs. sh-NC. (E) miR-195-5p expression levels in glioma tissues and para-carcinoma tissues were determined by RT-qPCR. n=40; **P<0.01). (F) miR-195-5p expression levels in high-grade glioma (WHO grade III and IV) and low-grade glioma (WHO grade I and II) tissues were detect by RT-qPCR. *P<0.05, **P<0.01. (G) Negative correlation between LINC00473 and miR-195-5p in glioma tissues. Ago2, argonaute 2; IgG, immunoglobulin G; miR, microRNA; MUT, mutant; NC, negative control; RIP, RNA immunoprecipitation; RT-qPCR, reverse transcription-qPCR; SNRNP70, U1 small nucleoprotein 70 kDa; WT, wild-type.
The effects of LINC00473 on miR-195-5p expression were evaluated by gain- and loss-of-function assays. The transfection efficiency of pcDNA 3.1-LINC00473 in U251 and U87 cells was confirmed by RT-qPCR (Fig. 4D). miR-195-5p expression levels were significantly increased upon sh-LINC00473 trans-fection, whereas expression was decreased upon LINC00473 overexpression, compared with the respective controls (Fig. 4D). Decreased miR-195-5p expression levels were also observed in human glioma tissues (Fig. 4E), and miR-195-5p expression was determined to be negatively related to the WHO grade of glioma (Fig. 4E). Bivariate Pearson’s correlation analysis revealed miR-195-5P expression was negatively correlated with LINC00473 in glioma tissues (Fig. 4F).
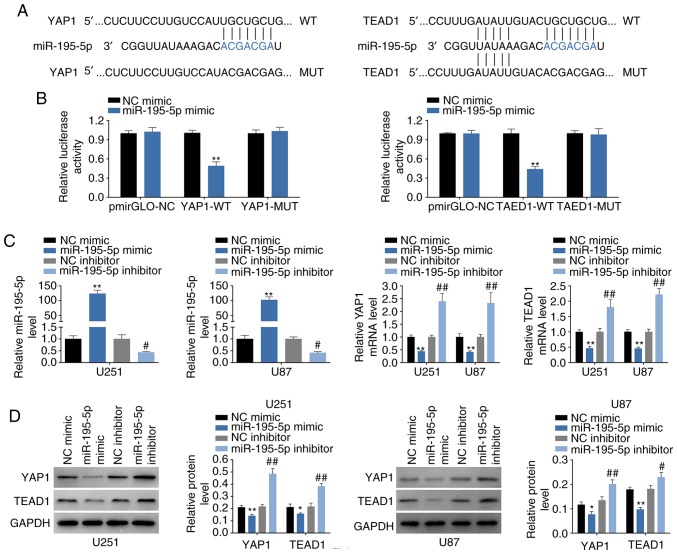
YAP1 and TEAD1 are direct targets of miR-195-5p
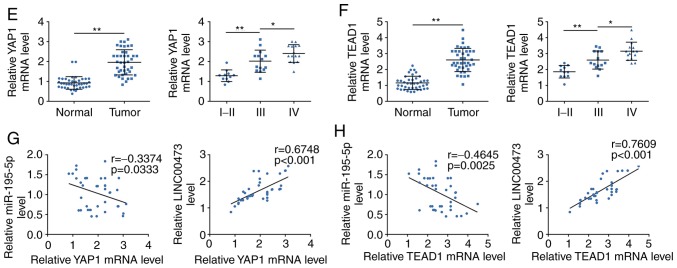
The potential targets for miR-195-5p were predicted as axin 2, cell division cycle 23, dynactin 5, importin 7, proline rich 15-like and sortilin 1 using TargetScan. YAP1 and TEAD1 were also identified as containing miR-195-5p target sites (Fig. 5A) and were selected for the following experiments. The targeting of miR-195-5p to YAP1 or TEAD1 3′UTRs were confirmed by dual luciferase reporter assay (Fig. 5B). Furthermore, the up- or downregulation efficiency of miR-195-5p mimics or inh were determined by RT-qPCR (Fig. 5C). Similar to the bioinformatics results, miR-195-5p mimics could decrease both mRNA (Fig. 5C) and subsequent protein (Fig. 5D) expression levels of YAP1 and TEAD1, whereas miR-195-5p inh resulted in significantly increased YAP1 and TEAD1 expressions compared with the respective controls (Fig. 5C and D). The elevated of YAP1 and TEAD1 mRNA expression levels were also observed in glioma tissues (Fig. 5E and F, respectively), and both of the YAP1 and TEAD1 expressions were positively related to the WHO grade of glioma (Fig. 5E and F, respectively). Bivariate Pearson’s correlation analysis indicated that YAP1 and TEAD1 expression were negatively correlated with miR-195-5p, but positively correlated with LINC00473 (Fig. 5G and H).
