Abstract
Thymic epithelial cells (TECs) play multiple essential roles in T-cell development and the establishment of immune tolerance, but their isolation can be challenging, and their low viability upon isolation complicates downstream experiments. A method that allows TECs to be isolated easily and to survive afterward will be useful for elucidating key questions in TEC biology. Here, we demonstrate a simple method to isolate highly viable TECs. Primary TECs isolated using papain together with collagenase IV and DNase I survive and proliferate in vitro. Moreover, these primary TECs functionally engraft after intrathymic transplantation into recipient mice. Thus, the methods described herein will be useful for elucidating the roles of TECs and TEC subsets in T-cell development and immune tolerance.
Keywords: Thymic epithelial cells, Intrathymic transplantation, Papain, Thymus
1. Introduction
TECs, the major stromal component of thymus, are essential mediators of T-cell development [1]. The interaction between TECs and developing T-cells ensures the development of self-tolerant, functional T cells [2]. The thymus reaches its maximal size early in life, and then begins a poorly understood process of involution [3, 4]. Thymic involution is accompanied by decreased T-cell production, which contributes to immune dysfunction in aged individuals [5]. Loss of TECs likely contributes to age-associated thymic involution, though the mechanisms underlying TEC loss are not well understood [6]. Furthermore, there are multiple subsets of TECs, both in the cortex and in the medulla of the thymus. The functions of some of the TEC subsets have been characterized using genetic methods, but functional characterization of isolated subsets of TECs has been difficult to achieve, partly because of challenges associated with isolation of highly viable TEC subsets. Improvements in the isolation and manipulation of TEC subsets have the potential to prospectively identify TEC subsets that play specific roles in T-cell development and immune tolerance.
TECs, unlike epithelial cells from other tissues such as the skin and intestine, form a three-dimensional mesh-like network which provides freedom of movement for highly migratory developing thymocytes, while still enabling extensive thymocyte-TEC contact [7]. This complex 3-D network of TECs occupies the entire thymus; however, TECs still comprise less than 1% of total thymic cells. The predominant cells within the thymus are developing T cells, but the thymus also contains multiple other hematopoietic lineages, most notably dendritic cells and B cells. The thymus also contains rare endothelial cells and mesenchymal cells. TECs form junctions with other TECs and are also supported by extracellular matrix (ECM) components, collagen type I, collagen type IV, fibronectin, and laminin [8, 9]. Thus, in contrast to developing T cells, which can be isolated from the thymus by simply dispersing the thymus (for example, by grinding between frosted slides), isolation of TECs requires an enzymatic digestion to break down TEC-TEC and TEC-ECM adhesions.
Many methods for TEC isolation have been described in the literature, however improved methods are needed for isolating highly viable TECs so that their functions can be assessed in vitro and in vivo. In this chapter, we demonstrate a simple, rapid TEC isolation method, which includes papain (a cysteine proteinase of the peptidase C1 family), collagenase IV and DNase I. Papain has been used to isolate various cell types including neurons, vascular smooth muscle cells, and ruminal epithelial cells [10–13], and we find papain facilitates the isolation of mouse TECs as well. This papain-based method is not only simple and fast, but also generates highly viable TECs; in vitro culture and intrathymic transplantation are shown here as examples of downstream applications.
2. Materials
2.1. Thymus Dissection
Mice of any age; in this chapter, green fluorescent protein (GFP) mice are used to visualize and track isolated TECs in vitro and in vivo.
Dissection board.
Dissection scissors.
Dissection forceps.
70% ethanol.
35 mm diameter petri dishes.
DMEM-F12 (Sigma), supplemented with sodium bicarbonate.
2.2. Enzymatic Dissociation of Thymic Epithelial Cells
DMEM-F12 (Sigma), supplemented with sodium bicarbonate.
0.25–0.5 mg/ml Papain (Worthington Biochemicals); aliquots stored at −80 °C, do not repeat freezing and thawing.
0.25 mg/ml Collagenase IV (Roche Life Science).
0.1 mg/ml DNase I (Roche Life Science).
35 mm diameter petri dishes.
37 °C CO2 incubator.
5 ml round-bottom tubes.
15 ml tubes.
2% bovine calf serum/PBS (2% Buffer).
Scalpel.
Forceps.
70 μm nylon mesh.
