Abstract
Introduction:
Dementia is the 7th leading cause of death that imposes a significant financial and service burden on the global population. Presently, only symptomatic care exists for cognitive loss, such as Alzheimer’s disease.
Areas Covered:
Given the advancing age of the global population, it becomes imperative to develop innovative therapeutic strategies for cognitive loss. New studies provide insight to the association of cognitive loss with metabolic disorders, such as diabetes mellitus.
Expert Opinion:
Diabetes mellitus is increasing in incidence throughout the world and affects 350 million individuals. Treatment strategies identifying novel pathways that oversee metabolic and neurodegenerative disorders offer exciting prospects to treat dementia. The mechanistic target of rapamycin (mTOR) and circadian clock gene pathways that include AMP activated protein kinase (AMPK), Wnt1 inducible signaling pathway protein 1 (WISP1), erythropoietin (EPO), and silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) provide novel strategies to treat cognitive loss that has its basis in metabolic cellular dysfunction. However, these pathways are complex and require precise regulation to maximize treatment efficacy and minimize any potential clinical disability. Further investigations hold great promise to treat both the onset and progression of cognitive loss that is associated with metabolic disease.
Keywords: Alzheimer’s disease, AMPK, circadian clock genes, diabetes mellitus, dementia, erythropoietin, mTOR, SIRT1, WISP1
1.0. Introduction and Background
1.1. Neurodegenerative Disorders and the Impact of Dementia
As a result of improvements in global healthcare and the progressive increase in life span, neurodegenerative disorders will continue to increase in prevalence among the world’s population. If one focuses on cognitive disorders, it is interesting to note that dementia is now considered to be the 7th leading cause of death. According to the World Health Organization [1], the present numbers for the prevalence and treatment costs for dementia are significant and affect all countries. For example, the incidence of sporadic cases of Alzheimer’s disease (AD) is expected to significantly increase throughout the globe [2,3]. Cognitive disorders such as AD affect more than 5 million individuals in the United States (US) alone [4,5]. At least sixty percent of dementia cases are believed to result from AD [5-8]. At minimum, five percent of the world’s elderly population suffer from dementia. This is equal to almost 50 million individuals and new cases each year are increasing at an alarming rate. By the year 2030, 82 million people are expected to have dementia. Projected out another twenty years to 2050, 152 million will suffer from dementia.
The financial and service burdens for dementia are equally staggering. More than $800 billion United States dollars (USD) are spent to care for individuals with dementia on an annual basis. These costs are close to two percent of the global gross domestic product. By the year 2030, medical and social services could reach in the US to two trillion USD annually with the ability to easily overwhelm the system. These projections do not include the significant financial costs that involve social and adult living care as well as informal and companion care for individual families. In addition, the World Health Organization estimates the need for close to sixty million new health and social care workers. When to address the need for these healthcare workers in a timely and efficient manner can be a difficult consideration since the onset and progression of dementia in individuals is not always well recognized. Furthermore, dementia and cognitive loss are considered to be under diagnosed throughout the world. Once diagnosis is correctly performed, it can be in the late or end stages of the disease, leaving little utility for treatment and possibly offering fragmented care.
1.2. Metabolic Disease, Diabetes Mellitus, and Dementia
Recent studies highlight the previously unrecognized link between metabolic disorders and cognitive loss. Disorders such as diabetes mellitus (DM) hold an increased risk for the onset and progression of AD and cognitive loss [3,9-12]. Similar to neurodegenerative disorders and dementia, DM is increasing in incidence throughout the world. Approximately 350 million individuals currently have DM [13-17]. An additional 8 million individuals are believed to suffer from metabolic disorders but remain undiagnosed at present [18-20]. The care for patients with DM also extracts a significant portion of healthcare resources. In the US, DM care accounts for seventeen percent of the Gross Domestic Product per the Centers for Medicare and Medicaid Services (CMS) [21]. Almost $176 billion is required for direct medical costs and another $69 billion in lost finances results from reduced productivity tied to DM.
Equally as important to recognize is that DM affects multiple systems of the body [3,10,12,22-25]. DM has been tied to mental illness [26,27], vascular brain injury [7,28-32], cardiovascular disease, immune function , and stem cell regulation [7,32-36]. In relation to cognitive loss and AD, diabetes can affect multiple pathways in these disorders and lead to disease progression [7,32-36].
2.0. Exploring Novel Targets for Dementia and Cognitive Loss
Neurodegenerative disorders, and in particular, cognitive loss can have multiple origins that lead to disease onset and progression. Risk factors for cognitive loss include tobacco use, low education in early life, and hypertension. Yet, new insights point to DM as a significant risk factor that affects large numbers of the global population. Early diagnosis of DM with rapid induction of available therapies for DM can offer some degree of improvement and slow the progression of DM. However, tight serum glucose control does not always lead to the resolution of complications from DM [14,37]. Use of diet and body mass control treatments may be effective to prevent hyperglycemic events, but these strategies also can potentially decrease organ mass through processes that involve autophagy [38]. Furthermore, most available treatments that are directed to treat AD alone involve the use of cholinesterase inhibitors [39]. Dementia that may be caused by vascular disease may be treated with therapies that focus on vascular and metabolic disorders, such as DM [40]. Yet, these treatments for the most part are symptomatic. As a result of these severe limitations to target cognitive loss during metabolic dysfunction, addressing novel pathways for future clinical work that can oversee both metabolic disease and neurodegenerative disorders may offer extremely valuable and exciting avenues to overcome cognitive loss. These innovative strategies involve the mechanistic target of rapamycin (mTOR) and circadian clock gene pathways that include AMP activated protein kinase (AMPK), Wnt1 inducible signaling pathway protein 1 (WISP1), erythropoietin (EPO), and silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1).
3.0. Cellular Pathways of the Mechanistic Target of Rapamycin
One possible target for innovative strategies to treat cognitive loss through metabolic pathways is the mechanistic target of rapamycin (mTOR), a 289-kDa serine/threonine protein kinase that is encoded by a single gene FRAP1 [10,41-43] (Figure 1). mTOR also is known as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 [8]. The target of rapamycin (TOR) was initially described in Saccharomyces cerevisiae with the genes TOR1 and TOR2 [8]. Using rapamycin-resistant TOR mutants, TOR1 and TOR2 were found to encode the Tor1 and Tor2 isoforms in yeast [44]. Rapamycin is a macrolide antibiotic in Streptomyces hygroscopicus that blocks TOR and mTOR activity [45]. Subsequently it was found that mTOR forms the principal component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [46-48]. Rapamycin can prevent mTORC1 activity by binding to immunophilin FK-506-binding protein 12 (FKBP12) that attaches to the FKBP12 -rapamycin-binding domain (FRB) at the carboxy (C) -terminal of mTOR to interfere with the FRB domain of mTORC1. mTORC1 appears to be more sensitive to inhibition by rapamycin than mTORC2, but chronic administration of rapamycin can inhibit mTORC2 activity as a result of the disruption of the assembly of mTORC2 [49,50].
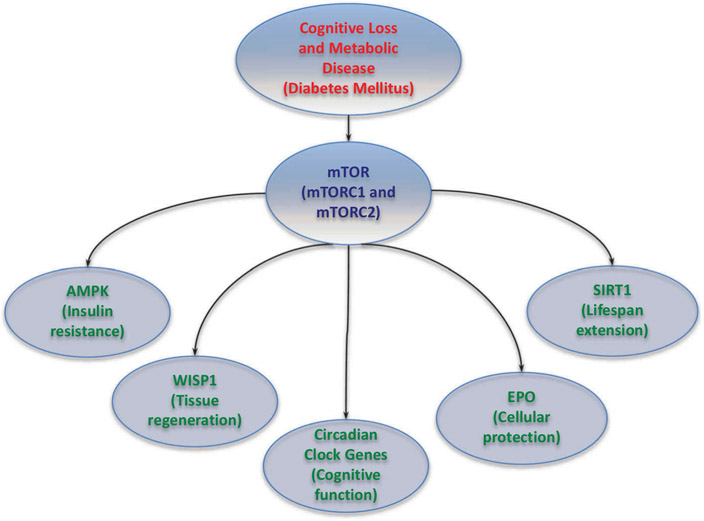
Figure 1: Innovative Strategies for Cognitive Loss.
