Abstract
This study intends to analyze the effects of doxorubicin and sodium bicarbonate release with polycaprolactone (PCL) coating on calcium phosphate system which is a bone like material, on the cell viability and proliferation of osteosarcoma and osteoblast. Increased systematic pH concentrations locally by the release of sodium bicarbonate diminished acidosis and hence, alleviated malignancy. In our studies, we have shown that the same of dosage of doxorubicin inhibited both osteoblast and osteosarcoma cell attachment and viability whereas, sodium bicarbonate abated osteosarcoma cell proliferation. Sodium bicarbonate also inhibited osteoblast cell proliferation in the early time points, however, the cell viability increased after the initial burst release of the molecule. Polymer coating on calcium phosphate-based implants, as carriers of drug, can minimize chances of toxic effects of higher oral drug dosage in the body, and also help in delivering effective doses of drugs, locally to the target tissues, as compared to the oral drug delivery approach. A coating of PCL was thus incorporated to control the initial burst release of bicarbonate, which enhanced the osteoblast cell viability, but was capable of diminishing osteosarcoma cell proliferation. The novelty and clinical significance of this study lies in the understanding of unique delivery using encapsulated naturally occurring and more benign sodium bicarbonate, for usage after excision of the cancerous bone, without any adverse effects on normal bone cells.
Keywords: HA-polymer composite, doxorubicin, bicarbonate, anti-cancer drug, sustained release, local drug delivery, in vitro, bone tissue engineering
Graphical Abstract
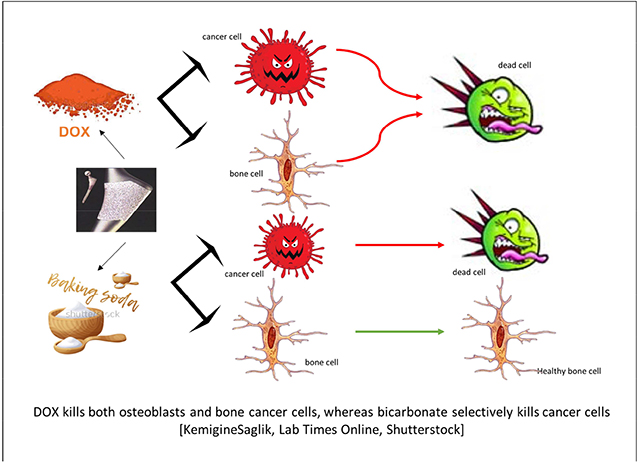
DOX kills both osteoblasts and bone cancer cells, whereas bicarbonate selectively kills cancer cells [KemigineSaglik, Lab Times Online, Shutterstock]
1. Introduction
The pH in normal non-malignant tissues remains slightly alkaline in the range of around 7.2 – 7.5. However, it decreases significantly to around 6.5 – 6.9 in the extra cellular matrix in the vicinity of a malignant solid tumor [1], [2], [3]. A variety of different mathematical models [4] and extensive in vitro [5] and in vivo studies [6] had shown decreased pH by the exportation of acids and stimulation of tumors by alterations in the acidic conditions by lysosomal proteases [7] in the surrounding matrix of a malignant tumor. Also, enhanced metastases have been observed from pretreatment of tumor cells with acids [8], which have further substantiated the belief of upregulation of tumor survival by acidic pHs.
The current study intends to test the hypothesis if the neutralization of acidic pHs in the vicinity of remnant bone tumor cells after excision of the tumor will inhibit the invasion of the tumor, and decrease possibilities of the further lung metastases [9], commonly observed in osteosarcoma incidences. Previous studies have shown the efficacy of oral bicarbonate dosages to alter the pH around tumor cells without adversely affecting the healthy normal tissue pHs. Conversely, oral dosages of doxorubicin (adriamycin) have been well studied for breast cancer treatments [10], [11], in vivo as well as clinical trials on osteosarcoma cells [12], and bone cancer metastases developing from breast cancer [13], [14]. Based on the variety of literature available showing the efficacy of oral doxorubicin dosage on cancer cell treatment, it was chosen as an ideal candidate to compare the effects of bicarbonate on the viability of mono-cultures of osteosarcoma and osteoblast cells in vitro.
Local drug delivery systems have been accepted as a potential alternative [15] to the conventional oral delivery systems to ensure targeting, reduce chances of toxicity in non-targeted cells and controlled pharmacokinetics [16], [17]. Calcium phosphate (CaP) coated metallic implants have been widely used as load bearing implants [18], [19] and as an accepted alternative to cemented implants for younger patients, owing to its compositional similarity and enhanced durability [20], [21]. They also have been a popular choice for tissue integration purposes and also, as reservoirs for desired rates of drug elution by a variety of surface modifications [16]. Amongst those, coating with a biocompatible, and biodegradable polymer, ensuring control over matrix solubility, drug-polymer interactions and surface microstructure has been exploited as one of the very favored choices for calcium phosphate cased scaffolds [22], [23], [16]. Polycaprolactone (PCL) coating has been used as a potential drug carrier for its inherent biodegradability and processing ease [23].
Delivery of the biomolecules through bioactive polymers coatings on calcium phosphate ceramics is an endeavor put forward to clinch desired delivery rates and limit toxicity to bystander cells. Based on our knowledge, no other literature compares the effects of sustained bicarbonate and doxorubicin release on the mono-cultures of osteoblasts and osteosarcoma cells for reduction of tumor metastasis. This study reports the comparison of the effects of bicarbonate and doxorubicin in PCL and calcium phosphate coated titanium alloys on release kinetics and in vitro osteoblast and osteosarcoma cell viability. We believe the study based on the effects of chemistry, environment and polymer-drug interactions on bone cancer cells would lead to a beneficial understanding for designing in vivo drug delivery vehicles to reduce metastases after tumor excision.