1000 μl pipette tips with wide-bore ends (commercially available, and also can be prepared by simply cutting the end with scissors and autoclaving) and regular ends.
2.3. Fluorescence-Activated Cell Sorting of Thymic Epithelial Cells
2% Buffer.
Ice.
5 ml round-bottom tubes.
Anti-Cy7 MicroBeads (Miltenyi Biotec; catalogue number 130–091-652), MACS Separation MS or LS Columns (Miltenyi Biotech; catalogue number 130–042-201 (MS), 130–042-401 (LS)), MiniMACS or MidiMACS Separator (Miltenyi Biotec; catalogue number 130–042-102 (Mini), 130–042-302 (Midi)), and MACS MultiStand (Miltenyi Biotec; catalogue number 130–042-303) are used for the positive selection of EpCAM+ TECs.
Antibodies against mouse antigens and lectin are as follows: CD16/32 (clone 93, Biolegend), CD45 (clone 30-F11, Bio-legend), EpCAM (clone G8.8, Biolegend), Ly-51 (clone 6C3, BD Biosciences), Ulex Europaeus Agglutinin 1 (UEA1, Vector laboratories), MHCII I-A/l-E (clone M5/114.15.2, Biolegend), and CD80 (clone 16–10A1, Biolegend). Propidium iodide (PI, 1 μg/ml, Sigma) is used to distinguish live and dead cells.
FACSAria (BD Biosciences) or a similar cell sorter.
FlowJo software (Tree Star) or a similar software to analyze flow cytometry data.
1.5 ml tubes for cell collection.
10% bovine calf serum/PBS (10% Buffer) to collect sorted cells for in vivo experiments.
MCDB153 medium containing 10% fetal calf serum (Hyclone), 50 units/ml penicillin (Life Technologies), 50 μg/ml strepta-vidin (Life Technologies), 2 mM L-glutamine (Life Technologies), 1 mM sodium pyruvate (Life Technologies), and 10 μM p160R0CK inhibitor Y-27632 (TOCRIS Bioscience) is used to collect sorted cells for in vitro culture pH adjusted to 7.2.
2.4. In Vitro Culture of Sorted Thymic Epithelial Cells
Tissue culture (TC) treated plates.
Mouse embryonic fibroblasts (MEFs): prepared from fetuses of embryonic stage ~13.5 (E13.5), and aliquots of propagated MEFs (passage 1 or 2) are stored at ≤−80 °C. Irradiated MEFs are prepared by exposing MEFs to 60 Gray of irradiation, before freezing or after thawing MEFs.
The same medium described in Subheading 2.3, step 10 is used to culture TECs in vitro.
Inverted fluorescence microscope (Olympus) is used to observe GFP-positive TECs growing in plates.
4% paraformaldehyde (PFA) in PBS, freshly prepared.
1% Rhodamine B (Sigma) in PBS is used to stain fixed TECs in plates.
Heating plate or a similar device, which can incubate PFA-fixed and Rhodamine B-stained TECs in plates.
PBS.
A camera to capture Rhodamine B-stained TECs in plates.
2.5. Intrathymic Transplantation of Sorted Thymic Epithelial Cells
Sterile Saline (0.9% sodium chloride in water, autoclaved).
A centrifuge with swinging buckets to spin down sorted cells in 1.5 ml microcentrifuge tubes.
Hamilton syringe (Hamilton Company; catalogue number 7654–01, model 1702RN).
Hamilton 30 gauge needle (Hamilton Company; catalogue number 7803–07, Small Hub RN; needle length of 1 in.).
1.5 ml tubes that can fit a Hamilton syringe (the outer barrel diameter of Hamilton syringe 1702RN is 7.75 mm (0.305 in.)).
10% Buffer to resuspend spun down cells in microcentrifuge tubes for intrathymic transplantation. AUTOCLIP 9 mm Wound Clips (Becton Dickson; catalogue number 427631).
AUTOCLIP 9 mm Wound Clip Applier (Becton Dickson; catalogue number 427630).
2,2,2,-Tribromoethanol (Avertin, Sigma).
Ice.
27-gauge needle for intraperitoneal injection.
1 ml syringe.
Surgical board (styrofoam or any appropriate board).
Rubber bands and needles to fix anesthetized animals on the surgical board.
