Abstract
Purpose:
Sleep disturbance and cancer-related fatigue (CRF) are among the most commonly reported symptoms associated with breast cancer and its treatment. This study identified symptom cluster groups of breast cancer patients based on multidimensional assessment of sleep disturbance and CRF prior to and during chemotherapy.
Methods:
Participants were 152 women with stage I-IIIA breast cancer. Data were collected before chemotherapy (T1) and during the final week of the fourth chemotherapy cycle (T2). Latent profile analysis was used to derive groups of patients at each timepoint who scored similarly on percent of the day/night asleep per actigraphy, the Pittsburgh Sleep Quality Index global score, and the five subscales of the Multidimensional Fatigue Symptom Inventory-Short Form. Bivariate logistic regression evaluated if sociodemographic/medical characteristics at T1 were associated with group membership at each timepoint.
Results:
Three groups (Fatigued with sleep complaints, Average, Minimal symptoms) were identified at T1, and five groups (Severely fatigued with poor sleep, Emotionally fatigued with average sleep, Physically fatigued with average sleep, Average, Minimal symptoms) at T2. The majority of individuals in a group characterized by more severe symptoms at T1 were also in a more severe symptom group at T2. Sociodemographic/medical variables at T1 were significantly associated with group membership at T1 and T2.
Conclusions:
This study identified groups of breast cancer patients with differentially severe sleep disturbance and CRF symptom profiles prior to and during chemotherapy. Identifying groups with different symptom management needs and distinguishing groups by baseline sociodemographic/medical variables can identify patients at risk for greater symptom burden.
Keywords: cancer, cancer-related fatigue, latent profile analysis, sleep disturbance, symptom cluster, oncology
Introduction
Sleep disturbance and fatigue are commonly reported in breast cancer. Up to 80% of breast cancer patients report sleep disturbance (e.g., difficulty initiating or maintaining sleep, waking up earlier than desired and being unable to fall back to sleep, excessive daytime sleepiness) [1], and up to 99% report cancer-related fatigue (CRF) [2]. Unlike fatigue typically experienced by individuals without cancer, CRF is relatively more severe, disabling, and challenging to relieve [2-4]. In fact, CRF has been described as more closely approximating chronic fatigue syndrome than it does non-disease-related fatigue [5]. Specifically, CRF involves persistent and distressing physical, emotional, and/or cognitive weakness, tiredness, and lack of energy associated with cancer and its treatment that does not subside with adequate sleep and rest, and is disproportionate to exertion [3]. Sleep disturbance and CRF are highly comorbid [6], and are associated with increased symptom burden and poorer health-related quality of life in cancer [7, 8]. Moreover, studies have linked poor sleep to cancer progression and mortality [9].
To better understand co-occurring symptoms such as sleep disturbance and CRF, research has recently focused on symptom clusters. Symptom clusters are sets of interrelated symptoms that occur simultaneously might share a common etiology or variance, and might contribute to outcomes different than those that would result from isolated symptoms [10]. Symptom cluster research has previously identified profiles/subtypes of cancer patients that include sleep disturbance and CRF among other variables [11-16]. However, most studies have measured sleep disturbance using only subjective assessments and have assessed CRF as a unidimensional construct. Subjective and objective assessments of sleep disturbance are often incongruous [17], and CRF may be better conceptualized as multidimensional [18]. Considering multidimensional assessments of both sleep disturbance and CRF in a symptom cluster could help inform more precise interventions. For example, patients who experience notable physical fatigue combined with poor objectively-measured sleep disturbance might benefit from a physical activity intervention. Alternatively, patients who experience elevated emotional fatigue combined with increased subjectively-reported distress related to sleep might be better served by a psychosocial intervention. Recognizing the potential benefits of personalized treatments, three known studies identified typologies of cancer survivors based on multiple dimensions of CRF [18-20], and one based on subjectively measured sleep disturbance [21], but no studies were found with concurrent assessments of just sleep disturbance and CRF. Given the known interrelationship between sleep disturbance and CRF [6], evaluating a symptom cluster informed by more nuanced assessment of these two highly prevalent symptoms can enhance patient care.
The present study had two aims. The first was to identify groups of breast cancer patients by simultaneously evaluating multiple assessments of sleep disturbance and CRF prior to chemotherapy and again during treatment. Group stability over time was also explored. The second aim was to determine the association between pre-treatment sociodemographic and medical variables and group membership to identify patients who may benefit from intervention to prevent or reduce these symptoms. Given the exploratory nature of this study, no specific hypotheses were made a priori.
Methods
Participants and Procedures
Participants (N = 152) were newly diagnosed stage I-IIIA breast cancer patients scheduled to receive at least four cycles of anthracycline-based chemotherapy. Recruitment took place through two separate prospective studies of breast cancer patients (Study 1: 83 participants [collected 2000-2005] [22]; Study 2: 69 participants [collected 2005-2010] [23]) with identical recruitment procedures and inclusion criteria. Women were ineligible if they had: metastatic or stage IIIB (including inflammatory) breast cancer, undergone bone marrow transplant, received radiotherapy, any physical or psychological diagnoses/impairments that could confound study results (e.g., anemia); or were pregnant. Men were ineligible.
