Abstract
Aim
EphB3 and dysadherin are involved in tumorigenesis and progression of many neoplasms. However, the roles of EphB3 and dysadherin in extrahepatic cholangiocarcinoma (ECC) remain to be revealed. In this study, we aimed to evaluate the expression of EphB3 and dysadherin, and investigate their clinicopathological significance in ECC.
Methods
We examined EphB3 and dysadherin expression in 100 ECC, 30 peritumoral tissues, 10 adenoma and 15 normal biliary tract tissues using EnVision immunohistochemistry. The relationship between EphB3 or dysadherin expression and clinicopathological features was evaluated using the χ2 test or Fisher’s exact test. The overall survival of ECC patients was analyzed using Kaplan-Meier univariate survival analysis and Log rank tests.
Results
We found that EphB3 expression was significantly down-regulated and dysadherin expression was significantly up-regulated in ECC tissues compared with normal tissues (P < 0.01). EphB3 expression was negatively correlated with dysadherin expression in ECC (P < 0.01). The positive rate of EphB3 expression and negative rate of dysadherin expression was significantly higher in patients with well-differentiated type, no lymph node metastasis, no surrounding tissues and organs invasion, early TNM stages (I + II) and radical resection (P < 0.01). The survival of ECC patients with positive EphB3 or negative dysadherin expression was significantly longer than patients with negative EphB3 or positive dysadherin expression (P < 0.01). Cox multivariate analysis demonstrated that negative EphB3 or positive dysadherin expression were independent poor prognostic factors in ECC patients. The ROC curves suggested that EphB3 and dysadherin combined diagnostic efficacy (AUC=0.688, 95%CI: 0.603-0.772) was significantly higher EphB3 diagnostic efficacy (AUC=0.654, 95%CI: 0.564-0.743) or dysadherin diagnostic efficacy (AUC=0.648, 95%CI: 0.558-0.737) alone.
Conclusion
EphB3 and dysadherin are involved in the carcinogenesis and progression of ECC, and ECC patients with negative EphB3 or positive dysadherin expression have a poor prognosis.
Keywords: extrahepatic cholangiocarcinoma, EphB3, dysadherin, prognosis, clinicopathological significance
Introduction
Cholangiocarcinoma (CCA), a malignant neoplasm arising from epithelial cells of the biliary tract, occurs at any location along the biliary tree.1 CCA is an aggressive malignancy and has a poor prognosis with an only 10% 5-year survival rate.2 There are a number of established risk factors associated with CCA tumorigenesis, such as primary sclerosing cholangitis, congenital hepatic fibrosis, Caroli disease, choledochal cysts, biliary stone disease, chronic infection with liver flukes.3 Based on arising anatomical location of the tumor, CCA is classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC). ECC is the most common CCA and accounts for approximately 90% of CCA.1,4 The typical clinical symptom of ECC is features of biliary obstruction including jaundice, pale stool, dark urine and pruritus.5–7 The diagnosis of ECC is based on clinical manifestation, blood test, imaging, and histology and cytology.5 Imaging is the main diagnostic method for ECC, including ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), endoscopic retrograde cholangiopancreatography (ERCP).6 Current treatment strategies for ECC include surgery, systemic chemotherapy and targeted radiation.8 Surgical resection is the only curative treatment approach for ECC. Chemotherapy and radiotherapy have no definitive therapeutic effect for unresectable ECC. Due to the lack of early clinical manifestations and reliable diagnostic biomarkers, most of ECC patients are diagnosed at an advanced stage and lost the opportunity to receive radical surgery so that these patients have a poor clinical outcome.9 Therefore, it is imperative to discover new specific diagnostic biomarkers for the early diagnosis of ECC.
The Ephrin (Eph) receptors are the largest subfamily of receptor tyrosine kinase superfamily in humans. According to their structure and their affinity for the corresponding ephrin ligands, these receptors are divided into A- and B-types, consisting of EphA1-8, EphA10, EphB1-4 and EphB6.10 All Eph receptors belong to single transmembrane protein with intrinsic tyrosine activity. The Eph receptors play an important role in regulating angiogenesis, tumorigenesis, cell attachment, shape and motility.11 As a member of the Eph family, EphB3 is also involved in many physiological and pathological processes in different organ systems.12,13 EphB3 expresses in a variety of organ systems, including lung, liver, kidney, intestine muscle, heart, and brain. Recently, several studies have demonstrated that EphB3 is associated with tumorigenesis of various types of human cancers, such as colorectal cancer, gastric cancer, head and neck tumor, non-small-cell lung cancer (NSCLC), ovarian cancer and prostate cancer.14–20 These studies revealed that EphB3 is closely related to pathogenesis, progression and biological behaviors of above malignant lesions. Nevertheless, there has been no study regarding the role of EphB3 in ECC.
Dysadherin, also known to FXYD5 that interacts with Na, K-ATPase and regulates its properties, is a cancer-associated membrane glycoprotein composed of 178 amino acids.21,22 Dysadherin expresses in a limited number of normal cell types, including lymphocytes, endothelial cells, and basal cells of stratified squamous epithelium.23 Dysadherin is involved in modulating ion transport and its expression is found in kidney, duodenum, spleen, and lung.21 Dysadherin expression is up-regulated in a variety of human cancer cells, and dysadherin can promote cancer metastasis and progression via down-regulating E-cadherin mediated cell-cell adhesion and up-regulating vimentin.22,24–28 Previous studies have revealed that overexpression of dysadherin is related to metastasis and poor clinical outcome of many human different cancer types.22,24–28 However, the relationship between dysadherin expression and ECC is never reported.
Therefore, we examined EphB3 and dysadherin expression in ECC using immunohistochemistry and analyzed their clinicopathological significance and prognostic values in this study.