2. Materials and Methods
2.1. Hydroxyapatite (HA) coating preparations by plasma spray method
Analytical grade HA granules (particle size >350 μm and <500 μm), purchased from Monsanto, USA was used to coat titanium alloy (Ti6Al4V) (Grade 5, President Titanium, MA, USA) discs of 12 mm in diameter and 2 mm thick. The powder granules were dry milled for couple hours in a 2:1 powder to alumina milling media ratio to achieve particle sizes ~150 μm for the coating. After sandblasting, the discs were washed ultrasonically in deionized water and acetone respectively to remove any organic residues. A 30-kW inductively coupled RF plasma spray system (Tekna Plasma Systems, Canada), equipped with an axial powder feeding system, at 25 kW and at 110 mm working distance using supersonic plasma nozzles, in an inert argon environment, was used for the HA coating preparation. The HA coated discs were sterilized by autoclaving at 121°C for 30 mins before using them for cell viability studies.
2.2. Doxorubicin, bicarbonate and PCL coating
10 mg of doxorubicin purchased from Sigma Aldrich, St. Louis, MO, USA was mixed in a solution with 10ml of water. The same dosage of sodium bicarbonate, also purchased from Sigma Aldrich, St. Louis, MO, USA was mixed in a 10 ml water solution to maintain consistency of drug dosage. The drugs were individually filter sterilized and then 50 μl of the drug solution drop-casted on the sterilized discs so that each disc contains 50 μg of Doxorubicin (lower dosage approved by FDA to be administered every 3–4 weeks [24]). Similar concentrations to doxorubicin had been utilized for the bicarbonate coating as well. The drug incorporated discs were left to dry in a sterilized chamber for 2 hours before the polymer incorporation. 5 wt % PCL (Mw=14000) (Sigma, St. Louis, MO, USA) was dissolved in 10ml of ethanol and kept in water bath at 60°C for 15–20 minutes. Similarly, 50 μl of the polymer solution was then drop-castedon the drug coated samples to ensure controlled release of the drugs. The samples were further dried overnight in the sterile environment before continuing with cell culture procedures.
2.3. Doxorubicin and bicarbonate loading and release
Release of doxorubicin and bicarbonate was measured by using 0.1 M pH buffers and distilled water respectively. A pH 7.4 PBS (phosphate buffer saline) was utilized to imitate the body’s physiological pH. Three samples from each concentration, with or without 5 wt% of PCL, with 50 μg of DOX or 12.5mg, 25mg and 50mg of bicarbonate incorporated were placed in 4ml of pH 7.4 phosphate buffer in individual vials. Higher dosage of bicarbonate was utilized to analyze the kinetics for the ease of measurements and avoid noises from the measurements. HA coated scaffold without sodium bicarbonate coating has been used as a control. The vials were placed at 37°C under constant shaking conditions of 150 rotations per minute. For the study, buffer and water solutions were changed at 3, 8, 18 and 28 days. At each time point, 4ml of the buffer solution was pipetted out and was restored with a new 4ml of the buffer solution for volume make-up. The doxorubicin concentration was investigated at 479 nm [25] wavelength using a BioTek Synergy 2 SLFPTAD microplate reader (BioTek, Wiooski, VT, USA). Standard curve was utilized to find the final cumulative percentage release of the drug. The Na+ ion release was analyzed using an Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS, Agilent model 7700 ICP-MS, Santa Clara, CA, USA).
2.4. Investigation of the surface properties before and after release
The surface morphologies of the discs were studied under a Field Emission Scanning Electron Microscope (FEI, Inc., OR, USA) following gold coating (Technics Hummer V, CA, USA) before and after the release study to investigate the degradation of the coating matrix.
2.5. In vitro cell materials interaction
Human fetal osteoblast cells (hFOB) (ATCC, Manassas, VA) and human osteosarcoma cells (ATCC, Manassas, VA) were incubated for 28 days with the aim of investigating the in vitro materials and bone cell interactions and analyzing the efficacy of doxorubicin and bicarbonate on minimizing targeted sarcoma cell viability. HA coated scaffolds, with bicarbonate and doxorubicin with or without PCL coating were seeded directly with 1.5 * 105 cells. After appropriate cell seeding procedure, 1 ml of Dulbecco’s modified Eagle’s medium (DMEM) enriched with 10% fetal bovine serum was added carefully to each of the samples as per ATCC’s hFOB cell culture protocols. The samples have been referred to as HA as control, HA+DOX, HA+DOX+PCL, HA+NaHCO3 and HA+NaHCO3+PCL. For the osteosarcoma cells, the similar samples were added with 1 ml of Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum. The cell media was altered every second day throughout the span of the experiment to provide fresh media to aid in enhancing cell growth and proliferation. The osteoblast cultures were incubated in a sterilized environment at 34 °C under 5 % CO2, whereas the osteosarcoma cell cultures were incubated at 37 °C under 5 % CO2 and 95 % humidified air atmosphere following the protocols of ATCC.
2.6. Cell morphology imaging by SEM
To observe the cell morphology, the samples were fixed with 1ml of 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer and refrigerated overnight at 4 °C after 3, 8, 18 and 28 days of culture. Following fixation, the samples were then treated with 2% osmium tetroxide (OsO4) and maintained for 2 hours at room temperature. Then, the samples were dehydrated by rinsing them in a consecutive series of ethanol drying and further by a hexamethyldisilane (HMDS) drying procedure. The dehydrated samples then were gold coated using Technics Hummer V, San Jose, CA, USA to ensure elimination of any kind of scanning faults arising from electrostatic charges on the sample surface. Following gold coating, the samples were observed under an FESEM, equipped with an ETD (Everhart-Thornley Detector), under high vacuum at 30 kV acceleration voltages.
2.7. Cell viability demonstration by MTT assay
The MTT assay (Sigma, ST. Louis, MO) was executed after 3, 8, 18 and 28 days of incubation to assess the cytotoxicity of the drugs on early and late stage hFOB and osteosarcoma cell proliferation. 5 mg ml−1 of MTT solution was made by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide in phosphate buffer and sterilized by filter sterilization. Serum free, phenol-red free Dulbecco’s Minimum Essential (DME) medium was used to dilute the 50 μl MTT solution thus made into 450 μl. 100 μl of the diluted MTT solution was then added carefully on the surface of each sample in the 24-well plates and topped with 900 μl of the DMEM culture media. These were incubated for 2 hours in their respective culture conditions. Following incubation, the samples were transferred to a new well plate and added with 600 μl of MTT solubilizer composed of 10% Triton X-100, 0.1 N HCl, and isopropanol to dissolve the formazan crystals, responsible for the purple color on the sample surface. 100 μl aliquots of this resulting solution were taken from each group in triplicates and measured at 570 nm by a BioTek Synergy 2 SLFPTAD microplate reader (BioTek, Wiooski, VT, USA).
