Abstract
Ophiopogonin D' (OPD') is a natural compound extracted from Ophiopogon japonicus, which is a plant used in traditional Chinese medicine. Our previous study has indicated that OPD' exhibits antitumor activity against androgen-independent prostate cancer (PCa), but the effects and the underlying molecular mechanism of action of OPD' in androgen-dependent PCa were unclear. In the present study, OPD' induced significant necroptosis in androgen-dependent LNCaP cancer cells by activating receptor-interacting serine/threonine-protein kinase 1 (RIPK1). Exposure to OPD' also increased Fas ligand (FasL)-dependent RIPK1 protein expression. The OPD'-induced necroptosis was inhibited by a RIPK1 inhibitor necrostatin-1, further supporting a role for RIPK1 in the effects of OPD´. The antitumor effects of OPD' were also inhibited by a mixed lineage kinase domain-like protein (MLKL) inhibitor necrosulfonamide. Following treatment with inhibitors of RIPK1 and MLKL, the effects of OPD' on LNCaP cells were inhibited in an additive manner. In addition, co-immunoprecipitation assays demonstrated that OPD' induced RIPK3 upregulation, leading to the assembly of a RIPK3-MLKL complex, which was independent of RIPK1. Furthermore, OPD' increased the expression of Fas-associated death domain, which is required to induce necroptosis in LNCaP cells. OPD' also regulated the expression levels of FasL, androgen receptor and prostate-specific antigen in a RIPK1-dependent manner. These results suggested that OPD' may exhibit potential as an anti-PCa agent by inducing RIPK1- and MLKL-dependent necroptosis.
Keywords: Ophiopogonin D', necroptosis, receptor-interacting serine/threonine-protein kinase 1, Fas-associated death domain, prostate cancer
Introduction
Although China in part of the low prostate cancer (PCa) incidence area, the incidence of PCa increased by 4.7% annually between 2005 and 2011, and the mortality increased by 5.5% annually between 2000 and 2011, making it a burden on the healthcare system (1). The early stages of PCa are typically androgen receptor (AR)-dependent, and the disease can be controlled by active monitoring, androgen deprivation/hormone therapy (ADT) and radical prostatectomy. However, ADT causes weight gain, lean muscle loss, diabetes and osteopenia (2,3). ADT has also been demonstrated to induce apoptosis resistance and may lead to the development of AR-independent PCa (4). Non-hormonal, non-toxic treatments that inhibit AR-dependent PCa are highly desirable.
Previous studies have reported that certain natural products, including lycopene, soy isoflavones, resveratrol and green tea extract can inhibit the expression of the AR and prostate-specific antigen (PSA) in androgen-dependent PCa LNCaP cells (5-7). These cells represent the most commonly used cellular model of AR-dependent PCa. The combination of ADR with a herbal supplement including cholecalciferol, α-tocopherol, L-selenomethionine and epigallocatechin was beneficial in a phase II clinical trial, leading to a decrease or stabilization of the level of PSA (6).
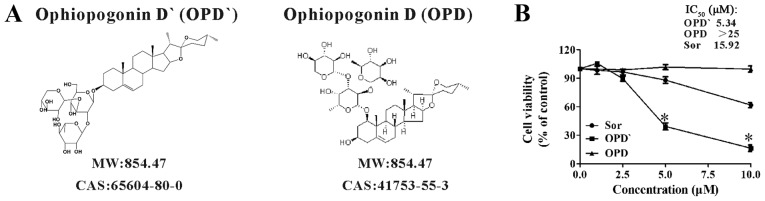
Ophiopogonin D' (OPD') and Ophiopogonin D (OPD) are two triterpene saponins derived from Ophiopogon japonicus, which is a plant used in traditional Chinese medicine. OPD and OPD´ have the same molecular weight (854.47 kDa), central structure (platycodigenin) and glycosylradical, but different glycosideradicals. Previous studies have demonstrated that OPD exhibits antitumor effects (8,9). However, little is known about the effects of OPD'. Our previous study revealed that OPD' induced apoptosis in androgen-independent PCa cells through a receptor-interacting serine/threonine-protein kinase 1 (RIPK1)-mediated mechanism (10).
RIPK1 serves an important role in cell death and inflammation. Activation of RIPK1 promotes cell death in radio resistant breast cancer and enhances the antitumor activity of docetaxel in patient-derived breast cancer xenografts (11,12). Abnormal expression of RIPK1 is associated with a range of human degenerative and inflammatory diseases, including central nervous system pathologies, amyotrophic lateral sclerosis, Alzheimer's disease, Parkinson's disease, traumatic brain injury, stroke and lysosomal storage diseases (13). In prostate cancer, RIPK1 interacts directly with the AR to suppress PCa cell proliferation in vitro and tumor growth in vivo (14). Inhibitor of apoptosis protein antagonists and sorafenib (a RIPK1 inhibitor) induce apoptosis or necroptosis in castration-resistant (AR-independent) or autophagy-deficient PCa cells (15-17).
Necroptosis is different from apoptosis in terms of the underlying mechanisms and the characteristics of cell death and is important to eliminate apoptosis-resistant tumor cells (18). Apoptosis involves the formation of apoptotic bodies and requires the activation of endogenous and/or exogenous caspase-dependent apoptosis pathways (19). Necroptosis is characterized by increased membrane permeability, swelling organelles and cleavage of the cell nucleus (20). Necroptosis is regulated by multiple pathways, including the RIPK1/RIPK3/mixed lineage kinase domain-like protein (MLKL) pathway. Recent studies suggest that splenomegaly is largely dependent on RIPK3-MLKL-mediated necroptosis, but is independent of RIPK1 kinase activity (21). By contrast, monocytosis is dependent on RIPK1 kinase activity, but not RIPK3-MLKL (21).
Our previous study demonstrated that OPD' inhibited the in vitro and in vivo growth of AR-independent PCa via RIPK1 with no significant effects on the body weight of nude mice (10). The aim of the present study was to explore the effects and mechanisms of action of OPD' in an AR-dependent PCa cell line LNCaP.