Materials and Methods
Case Selection
This study was approved by the Ethics Committee for Human Research, Central South University, and was performed according to the Declaration of Helsinki. This study is exempt from informed consent since it is a retrospective study and the data collection and analysis were carried out without disclosing patients’ identities. Tissue specimens were collected from the Second and Third Xiangya Hospitals, Central South University from January 2001 to December 2013. These specimens included 100 ECC, 30 peritumoral tissues, 10 bile tract adenoma, and 15 normal bile tract tissues. The 15 normal bile tract tissues were obtained from contributors of liver transplantation who were voluntary civilian organ donors. All specimens were histologically confirmed by two pathologists. Tumors were restaged based on the 7th TNM Classification of Malignant Tumors and classified following the World Health Organization tumor classification system.29 Tumor differentiation degrees were defined based on the World Health Organization criteria. We collected the survival information of the 100 patients with ECC via letter or telephone interviews. The follow-up time was 30 months, and patients who survived over 30 months were included in the analysis as censored cases.30
Main Reagents
Rabbit anti-human EphB3 and dysadherin polyclonal antibodies were purchased from Dako Corporation (Carpentaria, CA, USA). EnVisionTM Detection Kit was purchased from Dako Laboratories (CA, USA).
Immunohistochemistry
EnVision immunohistochemistry was conducted in accordance with the user manual. Briefly, 4 μm-thick paraffin slices were cut and then dewaxed. The slices were treated with 3% H2O2 for 15 min. Next, Heat-induced epitope retrieval was performed with sodium citrate buffer at 96°C for 30 min. The slices were soaked in PBS for 3 × 5 min and then incubated with the primary antibody (1:100 dilution) at 37°C for 2 hrs. Then, the slices were incubated with solution A for 30 min, followed by DAB staining and haematoxylin counter-staining. Finally, the slices were dehydrated, soaked in xylene, and mounted with neutral balsam. Two observers independently examined five hundred cells from ten random fields of per section, and the percentage of positive cells was counted. The staining evaluation was based on an average percentage of positive cells from these two observers. Cases with an average percentage of positive cells ≥25% were classed as positive expression, while other cases were classed as negative expression.30–32
Statistical Analysis
We analyzed data with the SPSS 17.0 (statistical package for the Social Sciences, Version 17.0). We used the Chi-squared test or Fisher’s exact test to evaluate the relationships between EphB3 and dysadherin and clinicopathological factors. Kaplan-Meier univariate survival analysis and Log rank tests were used to analyze the overall survival of ECC patients. Univariate analysis and multivariate analysis with the Cox proportional hazards model were applied, and the 95% confidence interval was calculated. A probability level of P < 0.05 was considered statistically significant.
Results
Characteristics of Patients
As shown in Table 1, the 100 ECC patients included 61 men and 39 women, and their ages varied from 35 to 80 (58.8 ±10.2) years. Histologically, the 100 ECCs consisted of 31 well-differentiated tumors (31.0%), 34 moderately differentiated tumors (34.0%) and 35 poorly differentiated tumors (35.0%). Among the 100 patients with ECC, 67% patients occurred invasion of region tissues and/or organs; 38.0% patients presented regional lymph node metastasis; and 31.0% patients had bile stone. Based on TNM staging, 35 ECC patients were classified as stage I + II, 38 ECC patients were classified as stage III and 27 ECC patients were classified as stage IV. Among the 100 ECC patients, 54 patients (54%) received radical resection; 36 patients (36%) received palliative resection; and 10 patients (10%) only received a biopsy.
Table 1.
Correlations of EphB3 and Dysadherin Protein Expression with the Clinicopathological Characteristics of ECC
| CPC | Number of Patients(n) | EphB3 | Dysadherin | ||||
|---|---|---|---|---|---|---|---|
| Pos No. (%) | χ2 | P | Pos No. (%) | χ2 | P | ||
| Age (year) | |||||||
| ≤45 years | 17 | 10 (58.8) | 2.380 | 0.123 | 7 (41.2) | 1.581 | 0.209 |
| >45 years | 83 | 32 (38.6) | 48 (57.8) | ||||
| Gender | |||||||
| Male | 61 | 24 (39.3) | 0.453 | 0.501 | 34(55.7) | 0.034 | 0.853 |
| Female | 39 | 18 (46.2) | 21 (53.8) | ||||
| Differentiation | |||||||
| Well | 31 | 21 (67.7) | 17.373 | 0.000 | 9(29.0) | 15.390 | 0.000 |
| Moderately | 34 | 15 (44.1) | 19 (55.9) | ||||
| Poorly | 35 | 6 (17.1) | 27 (77.1) | ||||
| Tumor size | |||||||
| ≤3cm | 62 | 27 (43.5) | 0.161 | 0.689 | 33 (53.2) | 0.208 | 0.649 |
| >3cm | 38 | 15 (39.5) | 22 (57.9) | ||||
| Tumor location | |||||||
| Hilar site | 27 | 9 (33.3) | 1.850 | 0.397 | 21 (77.8) | 9.135 | 0.010 |
| Hepatic duct | 4 | 1 (25.0) | 3 (75.0) | ||||
| Distal duct | 69 | 32 (46.4) | 31 (44.9) | ||||
| Bile stone | |||||||
| Absent | 69 | 31 (44.9) | 0.783 | 0.376 | 36 (52.2) | 0.718 | 0.397 |
| Present | 31 | 11 (35.5) | 19 (61.3) | ||||
| Lymph node metastasis | |||||||
| Negative | 62 | 37 (59.7) | 20.930 | 0.000 | 24 (38.7) | 17.494 | 0.000 |
| Positive | 38 | 5 (13.2) | 31 (81.6) | ||||
| Invasion | |||||||
| Negative | 33 | 21 (63.6) | 9.465 | 0.002 | 9 (27.3) | 15.300 | 0.000 |
| Positive | 67 | 21 (31.3) | 46 (68.7) | ||||
| TNM stage | |||||||
| I + II | 35 | 23 (65.7) | 21.460 | 0.000 | 8 (22.9) | 27.608 | 0.000 |
| III | 38 | 17 (44.7) | 23 (60.5) | ||||
| IV | 27 | 2 (7.4) | 24 (88.9) | ||||
| Surgery | |||||||
| Radical | 54 | 32 (59.3) | 14.371 | 0.001 | 20 (37.0) | 15.383 | 0.000 |
| Palliative | 36 | 8 (22.2) | 27 (75.0) | ||||
| Biopsy | 10 | 2 (20.0) | 8 (80.0) | ||||
Abbreviations: CPC, Clinicopathological characteristics; Pos No., Positive Number.