With the advancing age of the global population and the increased prevalence of dementia, it is critical to develop innovative therapeutic strategies for cognitive loss. New work and the identification of novel pathways provide insight into the increased risk for the onset of cognitive loss associated with metabolic disorders. mTOR forms the complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) and is integrated with a number of pathways that include AMP activated protein kinase (AMPK) (affects insulin resistance), Wnt1 inducible signaling pathway protein 1 (WISP1) (associated with tissue regeneration), circadian clock genes (oversee cognitive function), erythropoietin (EPO) (controls cellular protection), and silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) (governs lifespan extension). These pathways with careful biological control hold great promise for the successful and safe treatment of cognitive loss that is associated with metabolic dysfunction such as diabetes mellitus.
3.1. mTORC1
mTORC1 and mTORC2 are divided into subcomponents (Figure 1). mTORC1 is composed of Raptor, the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8/G□L) [8]. mTORC1 can bind to its constituents through the protein Ras homologue enriched in brain (Rheb) that phosphorylates the Raptor residue serine863 and other residues that include serine859, serine855, serine877, serine696, and threonine706 [51]. The inability to phosphorylate serine863 limits mTORC1 activity, as shown using a site-direct mutation of serine863 [52]. mTOR can control Raptor activity and this activity can be blocked by rapamycin [52]. Deptor, an inhibitor as well, blocks mTORC1 activity by binding to the FAT (FKBP12 -rapamycin-associated protein (FRAP), ataxia-telangiectasia (ATM), and the transactivation/transformation domain-associated protein) domain of mTOR. If the activity of Deptor is diminished, protein kinase B (Akt), mTORC1, and mTORC2 activities are increased [53]. PRAS40 also blocks mTORC1 activity by preventing the association of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor [54,55]. mTORC1 becomes active once PRAS40 is phosphorylated by Akt. This releases PRAS40 from Raptor to sequester PRAS40 in the cell cytoplasm with the docking protein 14-3-3 [56-60]. mLST8, in contrast to Deptor and PRAS40, promotes mTOR kinase activity. This involves the binding of p70S6K and 4EBP1 to Raptor [61]. Interestingly, mLST8 also controls insulin signaling through the transcription factor FoxO3 [62,63], is necessary for Akt and protein kinase C-α (PKCα) phosphorylation, and is required for Rictor to associate with mTOR [62].
3.2. mTORC2
mTORC2 has both similarities and differences to mTORC1. mTORC2 is composed of Rictor, mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) [54]. mTORC2 controls cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling [64]. mTORC2 activates protein kinases that includes glucocorticoid induced protein kinase 1 (SGK1), a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases. Protor-1, a Rictor-binding subunit of mTORC2, activates SGK1 [65,66]. The kinase domain of mTOR phosphorylates mSIN1 and prevents lysosomal degradation of this protein. Rictor and mSIN1 also can phosphorylate Akt at serine473 and foster threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) to enhance cell survival.
3.3. AMP activated protein kinase (AMPK)
In regards to metabolic disease, the AMP activated protein kinase (AMPK) is closely tied to the mTOR pathway through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that inhibits mTORC1 [5,67] (Figure 1). Control of the TSC1/TSC2 complex also is overseen through Akt and its phosphorylation of TSC2. Extracellular signal-regulated kinases (ERKs), protein p90 ribosomal S6 kinase 1 (RSK1), and glycogen synthase kinase −3β (GSK-3β) also can modulate the activity TSC1/TSC2 complex. AMPK can inhibit mTORC1 activity through the activation of the TSC1/TSC2 complex [68,69]. TSC2 functions as a GTPase-activating protein (GAP) that converts G protein Rheb (Rheb-GTP) into the inactive GDP-bound form (Rheb-GDP). Once Rheb-GTP is active, Rheb-GTP associates with Raptor to oversee the binding of 4EBP1 to mTORC1 and increase mTORC1 activity [70]. AMPK phosphorylates TSC2 to increase GAP activity to change Rheb-GTP into the inactive Rheb-GDP and to block mTORC1 activity [71].
AMPK has been shown to reduce insulin resistance, since the loss of AMPK results in reduced tolerance to the development of insulin resistance [72]. AMPK also is involved in the protection of endothelial progenitor cells during periods of hypeglycemia [73]. During periods of dietary restriction that may increase lifespan, AMPK can be one factor to shift to oxidative metabolism [74]. AMPK can reduce ischemic brain damage in diabetic animal models [75]. In addition, during periods of hyperglycemia, AMPK activity may be necessary to increase basal autophagy activity [76,77] and maintain endothelial cell survival [78,79].
AMPK activation can improve memory retention in models of AD and DM [80], limit cardiac ischemia in animal models, and prevent adipocyte differentiation, lipid accumulation, and obesity [45]. Metformin, an agent that controls hyperglycemia in DM, also inhibits mTOR activity and leads to the induction of autophagy. Metformin can activate AMPK [81] and block mTOR activity through additional pathways independent of AMPK [82]. Metformin prevents cell loss during hypoxia through increased AMPK activity [83], provides neuroprotection [29], limits cardiomyopathy in experimental models of DM [84] and prevents endothelial cell senescence [85]. Yet, the necessary level of AMPK activity to offer cellular protection during metabolic activity is not completely understood. In some cases, limited AMPK activity may be better for cellular protection in DM. Reduced AMPK activity can promote the protection of pancreatic islet cells in mice [86], limit amyloid (Aβ) toxicity [87], and prevent inflammation in the nervous system [88].
3.4. mTOR, Wingless pathway, and Wnt1 inducible signaling pathway protein 1 (WISP1)
The wingless pathway of Wnt proteins represents cysteine-rich glycosylated proteins that control processes involving metabolism, neuronal development, angiogenesis, immunity, tumorigenesis, fibrosis, and stem cell proliferation [89-92]. In the nervous system, Wnt signaling may be instrumental in the pathogenesis of neurodegenerative disorders [6,93-95]. Wnt signaling and its family member Wnt1 can block autophagy [96-99] and apoptotic endothelial cell injury during elevated glucose exposure [100]. Wnt signaling also promotes human β-cell proliferation [101], fosters the repair of diabetic wounds [102], impacts the vasculature of the brain [103], and prevents cognitive decline during aging and during DM [104]. Components of the Wnt pathway also have increased expression in the brain during periods of exercise [105] that may assist against insulin resistance.
A downstream target in the Wnt1 pathway is the Wnt1 inducible signaling pathway protein 1 (WISP1) (Figure 1). The CCN family member WISP1 has a significant role in cellular metabolism [24,90-92] that is dependent upon mTOR signaling pathways [106,107]. The CCN family of proteins has six secreted extracellular matrix associated proteins. They are defined by the first three members of the family that include Cysteine-rich protein 61, Connective tissue growth factor, and Nephroblastoma over-expressed gene [108,109]. WISP1 is a matricellular protein and a downstream target of the wingless pathway Wnt1 that can oversee metabolism [89]. WISP1 expression is affected by weight change in humans and increases during insulin resistance in children and adolescents [92]. WISP1 production also is increased during gestational diabetes [91]. As a result, WISP1 may represent an important reparative process in individuals with DM [63]. WISP1 can modulate cellular senescence [110] to a degree that does not promote excessive cellular proliferation in aging vascular cells [111] that could lead to atherosclerosis during DM. WISP1 also is one of several genes that are over-expressed during pancreatic regeneration, indicating that WISP1 may assist with protection of tissues necessary for metabolic homeostasis [112].