Materials and methods
Test compound, chemicals and reagents
The OPD and OPD' used in the present study (Fig. 1A) were obtained from Chengdu Must Bio-Technology and had a purity of >96%. The anti-human RIPK1 (1:1,000; cat. no. 3493), RIPK3 (1:1,000; cat. no. 13526), caspase 8 (1:1,000; cat. no. 9746), Fas-associated death domain (FADD; 1:500; cat. no. 2782) and mouse anti-rabbit IgG (light-chain specific; 1:1,000; cat. no. 45262) antibodies were obtained from Cell Signaling Technology, Inc. The RIPK3 (1:50; cat. no. ab56164), anti-MLKL (1:1,000; cat. no. ab184718) and anti-p-MLKL antibodies (1:1,000; cat. no. ab187091) were purchased from Abcam. The anti-β-actin (1:1,000; cat. no. TA-09), horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000, cat. no. ZB-2306) and horseradish peroxidase-conjugated anti-mouse IgG (1:2,000; cat. no. ZB-2305) antibodies were obtained from OriGene Technologies, Inc. Sorafenib (positive control), Necrostatin-1 (Nec-1, a RIPK1 inhibitor) and Z-VAD-FMK (a caspase inhibitor) were purchased from Selleck Chemicals. Necrosulfonamide (NSA) was purchased from Santa Cruz. N-acetylcysteine (NAC) was purchased from Beyotime Institute of Biotechnology.
Figure 1.
The chemical structure and biological activity of OPD'. (A) The chemical structures of OPD' and OPD. (B) The viability of LNCaP cells was examined by Cell Counting Kit-8 assay after treatment with OPD' or Sor (positive control) for 24 h. The concentrations that induced 50% growth inhibition (IC50) were calculated (n=3). * P<0.05 vs. Sor. OPD, Ophiopogonin D; Sor, sorafenib.
Cell lines and cell culture
The LNCaP, PC3 and DU145 cell lines were purchased from the American Type Culture Collection. Cells were incubated in a stable, humidified environment at 37°C with 5% CO2 and were passaged every 2-3 days when they became confluent. The three cell lines were cultured in their own special medium supplemented with 10% FBS as previously described (22).
Cell survival assay
The Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology) assay was used to examine the effects of OPD' on the survival of human LNCaP cells. LNCaP cells (8,000 cells/well) were treated with 0-25 µM OPD' or sorafenib (used as a positive control) for 24 h, and 10 µl CCK8 was added in per well for 3 h at 37°C. The absorbance of the sample at 450 nm was measured using a Tecan Infinite M200 microplate reader (Tecan Group Ltd.). The proportion of viable cells was calculated based on the following formula: Viable cells = [OD (OPD')-OD(blank)] / [OD(DMSO)-OD(blank)] × 100%. IC50 was calculated by the LOGIT method (23).
Apoptosis assay
The effects of OPD' or sorafenib on the proportion of LNCaP cells undergoing apoptosis and necroptosis were examined using an Annexin V-FITC/propidium iodide (PI) Apoptosis Detection kit (BestBio, Ltd.). LNCaP cells (2×105 cells/well) were treated with 2.5-10 µM OPD' or sorafenib for 18 h. The samples were collected for FITC/PI staining for 15 min according to the manufacturer's instructions and analyzed by a FACSCalibur flow cytometer (BD Biosciences) and FlowJo 7.6.1 software (BD Biosciences).
Ultrastructural study
LNCaP cells (1×106) were treated with 5 µM OPD' for 24 h. The cultured cells were collected, fixed with 2% glutaraldehyde in 0.1 M PBS overnight, post-fixed with 1% osmium tetroxide for 2 h at 4°C and observed under a FEITecnai 10 electron microscope (Thermo Fisher Scientific, Inc.) at ×12,000 magnification.
Co-immunoprecipitation of RIPK3- and MLKL-bound complexes
LNCaP cells were treated with OPD' for 6 h, and protein lysates were collected using ice-cold NP-40 cell lysis buffer (Beyotime Institute of Biotechnology) with 1 mM PMSF. A 50% protein A/G agarose mixture (cat. no. P2055; Beyotime Institute of Biotechnology) was added (100 µl per 1 ml sample solution), and the sample was agitated on a horizontal shaker for 60 min at 4°C. The sample was centrifuged at 825 × g for 2 min at 4°C, and the supernatant was divided into two parts. Subsequently, 10 µl anti-RIPK3 antibody (cat. no. ab56164; Abcam) or rabbit-IgG (cat. no. A0716; Beyotime Institute of Biotechnology) was added to yield ~500 µl total volume, and the sample was incubated at 4°C overnight to allow antibody binding. New 50% protein A/G agarose was added to the sample solution, which was incubated at 4°C for 1 h. The sample was washed with NETN lysis buffer (20 mM Tris, pH 8.0; 150 mM NaCl; 0.5% NP-40; 1 mM DTT; 0.5 mM EDTA; protease inhibitor mixture) 5 times at 4°C. The resulting complexes were collected and used for western blotting analysis.
Drug treatments
The cells were pre-treated with inhibitors (10 µM Nec-1, 10 µM NSA, 20 µM Z-VAD-FMK or 5 mM NAC) for 2 h and treated with OPD' for 24 or 18 h (total inhibitor treatment duration was 26 or 20 h). Cell survival rate and flow cytometry analyses were performed using CCK-8 and Annexin V-FITC/PI staining, respectively.
Small interfering (si)RNA transfection
siRNA sequences against FADD were purchased from Guangzhou RiboBio Co., Ltd. The siRNA sequences were as follows: siRNA-FADD#1, GACCGAGCTCAAGTTCCTA; and siRNA-FADD#2, CTGAGAATCTGGAAGAACA. A total of 2×105 LNCaP cells were transfected with 50 nM siRNA-FADD with Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and Opti-MEM I reduced serum medium (Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h at 37°C. Subsequently, the cells were incubated in 5 µM OPD' for another 6 or 24 h. Western blotting and flow cytometry analyses were performed using anti-FADD antibodies and Annexin V-FITC/PI staining, respectively.