Thirty peritumoral tissues were obtained from 20 male patients (66.6%) and 10 female patients (33.3%), their ages varied from 35 to 72 (48.5 ± 9.2) years. Histologically, among the 30 peritumoral tissues, 12 were normal tissues, 8 presented mild dysplasia, 6 presented moderately dysplasia and 4 presented severe dysplasia. Ten bile tract adenoma tissues were obtained from 6 male patients (60.0%) and 4 female patients (40.0%) whose ages varied from 33 to 70 (46.7 ± 10.2) years. Histologically, among the 10 bile tract adenoma tissues, 6 were simple adenoma tissues, 2 presented mild dysplasia and 2 presented moderate to severe dysplasia. Fifteen normal biliary tract tissues were obtained from contributors of liver transplantation and were all normal biliary tract tissues based on pathological examination.
EphB3 and Dysadherin Protein Expression in ECC, Peritumoral Tissues, Adenoma, and Normal Tissues
To study the expression of EphB3 and dysadherin in ECC, peritumoral tissues, adenoma, and normal tissues, EnVision immunohistochemistry was performed. As shown in Figures 1 and 2, immunohistochemical staining revealed that positive EphB3 expression was observed at the cytoplasm and positive dysadherin expression was observed at the cytomembrane and cytoplasm. Among the 100 cases of ECC, EphB3 and dysadherin were positively expressed in 42 (42%) cases and 55 (55%) cases, respectively (Table 2). Among the 10 cases of adenomas, EphB3 and dysadherin were positively expressed in 8 (80%) cases and 3 (30%) cases, respectively. All 15 normal tissues showed EphB3 positive expression and dysadherin negative expression. As presented in Table 2, ECC tissues exhibited a significantly lower positive rate of EphB3 expression and higher positive rate of dysadherin expression compared with normal tissues (P < 0.01). Moreover, Peritumoral tissues and adenoma with negative EphB3 and/or positive dysadherin expression exhibited moderate to severe dysplasia.
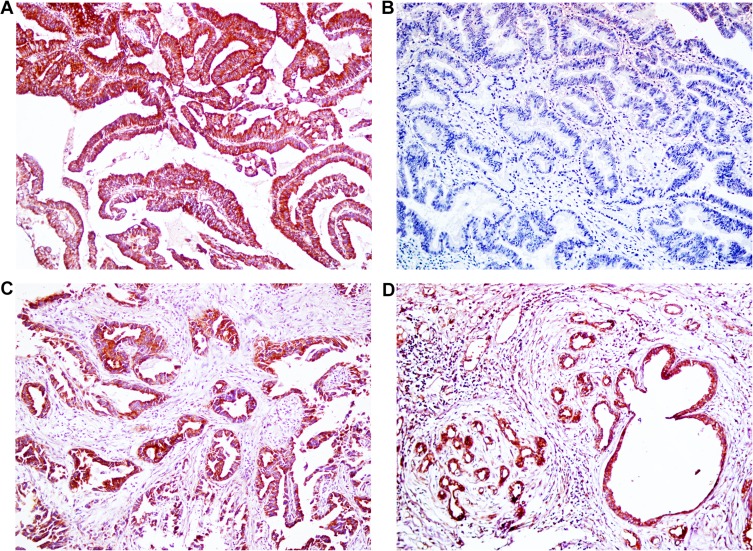
Figure 1.
Immunohistochemical staining of EphB3, ×200. (A) Positive expression of EphB3, well differentiated ECC. (B) Negative expression of EphB3, moderately- differentiated ECC. (C) Positive expression of EphB3, peritumoral tissues. (D) Positive expression of EphB3, adenoma.
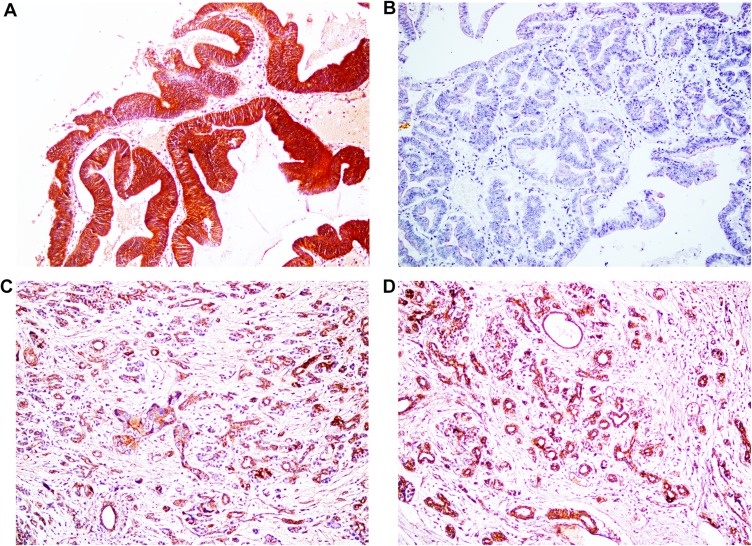
Figure 2.
Immunohistochemical staining of dysadherin, ×200. (A) Positive expression of dysadherin, moderately differentiated ECC. (B) Negative expression of dysadherin, well differentiated ECC. (C) Positive expression of dysadherin, peritumoral tissues. (D) Positive expression of dysadherin, adenoma.
Table 2.