Blunt-end dissection scissors (Fisher; catalogue number 08–950).
Forceps.
Buprenorphine hydrochloride (Sigma).
Headlight to aid the surgery.
Heating pad.
Sterile alcohol prep pads (Fisher; catalogue number 06–669-62).
Inverted fluorescence microscope (Olympus) is used to track down intrathymically transplanted GFP donor cells ex vivo.
3. Methods
3.1. Isolation of Mouse Thymus
Euthanize a mouse according to institutional guidelines.
Place the animal’s dorsal side onto the dissection board, and fix four limbs using needles or appropriate pins.
Spray the ventral surface with 70% ethanol.
Using forceps, lift up the midline skin right under the rib cage, and cut through the skin longitudinally, starting from the center of the midline all the way up to the neck. Make two more cuts from the center of the midline to left and right, and peel the skin over. This exposes the peritoneum and rib cage.
While holding the bottom tip of sternum with forceps, use scissors to cut through the peritoneum and thoracic diaphragm. Cut the far left and right sides of the rib cage longitudinally, and lift up the loosened rib cage all the way to expose thoracic viscera. The two-lobed thymus is located just above heart.
Use forceps to carefully remove any surrounding connective tissue (especially adipose tissue) around thymus.
Hold the middle part of thymus using a dissection blunt-end forceps, and then place another forceps with strong tips at the top end of thymus and gently pull out thymus towards you. Place the organ in a 35 mm petri dish containing DMEM/F12 medium (see Note 1).
3.2. Dissociation of Thymic Epithelial Cells
Using a scalpel, dice thymus into small pieces (0.1–0.2 cm in length and width) in 3 ml of DMEM/F12 medium (from Subheading 2.1, step 7, room temperature) in a 35 mm petri dish.
Transfer tissue fragments and medium into a 15 ml tube, and add DMEM/F12 medium up to 15 ml.
Rock the tube at room temperature for 3 min.
Stand the tube for 3 min, until tissue fragments settle down to the bottom.
Discard released thymocytes.
Add fresh DMEM/F12 medium up to 15 ml.
Rock the tube at room temperature for another 3 min.
Stand the tube for 3 min, until tissue fragments settle down to the bottom.
Discard the supernatant (see Note 2).
Transfer the remaining tissue fragments into a new 35 mm petri dish.
Add 500 μl of fresh DMEM/F12 medium (see Note 3).
Add enzymes to the dish: final concentrations at papain 0.25–0.5 mg/ml, collagenase IV 0.25 mg/ml, and DNase I 0.1 mg/ml.
Incubate tissue fragments with enzymes at 37 °C CO2 incubator, for 30 min (Fig. 1; see Note 4).
Add 1 ml of 2% Buffer to stop the enzymatic reaction.
Pipet up and down using first a wide-bore end 1 ml tip and then a regular end 1 ml tip.
Filter the dissociated cells in medium through 70 μm nylon mesh and transfer to a 5 ml round-bottom tube (see Note 5).
Add 2% Buffer up to 5 ml.
Centrifuge at 300 × g for 5 min.
Discard the supernatant.
Resuspend cells in ~200 μl of 2% Buffer and keep cells on ice.
Fig. 1.
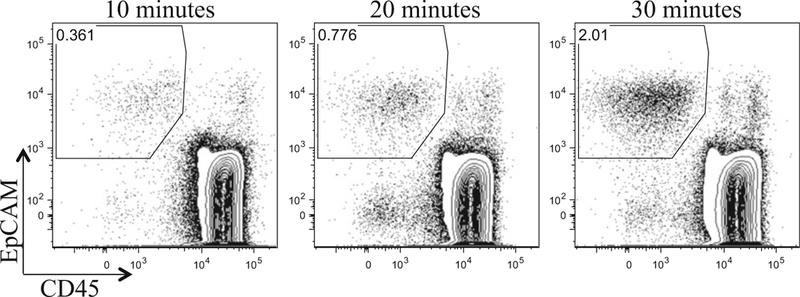
The time course of papain digestion on thymic epithelial cells. Three of half thymuses (~35 mg net mass/a half thymus) from 3-week old male C57BL/6 mice were digested in 250 μl of DMEM/F12 medium with 0.25 mg/ml papain/0.25 mg/ml collagenase IV/0.1 mg/ml DNase I, for 10, 20, and 30 min. Cells were pre-gated on live, PI− cells, and further gated on EpCAM vs. CD45 (hematopoietic cells). The percentage of EpCAM+ TECs increases as the digestion time increases
3.3. Fluorescence-Activated Cell Sorting of Thymic Epithelial Cells (See Note 6)
Add EpCAM-APC/Cy7 antibody (1 μl per 108 cells) and Fc blocking antibody (CD16/CD32) to cells in 2% Buffer (prepared in Subheading 3.2, step 14) and vortex the tube briefly (see Note 7).