Western blotting assays
LNCaP, DU145 and PC3 cells were treated with OPD', and the total protein was extracted with a protein extraction kit including RIPA, protease inhibitor mixture and phosphorylase inhibitor mixture (BestBio, Ltd.). The samples were centrifuge at 13,200 × g for 15 min at 4°C, and the supernatant was transferred to the new Eppendorf tube. Protein concentration was determined by the bicinchoninic assay method, and the samples were balanced with RIPA, protease inhibitor mixture, phosphorylase inhibitor mixture (250:1:1) and 5X sample loading buffer (cat. no. P0015; Beyotime Institute of Biotechnology) and denatured for 10 min at 100°C. A total of 50 µg protein sample per lane was separated by 8-12% SDS-PAGE at 80 V for 30 min and 120 V for 60 min, transferred to a PVDF membrane at 200 mA and 4°C for 150 min and blocked with 5% skimmed milk for 120 min at room temperature. The membrane was incubated with the primary antibodies overnight at 4°C with gentle agitation and washed with TBS + 0.1% Tween-20. The secondary antibodies were added and incubated for 2 h at room temperature. The membrane was visualized using a chemiluminescence kit (EMD Millipore), detected using a Fusion FX5 Spectra instrument (Vilber Lourmat Sté) and analyzed by Image Lab (Bio-Rad Laboratories, Inc.).
Statistical analysis
Continuous variables are presented as the means ± SEM. Statistical analysis was performed by one-way ANOVA and Tukey's test. All tests were performed using SPSS 13.0 (SPSS, Inc.) and were two-sided. P<0.05 was considered to indicate a statistically significant difference.
Results
OPD' inhibits LNCaP cell proliferation
The inhibitory effects of OPD' on LNCaP cell survival were assessed using the CCK-8 assay. Sorafenib has been recommended for the treatment of refractory PCa, and is also a RIPK1 inhibitor (16,20,24); thus, it was used as a positive control in the present study. OPD' exhibited stronger proliferation inhibitory effects compared with OPD and sorafenib in LNCaP cells at 24 h, with IC50 values of 5.34 µM for OPD', 15.92 µM for sorafenib and >25 µM for OPD (Figs. 1B and S1).
OPD' induces necrosis in LNCaP cells
As indicated in Fig. 2A, 24-h treatment with OPD' led to an increase in the proportion of FITC-positive and FITC/PI dual-positive cells (0 vs. 5 µM OPD', 3.9±1.3 vs. 14.2±3.6 and 3.5±2.6 vs. 51.0±7.5, respectively). Sorafenib treatment (10 μM) resulted in a greater increase in the proportion of FITC-positive cells and increased the proportion of FITC/PI dual-positive cells, but this effect was less potent compared with that of OPD' (Fig. 2A). The proportion of FITC/PI dual-positive cells is considered to reflect late apoptosis or necrosis (25), and apoptosis is mainly mediated by caspase pathways (19); the effects of OPD' were not reversed following pre-treatment with 20 µM Z-VAD-FMK (a pan-caspase inhibitor) for 2 h (Fig. 2B), suggesting that these cells were not undergoing apoptosis.
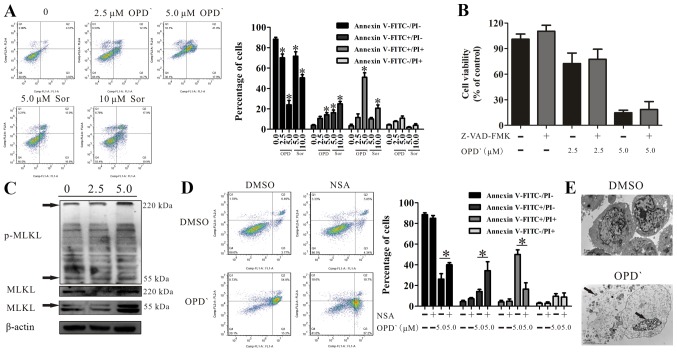
Figure 2.
OPD' induces necrosis in LNCaP cells. (A) Following LNCaP cell treatment with 2.5 or 5 µM OPD' for 24 h, the apoptotic rates were assessed by Annexin V-FITC/PI assay (n=3). *P<0.05 vs. vehicle. (B) The Cell Counting Kit-8 assay was used to analyze the effects of OPD' on cell survival following pre-treatment of LNCaP cells with Z-VAD-FMK (n=3). (C) Western blotting was used to examine the protein expression of MLKL and p-MLKL following 6-h treatment with OPD'. (D) Annexin V-FITC/PI staining was used to analyze the apoptotic rates of LNCaP cells following treatment with OPD' and NSA. (E) Treatment with 5 µM OPD' induced changes in cell morphology, as indicated by transmission electron microscopy. Arrows indicate leaked contents of the cell and cleavage of the cell nucleus. *P<0.05. OPD', Ophiopogonin D'; Sor, sorafenib; PI, propidium iodide; MLKL, mixed lineage kinase domain-like protein; NSA, necrosulfonamide; p, phosphorylated.
MLKL is a marker of necrosis (26). The results of western blot analysis demonstrated that OPD' exposure increased the protein expression levels of MLKL (55 and 220 kDa) and p-MLKL (220 kDa) (Figs. 2C and S2). Treatment of cells with OPD' and NSA, a MLKL inhibitor, reversed the impact of OPD' on the proportion of FITC−/PI− and FITC+/PI+ cells (5 µM OPD' vs. NSA + 5 µM OPD', 26.2±9.5 vs. 40.1±3.5 and 50.1±7.8 vs. 16.6±10.5, respectively; Fig. 2D). In addition, transmission electron microscopy images demonstrated changes in the cell morphology following treatment with 5 µM OPD' for 24 h, with leakage of cell contents, increased permeability of the cell membranes and cleavage of the cell nucleus, but no apoptotic bodies, which suggested that the cells were undergoing necrosis (Fig. 2E) (20).
OPD'-induced necroptosis is dependent on RIPK1
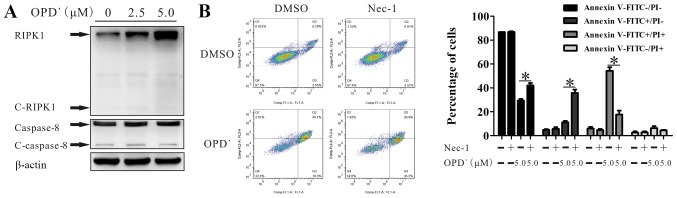
RIPK1 is involved in programmed cell death (27), and is cleaved by cleaved-caspase 8 (C-caspase 8). Western blot analysis indicated that treatment with 5 µM OPD' for 6 h increased the protein expression levels of RIPK1 and caspase 8, without any effect on the levels of cleaved-RIPK1 (C-RIPK1) or C-caspase 8 in LNCaP cells (Fig. 3A). Treatment with 2.5 and 5 µM OPD' increased RIPK1 without any effects on c-caspase 8 and possible slight increases in C-RIPK1 and caspase 8 at 2.5 µM (Fig. 3A). When the cells were co-treated with OPD' and Nec-1, the effects of OPD' on the proportion of FITC+/PI+ cells(late apoptosis and necrosis) were reversed (Fig. 3B), suggesting that RIPK1 was necessary for OPD'-induced necrosis. Compared with cells treated with only OPD', the proportion of FITC+/PI- cells was increased following co-treatment with OPD' and Nec-1 (Fig. 3B). Thus, early apoptosis was still induced, but late apoptosis/necrosis was not induced when cells were treated with Nec-1.