Comparison of EphB3 and Dysadherin Expression in Normal Tissue, Adenoma, Peritumoral Tissue and ECC
| Tissue Type | Number of Patients (N) | EphB3 Positive (%) | Dysadherin Positive (%) |
|---|---|---|---|
| ECC | 100 | 42 (42.0) | 55 (55.0) |
| Peritumoral tissues | 30 | 17(56.7) | 11 (36.7) |
| adenoma | 10 | 8 (80.0)* | 3(30.0) |
| Normal tissues | 15 | 15 (100.0)** | 0 (0.0)** |
Notes: Compared to ECC: *P < 0.05; **P < 0.01.
Abbreviation: ECC, extrahepatic cholangiocarcinoma.
We further analyzed the relationship between EphB3 expression and dysadherin expression in ECC by χ2 test. Among the 42 cases with positive EphB3 expression, 10 cases showed positive dysadherin expression. Among the 58 cases with negative EphB3 expression, 13 cases exhibited negative dysadherin expression. EphB3 expression was negatively correlated with dysadherin expression in ECC (Table 3, P < 0.01).
Table 3.
The Association Between EphB3 Expression and Dysadherin Expression in ECC
| EphB3 | Dysadherin | Total | ||
|---|---|---|---|---|
| − | + | |||
| − | 13 | 45 | 58 | |
| + | 32 | 10 | 42 | |
| Total | 45 | 55 | 100 | |
Notes: χ2 =28.464, P = 0.000.
Abbreviations: −, negative expression; +, positive expression.
Association of EphB3 and Dysadherin Expression with Clinicopathological Features in ECC
We further evaluated the potential correlation between EphB3 or dysadherin expression and clinicopathological parameters of the 100 patients with ECC. EphB3-positive expression was significantly correlated to well-differentiated type, the negativity of lymph node metastasis, the negativity of surrounding tissues and organs invasion and early TNM stage (I + II) (P < 0.01). The patients received radical resection showed a higher positive rate of EphB3 expression than the patients underwent no resection (biopsy only) (P < 0.01). Inversely, dysadherin-positive expression was significantly correlated to poorly differentiated type, the positivity of lymph node metastasis, the positivity of surrounding tissues and organs invasion, and advanced TNM stage (III or IV) (P < 0.01). The patients received radical resection showed a lower positive rate of dysadherin expression than the patients underwent no resection (biopsy only) (P < 0.01). However, there was no significant correlation between expression of EphB3 or dysadherin and other clinicopathological parameters including gender, age, tumor size, and the existence of biliary stone (P > 0.05) (Table 1).
EphB3 and Dysadherin Protein Expression Correlated with Overall Survival in Patients with ECC
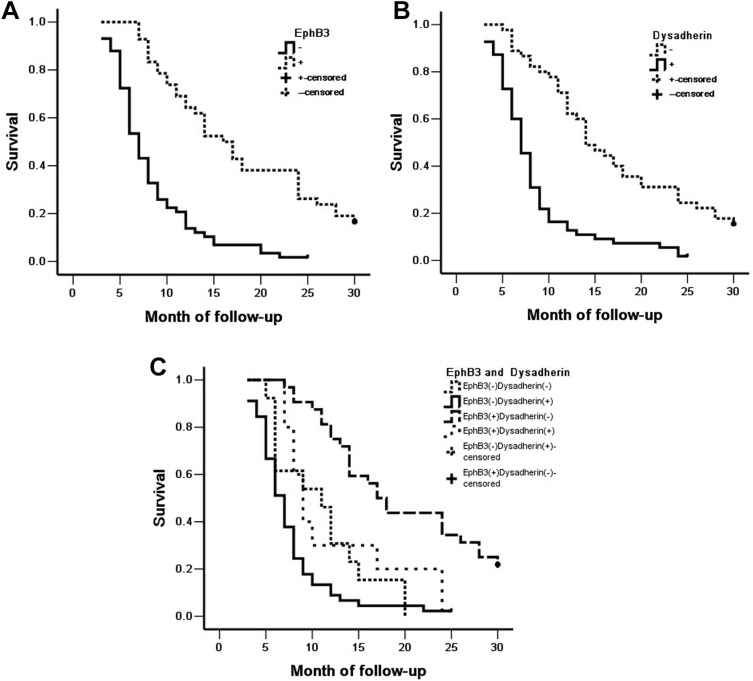
Overall survival was analyzed in the 100 patients with ECC. Among the 100 patients, 59 patients survived no longer than 12 months, 24 patients survived no longer than 24 months, 9 patients survived no longer than 30 months, and 8 patients who survived over 30 months were included in the analysis as censored cases. As shown in Table 4, the average overall survival time of ECC patients was closely related to several clinicopathological factors, including tumor differentiation, lymph node metastasis, invasion of surrounding tissues and organs, TNM stage and surgical procedure (P < 0.01) (Table 4). Kaplan-Meier survival curves showed that the overall survival time of patients with EphB3 positive or dysadherin negative expression was significantly longer than patients with negative EphB3 or positive dysadherin expression (P < 0.01) (Table 4, Figure 3). Furthermore, we defined four groups by the expression of EphB3 and dysadherin; positive expression of both EphB3 and dysadherin (+/+), positive and negative (+/−); negative and positive (−/+), and both negative (−/−). Kaplan-Meier survival curves revealed that the group with EphB3 positive and dysadherin negative expression had longest overall survival time than other groups, and the group with EphB3 negative and dysadherin positive expression had shortest overall survival time than other groups (Table 4, Figure 3).
Table 4.