3. Results
3.1. Doxorubicin and bicarbonate release
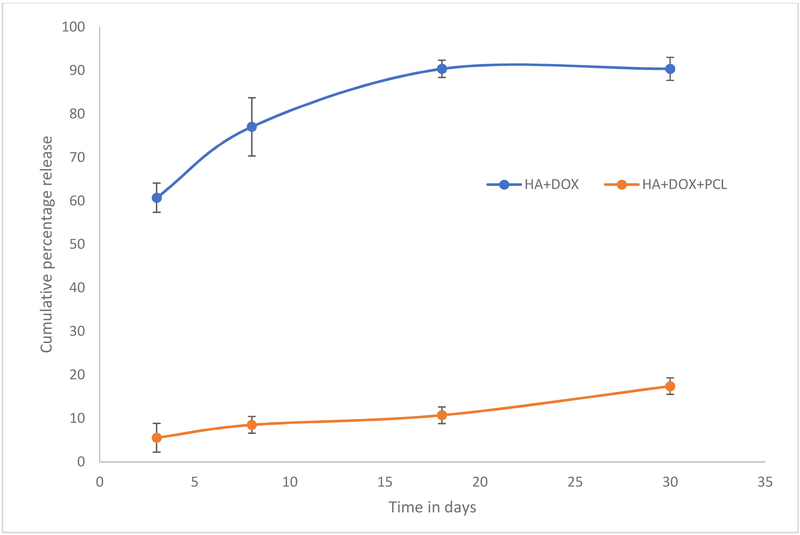
The release study is carried out to correlate the osteosarcoma and osteoblast cell viability after 3, 8, 18 and 28 days of incubation. Figure 1 shows the cumulative percent release of doxorubicin at pH 7.4 phosphate buffer. Burst release of around 60% doxorubicin is noted from the HA coated Ti6Al4V discs within the first 3 days at pH 7.4. Almost 90 % of the drug has been observed to be released within 18 days, which explains the enhancement of sarcoma cell viability in doxorubicin incorporated samples at day 28. PCL coating controls the drug release leading to a release of only 20% over the span of 28 days.
Figure 1.
shows the cumulative percentage release of doxorubicin from HA, and HA and PCL composite at pH 7.4 maintained at 37°C to mimic the physiological conditions more accurately. DOX has been observed to be almost plateauing after 18 days, which indicates the release of the doxorubicin is almost over. This is in accordance to the increment of both osteoblast and osteosarcoma cell viability after 18 days of culture.
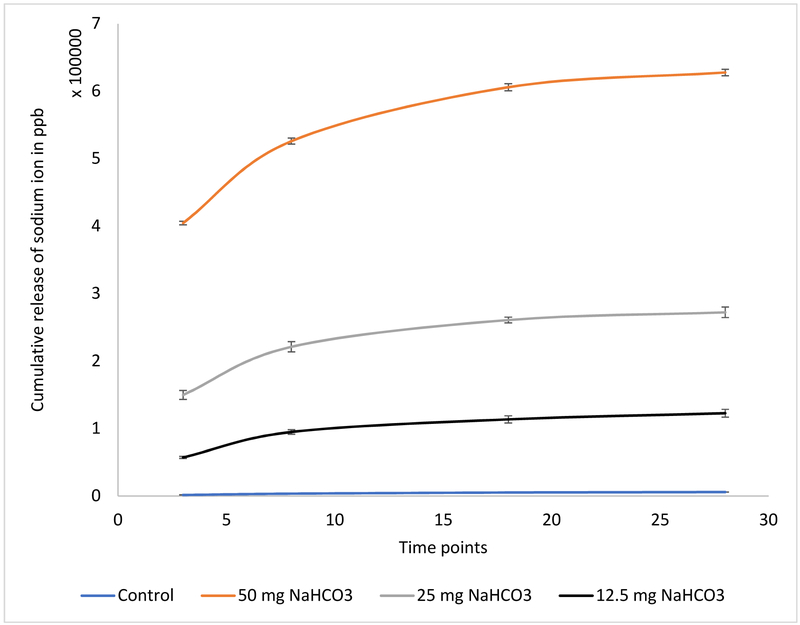
Figure 2 demonstrates the release of bicarbonate from the HA coated Ti alloys in distilled water. In all cases without polymer coating, a burst release of the biomolecule is observed which is attributed to the hydrophilicity of the molecule. This can be correlated to the initial reduction of osteoblast cell viability, which stabilize and witness enhanced growth after 8 days of incubation. Figure 2 shows a more controlled release of the bicarbonate release from polymer coated discs. This report shows the potency of the polymers in controlling the burst release of doxorubicin and bicarbonate from HA coated titanium implants.
Figure 2.
shows the cumulative percentage release of Na ion from HA coating with PCL in distilled water maintained at 37°C to mimic the physiological conditions more accurately (measured by ICP-MS). HA coated scaffolds without any sodium bicarbonate coating have been used as control. Sustained and controlled release is noted from the scaffolds with polymer.