Figure 3.
OPD' induces necroptosis in a RIPK1-dependentmanner. (A) Western blotting was used to examine the protein expression of RIPK1, cleaved-RIPK1, caspase 8 and cleaved-caspase 8 following treatment with OPD' for 6 h. (B) Annexin V-FITC/PI staining was used to analyze the LNCaP cell status after treatment with OPD' and/or Nec-1 (n=3). *P<0.05. OPD', Ophiopogonin D'; Nec-1, necrostatin-1; RIPK1, receptor-interacting serine/threonine-protein kinase 1; PI, propidium iodide; C, cleaved.
OPD' induces necroptosis by multiple pathways involving RIPK1 and RIPK3/MLKL
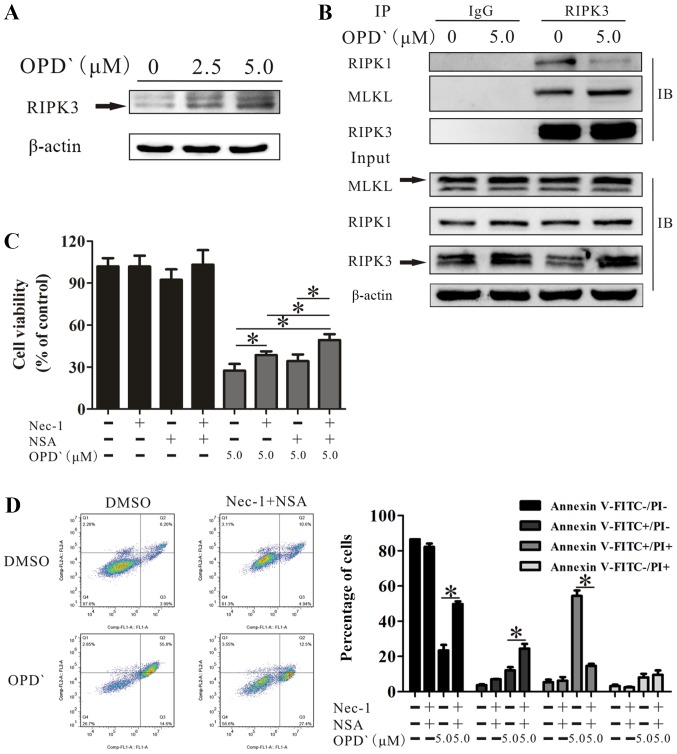
The RIPK1/RIPK3/MLKL pathway is a classical necroptosis pathway (28). Therefore, the effects of OPD' on the expression levels of these three proteins were examined. OPD' exposure increased the protein expression level of RIPK3 (Fig. 4A). Co-immunoprecipitation analysis results revealed that RIPK3, but not RIPK1, interacted with MLKL. This indicated that RIPK3 interacted with RIPK1 and MLKL, but RIPK3 did not require RIPK1 for binding MLKL (Fig. 4B).
Figure 4.
OPD' induces necroptosis by multiple pathways involving RIPK1 and RIPK3/MLKL. (A) Western blotting was used to examine the protein expression of RIPK3 following treatment with OPD' for 6 h. (B) LNCaP cells were exposed to 5 µM OPD' for 6 h, then the protein lysates were immunoprecipitated using anti-RIPK3 antibodies. The target proteins RIPK1, MLKL and RIPK3 were examined by western blotting. (C) The Cell Counting Kit-8 assay was used to analyze the survival of LNCaP cells in response to OPD' following pre-treatment with Nec-1 and/or NSA (n=3). (D) Annexin V-FITC/PI staining was used to analyze the apoptotic rates of LNCaP cells exposed to OPD' for 24 h following combined pre-treatment with Nec-1 and NSA for 2 h. *P<0.05. OPD', Ophiopogonin D'; Nec-1, necrostatin-1; NSA, necrosulfonamide; RIPK, receptor-interacting serine/threonine-protein kinase; MLKL, mixed lineage kinase domain-like protein; PI, propidium iodide.
Based on the above results, cells treated with only OPD', OPD' plus Nec-1, OPD' plus NSA or OPD' plus Nec-1 and NSA were compared; the results demonstrated that the effects of OPD' on cell viability were decreased by co-treatment with Nec-1 or Nec-1 and NSA (Fig. 4C). The effect was not reversed by co-treatment with NSA (P=0.109; Fig. 4C). However, the combination of Nec-1 and NSA was significantly more effective compared with the Nec-1 alone at inhibiting the effects of OPD' (Fig. 4C). The FICT/PI double staining analysis results demonstrated that the co-treatment of cells with OPD', Nec-1 and NSA inhibited the effects of OPD' on the proportions of FITC−/PI− and FITC+/PI+ cells (5 µM OPD' vs. Nec-1+NSA + 5 µM OPD', 23.3±7.1 vs. 49.8±3.4 and 54.3±7.0 vs. 14.6±2.7, respectively; Fig. 4D), resulting in an increase in the proportion of FITC+/PI- cells (12.1±3.9 vs. 24.4±6.1; Fig. 4D). Thus, Nec-1 and NSA attenuated the effects of OPD' on LNCaP cells.