Incubate cells on ice for 15 min, and protect cells from light.
Add 2% Buffer up to 5 ml.
Centrifuge at 300 × g for 5 min.
Resuspend cells in ~200 μl of 2% Buffer.
Add anti-Cy7 microbeads (5 μlper 108 cells) to cells and vortex the tube briefly (see Note 7).
Incubate cells on ice for 15 min, and protect cells from light.
Add 2% Buffer up to 5 ml.
Centrifuge at 300 × g for 5 min.
Resuspend cells in 1 ml of 2% Buffer.
Set up MACS Separation Columns following the manufacturer’s (Miltenyi Biotech) instructions.
Apply cells in solution from Subheading 3.3, step 10 onto a MACS column.
Collect EpCAM+ cells into a new 5 ml tube.
Add 2% Buffer up to 5 ml.
Centrifuge at 300 × g for 5 min.
Resuspend cells in ~200 μl of 2% Buffer.
Make a master mixture of antibodies in 20 μl of 2% Buffer (listed in Subheading 2.3, step 5) (see Note 7).
Add the antibody mixture to cells in solution (from Subheading 3.2, step 16) and vortex the tube briefly.
Incubate cells on ice for 15 min, and protect cells from light.
Add 2% Buffer up to 5 ml.
Centrifuge at 300 × g for 5 min.
Resuspend cells in ~200 μl of 2% Buffer.
Add secondary antibody to cells and vortex the tube briefly (see Note 7).
Incubate cells on ice for 15 min, and protect cells from light.
Add 2% Buffer up to 5 ml.
Resuspend cells in 2% Buffer containing 1 μg/ml PI (the concentration of cells is up to 107/ml).
Filter cells in solution through 70 μm nylon mesh.
Run and sort cells on a FACSAria, using a 70 μm nozzle, at speed of ~3000 cells per second. Gate PI– live cells and then select CD45– EpCAM+ cells. TECs are divided using UEA1 anti-Ly-51, which stain medullary TECs (mTECs) and cortical TECs (cTECs), respectively. mTECs and cTECs are further gated based on MHCII and CD80 expression (Fig. 2). The yield of sorted TECs varies depending on the age of the mouse: from one 3-day old mouse, 80,000 cTECs and 20,000 mTECs can be sorted; from one 3-month old mouse, 60,000 cTECs and 170,000 mTECs can be sorted.
Sort cTECs and mTECs into collection tubes containing 1 ml of 10% Buffer for in vivo or MCDB153 medium (from Subheading 2.3, step 10) for in vitro culture.
Fig. 2.
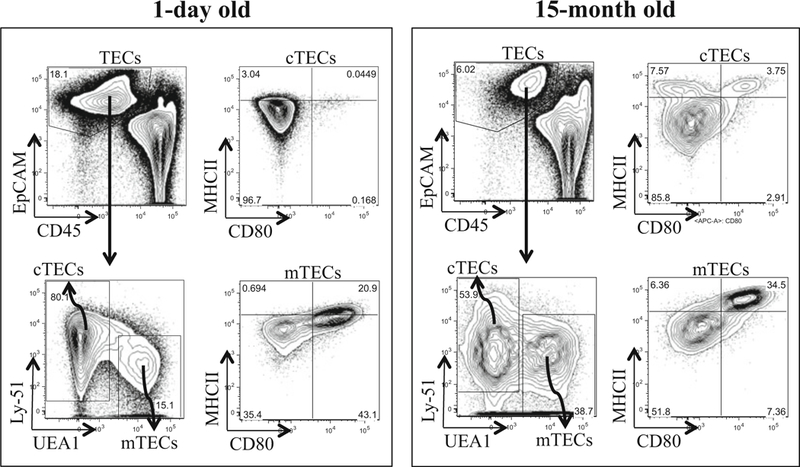
Flow Cytometry and cell sorting of thymic epithelial cells. Thymuses from 1-day old (n = 6) and 15-month old (n = 1) GFP mice were digested by 0.25 mg/ml papain/0.25 mg/ml collagenase IV/0.1 mg/ml DNase I, followed by EpCAM-positive selection through MACS columns. Positively selected cells were further stained with TEC markers and subjected to analysis and sorting. Live cells were divided into cTECs and mTECs based on Ly-51 and UEA1 expressions, respectively. cTECs and mTECs show different expression levels for functional markers such as CD80 and MHCII. This analysis also shows differences in cTECs and mTECs between 1-day old and 15-month old mice
3.4. In Vitro Culture of Sorted Thymic Epithelial Cells
Prior to cell sorting, prepare irradiated MEFs in MCDB153 medium (from Subheading 2.3, step 10, without Y-27632) onto a tissue-treated 96 flat-bottom plate, at a density around 3 × 104−4 × 104 per well (see Note 8).