Figure 5.
YAP1 and TEAD1 are direct targets of miR-195-5p. (A) Predicted miR-195-5p target sites in the WT 3′UTR of in YAP1 and TEAD1; MUT target sites are also shown. (B) Effect of miR-195-5p on luciferase activity of reporter gene with WT or MUT 3′UTR of YAP1 and TEAD1. **P<0.01 vs. miR-NC. (C) Transfection efficiency of miR-195-5p mimics or inh in U251 and U87 cells; and the influence of miR-195-5p mimics or inh on mRNA expression levels of YAP1 and TEAD1 were detected by RT-qPCR. **P<0.01 vs. miR-NC; #P<0.05 and ##P<0.01 vs. inh NC. (D) The influence of miR-195-5p mimics or inh on protein expression levels of YAP1 and TEAD1 was detected by western blotting. *P<0.05 and **P<0.01 vs. miR-NC; #P<0.05 and ##P<0.01 vs. inh NC. (E) YAP1 mRNA expression levels in glioma tissues (Tumor) and para-carcinoma tissues (Normal), n=40; and in high-grade glioma (WHO grade III and IV) and low-grade glioma (WHO grade I and II) tissues were detected by RT-qPCR. *P<0.05 and **P<0.01. (F) TEAD1 mRNA expression levels in glioma tissues and para-carcinoma tissues (n=40) and in high-grade glioma (WHO grade III and IV) and low-grade glioma (WHO grade I and II) tissues were detected by RT-qPCR. *P<0.05 and **P<0.01. (G) Negative correlation between miR-195-5p and YAP1 in glioma tissues; and positive correlation between LINC00473 and YAP1 in glioma tissues. (H) Negative correlation between miR-195-5p and TEAD1 in glioma tissues, and positive correlation between LINC00473 and TEAD1 in glioma tissues. Inh, inhibitor; miR, microRNA; MUT, mutant; RT-qPCR, reverse transcription-qPCR; TEAD1, TEA domain family member 1; UTR, untranslated region; WT, wild-type; YAP1, Yes-associated protein 1.
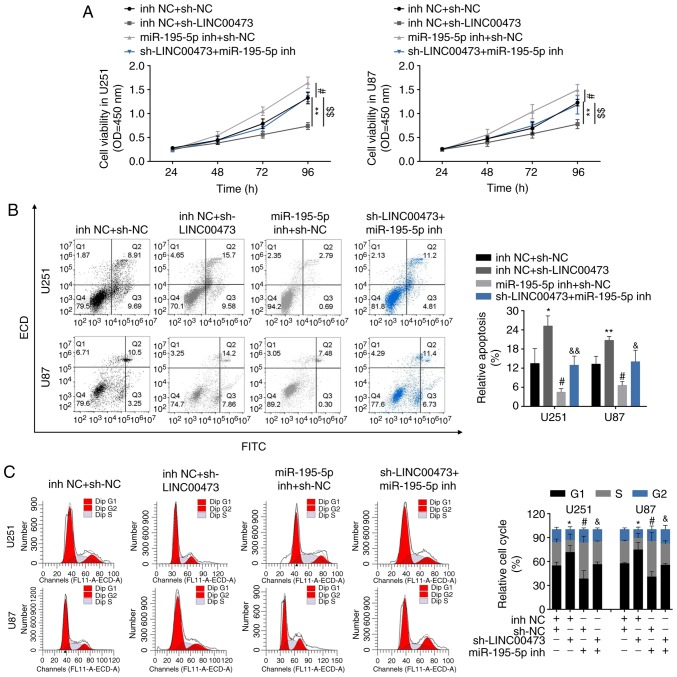
LINC00473 promotes glioma progression through miR-195-5p-mediated YAP1-TEAD1-Hippo signaling
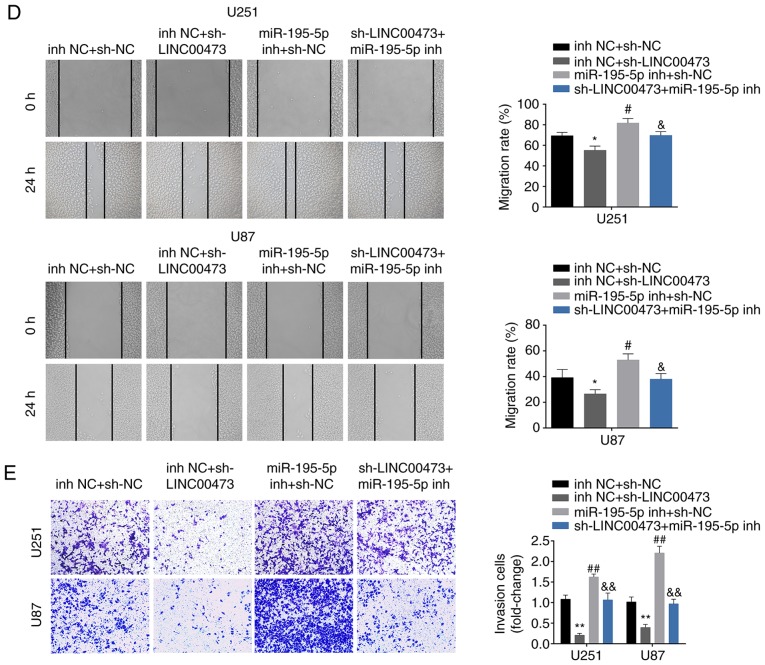
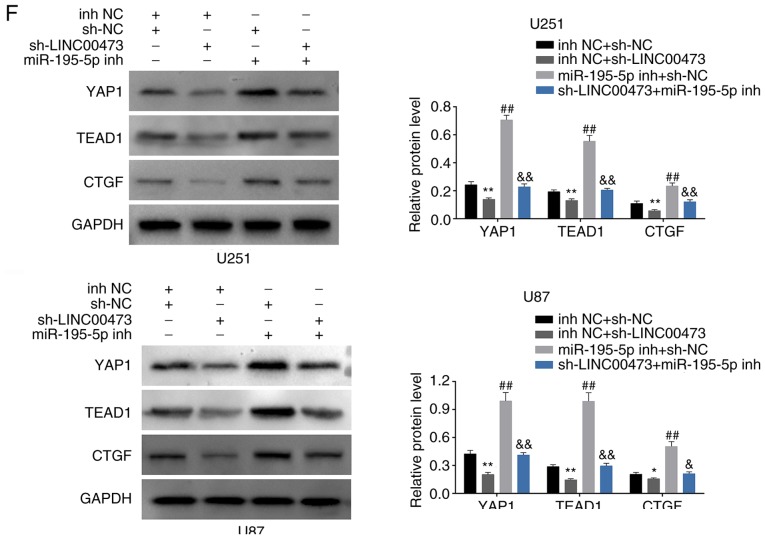