3.2. Surface morphologies micrographs and surface energy analysis
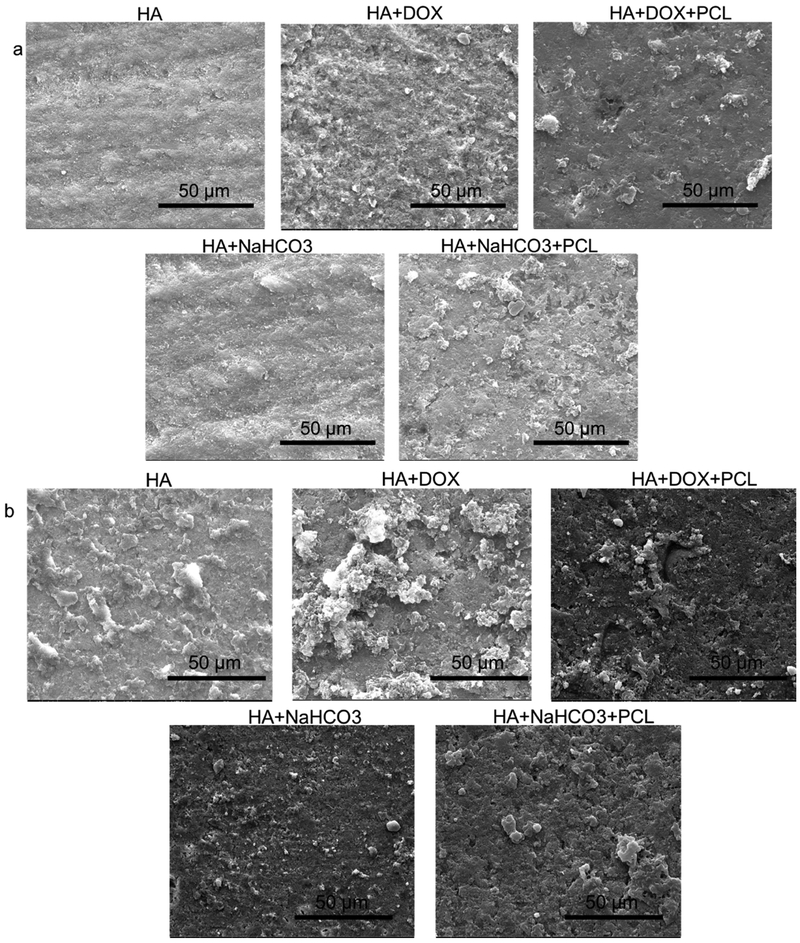
Figure 3 shows the surface morphologies of discs before (3a) and after (3b) the release study is conducted. A more porous structure caused by the degradation of the coating matrix is noted for all compositions after the release.
Figure 3.
investigates the surface properties of the discs before and after the release study was conducted for 28 days. The surface morphologies by SEM before (a) and after (b) the release reveal a more porous structure after the release was conducted, attributed to the coating matrix degradation over the study period.
3.3. Osteoblast cell material interaction by SEM and MTT
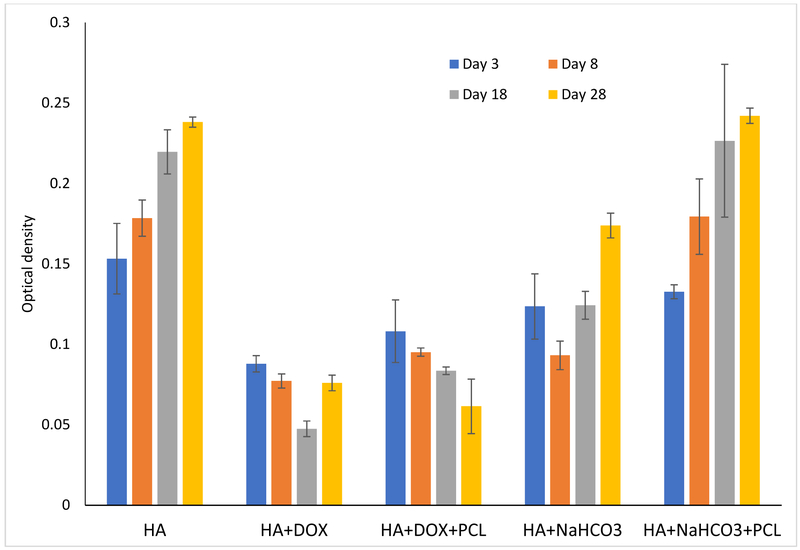
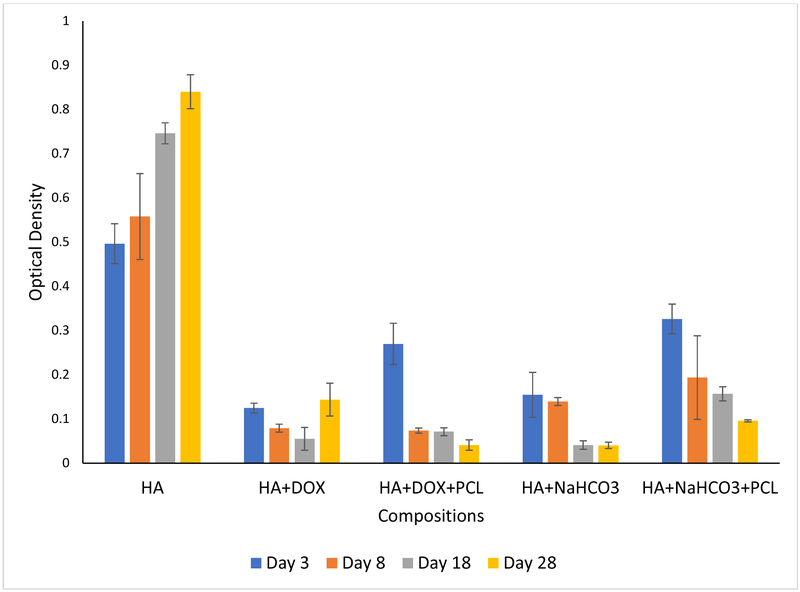
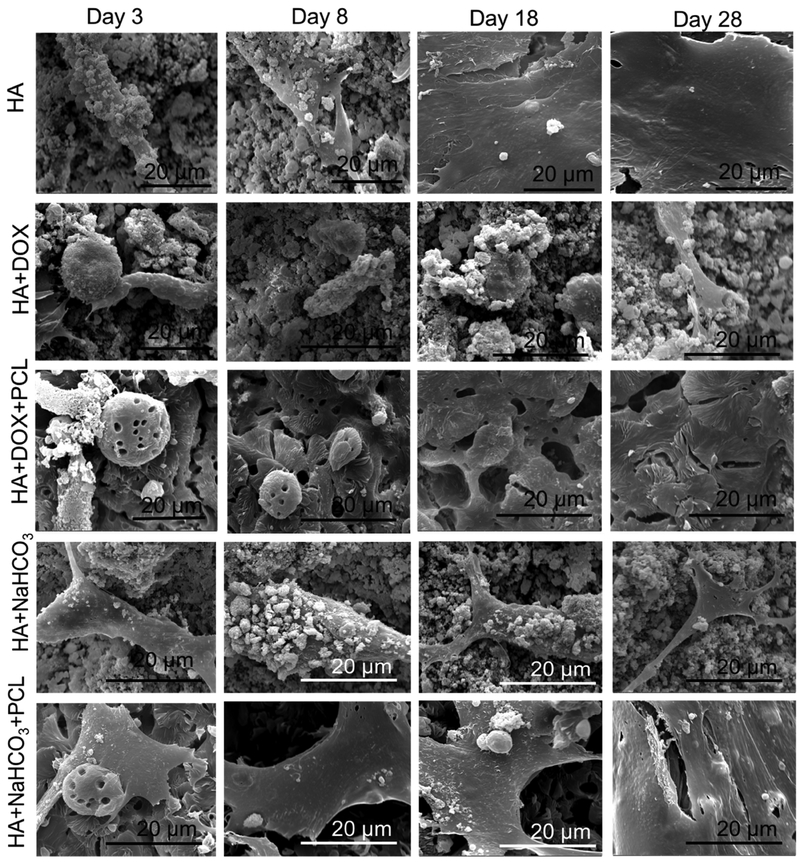
Figure 4 shows the growth and proliferation of human osteoblast cells on the samples after 3, 8, 18 and 28 days of cell seeding. Flattened adhered cells has been noted on the control coated HA discs, which are observed to form layers through the entire study length of 28 days. Figure 4(a) demonstrates reduced cell viability due to the doxorubicin incorporation. The polymer incorporation on doxorubicin coating does not have any statistical effect on the osteoblast cell viability compared to no polymer coating. Cell viability has been observed to enhance with only doxorubicin incorporation with no polymer coating after 28 days of incubation. This might be explained by the completion of 90% of release of doxorubicin from the coated samples by the first 18 days. A coating of PCL is in turn able to control the release kinetics at a desired rate and reduced cell viability has been noted consistently. Circular dead cells have been presented on the surface containing doxorubicin.
Figure 4 (a).