Plate sorted TECs (from Subheading 3.3, step 29) onto the MEFs layer, and add 200 μl of MCDB153 medium (from Subheading 2.3, step 10) per well (see Note 9).
Change medium every 2–3 days (see Note 10).
Stop the culture when TECs are confluent or at a desired time point (see Note 11) (Fig. 3).
Remove medium and fix TECs in 4% PFA for 10 min.
Wash fixed TECs with PBS.
Stain fixed TECs with 1% Rhodamine B, at 60 °C for 15 min.
Remove Rhodamine B solution.
Capture the image of the entire plate (Fig. 3).
Fig. 3.
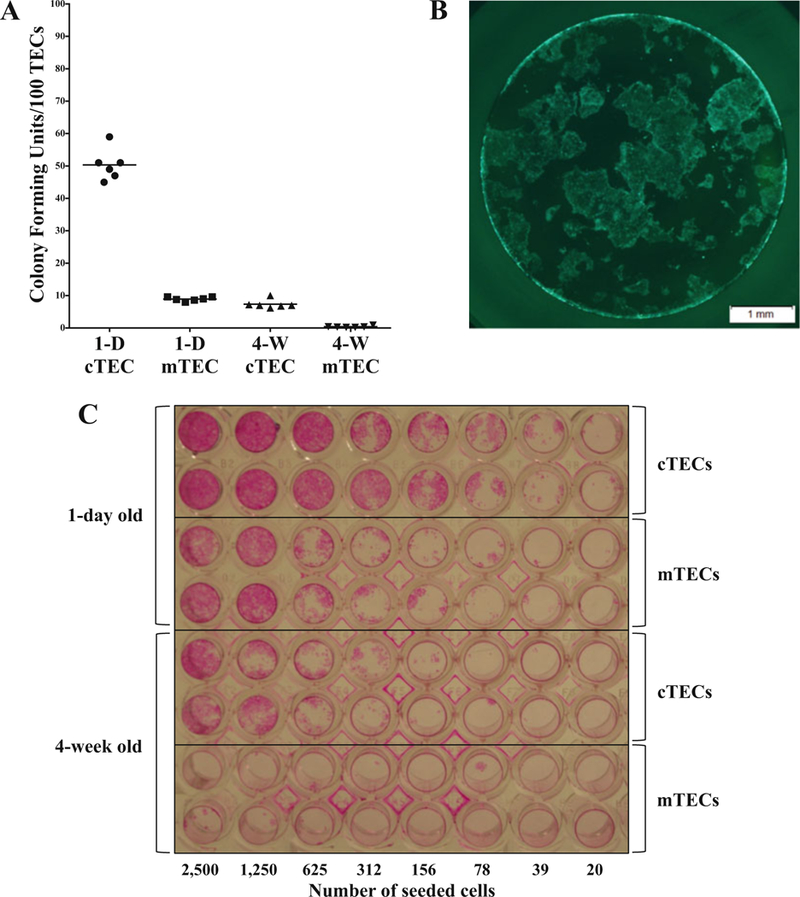
Proliferation of thymic epithelial cells in vitro. cTECs and mTECs were sorted from 1-day old and 4-week old GFP mice and the indicated numbers of cells were plated onto a TC-treated 96-well plate. (a) At day 3 post-seeding, GFP+ TEC colonies were manually counted under a fluorescence microscope and plotted (1-D: 1-day old; 4-W: 4-week old). (b) At day 7 post-seeding, proliferating GFP cTECs (from 1-day old mice; 1000 cTECs were seeded) are seen under an inverted fluorescence microscope. (c) At day 10 post-seeding, TECs were fixed in 4% PFA and stained with Rhodamine B solution. This shows the difference in colony forming units between 1-day old and 4-week old, and also between cTECs and mTECs. This assay is useful to assess the differentiation and function of TECs in vitro
3.5. Intrathymic Transplantation of Sorted Thymic Epithelial Cells
Prepare all the tools described in Subheading 2.5 and sterilize by autoclaving or soaking in 70% ethanol.