We designed four groups: U251 and U87 cells transfected with i) inh NC and sh-NC, ii) sh-LINC00473 and inh NC, iii) miR-195-5p inh and sh-NC, and iv) sh-LINC00473 and miR-195-5p inh, to determine the functional roles of LINC00473/miR-195-5p/YAP1-TEAD1-Hippo axis in regulating glioma progression. CCK-8 assay results revealed that the inhibition of cell viability by sh-LINC00473 was reversed in cells co-transfected with miR-195-5p inh (Fig. 6A); cells trans-fected with miR-195-5p inh exhibited higher viability compared with cells co-transfected sh-LINC00473 and miR-195-5p inh. Flow cytometry results revealed that induction of cell apoptosis (Fig. 6B) and inhibition of cell cycle (Fig. 6C) by sh-LINC00473 was also reversed in cells co-transfected with miR-195-5p inh; cells transfected with miR-195-5p inh exhibited lower apoptotic rates and higher cell cycle ratio (cell number of G1: All the cell number) compared with cells co-transfected sh-LINC00473 and miR-195-5p inh. Lastly, the suppressed migratory and invasive abilities of cells expressing sh-LINC00473 could be reversed by miR-195-5p inh co-transfection (Fig. 6D and E, respectively); cells transfected miR-195-5p inh exhibited significant increases in migration and invasion compared with cells co-transfected sh-LINC00473 and miR-195-5p inh (Fig. 6D and E, respectively). Western blot analysis demonstrated significantly decreased YAP1, TEAD1 and CTGF (connective tissue growth factor) levels upon sh-LINC00473 transfection compared with the control group (Fig. 6F), which were reversed in cells co-transfected with sh-LINC00473 and miR-195-5p inh (Fig. 6F). Cells transfected with miR-195-5p inh alone exhibited notably higher YAP1 and TEAD1 protein expression levels compared with cells co-expressing sh-LINC00473 and miR-195-5p inh (Fig. 6F).
Figure 6.
LINC00473 promotes glioma progression through miR-195-5p-mediated YAP1-TEAD1-Hippo signaling pathway. (A) The influence of sh-LINC00473 and miR-195-5p inh on cell viability was detected by Cell Counting Kit-8. (B) The influence of sh-LINC00473 and miR-195-5p inh on apoptosis was detected by flow cytometry. (C) The influence of sh-LINC00473 and miR-195-5p inh on cell cycle progression, specifically G1, was detected by flow cytometry. LINC00473 promotes glioma progression through miR-195-5p-mediated YAP1-TEAD1-Hippo signaling pathway. (D) The influence of sh-LINC00473 and