Correlations of Clinicopathological Characteristics, EphB3 and Dysadherin Expression with the Mean Survival in Patients with ECC
| Group | Number of Patients (n) | Median Survival (Month) | Log-Rank χ2 | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 61 | 12.67 (3–30) | 0.001 | 0.980 |
| Female | 39 | 12.59 (4–30) | ||
| Age (year) | ||||
| ≤45 | 17 | 13.82 (3–30) | 0.667 | 0.414 |
| >45 | 83 | 12.10 (3–30) | ||
| Differentiation | ||||
| Well | 31 | 18.46 (5–30) | 27.655 | 0.000 |
| Moderately | 34 | 11.41 (3–30) | ||
| Poorly | 35 | 7.97 (3–30) | ||
| Tumor size | ||||
| ≤3cm | 62 | 12.62 (3–30) | 0.235 | 0.628 |
| >3cm | 38 | 12.03 (5–30) | ||
| TNM stage | ||||
| I + II | 35 | 18.57 (7–30) | ||
| III | 38 | 11.05 (3–30) | 57.569 | 0.000 |
| IV | 27 | 6.26 (3–13) | ||
| Lymph node metastasis | ||||
| No | 62 | 15.52 (4–30) | 39.001 | 0.000 |
| Yes | 38 | 7.18 (3–25) | ||
| Invasion | ||||
| No | 33 | 17.52 (4–30) | 17.399 | 0.000 |
| Yes | 67 | 9.87 (3–30) | ||
| Surgery | ||||
| Radical | 54 | 16.62 (3–30) | 48.388 | 0.000 |
| Palliative | 36 | 7.58 (4–24) | ||
| Biopsy | 10 | 6.90 (3–14) | ||
| EphB3 | ||||
| − | 58 | 8.35 (3–25) | 37.806 | 0.000 |
| + | 42 | 17.88 (7–30) | ||
| Dysadherin | ||||
| − | 45 | 17.11 (5–30) | 32.224 | 0.000 |
| + | 55 | 8.46 (3–25) | ||
| EphB3 and Dysadherin | ||||
| EphB3(−) and Dysadherin (−) | 13 | 10.92(5–20) | 48.278 | 0.000 |
| EphB3(+) and Dysadherin (−) | 32 | 19.63(7–30) | ||
| EphB3(−) and Dysadherin (+) | 45 | 7.60(3–25) | ||
| EphB3(+) and Dysadherin (+) | 10 | 12.30(7–24) |
Abbreviations: −, negative expression; +, positive expression.
Figure 3.
Kaplan-Meier curves for ECC. (A) Positive and negative expression of EphB3 in ECC. (B) Positive and negative expression of dysadherin in ECC. (C) EphB3 and dysadherin expression in ECC.
According to univariate analysis and multivariate analysis using Cox’s proportional hazards model, this study found that several clinicopathological parameters negatively correlated with overall survival and positively correlated with mortality, including poorly differentiated type, the positivity of lymph node metastasis, the positivity of surrounding tissues and organs invasion, and advanced TNM stages (III or IV), which are risk factors and independent prognostic predictors (Tables 5 and 6). Negative EphB3 expression or positive dysadherin expression negatively correlated with overall survival and positively correlated with mortality, which are risk factors and independent prognostic predictors (Tables 5 and 6).
Table 5.
Univariate Cox Regression Analysis of Survival Rate in Patients with ECC and EphB3 and Dysadherin Expression
| Groups | Factors | B | SE | Wald | P | HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Differentiated degree | Well/moderately/poorly | 0.659 | 0.136 | 23.466 | 0.000 | 1.933 | 1.480 | 2.523 |
| Tumor size | ≤3cm/>3cm | 0.099 | 0.214 | 0.212 | 0.645 | 1.104 | 0.725 | 1.680 |
| Lymph node metastasis | No/Yes | 1.285 | 0.228 | 31.705 | 0.000 | 3.615 | 2.311 | 5.655 |
| Invasion | No/Yes | 0.912 | 0.237 | 14.841 | 0.000 | 2.489 | 1.565 | 3.957 |
| TNM stage | I/II/III/IV | 1.023 | 0.158 | 41.673 | 0.000 | 2.782 | 2.039 | 3.795 |
| Surgery | Radical/Palliative/Biopsy | 0.883 | 0.149 | 35.301 | 0.000 | 2.417 | 1.807 | 3.234 |
| EphB3 | −/+ | −1.323 | 0.239 | 30.720 | 0.000 | 0.266 | 0.167 | 0.425 |
| Dysadherin | −/+ | 1.181 | 0.229 | 26.595 | 0.000 | 3.257 | 2.079 | 5.102 |
Abbreviations: −, negative expression; +, positive expression; HR, hazard ratio; CI, confidence interval.
Table 6.
Multivariate Cox Regression Analysis of Survival Rate in Patients with ECC and EphB3 and Dysadherin Expression
| Groups | Factors | B | SE | Wald | P | HR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Differentiated degree | Well/moderately/poorly | 0.615 | 0.155 | 15.724 | 0.000 | 1.849 | 1.364 | 2.505 |
| Tumor size | ≤3cm/>3cm | 0.202 | 0.233 | 0.756 | 0.385 | 1.224 | 0.776 | 1.932 |
| Lymph node metastasis | No/Yes | 1.181 | 0.277 | 18.216 | 0.000 | 3.258 | 1.894 | 5.604 |
| Invasion | No/Yes | 0.829 | 0.349 | 5.659 | 0.017 | 2.292 | 1.157 | 4.539 |
| TNM stage | I/II/III/IV | 0.721 | 0.244 | 8.695 | 0.003 | 2.056 | 1.273 | 3.318 |
| Surgery | Radical/Palliative/Biopsy | 0.495 | 0.182 | 7.395 | 0.007 | 1.640 | 1.148 | 2.343 |
| EphB3 | −/+ | −0.826 | 0.290 | 8.131 | 0.004 | 0.438 | 0.248 | 0.772 |
| Dysadherin | −/+ | 0.739 | 0.264 | 7.824 | 0.005 | 2.093 | 1.247 | 3.513 |
Abbreviations: −, negative expression; +, positive expression; HR, hazard ratio; CI, confidence interval.