WISP1 leads to mTOR activation to block PRAS40 [58] and TSC2 [87] to protect cells against oxidative stress. WISP1 controls the post-translational phosphorylation of AMPK for glucose homeostasis [8,48,113,114]. It is the ability of WISP1 to control AMPK activity that becomes vital to control cellular metabolism during DM [48]. WISP1 modulates AMPK activation by differentially decreasing phosphorylation of TSC2 at serine1387, a target of AMPK, and increasing phosphorylation of TSC2 at threonine1462, a target of Akt [87]. This enables WISP1 to provide a minimal level of TSC2 and AMPK activity to control in dual fashion both cell survival and cell metabolism. AMPK activity levels can become an important factor for cellular survival. Increased AMPK activity can reduce insulin resistance and oxidative stress mediated through the activation of autophagy [72]. AMPK activation can correct metabolic parameters of cells and prevent adipocyte differentiation, lipid accumulation, and obesity [115]. However, under some conditions, increased AMPK activity can be detrimental. As previously noted, reduced AMPK activity is necessary to promote the protection of pancreatic islet cells in mice [86], limit amyloid (Aβ) toxicity [87], and prevent nervous system inflammation [88].
3.5. mTOR, Metabolism, and Erythropoietin
mTOR activation can control cellular metabolism and insulin signaling. mTOR pathways that include p70S6K and 4EBP1 can improve insulin secretion in pancreatic β-cells and increase resistance to β-cell streptozotocin toxicity and obesity in mice [116]. Loss of p70S6K activity results in hypo-insulinemia, insulin insensitivity to glucose secretion, glucose intolerance, and decreased pancreatic β-cell size [117]. Rapamycin administration leads to reduced β-cell function and mass, insulin resistance, decreased insulin secretion, and the induction of DM [118]. Although inhibition of mTOR activity with rapamycin can limit food intake and prevent fat-diet induced obesity in mice [119] and sometimes offer protection [120], rapamycin can impair glucose uptake and increase mortality in models of Type 2 DM [121]. Rapamycin and inhibition of mTOR blocks insulin generated Akt activation and alters the translocation of glucose transporters to the plasma membrane in skeletal muscle [119]. Activation of mTOR can protect pancreatic β- cells against cholesterol-induced apoptosis [122] and glucolipotoxicity [123]. Recently, the protective role of mTOR in areas such as the Mediterranean diet has been tied to a reduction in Aβ toxicity in astrocytes through enhanced Akt activity by consumption of polyphenol of olives and olive oil that ultimately could prevent the onset or progression of AD [124].
Yet, there appears to be feedback systems “built-in” with mTOR and cellular metabolic regulation. mTOR can function in a negative feedback loop and potentially produce glucose intolerance by inhibiting the insulin receptor substrate 1 (IRS-1). In studies with high fat fed obese rats, mTOR leads to inhibitory phosphorylation of IRS-1, impaired Akt signaling, and insulin resistance [125]. Activation of mTOR signaling with p70S6K can phosphorylate IRS-1 in the renin-angiotensin-aldosterone system during consumption of high fat diets that results in high circulating angiotensin II (ANG II) and insulin resistance [126].
mTOR also can rely upon growth factors that have defined clinical utility, such as erythropoietin (EPO) (Figure 1), to provide cellular protection during DM. The EPO gene is located on chromosome 7 and is a single copy in a 5.4 kb region of the genomic DNA [127]. This gene encodes for a polypeptide chain protein that has initially 193 amino acids [128]. EPO is then processed with the removal of a carboxy-terminal arginine166 in the mature human and recombinant human EPO (rhEPO). A protein of 165 amino acids with a molecular weight of 30.4 kDa is subsequently generated [129-132]. EPO, an erythropoiesis-stimulating agent, is approved for the treatment of anemia during chronic kidney failure, human immunodeficiency virus, and chemotherapy. EPO is present in the brain, uterus, and liver [133-137], but the primary site for the production and secretion of EPO is the kidney peritubular interstitial cells. EPO expression is controlled by changes in oxygen tension and not by the concentration of red blood cells [19,133,138].
EPO has a number of neuroprotective functions [139-141], uses multiple novel pathways to affect biological systems [6,128,142,143], may limit cognitive decline and AD [144], and affects metabolic pathways [145,146]. EPO controls pathways of apoptosis and autophagy through mTOR that can affect neuronal regeneration [147]. EPO prevents apoptotic cell death during Aβ exposure through mTOR to prevent caspase activation [58]. In addition, EPO can promote microglial survival during oxidative stress through mTOR signaling [148]. EPO oversees mTOR, Akt [142,149,150], and down-stream signaling pathways that involve proline rich Akt substrate 40 kDa (PRAS40) to enhance neuronal survival during oxygen-glucose deprivation [56]. EPO also relies upon mTOR during hypoxia-reoxygenation stress to protect hippocampus-derived neuronal cells [151].
In relation to cellular metabolism [15,47], EPO can reduce blood glucose levels in animal models of DM and obesity [152], promote wound healing during DM [153], and protect endothelial cells during experimental models of DM [100,154]. EPO can block the detrimental effects of obesity in animal models [132], preserve cellular mitochondrial function [148,155-158] and energy metabolism [131], and limit oxidative stress and apoptosis in Schwann cells mediated by advanced glycation end products (AGEs) [159].
EPO also governs the AMPK pathway and autophagy [160]. In some cases, EPO can protect against neuronal injury through increased AMPK activity and enhanced autophagy activity [161]. EPO can control AMPK and mTOR activities to protect cells under conditions of oxidative stress [87]. EPO also relies upon AMPK pathways for anti-oxidant gene expression [162] and oversees inflammation in the nervous system through AMPK [47]. EPO blocks apoptotic cell injury through AMPK by increasing autophagy-related signaling pathways [161]. The oversight of inflammation in the nervous system by EPO is intimately connected to AMPK [163]. It is the concentration and activity of EPO that can influence the protective actions of mTOR and signaling pathways associated with AMPK. EPO modulates a specific level of AMPK and mTOR activity to alleviate detrimental effects of oxidative stress [56,162]. This fine control over mTOR is important since high concentrations of EPO can lead to cellular damage and actually diminish the activity of mTOR [164].
PI 3-K and Akt that enhance mTOR activity also are critical pathways that provide cellular protection through EPO. EPO can phosphorylate Akt at serine473 to activate Akt [128,142,165,166]. EPO signaling through Akt activation has been shown to protect against hypoxia-reoxygenation stress [151] and EPO may control intracellular calcium levels to preserve mitochondrial function [45,131,155,167,168]. EPO also can increase cell survival through Akt activation during Aβ toxicity [169-171] and oxidative stress [56,148,172-174].
4.0. Clock Genes and Cellular Metabolism
Circadian rhythm clock genes play a significant role with neurodegenerative disease and cognitive loss [175,176] (Figure 1), as well as other disorders such as cancer [2,177-179]. The master mammalian circadian clock is in the suprachiasmatic nucleus (SCN) located above the optic chiasm and receives light input from photosensitive ganglion cells in the retina. The SCN controls most overt circadian rhythms and depends upon the pineal gland, hypothalamic nuclei, and vasoactive intestinal peptide to oversee processes that involve the sleep wake cycle, release of hormones cortisol and melatonin, oxidative stress responses [180], and the regulation of body temperature [181]. In the clock gene family, members of the basic helix-loop-helix -PAS (Period-Arnt-Single-minded) transcription factor family, such as CLOCK and BMAL1 [182], control the expression of the genes Cryptochrome (Cry1 and Cry2) and Period (Per1, Per2, and Per3). Feedback is provided by PER:CRY heterodimers that translocate to the nucleus to block the transcription activated by CLOCK:BMAL1 complexes. Additional regulatory loops consist of retinoic acid-related orphan nuclear receptors REV-ERBα, also known as NR1D1 (nuclear receptor subfamily 1, group D, member 1), and RORα that are activated by CLOCK:BMAL1 heterodimers. The REV-ERBα and RORα receptors bind retinoic acid-related orphan receptor response elements (ROREs) present in the BMAL1 promoter to repress and activate rhythmic transcription of BMAL1 by RORs and REV-ERBs, respectively. REV-ERBs can repress transcription to result in circadian oscillation of BMAL1 [183,184].