presents osteoblast cell viability on HA, HA and DOX, HA and bicarbonate samples, with or without PCL after 3, 8, 18 and 28 days of culture. Cell viability reduced a little bit by the burst release of bicarbonate which stabilized over the course of time, eventually leading to enhancement of cell density. A coating of PCL controlled the burst release of bicarbonate, leading to gradual enhancement of cell density. Whereas, cell continued to reduce by the addition of doxorubicin until day 18. It may be possible that release of doxorubicin was completed by day 18 after which cell proliferation improved. PCL coating on doxorubicin controlled the release and hence gradual decrement of cell density (n=9)
On the other hand, the burst release of bicarbonate after 3 days makes the pH far too alkaline (pH ~8.9) and hence, reduces the osteoblast cell viability. After the initial burst release, the pH stabilizes and a gradual increment in osteoblast cell viability has been observed. Very flattened adhered osteoblast cells have been demonstrated by presence of bicarbonate at all time points on the disc surface.
3.4. Osteosarcoma cell material interaction by SEM and MTT
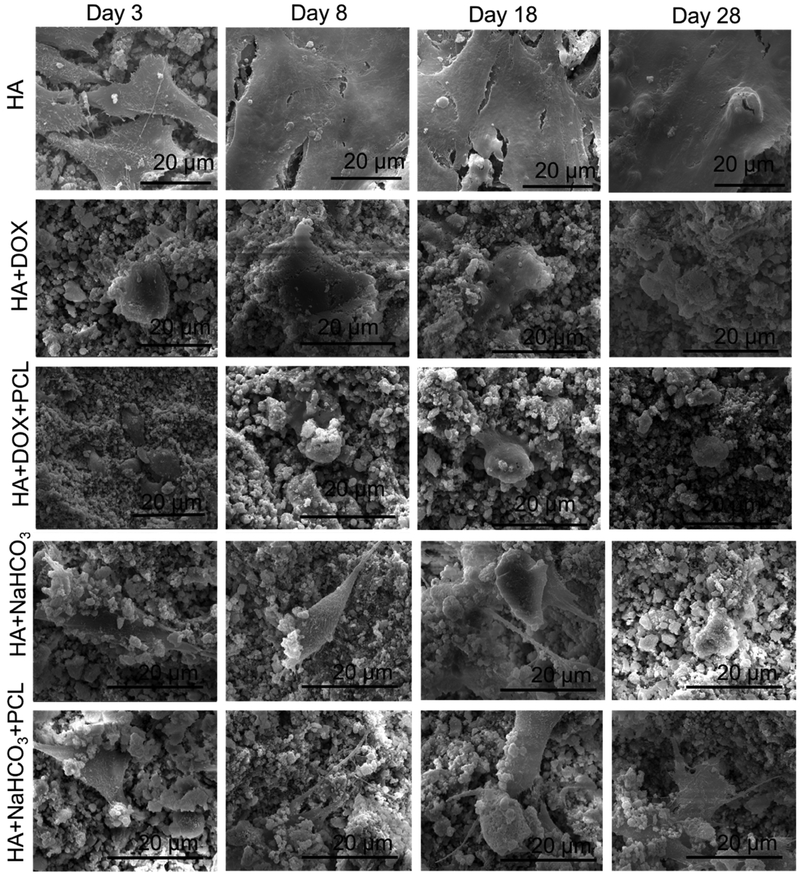
Figure 5 shows the proliferation and adherence of human osteosarcoma cells on the discs after 3, 8, 18 and 28 days of cell seeding. Flattened adhered layered cells have been noted on the control coated HA discs at all time points, showing the tendency of these cells to cause layering. Very similar to the osteoblast cell culture, reduced cell viability due to the doxorubicin incorporation is noted here too. The polymer incorporation on doxorubicin coating does not have any statistical effect on the osteoblast cell viability compared to no polymer coating. Here too, we observed enhanced cell density at 28-day time point which again can be attributed to almost completed doxorubicin release in the first 18 days. Circular dead cells have been presented on the surface of all discs containing doxorubicin.