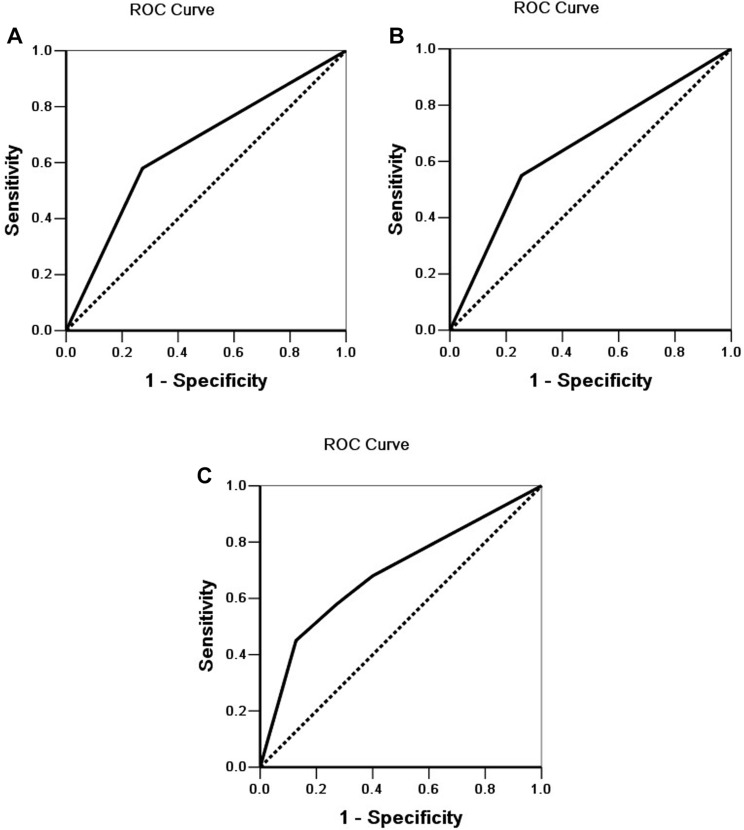
Lastly, we depicted the receiver operating characteristic (ROC) curves to evaluate the diagnostic efficacy of EphB3 expression, dysadherin expression, and EphB3 and dysadherin expression, respectively. The AUC of EphB3 was 0.654 (95% CI: 0.564–0.743), the AUC of dysadherin expression was 0.648 (95% CI: 0.558–0.737), and the AUC of EphB3 and dysadherin expression was 0.688 (95% CI:0.603–0.772) (Figure 4). Our results suggested that EphB3 and dysadherin combined diagnostic efficacy was significantly higher EphB3 diagnostic efficacy or dysadherin diagnostic efficacy alone.
Figure 4.
ROC of diagonal segments. (A) ROC of diagonal segments is produced by ties of EphB3 in ECC. (B) ROC of diagonal segments is produced by ties of dysadherin in ECC. (C) ROC of diagonal segments is produced by ties of EphB3 and dysadherin in ECC.
Discussion
ECC is an aggressive malignancy and has a poor prognosis. In this study, our data showed that the average survival time of patients with early TNM stages (I + II) is significantly longer than patients with advanced stages (III or IV). Additionally, the average survival time of patients received radical surgery are significantly longer than patients received a biopsy. These results demonstrated that early diagnosis is essential to improve the clinical prognosis of ECC. However, most patients with ECC are diagnosed at an advanced stage due to the lack of specific clinical manifestations and diagnostic biomarkers in the early stage. Hence, it is very urgent to find new specific diagnostic markers for early diagnosis of ECC.
Although EphB3 and dysadherin are related to the progression and prognosis of various human cancers, no studies have investigated their expression and biological roles in ECC. Thus, we assessed EphB3 and dysadherin expression in ECC and non-tumor tissues using immunohistochemistry and evaluated their correlations with clinicopathological parameters and survival. Significantly decreased EphB3 expression and significantly increased dysadherin expression was observed in ECC, compared with normal tissues. Additionally, negative EphB3 expression and positive dysadherin expression were closely associated with poorly differentiated type, the positivity of surrounding tissues and organs invasion, the positivity of lymph node metastasis, advanced TNM stages, and poor prognosis in ECC. To our knowledge, this is the first study to report the correlation between EphB3 or dysadherin expression and clinicopathologic characteristics and survival in ECC patients.
Many Eph receptors and ephrin ligands are associated with the development and progression of several human cancers. As one of Eph receptors family, EphB3 is also involved in tumorigenesis and progression of certain human cancers, such as colorectal cancer, ovarian serous carcinomas, prostate cancer, papillary thyroid cancer, head and neck squamous cell carcinoma, and NSCLC.15–20 It has been reported that EphB3 is over-expressed in normal prostate cell lines compared with prostate tumor cell lines.33 Previously studies demonstrated that EphB3 expression is down-regulated in ovarian serous carcinoma and colorectal cancer samples.15,18 Consistent with these previous studies, our study showed that EphB3 were significant down-expression in ECC compared with normal tissues, suggesting that EphB3 may be involved in tumorigenesis of ECC. Moreover, several studies have revealed that EphB3 is related to clinical prognosis and clinicopathological characteristics of several human cancers. EphB3 acts as a tumor suppressor in colorectal cancer and ovarian serous carcinomas.15,18 Chiu reported that overexpression of EphB3 in HT-29 colorectal cancer cells inhibits tumor growth and EphB3 expression levels is significantly decreased in advanced Dukes’ stage of human colorectal cancer.20 Xuan found that the EphB3 expression level was negatively correlated to the depth of tumor invasion, lymph node metastasis, TNM stage and differentiation of colorectal cancer, and the overall survival of patients with high EphB3 expression is significantly longer than patients with negative or weak EphB3 expression.15 Gao also reported that positive EphB3 expression is negatively related to histological grade and FIGO stage of ovarian serous carcinomas.18 Similarly, our study found that positive rates of EphB3 expression were significantly higher in cases with well-differentiation, no surrounding tissues and organs invasion, no lymph node metastasis and early TNM stages (I + II), and the patients with positive EphB3 expression exhibited longer survival time than patients with negative EphB3 expression. Thus, EphB3 may function as a tumor suppressor in ECC, which needs further study to identify its potential mechanism. However, EphB3 expression is significantly elevated in NSCLC and papillary thyroid cancer, and EphB3 can promote cell migration and metastasis of papillary thyroid cancer and lung cancer, which contradictory to our results.17,19 This may be due to the organ specificity. Therefore, the effect of EphB3 on development and progression of cancer is divergent and dependent on cancer types.