The clock gene pathway is affected during dementia disorders. Rhythmic methylation of BMAL1 is changed in the brains of patients with AD, suggesting that alterations in the DNA methylation of clock genes may contribute to cognitive loss and behavior changes [185]. Animal models of Parkinson’s disease with 6-hydroxydopamine (6-OHDA), a disorder also associated with cognitive loss, show that decreased BMAL1 and RORα persisted with levodopa treatment, indicating that chronic or long-term levodopa treatment may impair circadian rhythm function [186]. Clock genes also impact lifespan during neurodegenerative disorders. In studies with Drosophila melanogaster, lifespan was reduced in three arrhythmic mutants involving ClkAR, cyc0 and tim0. ClkAR mutants had significant faster age-related locomotor deficits that were similar to Parkinson’s disease. Restoring Clk function rescued Drosophila from the locomotor deficits. An increase in oxidative stress was noted with the mutant phenotypes, but deficits appeared to correlate best with loss of dopaminergic neurons, similar to Parkinson’s disease, rather than directly to the presence of oxidative stress in this case [187].
Circadian rhythm dysfunction during cognitive loss and aging has been associated with autophagy induction [188]. In animal models of AD, a basal circadian rhythm that governs macroautophagy may be necessary to limit cognitive decline and Aβ deposition [189]. Interestingly, changes in the external environment can affect circadian rhythm that affects cognition function [176]. For example, chronic sleep fragmentation has been shown to affect autophagy proteins in the hippocampus [190] that can impair memory and cognition [5,191-194]. Autophagy in the hippocampus also is blocked during the absence of the PER1 circadian clock protein that may worsen the pathology of cerebral ischemia [195], suggesting that PER1 circadian clock protein is necessary for neuroprotection.
4.1. Circadian Rhythm and mTOR
The control of circadian pathways are closely tied to mTOR [196-198] (Figure 1). Melatonin, a pineal hormone that is involved in regulating circadian rhythm, depends upon autophagy pathways and mTOR to control processes of aging and neurodegeneration [199]. Loss of mTOR activation has been shown to alter circadian rhythm and cognitive decline during prolonged space flight and microgravity [200]. Furthermore, cerebral ischemic infarction may be influenced by an alteration in circadian rhythm genes and fluctuations in mTOR activity [195,201].
4.2. Circadian Rhythm and SIRT1
The role of mTOR may extend beyond its internal pathways to oversee circadian rhythm and involve the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) [7,202-204] (Figure 1). SIRT1 may be a significant component to regulate the activity of mTOR during metabolic regulation and cognition. Genes with the greatest statistical change following caloric restriction in mice have included those associated with sirtuin activation and mTOR inhibition [205]. SIRT1 can increase lifespan in higher organisms [77,206-209], can be associated with neuroprotection and cognition [206,210], and provides protection against oxidative stress [24,211-214]. SIRT1 inhibits mTOR pathways through AMPK. SIRT1 has an inverse relationship with mTOR in embryonic stem cells [15,47] and can block mTOR to promote autophagy. In relation to circadian rhythm, SIRT1 has been associated with circadian rhythm dysfunction that affects the development of cognitive disorders such as AD [175]. SIRT1 control of circadian rhythm and melatonin also may affect glucose tolerance and DM [181] as well as inflammation during obesity [215]. Increased SIRT1 activity with a disruption in circadian rhythm results in additional disorders such as increased susceptibility to mammary carcinogenesis [216]. Yet, SIRT1 may be beneficial under specific circumstances to regulate circadian rhythm gene expression that can foster hepatocellular proliferation and liver regeneration following liver resection [217]. More recent work also suggests an important role for SIRT1 targets with aging and circadian gene expression in the liver [218].
These studies suggest that specifically controlled activities of mTOR and SIRT1 are required to achieve optimal control over metabolic and cognitive function. A decrease in SIRT1 activity that would mirror an increase in mTOR activity is associated with neural differentiation and the maturation of embryonic cortical neurons [219]. Differentiation of human embryonic stem cells into motoneurons also occurs with decreased SIRT1 activity. In contrast, increased activity of SIRT1 through microRNA-34a can promote the apoptotic cell death of mesenchymal stem cells [220].
5.0. Expert Opinion
With the significant increase in neurodegenerative disorders throughout the globe and especially those disease entities that affect cognition, it is imperative that innovative strategies are developed to provide treatment for individuals that suffer from cognitive loss and dementia (Article Highlights). Dementia is now considered to be the 7th leading cause of death with staggering financial and service burdens. At present, only limited symptomatic treatments exist for these individuals. New studies are now highlighting the previously unrecognized association between metabolic disorders and dementia, including AD. DM alone is increasing in incidence throughout the world and almost 350 million individuals currently have DM. Early diagnosis of DM with use of available therapies for DM can offer some degree of improvement. However, tight serum glucose control does not always prevent complications from DM [14,37]. Reduced diet and body mass control treatments may be effective to prevent hyperglycemic events, but these treatments may decrease organ mass through processes that involve autophagy [38]. To a similar extent, most available treatments that are directed to treat AD alone involve the use of cholinesterase inhibitors [39] which have limited efficacy. Given these severe limitations for dementia during metabolic dysfunction, addressing novel pathways that can oversee metabolic disease and cognitive loss may offer critical therapies to overcome dementia. The pathways of mTOR, AMPK, WISP1, EPO, circadian clock genes, and SIRT1 offer exciting prospects to treat cognitive loss that has its basis in metabolic cellular dysfunction. Interestingly, identification of mTOR activity in the Mediterranean diet has been tied to a reduction in Aβ toxicity in astrocytes through enhanced Akt activity by consumption of polyphenol of olives and olive oil. This work suggests that such a diet through mTOR control of cellular metabolism could potentially prevent the onset or progression of AD [124]. However, this course has a number of challenges. These pathways ultimately require fine biological control to prevent cellular demise and unwanted clinical disability. For example, mTOR can function in a negative feedback loop and potentially produce glucose intolerance by inhibiting the insulin receptor substrate 1 (IRS-1) [125]. In addition, AMPK activation can improve memory retention in models of AD and DM [80], prevent lipid accumulation and obesity [45], and promote neuroprotection [29]. Yet, in some scenarios, reduced AMPK activity is required to promote the protection of pancreatic islet cells in mice [86], limit Aβ toxicity [87], and prevent inflammation in the nervous system [88]. Additional work suggests that specifically controlled activities of mTOR and SIRT1 are required to control metabolic and cognitive function. At times, increased activity of SIRT1 through microRNA-34a can be detrimental, such as promoting the apoptotic cell death of mesenchymal stem cells [220]. Furthermore, these pathways can be proliferative in nature, such as WISP1, and lead to tumorigenesis [109]. As a result, continued work is required to further unravel the complex functions of these pathways for the promotion of drug development success and limit negative clinical outcomes during cognitive loss and metabolic dysfunction.
Article Highlights.