miR-195-5p inh on cell migration as determined by wound healing assay. (E) The influence of sh-LINC00473 and miR-195-5p inh on cell invasion was detected by Transwell assay. (F) The influence of sh-LINC00473 and miR-195-5p inh on protein expression levels of YAP1, TEAD1 and CTGF were determined by western blotting. *P<0.05 and **P<0.01 vs. inh NC + sh-NC; #P<0.05 and ##P<0.01 vs. inh NC + sh-NC; &P<0.05 and &&P<0.01 vs. inh NC + sh-LINC00473. CTGF, connective tissue growth factor; inh, inhibitor; miR, microRNA; NC, negative control; sh, short hairpin RNA; TEAD1, TEA domain family member 1; YAP1, Yes-associated protein 1.
Discussion
Glioma is considered as one of most aggressive malignancies in central nervous system worldwide; it is clinically complicated due to various pathogenic genes and proteins (28,29). lncRNAs are key regulators in glioma pathogenesis (7), and LINC00473 has been reported to be a novel oncogene of human cancers (10,17). Therefore, it is important to investigate the regulatory mechanisms of LINC00473 on prognosis with the aim to treat glioma.
Results from the present study indicated that LINC00473 was highly expressed in both glioma tissues and cell lines, which was in line with results from a previous study (30). Moreover, high LINC00473 expression was not only tightly associated with WHO grades of glioma, but also related to the OS of the patients, predicting a poor prognosis in patients with glioma. Poor tumor prognosis is related to the invasion and metastasis of cancer cells (22). LINC00473 has been correlated with poor prognosis in non-small cell lung cancer (9), gastric cancer (15), colorectal cancer (17) and head and neck squamous cell carcinoma (31) by promoting cancer cell migration and invasion. Therefore, LINC00473 was speculated to be involved in glioma progression. However, owing to the small sample size of our current clinical analysis (n=40), the significant correlation between high LINC00473 expression with other clinical parameters requires further investigations. A larger patient cohort is also needed to strengthen the clinical significance of LINC00473 in patients with glioma.