One hour before the intrathymic transplantation, inject a mouse intraperitoneally with preoperative analgesic, buprenorphine (0.075 mg/kg body), prepared in Saline.
Centrifuge sorted cells in a 1.5 ml tube (from Subheading 3.3, step 29) at 300 × g for 5 min (see Note 12).
Resuspend cells in 10% Buffer (concentration up to 400,000 cells/10 μl) and keep on ice until injection. Use ~10 μl of cells per intrathymic injection (see Note 13).
Inject the mouse intraperitoneally with Avertin (250 mg/kg body weight), prepared in Saline.
When the mouse is unconscious, place it in the supine position onto the surgical board and fix it with rubber bands and pins. Place the mouse head toward you (Fig. 4a).
Wipe the chest area with a sterile alcohol prep pad.
Lifting up the skin around the neck with forceps, make a small perpendicular cut in the neck using a blunt-end scissors (Fig. 4b). Be careful not to puncture blood vessels. Using forceps, flip over salivary glands toward you (Fig. 4c).
Using one tip of forceps, make a small incision through the serous membrane, right above the top of sternum (Fig. 4d).
Using the same blunt-end scissors (Fig. 4e), cut ~0.5 cm of the manubrium and sternum perpendicularly (Fig. 4f).
Draw out ~10 μl of cells in suspension into Hamilton syringe attached with 30-gauge needle. To prevent the needle going through the entire thymus, epoxy can be applied to the bottom tip of the needle, based on the thymus thickness (Fig. 4g).
Turn on the headlight (worn around your head) to look into the thoracic cavity better. Use forceps to keep the thoracic cavity area open (Fig. 4h).
While holding the tip of the rib cage, inject cells into a thymic lobe (Fig. 4i).
Put back salivary glands and bring back the divided skin to the midline.
While holding the skin with forceps, apply 1–2 wound clips perpendicularly (Fig. 4j k and k).
Place the mouse on the heating pad.
When the mouse becomes conscious, e.g., reflexes in eyes or limbs, put it back to its original cage.
At a desired time point, euthanize the mouse according to the institutional guideline.
Open up the thoracic cavity.
Remove thymus as described in Subheading 3.1 and place it in a 35 mm petri dish with PBS.
Using a fluorescence microscope, check for donor fluorescent cells within the recipient thymus (Fig. 5).
Process the organ for further analysis, e.g., flow cytometry and histology.
Fig. 4.