Improvements in global healthcare and the progressive increase in life span have led to increased prevalence of neurodegenerative disorders and dementia, such as Alzheimer’s disease, in the world’s population
Recent studies highlight the previously unrecognized link between metabolic disorders, such as diabetes mellitus, and cognitive loss
The mechanistic target of rapamycin (mTOR), a principal component for mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2), and circadian clock genes are exciting novel targets to treat cognitive loss through metabolic pathways
mTOR and circadian clock genes are intimately linked to AMP activated protein kinase (AMPK), Wnt1 inducible signaling pathway protein 1 (WISP1), erythropoietin (EPO), and silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) to oversee cellular metabolic homeostasis and cognitive function
mTOR and circadian clock genes in association with their downstream pathways offer fruitful prospects to preserve neuronal and vascular function through apoptotic and autophagic pathways, but are complex in nature requiring fine biological control to further the development of effective treatment strategies and limit the potential for negative clinical outcomes
Acknowledgments
Funding
This paper was funded by U.S. Department of Health and Human Services, National Institutes of Health (NIH) grant NS053946.
Footnotes
Declaration of Interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Organization WH. Global action plan on the public health response to dementia 2017-2025. 2017:1–44. [Google Scholar]
- 2.Maiese K Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural regeneration research. 2019. May;14(5):773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su M, Naderi K, Samson N, et al. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol. 2019. January 25. [DOI] [PubMed] [Google Scholar]
- 4.Filley CM, Rollins YD, Anderson CA, et al. The genetics of very early onset Alzheimer disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007. September;20(3):149–56. [DOI] [PubMed] [Google Scholar]
- 5.Maiese K Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014. August 8;46(8):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005. February;75(3):207–46. [DOI] [PubMed] [Google Scholar]
- 7.Maiese K SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015. March 26;7(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese K, Chong ZZ, Shang YC, et al. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013. January;19(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh CF, Liu CK, Lee CT, et al. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Scientific reports. 2019. January 29;9(1):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiese K Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural regeneration research. 2016. March;11(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiese K Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr Neurovasc Res. 2018. November 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong WY, Wu YJ, Farooqui T, et al. Qi Fu Yin-a Ming Dynasty Prescription for the Treatment of Dementia. Mol Neurobiol. 2018. February 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G, Aroor AR, Martinez-Lemus LA, et al. Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol. 2014. September 24;307(10):R1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiese K, Chong ZZ, Shang YC, et al. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011. March 10;51(2):128–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiese K, Chong ZZ, Shang YC, et al. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2013. January;8(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutter MK, Massaro JM, Hoffmann U, et al. Fasting Glucose, Obesity, and Coronary Artery Calcification in Community-Based People Without Diabetes. Diabetes Care. 2012. July 6;35(9):1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu E, Schwab M, Marette A. Role of protein tyrosine phosphatases in the modulation of insulin signaling and their implication in the pathogenesis of obesity-linked insulin resistance. Rev Endocr Metab Disord. 2014. March;15(1):79–97. [DOI] [PubMed] [Google Scholar]
- 18.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000. Jul-Aug;16(4):230–6. [DOI] [PubMed] [Google Scholar]
- 19.Maiese K Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural regeneration research. 2015;10(4):518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007;14(16):1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2012-2022. wwwcmsgov. 2013.
- 22.Barchetta I, Cimini FA, Ciccarelli G, et al. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. Journal of endocrinological investigation. 2019. May 9. [DOI] [PubMed] [Google Scholar]
- 23.Gkogkolou P, Sarna M, Sarna T, et al. Protection of glucotoxicity by a tripeptide derivative of alpha-melanocyte-stimulating hormone in human epidermal keratinocytes. The British journal of dermatology. 2018. September 1. [DOI] [PubMed] [Google Scholar]
- 24.Maiese K New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015(2015:875961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong J, Lai Y, Yao YA, et al. Qiliqiangxin Rescues Mouse Cardiac Function by Regulating AGTR1/TRPV1-Mediated Autophagy in STZ-Induced Diabetes Mellitus. Cell Physiol Biochem. 2018. June 19;47(4):1365–1376. [DOI] [PubMed] [Google Scholar]
- 26.Hadamitzky M, Herring A, Kirchhof J, et al. Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. The international journal of neuropsychopharmacology. 2018. February 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignacio ZM, Reus GZ, Arent CO, et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2016. November 27;82(5):1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res. 2012. February;129(2):116–26. [DOI] [PubMed] [Google Scholar]
- 29.Jiang T, Yu JT, Zhu XC, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014. July;171(13):3146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese K, Chong ZZ, Hou J, et al. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008. May;5(2):125–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao FH, He YH, Li QG, et al. A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS One. 2015;10(3):e0120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu YJ, Tappia PS, Neki NS, et al. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart failure reviews. 2014. January;19(1):113–21. [DOI] [PubMed] [Google Scholar]
- 33.Maiese K, Chong ZZ, Shang YC, et al. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009. February;116(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang PC, Ng YF, Ho S, et al. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res. 2014. December;90:88–115. [DOI] [PubMed] [Google Scholar]
- 35.Xiang L, Mittwede PN, Clemmer JS. Glucose Homeostasis and Cardiovascular Alterations in Diabetes. Comprehensive Physiology. 2015. September 20;5(4):1815–39. [DOI] [PubMed] [Google Scholar]
- 36.Yao T, Fujimura T, Murayama K, et al. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic beta-Cells. Cells. 2017. October 18;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coca SG, Ismail-Beigi F, Haq N, et al. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012. May 28;172(10):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Lee JH, Jin M, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruhal P, Dhingra D. Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology. 2018. April 4;26(5):1317–1329. [DOI] [PubMed] [Google Scholar]
- 40.Maiese K The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans. 2018. March 9;46(2):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong ZZ, Maiese K. Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol. 2012. April 30;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esterline RL, Vaag A, Oscarsson J, et al. MECHANISMS IN ENDOCRINOLOGY: SGLT2 inhibitors; clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. 2018. January 25. [DOI] [PubMed] [Google Scholar]
- 43.Jesko H, Stepien A, Lukiw WJ, et al. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol Neurobiol. 2018. August 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991. August 23;253(5022):905–9. [DOI] [PubMed] [Google Scholar]
- 45.Maiese K mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015. March 15;6(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep. 2011. August;44(8):506–11. [DOI] [PubMed] [Google Scholar]
- 47.Maiese K Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res. 2016;13(4):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez de Morentin PB, Martinez-Sanchez N, Roa J, et al. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014. January;14(1):3–21. [DOI] [PubMed] [Google Scholar]
- 49.Maiese K Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies. Elsevier and Academic Press; 2016;ISBN 9780128027332. [Google Scholar]
- 50.Maiese K Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016. November;82(5):1245–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foster KG, Acosta-Jaquez HA, Romeo Y, et al. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010. January 1;285(1):80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Lawrence JC Jr., Sturgill TW, et al. Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem. 2009. May 29;284(22):14693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009. May 29;137(5):873–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiese K Driving neural regeneration through the mammalian target of rapamycin. Neural regeneration research. 2014. August 1;9(15):1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malla R, Ashby CR Jr., Narayanan NK, et al. Proline-rich AKT substrate of 40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys Res Commun. 2015. July 31;463(3):161–6. [DOI] [PubMed] [Google Scholar]