Figure 5(a).
presents osteosarcoma cell viability on HA, HA and DOX, HA and bicarbonate samples, with or without PCL after 3, 8, 18 and 28 days of culture. Cell viability reduced by the release of bicarbonate for the entire duration of the experiment duration. A coating of PCL controlled the burst release of bicarbonate, leading to gradual enhancement of cell density. Whereas, cell continued to reduce by the addition of doxorubicin until day 18. It may be possible that release of doxorubicin was completed by day 18 after which cell proliferation improved. PCL coating on doxorubicin controlled the release and hence gradual decrement of cell density (n=9)
Similarly, the bicarbonate release reduces the osteoblast cell viability by making the pH alkaline. This report shows the efficacy of both the drug molecules in diminishing sarcoma cell proliferation and viability.
4. Discussions
Metallic implants made out of titanium alloys have been in use for load bearing orthopedic and dental applications for around a decade. It has been envisioned that modification of surface of metallic implants can limit the intrinsic disadvantages of bio inertness of these implants [26]. Augmentation of tissue integration in vitro and in vivo for these implants have been achieved by the calcium phosphate coatings [16]. Moreover, these coatings can serve as drug delivery vehicle [16], [15] for targeted delivery of anticancer drugs for limiting the chances of regrowth of the tumor from the excised sites.
Most anti-cancer drugs explored by the researchers have toxic effects on the surrounding healthy osseous tissues. For targeted delivery of those drugs, the effects on the by-stander drugs become more aggravated. Thus, our study aims to compare the efficacy of the release of bicarbonate, a naturally available biomolecule and doxorubicin, a commercially used drug for cancer treatments, on the proliferation and viability of osteosarcoma and osteoblast cells.
A burst release of biomolecules is very commonly observed from calcium phosphate-based drug delivery systems. The initial burst release in an uncontrolled way is not desired and does not serve the purpose of targeted delivery for effective treatments for a desired period of time [22]. Weak electrostatic interactions often times keep the desired drug molecules adsorbed on the surface of the calcium phosphate coatings [23]. One of the other objectives of this study is to develop a sustained release kinetics of these anti-cancer drugs from the calcium phosphate coated titanium alloy implants for load bearing applications.
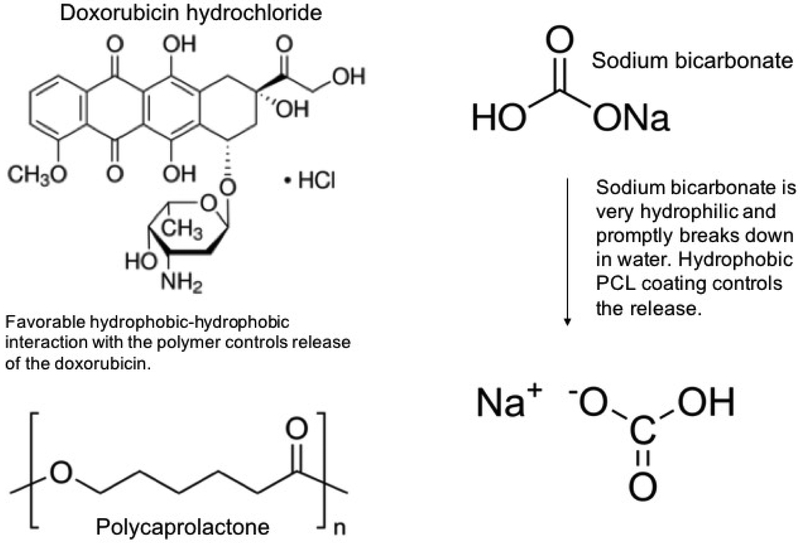
As observed from the figure 1, an initial burst release of 60% doxorubicin is noted after 3 days in PBS buffer at pH 7.4 at 37°C. After a gradual release of the drug until 18 days, most of the drug release is complete. It plateaus and hardly any release has been observed from day 18 to day 28. It leads us to conclude that the release is complete within the first 18 days. Biodegradable polymer coatings on calcium phosphate-based delivery systems have been quite useful in controlling release of drugs adsorbed by weak electrostatic interactions [16]. Figure 6 shows the chemical structure of the drug molecules and the expected interaction between PCL and doxorubicin in the physiological pH. A coating of PCL has been observed to sustain the release of doxorubicin to a great extent. The drug release often times is influenced by the matrix degradation, diffusion processes or by chemical mechanisms [22]. Usually drug releases from calcium phosphate-based delivery vehicles are diffusion based because diffusion has a faster kinetics than the process of matrix degradation [16]. Porous surfaces after the release shown in figure 3b reveal the degradation of the coating matrix compared to the before release morphologies (figure 3a). Thus, the doxorubicin release from the HA coatings is diffusion dominated in the presence of the polymer PCL owing to the interaction between the polymer and the drug. In the physiological pH, the doxorubicin drug remains in its’ hydrophobic undissociated form. This deprotonated form of the drug remains stable by forming a favorable hydrophobic-hydrophobic interaction with the hydrophobic polymer PCL, resulting in a controlled release in the presence of the polymer. Also, investigation of the surface roughness by analyzing the contact angles by a sessile drop method showed spread of solvent owing to the porous HA coating. However, the trend on different compositions showed an increase in contact angle from 18° for HA coating to 35° in presence of the hydrophobic drug, doxorubicin, whereas hydrophilic coating of sodium bicarbonate showed a decrease in contact angle to ∼6/8°. The polymer coating of polycaprolactone increased the contact angles for both the compositions, which also corelated with the release kinetics data, showed in figure 1.
Figure 6.
shows the structures of doxorubicin hydrochloride, polycaprolactone and sodium bicarbonate and their anticipated interaction in the physiological system. The hydrophobic drug doxorubicin interacts favourably with the hydrophobic polymer PCL and hence we observe a controlled release in the physiological system. On the other hand, the hydrophilic small drug molecule of sodium bicarbonate breaks down promptly in the presence of the aqueous surroundings to form carbonic acid. The hydrophobic polymer PCL, in this case, controls the interaction of sodium bicarbonate with the aqueous surroundings and hence controls the release [structures are taken from Sigma-Aldrich website].