Dysadherin is a protein that is involved in tumorigenesis by down-regulating cell-cell adhesion and up-regulating chemokine production.34 Dysadherin expression has been studied in various human cancer types and is a mark of poor prognosis of these cancers.22,26,28,35 In most human cancers studies, the increased dysadherin expression is associated with decreased E-cadherin expression and reflects tumor aggressiveness.34,35 Several studies showed that dysadherin was frequently expressed in cancer cells, but not expressed in the cells of corresponding normal tissues.22,26,36 In agreement with previous reports, we found that positive dysadherin expression in ECC was higher compared to normal tissues and dysadherin was not detected in normal biliary tract tissue, indicating that dysadherin may be involved in the oncogenesis of ECC. To further identify the role of dysadherin in ECC, we analyzed the relationship between dysadherin expression and several clinicopathological features including tumor differentiation, lymph node metastasis, invasion, TNM stage, and surgical procedure. Previous studies have shown that dysadherin can promote cancer metastasis and progression of several human cancers, such as colorectal cancer, ovarian carcinomas, gastric cancer.22,24,25,34 Likewise, our data demonstrated that dysadherin positive expression was higher in the cases with poor differentiation, lymph node metastasis, invasion, advanced TNM stages, suggesting that dysadherin may be contributed to metastasis and development of ECC. Furthermore, the current study also revealed that dysadherin positive expression had a significant effect on ECC patient survival and was significantly correlated to poor prognosis of ECC patient, which is consistent with previous reports.22,26,28,35 Thus, dysadherin may play an unneglected role in carcinogenesis and progression of ECC, which needs further in vivo and in vitro study to explore its underlying mechanism.
In this study, we found that EphB3 expression was significantly down-regulated and dysadherin expression was significantly up-regulated in ECC. In peritumoral tissues and adenoma tissues, negative EphB3 or positive dysadherin expression was positively related to dysplasia of biliary tract epithelia. Thus, down-expression of EphB3 or overexpression of dysadherin may be involved in the processes that benign lesions evolve into ECC. Furthermore, EphB3 negative expression or dysadherin positive expression was closely associated with several clinicopathological features of ECC, which could reflect aggressiveness and malignant degree of the tumor. The survival of patients with positive expression of EphB3 was significantly longer than patients with negative expression of EphB3, which was contrary to the relationship between dysadherin expression and patient survival. Cox multivariate analysis further demonstrated that negative EphB3 or positive dysadherin expression was an independent predictor for poor prognosis in patients with ECC. The AUC of EphB3 and dysadherin suggested that the expression of EphB3 and dysadherin may have potential clinicopathological diagnostic value. These results showed that EphB3 and dysadherin may function an important role in the tumorigenesis and progression of ECC. Therefore, detection of EphB3 or dysadherin expression in biliary duct tissues may have important clinical significance in the prevention or early finding of ECC.
Conclusions
EphB3 and dysadherin are involved in the carcinogenesis and progression of ECC, and ECC patients with negative EphB3 or positive dysadherin expression have a poor prognosis.
Funding Statement
This work was funded by the National Natural Science Foundation of China (grant number 81472738); Natural Science Foundation of Hunan Province, China (2019JJ10002); and Hunan Provincial Key Research and Development Program (2019SK2042).
Abbreviations
ECC, extrahepatic cholangiocarcinoma; ICC, intrahepatic cholangiocarcinoma; TNM, tumor-node-metastasis; PSC, Primary Sclerosing Cholangitis; CT, ultrasonography, computed tomography; MRI, magnetic resonance imaging; ERCP, endoscopic retrograde cholangiopancreatography; NSCLC, non-small-cell lung cancer; CPC, Clinicopathological characteristics; Pos No., Positive Number; RR, relative risk; CI, confidence interval.
Author Contributions
Zhengchun Wu, Rushi Liu, and Li Xiong carried out studies and wrote the paper; Zhulin Yang designed the study and revised the paper; Zhulin Yang and Xiongying Miao performed the statistical analysis; Daiqiang Li, Yuan Yuan, and Qiong Zou collected specimens and experimental materials. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest regarding this work.