- 56.Chong ZZ, Shang YC, Wang S, et al. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fonseca BD, Smith EM, Lee VH, et al. PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem. 2007. August 24;282(34):24514–24. [DOI] [PubMed] [Google Scholar]
- 58.Shang YC, Chong ZZ, Wang S, et al. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012. August 6;9(4):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Zhang Q, Wen Q, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012. January;24(1):17–24. [DOI] [PubMed] [Google Scholar]
- 60.Xiong X, Xie R, Zhang H, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis. 2014. February 27;66:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003. April;11(4):895–904. [DOI] [PubMed] [Google Scholar]
- 62.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006. December;11(6):859–71. [DOI] [PubMed] [Google Scholar]
- 63.Maiese K FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res. 2015. August 7;12(4):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004. November;6(11):1122–8. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J. 2008. December 15;416(3):375–85. [DOI] [PubMed] [Google Scholar]
- 66.Pearce LR, Sommer EM, Sakamoto K, et al. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011. May 15;436(1):169–79. [DOI] [PubMed] [Google Scholar]
- 67.Mi R, Ma J, Zhang D, et al. Efficacy of combined inhibition of mTOR and ERK/MAPK pathways in treating a tuberous sclerosis complex cell model. Journal of genetics and genomics = Yi chuan xue bao. 2009. June;36(6):355–61. [DOI] [PubMed] [Google Scholar]
- 68.Maiese K Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res. 2017;14(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peixoto CA, de Oliveira WH, da Rocha Araujo SM, et al. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol. 2017. August 24. [DOI] [PubMed] [Google Scholar]
- 70.Sato T, Nakashima A, Guo L, et al. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009. May 8;284(19):12783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003. November 26;115(5):577–90. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Palanivel R, Rai E, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014. July 28;64(1):36–48. [DOI] [PubMed] [Google Scholar]
- 73.Chiu SC, Chao CY, Chiang EI, et al. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. The Journal of nutritional biochemistry. 2017. April;42:172–181. [DOI] [PubMed] [Google Scholar]
- 74.Moroz N, Carmona JJ, Anderson E, et al. Dietary restriction involves NAD -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014. September 25;13(6):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu P, Yang X, Hei C, et al. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci. 2016;12(8):1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016. January 2;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maiese K FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst). 2015;2015:569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal PB, Sonowal H, Shukla K, et al. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019. April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weikel KA, Cacicedo JM, Ruderman NB, et al. Knockdown of GSK3beta Increases Basal Autophagy and AMPK Signaling in Nutrient-laden Human Aortic Endothelial Cells. Bioscience reports. 2016. August 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du LL, Chai DM, Zhao LN, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2015. August 11;43(3):775–84. [DOI] [PubMed] [Google Scholar]
- 81.Leclerc GM, Leclerc GJ, Kuznetsov JN, et al. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS One. 2013;8(8):e74420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010. May 5;11(5):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheng B, Liu J, Li GH. Metformin preconditioning protects Daphnia pulex from lethal hypoxic insult involving AMPK, HIF and mTOR signaling. Comp Biochem Physiol B Biochem Mol Biol. 2012. May 4;163(1):51–58. [DOI] [PubMed] [Google Scholar]
- 84.Xie Z, Lau K, Eby B, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011. June;60(6):1770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arunachalam G, Samuel SM, Marei I, et al. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014. January;171(2):523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guan FY, Gu J, Li W, et al. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 beta-cells. Life Sci. 2014. June 27;107(1-2):42–9. [DOI] [PubMed] [Google Scholar]
- 87.Shang YC, Chong ZZ, Wang S, et al. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013. February;10(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Russo E, Andreozzi F, Iuliano R, et al. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun. 2014. July 3;42:157–168. [DOI] [PubMed] [Google Scholar]
- 89.Maiese K, Li F, Chong ZZ, et al. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008. April;118(1):58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murahovschi V, Pivovarova O, Ilkavets I, et al. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015. March;64(3):856–66. [DOI] [PubMed] [Google Scholar]
- 91.Sahin Ersoy G, Altun Ensari T, Subas S, et al. WISP1 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2016. June 8:1–5. [DOI] [PubMed] [Google Scholar]
- 92.Wang AR, Yan XQ, Zhang C, et al. Characterization of Wnt1-inducible Signaling Pathway Protein-1 in Obese Children and Adolescents. Current medical science. 2018. October;38(5):868–874. [DOI] [PubMed] [Google Scholar]
- 93.Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med. 2013. March;19(3):144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L, Cen L, Qu S, et al. Enhancing Beta-Catenin Activity via GSK3beta Inhibition Protects PC12 Cells against Rotenone Toxicity through Nurr1 Induction. PLoS One. 2016;11(4):e0152931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng H, Jia L, Liu CC, et al. TREM2 promotes microglial survival by activating Wnt/beta-catenin pathway. J Neurosci. 2017. January 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y, Chang H, Peng X, et al. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One. 2014;9(7):e102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geng Y, Ju Y, Ren F, et al. Insulin receptor substrate 1/2 (IRS1/2) regulates Wnt/beta-catenin signaling through blocking autophagic degradation of dishevelled2. J Biol Chem. 2014. April 18;289(16):11230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ortiz-Masia D, Cosin-Roger J, Calatayud S, et al. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: relevance in IBD. Mucosal immunology. 2014. July;7(4):929–38. [DOI] [PubMed] [Google Scholar]
- 99.Wang S, Chong ZZ, Shang YC, et al. WISP1 (CCN4) autoregulates its expression and nuclear trafficking of beta-catenin during oxidant stress with limited effects upon neuronal autophagy. Curr Neurovasc Res. 2012. April 4;9(2):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007. August;4(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aly H, Rohatgi N, Marshall CA, et al. A novel strategy to increase the proliferative potential of adult human beta-cells while maintaining their differentiated phenotype. PLoS One. 2013;8(6):e66131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun TJ, Tao R, Han YQ, et al. Therapeutic potential of umbilical cord mesenchymal stem cells with Wnt/beta-catenin signaling pathway pre-activated for the treatment of diabetic wounds. European review for medical and pharmacological sciences. 2014;18(17):2460–4. [PubMed] [Google Scholar]
- 103.Guo S, Zhou Y, Xing C, et al. The vasculome of the mouse brain. PLoS ONE. 2012;7(12):e52665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bayod S, Felice P, Andres P, et al. Downregulation of canonical Wnt signaling in hippocampus of SAMP8 mice. Neurobiol Aging. 2014. September 28. [DOI] [PubMed] [Google Scholar]
- 105.Bayod S, Menella I, Sanchez-Roige S, et al. Wnt pathway regulation by long-term moderate exercise in rat hippocampus. Brain Res. 2014. January 16;1543:38–48. [DOI] [PubMed] [Google Scholar]
- 106.Chong ZZ, Shang YC, Wang S, et al. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012. August 15;99(2):128–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maiese K Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res. 2014. April 7;11(2):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Human genomics. 2015;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maiese K WISP1: Clinical Insights for a Proliferative and Restorative Member of the CCN Family. Curr Neurovasc Res. 2014. September 12;11(4):378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Du J, Klein JD, Hassounah F, et al. Aging increases CCN1 expression leading to muscle senescence. Am J Physiol Cell Physiol. 2014. January 1;306(1):C28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Marchand A, Atassi F, Gaaya A, et al. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell. 2011. April;10(2):220–32. [DOI] [PubMed] [Google Scholar]
- 112.Lim HW, Lee JE, Shin SJ, et al. Identification of differentially expressed mRNA during pancreas regeneration of rat by mRNA differential display. Biochem Biophys Res Commun. 2002. December 20;299(5):806–12. [DOI] [PubMed] [Google Scholar]