The efficacy of the doxorubicin is demonstrated on the proliferation and viability of osteosarcoma and osteoblast cells for a span of 28 days. Both cell densities have been observed to reduce by the release of doxorubicin, showing the non-targeted efficiency of the drug in inhibition of cell growth. Layered osteosarcoma cells, resembling the structure of tumor cells have been observed on the surface of the bare HA coated Ti discs, whereas, only rounded dead cells are witnessed on doxorubicin coated discs at all time points. PCL coating makes the coated surface roughened and the flake like PCL coating simulate a 3D micro-environment, noted in figure 4(b) [22]. The MTT assay, performed to investigate the cytotoxicity of the drug on the cell adhesion and viability, presents significantly lower cell density on the doxorubicin coated discs compared to the control ones at all pre-determined time points. After a consistent gradual reduction in cell viability in the first 18 days, a sudden increment in cell density is observed at the 28-day time point. This can be explained by the previously noted release data where it shows an almost complete release of doxorubicin in the first 18 days, without the presence of the polymer. With the presence of the polymer PCL, a gradual reduction in cell viability is observed which is in consistence to the controlled release of the drug over the span of 28 days.
Figure 4(b).
shows SEM micrographs of human osteoblast cell proliferation and attachment on the surface of HA coated Ti alloy incorporated by bicarbonate and DOX, with or without PCL after 3, 8, 18 and 28 days of culture. Osteoblast cell viability decreased by the addition of DOX coating, as revealed by circular non-attached cells. Good flattened cells are seen on the other hand on the samples with bicarbonate.
The bicarbonate release in the physiological pH and temperature is measured by the sodium ion measurement in the release media by ICP-MS measurements. Figure 2 presents the cumulative percentage release of sodium ion from different dosages of sodium bicarbonate in the release media with the presence of PCL. Bicarbonate is a small hydrophilic molecule and a burst release of bicarbonate has been observed from the calcium phosphate coating in the first 3 days from all the bicarbonate concentrations. The application of the PCL polymer as a coating is applied to control the burst release of the biomolecule for the 28 days span. The unfavorable hydrophobic interaction of the PCL polymer with the hydrophilic bicarbonate molecule controls the release of the biomolecule in the physiological pH. As indicated from the release kinetics, the bicarbonate release is primarily diffusion dominated.
Bicarbonate molecules reverse the pH gradient in bone tumors and reduce incidences of tumor reoccurrences [27]. The abundance of hydrogen ions in the acidic environment helps in the progression of the tumor. Alkaline environment by the release of sodium bicarbonate reduces the acidosis helping in tumor suppression [28]. The normal tissues remain unaffected by the change in the pH of the vicinity. The efficacy of selective killing of osteosarcoma cells by the change of pH in presence of same dosage of bicarbonate has been demonstrated by the mono-culture of both osteoblast and osteosarcoma cells in this study. A reduction in cell viability has been observed at day 3, without the polymer. This is explained by the sudden increment of the pH by the burst release of bicarbonate within the first couple days. The osteoblast cells adapted fast to the pH change and a gradual increment in cell viability is noted afterwards. In the presence of PCL, with controlled release of sodium bicarbonate, a gradual increment in the osteoblast cell density is noted. The flake like rough 3D micro-environment created by the PCL coating is observed to promote cell growth and healthy flattened osteoblast cells are observed to cover the surface of the discs coated with the polymer.
Osteosarcoma cell density, on the other hand, is shown to reduce by presence of bicarbonate molecule. The pH changes in the vicinity of the surrounding cancer cells by the presence of bicarbonate promote cell death. These results show the efficacy of the controlled release of bicarbonate from calcium phosphate coated Ti alloy discs on targeted death of osteosarcoma and osteoblast cells.
Thus, our results suggest that the same dosage of doxorubicin is more efficient to inhibit the osteosarcoma cell proliferation compared to the bicarbonate. However, it does not perform a selective cell killing and has adverse effects on the normal osseous tissues as well. The bicarbonate on the other hand, performs a targeted killing of the cancer cells, with minimal adverse effects on the by-stander cells.
Thus, our study was able to effectively develop a load bearing calcium phosphate coated Ti disc implant which has the potential to be used as a delivery vehicle of bicarbonate molecule for implantation after limp salvage surgeries to inhibit recurrences of osteosarcoma.
5. Conclusions
The efficacy of the effects of targeted drug delivery of doxorubicin and bicarbonate on osteoblast and osteosarcoma cell viability and proliferation has been compared. Our data shows that the incorporation of doxorubicin failed to have any targeted reduction on cancer cell viability. Doxorubicin reduces cell proliferation and viability of both the osteoblast and osteosarcoma cells over the experimental period. The increment of the osteosarcoma cell viability after 28 days is attributed to the completed doxorubicin release from the HA coated Ti alloy without a polymer. The hydrophobic coating of polycaprolactone controlled the release of doxorubicin in the physiological pH and hence show a consistent reduction in both osteoblast and osteosarcoma cell viability. On the other hand, bicarbonate has been observed to reduce the osteosarcoma cell viability by increasing the pH of the vicinity media over the span of chosen 28 days. Reduced osteoblast cell viability is observed in the first 3 days with the burst release of bicarbonate and a sudden increment in the pH of the vicinity. The osteoblast cells, however, is shown to adapt to the environment and an increment of cell viability is noted over the 28 days. Thus, our study is able to develop a unique load bearing implant incorporated with bicarbonate and polymer for targeted reduction of cancer cell viability.
Figure 5(b).
shows SEM micrographs of human osteosarcoma cell proliferation and attachment on the surface of HA coated Ti alloy incorporated by bicarbonate and DOX, with or without PCL after 3, 8, 18 and 28 days of culture. Osteosarcoma cell viability decreased by the addition of DOX and bicarbonate coating, as revealed by circular non-attached cells at all time points.
Highlights.
Commercial cancer drug, doxorubicin and bicarbonate were compared on cancer treatment.
Same dosage of doxorubicin is efficient on reducing osteosarcoma cell viability.
Doxorubicin inhibits osteoblast cell viability, thus adversely affecting healthy bone growth.
Sodium bicarbonate selectively inhibits osteosarcoma cell growth.
No long-term adverse effects of bicarbonate on osteoblast cell viability.