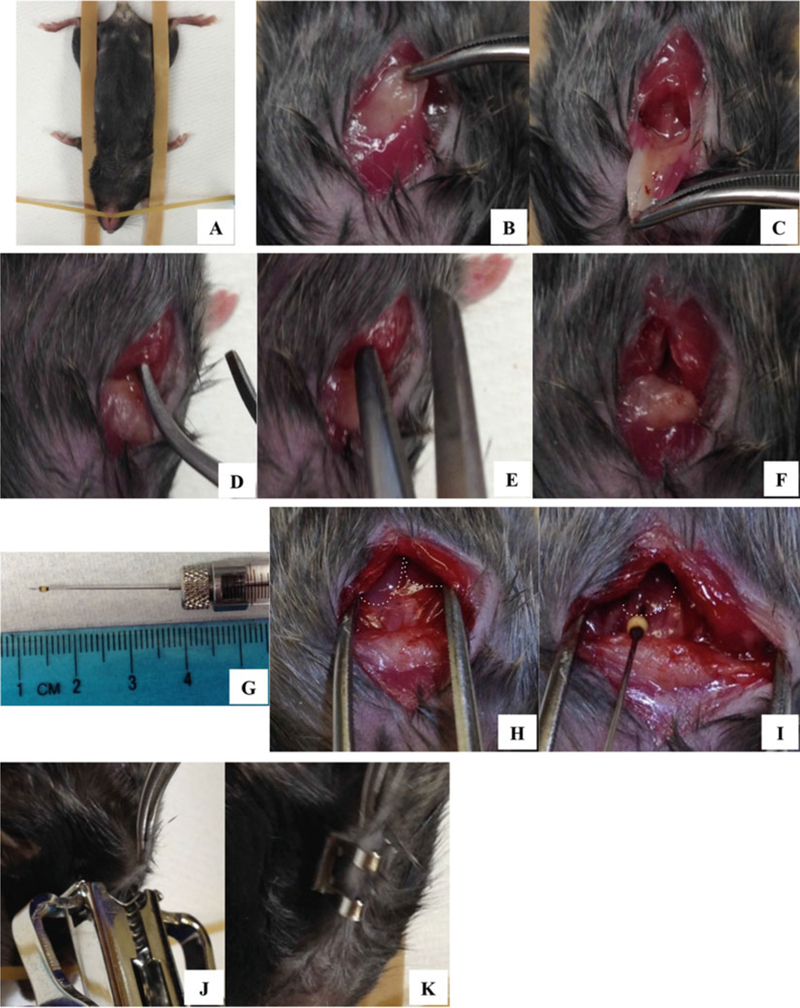
Intrathymic injection of donor cells into a recipient thymus. As described in Subheading 3.5, an anesthetized mouse is placed on the surgical board (a), and a small incision is made in the thoracic area (b–f). TECs drawn into a Hamilton syringe and needle (g) are injected into a thymic lobe (h and i dotted lines indicate thymic lobes). After intrathymic injection, the wound is closed with wound clips
Fig. 5.
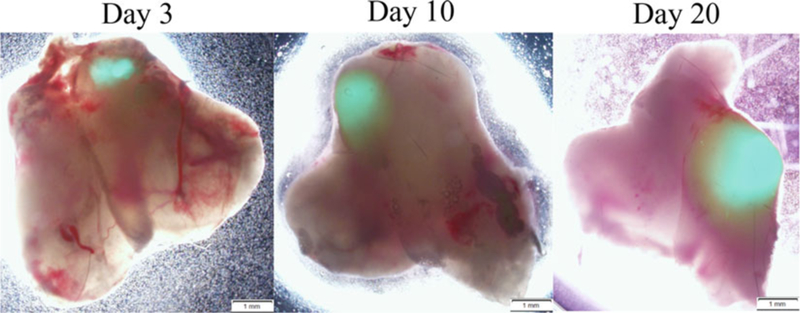
Intrathymically transplanted TECs engraft and proliferate within recipient thymuses. cTECs were sorted from 3-day old GFP transgenic mice and intrathymically transplanted into 5-month old male mice. Each mouse thymic lobe received 110,000 of cTECs. At day 3,10, and 20 posttransplantation, thymuses were recovered from recipient mice and examined under a fluorescence microscope. The donor GFP TECs are seen as green fluorescence. This intrathymic transplantation technique demonstrates that sorted TECs using the papain method are not only viable but also transplantable. According to our analysis by flow cytometry and histology, transplanted TECs proliferate within recipient thymuses. This will be useful to study TEC differentiation and function in vivo
Acknowledgments
MJK was funded by a Mary K. Iacocca Foundation Research Fellowship. TS received funding from a seed grant from the Harvard Stem Cell Institute. The authors gratefully acknowledge the Joslin Diabetes Center Flow Cytometry Core supported by an NIH Diabetes Research Center grant (NIH award P30DK036836) and the Harvard Stem Cell Institute. The authors acknowledge the excellent support of the Joslin Animal Facility.
4 Notes
If thymus mass needs to be measured, weigh thymus before putting it into medium.
Repeat the thymocyte releasing step if the supernatant is still turbid.
For one adult thymus of ~50 mg mass (measured upon tissue harvest from a mouse), use 500 μl of medium. The medium volume is adjusted depending on the tissue mass.
For fetal thymuses, incubate tissue fragments in enzyme solution for 10 min. Do not exceed the enzymatic digestion time over an hour; it will lead to cell death.