- 113.Chong ZZ, Shang YC, Zhang L, et al. Mammalian target of rapamycin: hitting the bull’s-eye for neurological disorders. Oxid Med Cell Longev. 2010. Nov-Dec;3(6):374–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kopp C, Hosseini A, Singh SP, et al. Nicotinic Acid Increases Adiponectin Secretion from Differentiated Bovine Preadipocytes through G-Protein Coupled Receptor Signaling. International journal of molecular sciences. 2014;15(11):21401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lai CS, Tsai ML, Badmaev V, et al. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARgamma and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. Journal of agricultural and food chemistry. 2012. February 1;60(4):1094–101. [DOI] [PubMed] [Google Scholar]
- 116.Hamada S, Hara K, Hamada T, et al. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes. 2009. June;58(6):1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pende M, Kozma SC, Jaquet M, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000. December 21-28;408(6815):994–7. [DOI] [PubMed] [Google Scholar]
- 118.Fraenkel M, Ketzinel-Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008. April;57(4):945–57. [DOI] [PubMed] [Google Scholar]
- 119.Deblon N, Bourgoin L, Veyrat-Durebex C, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012. April;165(7):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fletcher L, Evans TM, Watts LT, et al. Rapamycin treatment improves neuron viability in an in vitro model of stroke. PLoS One. 2013;8(7):e68281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sataranatarajan K, Ikeno Y, Bokov A, et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J Gerontol A Biol Sci Med Sci. 2015. October 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou J, Wu J, Zheng F, et al. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells −1mTORbetaTC-6. Journal of diabetes. 2015. March;7(2):231–9. [DOI] [PubMed] [Google Scholar]
- 123.Miao XY, Gu ZY, Liu P, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013. January;39:71–9. [DOI] [PubMed] [Google Scholar]
- 124.Crespo MC, Tome-Carneiro J, Pintado C, et al. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors (Oxford, England). 2017. March 20. [DOI] [PubMed] [Google Scholar]
- 125.Khamzina L, Veilleux A, Bergeron S, et al. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005. March;146(3):1473–81. [DOI] [PubMed] [Google Scholar]
- 126.Kim JA, Jang HJ, Martinez-Lemus LA, et al. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012. January;302(2):E201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maiese K Regeneration in the nervous system with erythropoietin. Frontiers in bioscience (Landmark edition). 2016;21:561–96. [DOI] [PubMed] [Google Scholar]
- 128.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005. January 5;293(1):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castaneda-Arellano R, Beas-Zarate C, Feria-Velasco AI, et al. From neurogenesis to neuroprotection in the epilepsy: signalling by erythropoietin. Frontiers in bioscience (Landmark edition). 2014;19:1445–55. [DOI] [PubMed] [Google Scholar]
- 130.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008. April;19(2):145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci. 2014;10(8):921–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Wang L, Dey S, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. International journal of molecular sciences. 2014;15(6):10296–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012. January;31(1):89–119. [DOI] [PubMed] [Google Scholar]
- 134.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002. December;11(6):863–71. [DOI] [PubMed] [Google Scholar]
- 135.Kato S, Aoyama M, Kakita H, et al. Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res. 2011. October;89(10):1566–74. [DOI] [PubMed] [Google Scholar]
- 136.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004. 11;25(11):577–583. [DOI] [PubMed] [Google Scholar]
- 137.Moore EM, Bellomo R, Nichol AD. Erythropoietin as a novel brain and kidney protective agent. Anaesth Intensive Care. 2011. May;39(3):356–72. [DOI] [PubMed] [Google Scholar]
- 138.Maiese K Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008. Apr-May;62(4):218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rey F, Balsari A, Giallongo T, et al. Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases. ASN Neuro. 2019. Jan-Dec;11:1759091419871420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simon F, Floros N, Ibing W, et al. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural regeneration research. 2019. August;14(8):1309–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xiong T, Yang X, Qu Y, et al. Erythropoietin induces synaptogenesis and neurite repair after hypoxia ischemia-mediated brain injury in neonatal rats. Neuroreport. 2019. August 7;30(11):783–789. [DOI] [PubMed] [Google Scholar]
- 142.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002. December 3;106(23):2973–9. [DOI] [PubMed] [Google Scholar]
- 143.Maiese K Erythropoietin and diabetes mellitus. World J Diabetes. 2015. October 25;6(14):1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun J, Martin JM, Vanderpoel V, et al. The Promises and Challenges of Erythropoietin for Treatment of Alzheimer’s Disease. Neuromolecular Med. 2019. January 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Entezari M, Flavarjani ZK, Ramezani A, et al. Combination of intravitreal bevacizumab and erythropoietin versus intravitreal bevacizumab alone for refractory diabetic macular edema: a randomized double-blind clinical trial. Graefes Arch Clin Exp Ophthalmol. 2019. August 10. [DOI] [PubMed] [Google Scholar]
- 146.Montesano A, Bonfigli AR, De Luca M, et al. Erythropoietin (EPO) haplotype associated with all-cause mortality in a cohort of Italian patients with Type-2 Diabetes. Scientific reports. 2019. July 17;9(1):10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barros Ribeiro da Silva V, Porcionatto M, Toledo Ribas V. The Rise of Molecules Able to Regenerate the Central Nervous System. J Med Chem. 2019. September 13. [DOI] [PubMed] [Google Scholar]
- 148.Shang YC, Chong ZZ, Wang S, et al. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011. October 19;8(4):270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chong ZZ, Lin SH, Kang JQ, et al. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003. March 1;71(5):659–69. [DOI] [PubMed] [Google Scholar]
- 150.Kwon MS, Kim MH, Kim SH, et al. Erythropoietin exerts cell protective effect by activating PI3K/Akt and MAPK pathways in C6 Cells. Neurol Res. 2014. March;36(3):215–23. [DOI] [PubMed] [Google Scholar]
- 151.Ryou MG, Choudhury GR, Li W, et al. Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress. Neuroscience. 2015. August 20;301:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Katz O, Stuible M, Golishevski N, et al. Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J Endocrinol. 2010. April;205(1):87–95. [DOI] [PubMed] [Google Scholar]
- 153.Hamed S, Bennett CL, Demiot C, et al. Erythropoietin, a novel repurposed drug: an innovative treatment for wound healing in patients with diabetes mellitus. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014. Jan-Feb;22(1):23–33. [DOI] [PubMed] [Google Scholar]
- 154.Chong ZZ, Hou J, Shang YC, et al. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3beta, and beta-Catenin to Foster Vascular Integrity During Experimental Diabetes. Curr Neurovasc Res. 2011. May 1;8(2):103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003. March;23(3):320–30. [DOI] [PubMed] [Google Scholar]
- 156.Costa DC, Alva N, Trigueros L, et al. Intermittent hypobaric hypoxia induces neuroprotection in kainate-induced oxidative stress in rats. J Mol Neurosci. 2013. July;50(3):402–10. [DOI] [PubMed] [Google Scholar]
- 157.Hou J, Wang S, Shang YC, et al. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res. 2011. August 1;8(3):220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parvin A, Pranap R, Shalini U, et al. Erythropoietin protects cardiomyocytes from cell death during hypoxia/reperfusion injury through activation of survival signaling pathways. PLoS One. 2014;9(9):e107453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu T, Li L, Chen T, et al. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of schwann cells in vitro. Neurochem Res. 2015. April;40(4):698–712. [DOI] [PubMed] [Google Scholar]
- 160.Maiese K Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr Neurovasc Res. 2017;14(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jang W, Kim HJ, Li H, et al. The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy. Mol Neurobiol. 2015. July 9;53(6):3812–3821. [DOI] [PubMed] [Google Scholar]
- 162.Wang L, Di L, Noguchi CT. AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes. Int J Biochem Cell Biol. 2014. September;54:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsai CF, Kuo YH, Yeh WL, et al. Regulatory Effects of Caffeic Acid Phenethyl Ester on Neuroinflammation in Microglial Cells. International journal of molecular sciences. 2015;16(3):5572–5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andreucci M, Fuiano G, Presta P, et al. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009. August;42(4):554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee HJ, Koh SH, Song KM, et al. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology. 2016. April 13;110(2):93–100. [DOI] [PubMed] [Google Scholar]