OPD' regulates Fas ligand (FasL), AR and PSA through RIPK1
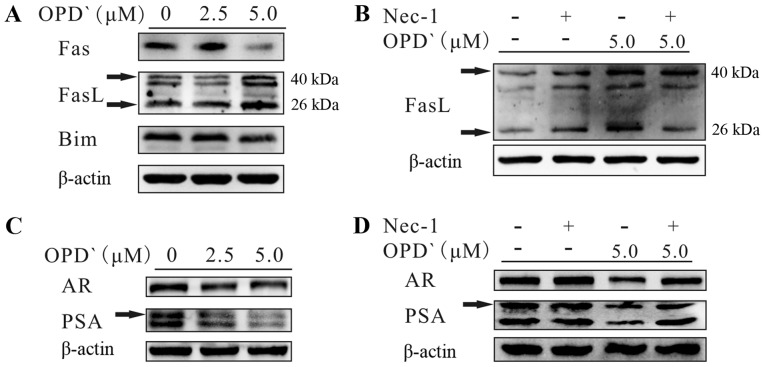
In addition to the RIPK1/RIPK3/MLKL pathway, RIPK1 also activates mitogen-activated protein kinases, such as JNK (29), which upregulate the expression of Fas, FasL and Bim (30) and interact with the AR to inhibit the development of PCa (14). As presented in Fig. 5A, exposure of LNCaP cells to 5 µM OPD' for 6 h increased the protein expression levels of FasL (40 kDa) and soluble FasL (26 kDa), whereas the protein expression levels of Fas and Bim were decreased. In addition, the effects of OPD' on soluble FasL were reversed by pre-treatment with Nec-1 for 2 h prior to OPD' treatment (Fig. 5B). Following treatment with OPD', the protein expression levels of the AR and PSA were decreased (Fig. 5C). This effect was reversed by pre-treatment with Nec-1 for 2 h (Fig. 5D). These results suggested that OPD' may affect FasL via RIPK1, thus inhibiting the AR and inhibiting PCa cell proliferation, while also inducing necroptosis.
Figure 5.
OPD' regulates FasL, androgen receptor and prostate-specific antigen through RIPK1. (A) Western blotting was used to detect the effects of OPD' on target proteins FasL, Fas and Bim in LNCaP cells. (B) Western blotting was used to detect the effects of OPD' on the protein expression of FasL following treatment with Nec-1 and/or OPD'. (C) Western blotting was used to detect the effects of OPD' on target proteins AR and PSA in LNCaP cells. (D) Western blotting was used to detect the effects of OPD' on the protein expression of AR and PSA following treatment with Nec-1 and/or OPD'. OPD', Ophiopogonin D'; FasL, Fas ligand; Nec-1, necrostatin-1; Bim, Bcl-2-like protein 11; AR, androgen receptor; PSA, prostate-specific antigen.
OPD' determines the pattern of cell death through FADD
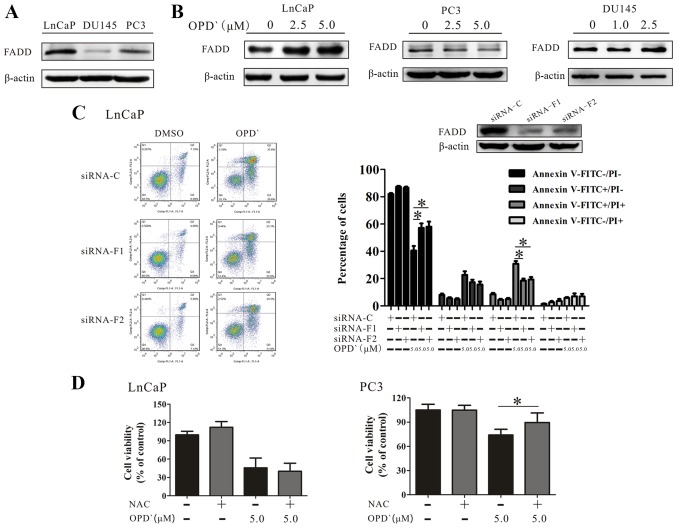
The type II tumor necrosis factor receptor 1-interacting protein complex (comprising RIPK1, caspase 8, FADD and tumor necrosis factor receptor type 1-associated death domain protein) requires FADD to induce apoptosis or necroptosis (31,32). As presented in Fig. 6A, the baseline FADD protein expression level in LNCaP cells was higher compared with that in PC3 and DU145 cell lines. Although OPD' treatment increased the protein expression of FADD in LNCaP cells(Fig. 6B), only a slight increase was observed in the protein expression level of FADD following exposure of DU145 cells to 1 µM and 2.5 µM OPD' for 6 h. Of note, the FADD protein level exhibited a decrease in PC3 cells (Fig. 6B). The effects of OPD' on the proportion of FITC−/PI− and FITC+/PI+ cells were reversed in LNCaP cells by pre-treatment with siRNA-FADD (5 µM OPD' vs. siRNA-F1 + 5 µM OPD' and siRNA-F2 + 5 µM OPD', 42.6±6.7 vs. 58.6±7.6 and 59.8±8.6, and 30.9±9.8 vs. 18.5±3.2 and 19.3±4.0, respectively; Fig. 6C).
Figure 6.
OPD' determines the pattern of cell death mediated by FADD. (A) The baseline FADD protein expression in LNCaP, PC3 and DU145 cells was examined by western blotting. (B) LNCaP, PC3 and DU145 cells were treated with OPD' for 6 h, and FADD protein expression was examined by western blotting. (C) Annexin V-FITC/PI staining was used to analyze the apoptotic rates of LNCaP cells following treatment with OPD' and siRNA-FADD (n=3). (D) The Cell Counting Kit-8 assay was used to analyze the viability of LNCaP and PC3 cells following treatment with OPD' and NAC (n=3). *P<0.05. OPD', Ophiopogonin D'; FADD, Fas-associated death domain; siRNA, small interfering RNA; C, siRNA-control; siRNA-F1, siRNA-FADD#1; siRNA-F2, siRNA-FADD#2; PI, propidium iodide; NAC, N-acetylcysteine.
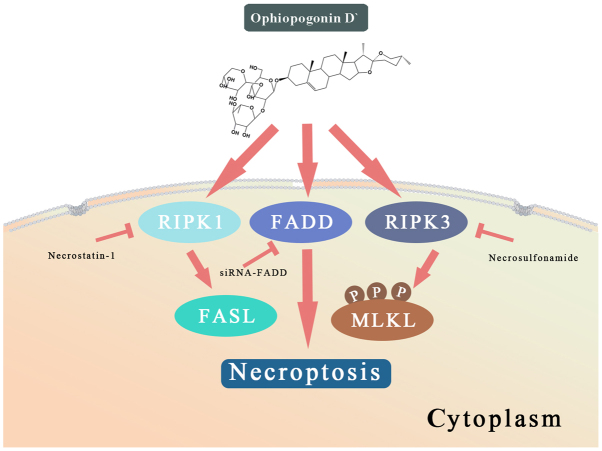
RIPK1 induces apoptosis by generating reactive oxygen species (ROS) in the absence of FADD (33). The effects of OPD' were reversed by pretreatment of PC3 cells with an antioxidant NAC, but this was not observed in LNCaP cells (Fig. 6D). These results suggested that OPD' induced necrop-tosis through multiple pathways, and that FADD served a key role in determining the form of cell death (Fig. 7).