Thymus from mice of age older than 2–3 weeks old leaves undigested tissue remnants. Make sure to filter out them before adding antibodies.
Keep all the reagents, cells, and centrifuge at 4 °C for Subheading 3.3.
Antibodies need to be titrated in each laboratory. If EpCAM-positive selection is not necessary, skip steps 1–14 and proceed to the cell staining at step 15 and include EpCAM antibody to the antibody mixture at step 17.
The cell seeding density can be adjusted based on the surface area of the culture plate. TECs can be plated onto a TC-treated plate without MEFs layer, although the survival and growth decrease without MEFs.
Instead of dividing 1 ml of sorted cells into a 96-well plate (10 μl per well), it is more accurate to divide a larger volume of cells in solution; e.g., MCDB153 medium is added to 1 ml of sorted TECs up to 20 ml, and then 200 μl of medium that contains sorted cells can be plated into each well. Prepare at least two wells of each TEC type (e.g., cTEC and mTEC) as a technical replicate. In this system, TEC proliferation and function can be measured.
Ap160R0CKinhibitor, Y-27632, is only necessary for the first 2–3 days of TEC culture after sorting and plating.
During the culture, observe the growth of TECs in plates under a fluorescence microscope, if TECs are of GFP or other fluorescence.
It is better to spin down cells using a centrifuge with swing buckets so that cells form a nice pellet at the bottom tip of the tube.
All the steps from enzymatic digestion to cell sorting reduce the number of viable TECs; therefore for the successful readout after intrathymic injection, sorted TECs of at least 50,000 need to be injected into each thymic lobe. The younger the donor mice are, the better the transplantation works. Authors found that sorted TECs of mice aged from E13 (embryonic stage 13) up to P14 (post-natal 14) transplant well and they are also recoverable.
References
- 1.Anderson G, Jenkinson EJ (2001) Lymphostromal interactions in thymic development and function. Nat Rev Immunol 1:31–40 [DOI] [PubMed] [Google Scholar]
- 2.Palmer E (2003) Negative selection–clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol 5:383–391 [DOI] [PubMed] [Google Scholar]
- 3.Steinmann GG, Klaus B, Müller-Hermelink HK (1985) The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 22:563–575 [DOI] [PubMed] [Google Scholar]
- 4.Hale JS, Boursalian TE, Turk GL, Fink PJ (2006) Thymic output in aged mice. Proc Natl Acad Sci U S A 103:8447–8452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF (2002) T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol 38:841–848 [DOI] [PubMed] [Google Scholar]
- 6.Chinn IK, Blackburn CC, Manley NR, Sempowski GD (2012) Changes in primary lymphoid organs with againg. Semin Immunol 24:309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ewijk W, Wang B, Holländer G, Kawamoto H, Spanopoulou E, Ito M, Amagai T, Jian YF, Germeraad WTV, Chen WF, Katsura Y (1999) Thymic microenvironments, 3-D versus 2-D? Semin Immunol 11:57–64 [DOI] [PubMed] [Google Scholar]
- 8.Berrih S, Savino W, Cohen S (1985) Extracellular matrix of the human thymus: immunofluorescence studies on frozen sections and cultured epithelila cells. J Histochem Cytochem 33:655–664 [DOI] [PubMed] [Google Scholar]
- 9.Lannes-Vieira J, Dardenne M, Savino W (1991) Extracellular matrix components of the mouse thymus microenvironment: ontogenetic studies and modulation by glucocorticoid hormones. J Histochem Cytochem 39:1539–1546 [DOI] [PubMed] [Google Scholar]
- 10.Huettner JE, Baughman RW (1986) Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci 6:3044–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarthy PV, Balkema GW (1981) Retrograde labeling and dissociation of mouse retinal ganglion cells. Neurosci Lett 25:205–208 [DOI] [PubMed] [Google Scholar]
- 12.Bolzon BJ, Cheung DW (1989) Isolation and characterization of single vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension 14:137–144 [DOI] [PubMed] [Google Scholar]
- 13.Weekes TE (1974) The preparation of a viable suspension of epithelial cells from the rumen mucosa of cattle. Comp Biochem Physiol 49:407–413 [DOI] [PubMed] [Google Scholar]