- 166.Ma C, Cheng F, Wang X, et al. Erythropoietin Pathway: A Potential Target for the Treatment of Depression. International journal of molecular sciences. 2016;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003. March;138(6):1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Millet A, Bouzat P, Trouve-Buisson T, et al. Erythropoietin and Its Derivates Modulate Mitochondrial Dysfunction after Diffuse Traumatic Brain Injury. J Neurotrauma. 2016. March 15. [DOI] [PubMed] [Google Scholar]
- 169.Esmaeili Tazangi P, Moosavi SM, Shabani M, et al. Erythropoietin improves synaptic plasticity and memory deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacol Biochem Behav. 2015. March;130:15–21. [DOI] [PubMed] [Google Scholar]
- 170.Maurice T, Mustafa MH, Desrumaux C, et al. Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Abeta(2)(5)(−)(3)(5) non-transgenic mouse model of Alzheimer’s disease. J Psychopharmacol. 2013. November;27(11):1044–57. [DOI] [PubMed] [Google Scholar]
- 171.Shang YC, Chong ZZ, Wang S, et al. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012. March;4(3):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007. April;150(7):839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shen J, Wu Y, Xu JY, et al. ERK- and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest Ophthalmol Vis Sci. 2010. January;51(1):35–46. [DOI] [PubMed] [Google Scholar]
- 174.Wu Y, Shang Y, Sun S, et al. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur J Pharmacol. 2007. June 14;564(1-3):47–56. [DOI] [PubMed] [Google Scholar]
- 175.Bellanti F, Iannelli G, Blonda M, et al. Alterations of Clock Gene RNA Expression in Brain Regions of a Triple Transgenic Model of Alzheimer’s Disease. J Alzheimers Dis. 2017. June 24;59(2):615–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maiese K Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res. 2017;14(3):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Angelousi A, Kassi E, Nasiri-Ansari N, et al. Clock genes and cancer development in particular in endocrine tissues. Endocrine-related cancer. 2019. April 1. [DOI] [PubMed] [Google Scholar]
- 178.Maiese K Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr Neurovasc Res. 2018. March 19;15(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rossetti ML, Esser KA, Lee C, et al. Disruptions to the Limb Muscle Core Molecular Clock Coincide with Changes in Mitochondrial Quality Control following Androgen Depletion. Am J Physiol Endocrinol Metab. 2019. July 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Patel SA, Velingkaar NS, Kondratov RV. Transcriptional Control of Antioxidant Defense by the Circadian Clock. Antioxid Redox Signal. 2014. January 3;20(18):2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hardeland R Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J Pineal Res. 2016. October 20. [DOI] [PubMed] [Google Scholar]
- 182.Lin F, Chen Y, Li X, et al. Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct. 2013. March;31(2):166–72. [DOI] [PubMed] [Google Scholar]
- 183.Bunney BG, Li JZ, Walsh DM, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015. February;20(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hood S, Amir S. Neurodegeneration and the Circadian Clock. Frontiers in aging neuroscience. 2017;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cronin P, McCarthy MJ, Lim ASP, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2017. June;13(6):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li SY, Wang YL, Liu WW, et al. Long-term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-hydroxydopamine Rat Model of Parkinson’s Disease. Chin Med J (Engl). 2017. May 05;130(9):1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vaccaro A, Issa AR, Seugnet L, et al. Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function. PLoS Genet. 2017. January;13(1):e1006507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bondy S, Maiese K. Aging and Age-Related Disorders. Springer Science. 2010;ISBN: 978-1-60761-601-6. [Google Scholar]
- 189.Chen X, Kondo K, Motoki K, et al. Fasting activates macroautophagy in neurons of Alzheimer’s disease mouse model but is insufficient to degrade amyloid-beta. Scientific reports. 2015. July 14;5:12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.He Y, Cornelissen-Guillaume GG, He J, et al. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiology international. 2016;33(5):553–60. [DOI] [PubMed] [Google Scholar]
- 191.Dong W, Wang R, Ma LN, et al. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res. 2016. April;28(2):303–11. [DOI] [PubMed] [Google Scholar]
- 192.Maiese K Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci. 2016;2(4):241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Min JJ, Huo XL, Xiang LY, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Scientific reports. 2014;4:5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang ZH, Wu QY, Zheng R, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci. 2017. January 30;37(9):2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rami A, Rawashdeh O. The hippocampal autophagic machinery is depressed in the absence of the circadian clock protein PER1 that may lead to vulnerability during cerebral ischemia. Curr Neurovasc Res. 2017. June 18;14(3):207–214. [DOI] [PubMed] [Google Scholar]
- 196.Johnson SC, Sangesland M, Kaeberlein M, et al. Modulating mTOR in aging and health. Interdisciplinary topics in gerontology. 2015;40:107–27. [DOI] [PubMed] [Google Scholar]
- 197.Maiese K Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells. 2015. August 26;7(7):999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ramanathan C, Kathale ND, Liu D, et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018. May;14(5):e1007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jenwitheesuk A, Nopparat C, Mukda S, et al. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. International journal of molecular sciences. 2014. September 22;15(9):16848–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu X, Li D, Liu J, et al. Dammarane Sapogenins Ameliorates Neurocognitive Functional Impairment Induced by Simulated Long-Duration Spaceflight. Frontiers in pharmacology. 2017;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Beker MC, Caglayan B, Yalcin E, et al. Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol. 2017. April 18;Apr 18. [DOI] [PubMed] [Google Scholar]
- 202.Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008. July 25;134(2):317–28. [DOI] [PubMed] [Google Scholar]
- 203.Favero G, Franceschetti L, Rodella LF, et al. Sirtuins, aging, and cardiovascular risks. Age (Dordr). 2015. August;37(4):9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nakahata Y, Sahar S, Astarita G, et al. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009. May 1;324(5927):654–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Estep PW 3rd, Warner JB, Bulyk ML. Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS ONE. 2009;4(4):e5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Balan V, Miller GS, Kaplun L, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008. October 10;283(41):27810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.D’Onofrio N, Vitiello M, Casale R, et al. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim Biophys Acta. 2015. July;1852(7):1311–1322. [DOI] [PubMed] [Google Scholar]
- 208.Ma L, Dong W, Wang R, et al. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015. July;116:67–72. [DOI] [PubMed] [Google Scholar]
- 209.Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015. November;1852(11):2442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Khan M, Ullah R, Rehman SU, et al. 17beta-Estradiol Modulates SIRT1 and Halts Oxidative Stress-Mediated Cognitive Impairment in a Male Aging Mouse Model. Cells. 2019. August 19;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hung CH, Chan SH, Chu PM, et al. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Molecular nutrition & food research. 2015. July 23. [DOI] [PubMed] [Google Scholar]
- 212.Maiese K MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J Transl Sci. 2015. November;1(3):55–57. [PMC free article] [PubMed] [Google Scholar]
- 213.Yu L, Liang H, Dong X, et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res. 2015. October;59(3):376–90. [DOI] [PubMed] [Google Scholar]
- 214.Zhang F, Hu Y, Xu X, et al. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res. 2015. March;194(1):127–38. [DOI] [PubMed] [Google Scholar]
- 215.Liu Z, Gan L, Zhang T, et al. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res. 2017. November 17. [DOI] [PubMed] [Google Scholar]
- 216.Fang M, Ohman Strickland PA, Kang HG, et al. Uncoupling genotoxic stress responses from circadian control increases susceptibility to mammary carcinogenesis. Oncotarget. 2017. May 16;8(20):32752–32768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bellet MM, Masri S, Astarita G, et al. Histone Deacetylase SIRT1 Controls Proliferation, Circadian Rhythm, and Lipid Metabolism during Liver Regeneration in Mice. J Biol Chem. 2016. October 28;291(44):23318–23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sato S, Solanas G, Peixoto FO, et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell. 2017. August 10;170(4):664–677 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu DJ, Hammer D, Komlos D, et al. SIRT1 knockdown promotes neural differentiation and attenuates the heat shock response. J Cell Physiol. 2014. September;229(9):1224–35. [DOI] [PubMed] [Google Scholar]
- 220.Zhang F, Cui J, Liu X, et al. Roles of microRNA-34a targeting SIRT1 in mesenchymal stem cells. Stem cell research & therapy. 2015;6(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]