6. Acknowledgements
Authors would like to acknowledge financial support from the National Institutes of Health under grant numbers R01 AR066361. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].Griffiths JR, “Are cancer cells acidic?,” Br. J. Cancer, vol. 64, no. 3, p. 425, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaupel P, Kallinowski F, and Okunieff P, “Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review,” Cancer Res, vol. 49, no. 23, pp. 6449–6465, 1989. [PubMed] [Google Scholar]
- [3].Wike-Hooley JL, Haveman J, and Reinhold HS, “The relevance of tumour pH to the treatment of malignant disease,” Radiother. Oncol, vol. 2, no. 4, pp. 343–366, 1984. [DOI] [PubMed] [Google Scholar]
- [4].Gatenby RA and Gawlinski ET, “Mathematical models of tumour invasion mediated by transformation-induced alteration of microenvironmental pH,” in Novartis Foundation Symposium, 2001, pp. 85–95. [DOI] [PubMed] [Google Scholar]
- [5].Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu Y-W, Gillies RJ, and Hendrix MJ, “Acidic pH enhances the invasive behavior of human melanoma cells,” Clin. Exp. Metastasis, vol. 14, no. 2, pp. 176–186, 1996. [DOI] [PubMed] [Google Scholar]
- [6].Gillies RJ, Raghunand N, Karczmar GS, and Bhujwalla ZM, “MRI of the tumor microenvironment,” J. Magn. Reson. Imaging, vol. 16, no. 4, pp. 430–450, 2002. [DOI] [PubMed] [Google Scholar]
- [7].Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, and Bhujwalla ZM, “Extracellular acidification alters lysosomal trafficking in human breast cancer cells,” Neoplasia, vol. 5, no. 6, pp. 533–545, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rofstad EK, Mathiesen B, Kindem K, and Galappathi K, “Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice,” Cancer Res, vol. 66, no. 13, pp. 6699–6707, 2006. [DOI] [PubMed] [Google Scholar]
- [9].Hayashi K et al. , “Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhymurium,” Cell Cycle, vol. 8, no. 6, pp. 870–875, 2009. [DOI] [PubMed] [Google Scholar]
- [10].Paridaens R et al. , “Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over,” J. Clin. Oncol, vol. 18, no. 4, pp. 724–724, 2000. [DOI] [PubMed] [Google Scholar]
- [11].Sledge GW et al. , “Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1193),” J. Clin. Oncol, vol. 21, no. 4, pp. 588–592, 2003. [DOI] [PubMed] [Google Scholar]
- [12].Kamba SA, Ismail M, Hussein-Al-Ali SH, Ibrahim TAT, and Zakaria ZAB, “In vitro delivery and controlled release of doxorubicin for targeting osteosarcoma bone cancer,” Molecules, vol. 18, no. 9, pp. 10580–10598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paterson AH, Powles TJ, Kanis JA, McCloskey E, Hanson J, and Ashley S, “Double-blind controlled trial of oral clodronate in patients with bone metastases from breast cancer.,” J. Clin. Oncol, vol. 11, no. 1, pp. 59–65, 1993. [DOI] [PubMed] [Google Scholar]
- [14].Kang Y et al. , “A multigenic program mediating breast cancer metastasis to bone,” Cancer Cell, vol. 3, no. 6, pp. 537–549, 2003. [DOI] [PubMed] [Google Scholar]
- [15].Bigi A and Boanini E, “Calcium Phosphates as Delivery Systems for Bisphosphonates,” J. Funct. Biomater, vol. 9, no. 1, p. 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bose S and Tarafder S, “Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review,” Acta Biomater., vol. 8, no. 4, pp. 1401–1421, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benghuzzi H, Wilson G, White J, and Tucci M, “The effects of sustained delivery of estrogen and demineralized bone matrix proteins on bone in ovariectomized female rats,” Biomed Sci Instrum, vol. 49, pp. 85–93, 2013. [PubMed] [Google Scholar]
- [18].Katti KS, “Biomaterials in total joint replacement,” Colloids Surf. B Biointerfaces, vol. 39, no. 3, pp. 133–142, 2004. [DOI] [PubMed] [Google Scholar]
- [19].Bigi A and Boanini E, “Functionalized biomimetic calcium phosphates for bone tissue repair.,” J. Appl. Biomater. Funct. Mater, vol. 15, no. 4, 2017. [DOI] [PubMed] [Google Scholar]
- [20].Roy M, Bandyopadhyay A, and Bose S, “Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants,” Surf. Coat. Technol, vol. 205, no. 8, pp. 2785–2792, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roy M, Bandyopadhyay A, and Bose S, “Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant,” J. Biomed. Mater. Res. B Appl. Biomater, vol. 99, no. 2, pp. 258–265, 2011. [DOI] [PubMed] [Google Scholar]
- [22].Tarafder S, Nansen K, and Bose S, “Lovastatin release from polycaprolactone coated β-tricalcium phosphate: Effects of pH, concentration and drug–polymer interactions,” Mater. Sci. Eng. C, vol. 33, no. 6, pp. 3121–3128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tarafder S and Bose S, “Polycaprolactone-coated 3D printed tricalcium phosphate scaffolds for bone tissue engineering: in vitro alendronate release behavior and local delivery effect on in vivo osteogenesis,” ACS Appl. Mater. Interfaces, vol. 6, no. 13, pp. 9955–9965, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].“Doxorubicin FDA dosage.”.
- [25].Jeong Y-I et al. , “Doxorubicin-incorporated nanoparticles composed of poly (ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro,” Colloids Surf. B Biointerfaces, vol. 79, no. 1, pp. 149–155, 2010. [DOI] [PubMed] [Google Scholar]
- [26].Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, and Bose S, “In vivo response of laser processed porous titanium implants for load-bearing implants,” Ann. Biomed. Eng, vol. 45, no. 1, pp. 249–260, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Robey IF et al. , “Bicarbonate increases tumor pH and inhibits spontaneous metastases,” Cancer Res., vol. 69, no. 6, pp. 2260–2268, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Swietach P, Vaughan-Jones RD, Harris AL, and Hulikova A, “The chemistry, physiology and pathology of pH in cancer,” Phil Trans R Soc B, vol. 369, no. 1638, p. 20130099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]