Figure 7.
A schematic representation of the anti-cancer effects of OPD' in LNCaP and PC3 cells. RIPK, receptor-interacting serine/threonine-protein kinase; MLKL, mixed lineage kinase domain-like protein; FADD, Fas-associated death domain; FASL, Fas ligand; p, phosphorylated; siRNA, small interfering RNA.
Discussion
A previous study has suggested that promoting apoptosis may cure PCa (34). Apoptosis resistance frequently develops after ADT, leading to treatment-resistant PCa (4). The occurrence of apoptosis resistance is associated with the induction of autophagy by androgen deprivation, which reduces the efficacy of ADT (35,36). More specifically, upregulation of autophagy-associated proteins has been demonstrated to promote resistance to ADT (35,37,38). Another study has indicated that the transformation of cell death patterns between apoptosis and necroptosis is controlled by autophagy machinery (39). In prostate cells defective in autophagy, toxic agents induced necroptosis rather than apoptosis (16). Inhibition of autophagy has also been demonstrated to improve the efficacy of abiraterone (40). Thus, suppressing autophagy and/or inducing necroptosis may make ADT more effective (34). The results of the present study suggested that OPD' induced necroptosis in LNCaP cells.
RIPK1 is a crucial regulator of necroptosis (41). The results of the present study suggested that OPD' induced necroptosis via several RIPK1-dependent mechanisms. RIPK3 is known to interact with RIPK1 to induce necroptosis (19). The present study demonstrated that OPD' activated MLKL through RIPK3 to induce necroptosis. Of note, when MLKL was inhibited by NSA, the OPD'-induced necroptosis and apoptosis was increased. The results of the present study also revealed that RIPK1 did not directly interact with RIPK3, but was able to induce necroptosis in a RIPK3/MLKL-independent manner. Thus, although MLKL and RIPK3 maybe involved in the necroptotic effects of OPD', other regulators appear to be involved. RIPK3 may induce apoptosis by disrupting FUN14 domain-containing 1 activation (42) when MLKL is inhibited by NSA. RIPK1 also regulates FasL, which is required for the execution of necroptosis in red blood cells (43), as well as mitogen-activated protein kinases such as JNK (29), which promotes the protein expression of Fas, FasL and Bim (30). In the present study, OPD' treatment increased the protein expression levels of FasL (but not Bim or Fas) in LNCaP cells in a RIPK1-dependent manner, which supported the involvement of FasL.
The AR is a transcription factor that activates genes that promote PCa cell proliferation and survival (44). AR antagonists or surgery are typically used to treat patients with early-stage PCa. The results of the present study demonstrated that OPD' inhibited the AR protein expression via RIPK1. PSA is an important index used for the diagnosis and evaluation of PCa progression and is related to the expression of the AR (45,46). In the present study, OPD' decreased the protein expression of PSA via a mechanism dependent on RIPK1. Other saponin products, such asginsenosides, have also been demonstrated to inhibit the AR protein expression during testosterone stimulation (47). In addition to saponins, an ethanol extract of Algerian propolis was demonstrated to decrease androgen receptor transcriptional activity and the secretion of PSA (48). Thus, a body of evidence supports the use of plant-derived compounds to reduce the AR expression and activity.
Tumors are composed of genetically heterogeneous cellular clones, which undergo further genetic changes during disease progression and in response to clinical treatment (49). Prostate tumors contain functionally and phenotypically distinct cells (50). The androgen receptor status of prostate tumor cells is one of the major indicators used for the staging of PCa (51). Our previous study demonstrated that OPD' exhibited activity against androgen-independent PC3 and DU145 prostate cancer cells, which was mediated through RIPK1 (10). Although OPD' increased RIPK1 protein expression in these cells and androgen-dependent LNCaP cells, the treatment resulted in different effects. Interestingly, OPD' induced apoptosis in PC3 and DU145 cells, which are more sensitive compared with PC3 cells (10), but led to necroptosis in LNCaP cells. This may be due to the different baseline expression and activity of FADD in the different cell lines.
FADD is not only a crucial adaptor protein in death receptor-mediated apoptosis, but is also important for necroptosis (52). FADD, caspase 8 and RIPK1 form a death-inducing signaling complex that has the potential to initiate either apoptosis or necroptosis depending on the conditions and cell type (53). In the absence of FADD, RIPK1 induces apoptosis by generating ROS (33). The results of the present study demonstrated that OPD' increased the protein expression of FADD and induced necroptosis in LNCaP cells, whereas in our previous study, it reduced the protein expression of FADD and induced apoptosis in PC3 cells (10). Further experiments in the present study revealed that the effects of OPD' were reversed by pre-treatment of PC3 cells with NAC, which was not observed in LNCaP cells. The level of OPD'-induced apoptosis did not change following pre-treatment of cells with siRNA-FADD. This may be attributed to PC3 cells having a high mitochondrial oxygen concentration and generating more O2- compared with LNCaP cells (54). Although normal ROS levels maintain cell proliferation, excessive ROS induces cell death, and PC3 cells were already being exposed to high levels of ROS (55). FADD induces apoptosis in the absence of RIPK1 (56), and when RIPK1 was inhibited by Nec-1, the level of OPD'-induced apoptosis in LNCaP cells increased.
In summary, the results of the present study demonstrated that OPD' inhibited the proliferation of LNCaP cells, and that these effects were at least partially RIPK1-dependent. OPD' also affected the expression levels of FasL, AR and PSA in a RIPK1-dependent manner. OPD' may inhibit the viability of diverse types of prostate cancer cell and exert its effects via multiple mechanisms. Further studies are needed to replicate these results and mechanisms in vivo. However, the current results are novel, suggesting that OPD' may have potential as an anti-tumor agent in the treatment of PCa.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (grant nos. 81603347, 81673167 and 81171991).