Consistent with a previous hypothesis (30), our in vitro and in vivo loss-of-function assays indicated that LINC00473 knockdown not only inhibited cell proliferation, invasion and migration of glioma cells, but also blocked cell cycle and induced apoptosis. In vivo subcutaneous xenotransplanted tumor models revealed that interference of LINC00473 could suppress tumorigenic ability of glioma, as shown by the lower expression of Ki67 in sh-LINC00473 group than sh-NC group. Ki67 is a molecular marker that predicts poor prognosis of glioma patients (32), the reduced expression of Ki67 indicated the potential clinical application of LINC00473 in treatment of glioma. However, the underlying mechanism remains to be clarified.
Accumulating evidence suggest that lncRNAs function as ceRNAs to sponge miRNA, therefore titrating them off the binding sites on protein-coding mRNAs (33). LINC00473 was previously reported to sponge miR-15a in colorectal cancer (17), and the binding ability between LINC00473 and miR-195 was also reported in Wilms tumor (10). In the present study, miR-195-5p was identified as a target of LINC00473 in glioma cells by the means of dual luciferase reporter assay and RIP. Moreover, downregulation of miR-195-5p was found to be associated with poor prognosis of patients with glioma. In particular, miR-195-5p may inhibit cell proliferation and induce apoptosis in glioma cells through the regulation of cell apoptosis-related proteins including Caspase-3, -8, -9 and Bcl-2 (34). Direct targets of miR-195 in glioma cells were identified as Sal-like protein 4 (35), cyclin E1 (36) to affect cell cycle (37) in the previous studies. The present study also demonstrated that miR-195-5p expression is reduced in glioma tissues and cells, and inhibition of miR-195-5p promoted cell proliferation, migration and invasion of glioma cells, and inhibited apoptosis.
YAP1 and TEAD1 were identified as targets of miR-195-5p using online prediction databases and dual luciferase reporter assay. In vitro loss-of-function assays demonstrated that LINC00473 knockdown reduced YAP1 and TEAD1 expression, which may suppress glioma progression. In addition, CTGF, which was reported to promote glioma migration (38), was also decreased upon loss of LINC00473 expression, which indicated an oncogenic role of LINC00473. Activation of YAP increased the expression of downstream target CTGF, leading to stem cell phenotype of glioma (39). Effects of LINC00473 on epithelial-mesenchymal transition and stemness properties of glioma cells required further investigation. As LINC00473 is associated with various lncRNA-miRNA-mRNA regulatory axes and is involved in signaling pathways in regulation of tumors progression; additional potential miRNA targets and downstream signaling networks should be studied in the future. Recently, LINC00473 was reported to promote glioma progression via regulation of the miR-637/CDK6 axis (30), confirming various LINC00473-miRNA-mRNA regulatory axes in regulation of glioma.
In conclusion, the present study demonstrated that knockdown of lncRNA LINC00473 inhibited cell proliferation, migration and invasion of glioma cells, as well as blocked cell cycle and induced cell apoptosis, via regulation of miR-195-5p/YAP1-TEAD1 axis. These data revealed the relationship between LINC00473/miR-195-5p/YAP1/TEAD1 regulatory axis and glioma progression, and may shed light on the potential therapeutic application of LINC00473 in glioma treatment.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
XW and XBJ conceived and designed the experiments. XDL and ZYF analyzed and interpreted the results of the experiments. YZ and XH performed the experiments.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China; approval no. 2014-S034), and all the patients signed written informed consent. All studies involving animals were approved by Union Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology and conducted in accordance with the guidelines set out by Medical Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (approval no. 2014-S034).