References
- 1.Esnaola NF, Meyer JE, Karachristos A, et al. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349–1369. doi: 10.1002/cncr.29692 [DOI] [PubMed] [Google Scholar]
- 2.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labib PL, Goodchild G, Pereira SP. Molecular pathogenesis of cholangiocarcinoma. BMC Cancer. 2019;19(1):185. doi: 10.1186/s12885-019-5391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748 [DOI] [PubMed] [Google Scholar]
- 6.Alsaleh M, Leftley Z, Barbera TA, et al. Cholangiocarcinoma: a guide for the nonspecialist. Int J Gen Med. 2019;12:13–23. doi: 10.2147/IJGM.S186854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51(Suppl 6):I1–I9. doi: 10.1136/gut.51.suppl_6.vi1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2. doi: 10.1007/s11894-017-0542-4 [DOI] [PubMed] [Google Scholar]
- 9.Liang Z, Liu X, Zhang Q, et al. Diagnostic value of microRNAs as biomarkers for cholangiocarcinoma. Dig Liver Dis. 2016;48(10):1227–1232. doi: 10.1016/j.dld.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Aasheim HC, Patzke S, Hjorthaug HS, et al. Characterization of a novel Eph receptor tyrosine kinase, EphA10, expressed in testis. Biochim Biophys Acta. 2005;1723(1–3):1–7. doi: 10.1016/j.bbagen.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 11.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15(6):419–433. doi: 10.1016/j.cytogfr.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Baker RK, Vanderboom AK, Bell GW, et al. Expression of the receptor tyrosine kinase gene EphB3 during early stages of chick embryo development. Mech Dev. 2001;104(1–2):129–132. doi: 10.1016/S0925-4773(01)00363-X [DOI] [PubMed] [Google Scholar]
- 13.Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SY, Na YJ, Jeong YA, et al. Upregulation of EphB3 in gastric cancer with acquired resistance to a FGFR inhibitor. Int J Biochem Cell Biol. 2018;102:128–137. doi: 10.1016/j.biocel.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 15.Xuan Z, Huang J, Gao L, et al. Receptor tyrosine kinase EphB3: a prognostic indicator in colorectal carcinoma. Pathol Oncol Res. 2018. doi: 10.1007/s12253-018-0562-x [DOI] [PubMed] [Google Scholar]
- 16.Bhatia S, Griego A, Lennon S, et al. Role of EphB3 receptor in mediating head and neck tumor growth, cell migration, and response to PI3K inhibitor. Mol Cancer Ther. 2018;17(9):2049–2059. doi: 10.1158/1535-7163.MCT-17-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JJ, Sun ZJ, Yuan YM, et al. EphB3 stimulates cell migration and metastasis in a kinase-dependent manner through Vav2-Rho GTPase axis in papillary thyroid cancer. J Biol Chem. 2017;292(3):1112–1121. doi: 10.1074/jbc.M116.750349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao W, Zhang Q, Wang Y, et al. EphB3 protein is associated with histological grade and FIGO stage in ovarian serous carcinomas. APMIS. 2017;125(2):122–127. doi: 10.1111/apm.12646 [DOI] [PubMed] [Google Scholar]
- 19.Ji XD, Li G, Feng YX, et al. EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res. 2011;71(3):1156–1166. doi: 10.1158/0008-5472.CAN-10-0717 [DOI] [PubMed] [Google Scholar]
- 20.Chiu ST, Chang KJ, Ting CH, et al. Over-expression of EphB3 enhances cell-cell contacts and suppresses tumor growth in HT-29 human colon cancer cells. Carcinogenesis. 2009;30(9):1475–1486. doi: 10.1093/carcin/bgp133 [DOI] [PubMed] [Google Scholar]
- 21.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, et al. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem. 2005;280(45):37717–37724. doi: 10.1074/jbc.M506397200 [DOI] [PubMed] [Google Scholar]
- 22.Aoki S, Shimamura T, Shibata T, et al. Prognostic significance of dysadherin expression in advanced colorectal carcinoma. Br J Cancer. 2003;88(5):726–732. doi: 10.1038/sj.bjc.6600778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ino Y, Gotoh M, Sakamoto M, et al. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci U S A. 2002;99(1):365–370. doi: 10.1073/pnas.012425299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raman P, Purwin T, Pestell R, et al. FXYD5 is a marker for poor prognosis and a potential driver for metastasis in ovarian carcinomas. Cancer Inform. 2015;14:113–119. doi: 10.4137/CIN.S30565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada Y, Yamasaki S, Hashimoto Y, et al. Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res. 2004;10(8):2818–2823. doi: 10.1158/1078-0432.CCR-0633-03 [DOI] [PubMed] [Google Scholar]
- 26.Shimamura T, Sakamoto M, Ino Y, et al. Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol. 2003;21(4):659–667. doi: 10.1200/JCO.2003.06.179 [DOI] [PubMed] [Google Scholar]
- 27.Kyzas PA, Stefanou D, Batistatou A, et al. Dysadherin expression in head and neck squamous cell carcinoma: association with lymphangiogenesis and prognostic significance. Am J Surg Pathol. 2006;30(2):185–193. doi: 10.1097/01.pas.0000178090.54147.f8 [DOI] [PubMed] [Google Scholar]
- 28.Sato H, Ino Y, Miura A, et al. Dysadherin: expression and clinical significance in thyroid carcinoma. J Clin Endocrinol Metab. 2003;88(9):4407–4412. doi: 10.1210/jc.2002-021757 [DOI] [PubMed] [Google Scholar]
- 29.FT B, C F, RH H, et al. World Health Organization Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. [Google Scholar]
- 30.Liu R, Yang Z, Huang S, et al. The expressions of HMGA2 and Thy1 in extrahepatic cholangiocarcinoma and their clinicopathological significances. Surg Oncol. 2019;29:41–47. doi: 10.1016/j.suronc.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 31.He J, Yang Z, Wu Z, et al. Expression of FOXP1 and FOXO3a in extrahepatic cholangiocarcinoma and the implications in clinicopathological significance and prognosis. Onco Targets Ther. 2019;12:2955–2965. doi: 10.2147/OTT.S197001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu ZC, Xiong L, Wang LX, et al. Comparative study of ROR2 and WNT5a expression in squamous/adenosquamous carcinoma and adenocarcinoma of the gallbladder. World J Gastroenterol. 2017;23(14):2601–2612. doi: 10.3748/wjg.v23.i14.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox BP, Tabone CJ, Kandpal RP. Potential clinical relevance of Eph receptors and ephrin ligands expressed in prostate carcinoma cell lines. Biochem Biophys Res Commun. 2006;342(4):1263–1272. doi: 10.1016/j.bbrc.2006.02.099 [DOI] [PubMed] [Google Scholar]
- 34.Nam JS, Hirohashi S, Wakefield LM. Dysadherin: a new player in cancer progression. Cancer Lett. 2007;255(2):161–169. doi: 10.1016/j.canlet.2007.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura M, Ohta Y, Tsunezuka Y, et al. Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130(3):740–745. doi: 10.1016/j.jtcvs.2004.12.051 [DOI] [PubMed] [Google Scholar]
- 36.Park JR, Kim RJ, Lee YK, et al. Dysadherin can enhance tumorigenesis by conferring properties of stem-like cells to hepatocellular carcinoma cells. J Hepatol. 2011;54(1):122–131. doi: 10.1016/j.jhep.2010.06.026 [DOI] [PubMed] [Google Scholar]