Availability of data and materials
All data used or analyzed in this study are available from the corresponding author upon reasonable request.
Authors' contributions
ZL and HX organized, conceived, designed and supervised the study. CW, MZ, WS and JW designed and conducted the experiments and drafted the manuscript. YL, HW, JG, NL and JL participated in the study design and data interpretation. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 3.Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol. 2002;167:1952–1956. doi: 10.1016/S0022-5347(05)65060-4. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Wu W, Fiandalo MV. The role of intracrine androgen metabolism, androgen receptor and apoptosis in the survival and recurrence of prostate cancer during androgen deprivation therapy. Curr Drug Targets. 2013;14:420–440. doi: 10.2174/1389450111314040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J. 2004;18:1019–1021. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 6.Dorff TB, Groshen S, Tsao-Wei DD, Xiong S, Gross ME, Vogelzang N, Quinn DI, Pinski JK. A Phase II trial of a combination herbal supplement for men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:359–365. doi: 10.1038/pcan.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLarty J, Bigelow RLH, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res (Phila) 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Kim C, Lee SG, Sethi G, Ahn KS. Ophiopogonin D, a Steroidal Glycoside Abrogates STAT3 Signaling Cascade and Exhibits Anti-Cancer Activity by Causing GSH/GSSG Imbalance in Lung Carcinoma. Cancers (Basel) 2018;10:E427. doi: 10.3390/cancers10110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Kim C, Lee SG, Yang WM, Um JY, Sethi G, Ahn KS. Ophiopogonin D modulates multiple oncogenic signaling pathways, leading to suppression of proliferation and chemosensitization of human lung cancer cells. Phytomedicine. 2018;40:165–175. doi: 10.1016/j.phymed.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Lu Z, Wang H, Zhu M, Song W, Wang J, Wu C, Kong Y, Guo J, Li N, Liu J, et al. Ophiopogonin D', a Natural Product From Radix Ophiopogonis, Induces in Vitro and in Vivo RIPK1-Dependent and Caspase-Independent Apoptotic Death in Androgen-Independent Human Prostate Cancer Cells. Front Pharmacol. 2018;9:432. doi: 10.3389/fphar.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez-Añorve IX, Gonzalez-De la Rosa CH, Soto-Reyes E, Beltran-Anaya FO, Del Moral-Hernandez O, Salgado-Albarran M, Angeles-Zaragoza O, Gonzalez-Barrios JA, Landero-Huerta DA, Chavez-Saldaña M, et al. New insights into radioresistance in breast cancer identify a dual function of miR-122 as a tumor suppressor and oncomiR. Mol Oncol. 2019;13:1249–1267. doi: 10.1002/1878-0261.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newell M, Goruk S, Mazurak V, Postovit L, Field CJ. Role of docosahexaenoic acid in enhancement of docetaxel action in patient-derived breast cancer xenografts. Breast Cancer Res Treat. 2019;177:357–367. doi: 10.1007/s10549-019-05331-8. [DOI] [PubMed] [Google Scholar]
- 13.Degterev A, Ofengeim D, Yuan J. Targeting RIPK1 for the treatment of human diseases. Proc Natl Acad Sci USA. 2019;116:9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CL, Liu JS, Lin TW, Chang YH, Kuo YC, Lin AC, Ting HJ, Pang ST, Lee LY, Ma WL, et al. Characterization of a novel androgen receptor (AR) coregulator RIPK1 and related chemicals that suppress AR-mediated prostate cancer growth via peptide and chemical screening. Oncotarget. 2017;8:69508–69519. doi: 10.18632/oncotarget.17843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martens S, Jeong M, Tonnus W, Feldmann F, Hofmans S, Goossens V, Takahashi N, Bräsen JH, Lee EW, Van der Veken P, et al. Sorafenib tosylate inhibits directly necrosome complex formation and protects in mouse models of inflammation and tissue injury. Cell Death Dis. 2017;8:e2904. doi: 10.1038/cddis.2017.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharaziha P, Chioureas D, Baltatzis G, Fonseca P, Rodriguez P, Gogvadze V, Lennartsson L, Björklund AC, Zhivotovsky B, Grandér D, et al. Sorafenib-induced defective autophagy promotes cell death by necroptosis. Oncotarget. 2015;6:37066–37082. doi: 10.18632/oncotarget.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCann C, Crawford N, Majkut J, Holohan C, Armstrong CWD, Maxwell PJ, Ong CW, LaBonte MJ, McDade SS, Waugh DJ, Longley DB. Cytoplasmic FLIP(S) and nuclear FLIP(L) mediate resistance of castrate-resistant prostate cancer to apoptosis induced by IAP antagonists. Cell Death Dis. 2018;9:1081. doi: 10.1038/s41419-018-1125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2019 Oct 21; doi: 10.1038/s41580-019-0173-8. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Yuan J. SnapShot: Necroptosis. Cell. 2014;158:464–464.e1. doi: 10.1016/j.cell.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Polykratis A, Martens A, Eren RO, Shirasaki Y, Yamagishi M, Yamaguchi Y, Uemura S, Miura M, Holzmann B, Kollias G, et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol. 2019;21:731–742. doi: 10.1038/s41556-019-0324-3. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Zhou R, Kong Y, Wang J, Xia W, Guo J, Liu J, Sun H, Liu K, Yang J, et al. S-equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, Though Activating the Akt/FOXO3a Pathway. Curr Cancer Drug Targets. 2016;16:455–465. doi: 10.2174/1568009616666151207105720. [DOI] [PubMed] [Google Scholar]