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Reference
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Naderali E, Nikbakht F, Ofogh SN, Rasoolijazi H. The role of rosemary extract in degeneration of hippocampal neurons induced by kainic acid in the rat: A behavioral and histochemical approach. J Integr Neurosci. 2018;17:19–25. doi: 10.3233/JIN-170035. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. quiz 1 p following 516. [DOI] [PubMed] [Google Scholar]
- 4.Milano MT, Johnson MD, Sul J, Mohile NA, Korones DN, Okunieff P, Walter KA. Primary spinal cord glioma: A surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98:83–92. doi: 10.1007/s11060-009-0054-7. [DOI] [PubMed] [Google Scholar]
- 5.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 6.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiang KM, Zhang XQ, Leung GK. Long non-coding RNAs: The key players in glioma pathogenesis. Cancers (Basel) 2015;7:1406–1424. doi: 10.3390/cancers7030843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruunsild P, Bengtson CP, Bading H. Networks of cultured iPSC-derived neurons reveal the human synaptic activity-regulated adaptive gene program. Cell Rep. 2017;18:122–135. doi: 10.1016/j.celrep.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Li JL, Lin S, Cao C, Gimbrone NT, Yang R, Fu DA, Carper MB, Haura EB, Schabath MB, et al. cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. J Clin Invest. 2016;126:2267–2279. doi: 10.1172/JCI85250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu S, Fu W, Zhang L, Fu K, Hu J, Jia W, Liu G. LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKα. Cell Prolif. 2018;51 doi: 10.1111/cpr.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, Yang F, Li X, Gong ZJ, Wang LW. Long noncoding RNA LNC473 inhibits the ubiquitination of survivin via association with USP9X and enhances cell proliferation and invasion in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2018;499:702–710. doi: 10.1016/j.bbrc.2018.03.215. [DOI] [PubMed] [Google Scholar]
- 12.Shi C, Yang Y, Yu J, Meng F, Zhang T, Gao Y. The long noncoding RNA LINC00473, a target of microRNA 34a, promotes tumorigenesis by inhibiting ILF2 degradation in cervical cancer. Am J Cancer Res. 2017;7:2157–2168. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Lin S, Li JL, Ni W, Guo R, Lu J, Kaye FJ, Wu L. CRTC1-MAML2 fusion-induced lncRNA LINC00473 expression maintains the growth and survival of human muco-epidermoid carcinoma cells. Oncogene. 2018;37:1885–1895. doi: 10.1038/s41388-017-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinh TA, Vitucci EC, Wauthier E, Graham RP, Pitman WA, Oikawa T, Chen M, Silva GO, Greene KG, Torbenson MS, et al. Comprehensive analysis of the cancer genome atlas reveals a unique gene and non-coding RNA signature of fibrolamellar carcinoma. Sci Rep. 2017;7:44653. doi: 10.1038/srep44653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Song Y. LINC00473 predicts poor prognosis and regulates cell migration and invasion in gastric cancer. Biomed Pharmacother. 2018;107:1–6. doi: 10.1016/j.biopha.2018.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20:R858–R861. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Zhang X, Sheng L, Qiu C, Luo R. LINC00473 promotes the Taxol resistance via miR-15a in colorectal cancer. Biosci Rep. 2018;38 doi: 10.1042/BSR20180790. pii: BSR20180790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui W, Yuntao L, Lun L, WenSheng L, ChaoFeng L, HaiYong H, Yueyang B. MicroRNA-195 inhibits the proliferation of human glioma cells by directly targeting cyclin D1 and cyclin E1. PLoS One. 2013;8:e54932. doi: 10.1371/journal.pone.0054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang QQ, Xu H, Huang MB, Ma LM, Huang QJ, Yao Q, Zhou H, Qu LH. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro Oncol. 2012;14:278–287. doi: 10.1093/neuonc/nor216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilaz Susluer S, Biray Avci C, Dodurga Y, Ozlem Dogan Sigva Z, Oktar N, Gunduz C. Downregulation of miR-195 via cyclo-sporin A in human glioblastoma cells. J BUON. 2015;20:1337–1340. [PubMed] [Google Scholar]
- 21.Yu S, Jing L, Yin XR, Wang MC, Chen YM, Guo Y, Nan KJ, Han LL. MiR-195 suppresses the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by inhibiting YAP. Oncotarget. 2017;8:99757–99771. doi: 10.18632/oncotarget.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Wu K, Wang S, Wei G. Long non-coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR-195-5p. J Cell Biochem. 2018;119:5646–5656. doi: 10.1002/jcb.26743. [DOI] [PubMed] [Google Scholar]
- 23.Sun M, Song H, Wang S, Zhang C, Zheng L, Chen F, Shi D, Chen Y, Yang C, Xiang Z, et al. Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. J Hematol Oncol. 2017;10:79. doi: 10.1186/s13045-017-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chai J, Xu S, Guo F. TEAD1 mediates the oncogenic activities of Hippo-YAP1 signaling in osteosarcoma. Biochem Biophys Res Commun. 2017;488:297–302. doi: 10.1016/j.bbrc.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittig A, Moss RL, Sauerwein WA. Glioblastoma, brain metastases and soft tissue sarcoma of extremities: Candidate tumors for BNCT. Appl Radiat Isot. 2014;88:46–49. doi: 10.1016/j.apradiso.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 27.Tome-Garcia J, Erfani P, Nudelman G, Tsankov AM, Katsyv I, Tejero R, Bin Zhang, Walsh M, Friedel RH, Zaslavsky E, Tsankova NM. Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma. Nat Commun. 2018;9:4020. doi: 10.1038/s41467-018-06258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berntsson SG, Merrell RT, Amirian ES, Armstrong GN, Lachance D, Smits A, Zhou R, Jacobs DI, Wrensch MR, Olson SH, et al. Glioma-related seizures in relation to histopathological subtypes: A report from the glioma international case-control study. J Neurol. 2018;265:1432–1442. doi: 10.1007/s00415-018-8857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochiai Y, Sano E, Okamoto Y, Yoshimura S, Makita K, Yamamuro S, Ohta T, Ogino A, Tadakuma H, Ueda T, et al. Efficacy of ribavirin against malignant glioma cell lines: Follow-up study. Oncol Rep. 2018;39:537–544. doi: 10.3892/or.2017.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Wang G, Xu L, Yao Z, Song L. Long non-coding RNA LINC00473 promotes glioma cells proliferation and invasion by impairing miR-637/CDK6 axis. Artif Cells Nanomed Biotechnol. 2019;47:3896–3903. doi: 10.1080/21691401.2019.1671431. [DOI] [PubMed] [Google Scholar]
- 31.Han PB, Ji XJ, Zhang M, Gao LY. Upregulation of lncRNA LINC00473 promotes radioresistance of HNSCC cells through activating Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:7305–7313. doi: 10.26355/eurrev_201811_16267. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Y, Xiang W, Yanhui L, Ruofei L, Shuang L, Yingjun F, Qiao Z, Yanwu Y, Qing M. Ki-67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure. 2013;22:877–881. doi: 10.1016/j.seizure.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: The rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia Y, Tian Y, An S, Yang D. Effects of microRNA-195 on the prognosis of glioma patients and the proliferation and apoptosis of human glioma cells. Pathol Oncol Res. 2019 Feb 26; doi: 10.1007/s12253-019-00622-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Chen LP, Zhang NN, Ren XQ, He J, Li Y. miR-103/miR-195/miR-15b regulate SALL4 and inhibit proliferation and migration in glioma. Molecules. 2018;23 doi: 10.3390/molecules23112938. pii: E2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Ren S, Xu Y, Miao W, Huang X, Qu Z, Li J, Liu X, Kong P. MicroRNA-195 reverses the resistance to temozolomide through targeting cyclin E1 in glioma cells. Anticancer Drugs. 2019;30:81–88. doi: 10.1097/CAD.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo A, Donzelli S, Girgenti V, Sacconi A, Vasco C, Salmaggi A, Blandino G, Maschio M, Ciusani E. In vitro antineoplastic effects of brivaracetam and lacosamide on human glioma cells. J Exp Clin Cancer Res. 2017;36:76. doi: 10.1186/s13046-017-0546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072–2081. doi: 10.1158/1078-0432.CCR-0659-03. [DOI] [PubMed] [Google Scholar]
- 39.Zhu G, Wang Y, Mijiti M, Wang Z, Wu PF, Jiafu D. Upregulation of miR-130b enhances stem cell-like phenotype in glioblastoma by inactivating the Hippo signaling pathway. Biochem Biophys Res Commun. 2015;465:194–199. doi: 10.1016/j.bbrc.2015.07.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.