- 23.Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011;10:128–134. doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- 24.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, Eastham JA, Enke CA, Farrington TA, Higano CS, et al. National comprehensive cancer network: Prostate cancer, version 1.2014. J Natl Compr Canc Netw. 2013;11:1471–1479. doi: 10.6004/jnccn.2013.0174. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Cheng AC, Wang MS, Peng X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol. 2008;14:2174–2178. doi: 10.3748/wjg.14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Luan Q, Jin L, Jiang CC, Tay KH, Lai F, Liu XY, Liu YL, Guo ST, Li CY, Yan XG, et al. RIPK1 regulates survival of human melanoma cells upon endoplasmic reticulum stress through autophagy. Autophagy. 2015;11:975–994. doi: 10.1080/15548627.2015.1049800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell Death Dis. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 30.Cohen M, Pierredon S, Wuillemin C, Delie F, Petignat P. Acellular fraction of ovarian cancer ascites induce apoptosis by activating JNK and inducing BRCA1, Fas and FasL expression in ovarian cancer cells. Oncoscience. 2014;1:262–271. doi: 10.18632/oncoscience.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 32.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunovic M, Matic I, Nagy B, Caput Mihalic K, Skelin J, Stambuk J, Josipovic P, Dzinic T, Paradzik M, Marijanovic I. FADD-deficient mouse embryonic fibroblasts undergo RIPK1-dependent apoptosis and autophagy after NB-UVB irradiation. J Photochem Photobiol B. 2019;194:32–45. doi: 10.1016/j.jphotobiol.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang KX, Firus J, Prieur B, Jia W, Rennie PS. To die or to survive, a fatal question for the destiny of prostate cancer cells after androgen deprivation therapy. Cancers (Basel) 2011;3:1498–1512. doi: 10.3390/cancers3021498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HY, Ma YD, Zhang Y, Cui J, Wang ZM. Elevated levels of autophagy-related marker ULK1 and mitochondrion-associated autophagy inhibitor LRPPRC are associated with biochemical progression and overall survival after androgen deprivation therapy in patients with metastatic prostate cancer. J Clin Pathol. 2017;70:383–389. doi: 10.1136/jclinpath-2016-203926. [DOI] [PubMed] [Google Scholar]
- 36.Ziparo E, Petrungaro S, Marini ES, Starace D, Conti S, Facchiano A, Filippini A, Giampietri C. Autophagy in prostate cancer and androgen suppression therapy. Int J Mol Sci. 2013;14:12090–12106. doi: 10.3390/ijms140612090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berchem GJ, Bosseler M, Sugars LY, Voeller HJ, Zeitlin S, Gelmann EP. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer Res. 1995;55:735–738. [PubMed] [Google Scholar]
- 38.Bonkhoff H, Fixemer T, Remberger K. Relation between Bcl-2, cell proliferation, and the androgen receptor status in prostate tissue and precursors of prostate cancer. Prostate. 1998;34:251–258. doi: 10.1002/(SICI)1097-0045(19980301)34:4<251::AID-PROS2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Goodall ML, Fitzwalter BE, Zahedi S, Wu M, Rodriguez D, Mulcahy-Levy JM, Green DR, Morgan M, Cramer SD, Thorburn A. The Autophagy Machinery Controls Cell Death Switching between Apoptosis and Necroptosis. Dev Cell. 2016;37:337–349. doi: 10.1016/j.devcel.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Zou L, Li X, Chen Z, Lin Z, Wu X. Inhibition of Autophagy Improves the Efficacy of Abiraterone for the Treatment of Prostate Cancer. Cancer Biother Radiopharm. 2019;34:181–188. doi: 10.1089/cbr.2018.2559. [DOI] [PubMed] [Google Scholar]
- 41.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, Kőműves L, Webster JD, Dixit VM. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaRocca TJ, Stivison EA, Mal-Sarkar T, Hooven TA, Hod EA, Spitalnik SL, Ratner AJ. CD59 signaling and membrane pores drive Syk-dependent erythrocyte necroptosis. Cell Death Dis. 2015;6:e1773. doi: 10.1038/cddis.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 45.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW, Walsh PC, Carter HB. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 46.Lev A, Lulla AR, Ross BC, Ralff MD, Makhov PB, Dicker DT, Eldeiry WS. ONC201 Targets AR and AR-V7 Signaling, Reduces PSA, and Synergizes with Everolimus in Prostate Cancer. Mol Cancer Res. 2018;16:754–766. doi: 10.1158/1541-7786.MCR-17-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae JS, Park HS, Park JW, Li SH, Chun YS. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J Nat Med. 2012;66:476–485. doi: 10.1007/s11418-011-0609-8. [DOI] [PubMed] [Google Scholar]
- 48.Zabaiou N, Fouache A, Trousson A, Buñay-Noboa J, Marceau G, Sapin V, Zellagui A, Baron S, Lahouel M, Lobaccaro JA. Ethanolic extract of Algerian propolis decreases androgen receptor transcriptional activity in cultured LNCaP cells. J Steroid Biochem Mol Biol. 2019;189:108–115. doi: 10.1016/j.jsbmb.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, Ferreira A, Lambros MBK, Seed G, Yuan W, et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur Urol. 2019;76:469–478. doi: 10.1016/j.eururo.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Q, Tang DG. Androgen receptor and prostate cancer stem cells: Biological mechanisms and clinical implications. Endocr Relat Cancer. 2015;22:T209–T220. doi: 10.1530/ERC-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav N, Heemers HV. Androgen action in the prostate gland. Minerva Urol Nefrol. 2012;64:35–49. [PubMed] [Google Scholar]
- 52.Tourneur L, Chiocchia G. FADD: A regulator of life and death. Trends Immunol. 2010;31:260–269. doi: 10.1016/j.it.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Ang RL, Ting AT. Detection of RIPK1 in the FADD-Containing Death Inducing Signaling Complex (DISC) During Necroptosis. Methods Mol Biol. 2018;1857:101–107. doi: 10.1007/978-1-4939-8754-2_10. [DOI] [PubMed] [Google Scholar]
- 54.Wiese AK, Prior S, Chambers TC, Higuchi M. Intracellular Oxygen Concentration Determined By Mitochondrial Respiration Regulates Production of Reactive Oxygen Species. Integr Cancer Biol Res. 2017;1:006. [PMC free article] [PubMed] [Google Scholar]
- 55.Rasul A, Di J, Millimouno FM, Malhi M, Tsuji I, Ali M, Li J, Li X. Reactive oxygen species mediate isoalantolactone-induced apoptosis in human prostate cancer cells. Molecules. 2013;18:9382–9396. doi: 10.3390/molecules18089382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderton H, Bandala-Sanchez E, Simpson DS, Rickard JA, Ng AP, Di Rago L, Hall C, Vince JE, Silke J, Liccardi G, et al. RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development. Cell Death Differ. 2019;26:877–889. doi: 10.1038/s41418-018-0166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used or analyzed in this study are available from the corresponding author upon reasonable request.