Data used in the present analysis were collected prior to the first cycle of chemotherapy (T1) and during the last week of the fourth cycle of chemotherapy (T2). At each timepoint participants wore an actigraph for 72 hours and completed questionnaires. Institutional Review Board approval was obtained prior to enrollment. All participants provided written informed consent.
Measures
Sociodemographic and medical variables.
Age, ethnicity, education, and marital status were self-reported. Body mass index (BMI), cancer stage at diagnosis, first treatment received (i.e., lumpectomy, mastectomy, double mastectomy, or neoadjuvant chemotherapy), chemotherapy formulation, current medications, and medical comorbidities were extracted from medical records. Breast cancer staging was performed by the referring medical oncologist according to the American Joint Committee on Cancer Staging Manual 5th Edition
Objective sleep disturbance.
Sleep disturbance was objectively assessed with wrist actigraphy [24]. Actigraphs are small, wearable devices that measure sleep/wake patterns and circadian rhythms over multiple days. All Study 1 participants and 14 Study 2 participants wore an Actillume-II (Ambulatory Monitoring Inc., Ardsley, NY); the remaining Study 2 participants wore an Actiwatch-Light (Philips Respironics Mini Mitter, Bend, OR). To establish equivalency across devices, eight volunteers concurrently wore both on the same wrist for 72 hours. Activity count data and software-scored sleep/wake data from both devices were highly correlated (rs > 0.85), supporting equivalency.
A one-minute epoch setting was utilized. Data were manually edited based on sleep logs wherein participants recorded bedtime, wake time, and naps while wearing the actigraph, as recommended by the Society of Behavioral Sleep Medicine [25]. For the present analysis the percentage of the night spent asleep (from sleep onset to final awakening according to scored actigraphic records) and percentage of the day spent asleep (from final up time to bedtime according to sleep logs) were calculated. Higher percentage of the night spent asleep indicated better sleep efficiency, reflecting less sleep disturbance. This variable was used in place of sleep efficiency as it only considers the percent of time spent asleep after sleep onset and does not consider sleep onset latency, which has been shown to be less reliable when measured by wrist actigraphy [26]. Higher percentage of the day spent asleep indicated increased daytime napping, reflecting increased sleepiness, circadian dysregulation, and more sleep disturbance.
Subjective sleep quality.
Pittsburgh Sleep Quality Index.
The Pittsburgh Sleep Quality Index [PSQI] [27] is a 19-item self-report measure of sleep quality over the prior month. Each item is weighted on a 0-3 interval scale. Items contribute to seven component scores, which are in turn summed to yield a global score ranging from 0 to 21; higher scores indicate worse subjective sleep quality. A global score above 5 indicates poor sleep [27].
Cancer-related fatigue.
Multidimensional Fatigue Symptom Inventory – Short Form.
The Multidimensional Fatigue Symptom Inventory-Short Form [MFSI-SF] [28, 29] is a 30-item self-report assessment composed of five, six-item subscales (General fatigue, Physical fatigue, Emotional fatigue, Mental fatigue, Vigor). Subscale scores, ranging from 0 to 24, were computed by summing items. For the four Fatigue subscales, higher scores indicate more cancer-related fatigue; for the Vigor subscale, higher scores indicate less cancer-related fatigue. There are no published clinical cutoffs for the MFSI-SF.
Data Analytic Plan
Exploratory Latent Profile Analysis (LPA) was used to derive categorical latent variables representing groups of individuals who scored similarly, relative to the rest of the sample, on two measures of objective sleep disturbance (percentage of night spent asleep, percentage of day spent asleep), one measure of subjective sleep quality (PSQI global score), and five dimensions of CRF (MFSI-SF subscales). Two separate cross-sectional LPAs were conducted (T1 and T2).
In LPA the probability that an individual is properly classified is estimated simultaneously within the overall model [30]. Models are estimated with classes added iteratively to determine which model best fits the data. For this study, LPA was conducted using the maximum likelihood robust (MLR) estimation procedure, to account for missing data, in MPlus 7.2 [31, 32]. To determine the optimal number of groups, models were evaluated using the Akaike information criterion (AIC) [33], sample size-adjusted Bayesian information criterion (sBIC) [34], Bootstrapped Likelihood Ratio Test (BLRT) [35, 36], and Entropy [37] The AIC and sBIC are descriptive fit indices wherein smaller values indicate better model fit. The BLRT compares the fit of a target model (e.g., a two-profile model) to a comparison model that specifies one less profile (e.g., a one-profile model). The p-values generated for the BLRT indicate whether the solution with more profiles (p < .05) or fewer profiles (p > .05) is a superior fit to the data. Entropy is a measure of how well profiles can be distinguished, with higher values indicating that more individuals in the sample have been correctly classified in the specified model. Each model was also evaluated on interpretability and sample size, with profiles containing less than 5% of the sample considered spurious [38].
After best-fitting models were determined, descriptive statistics were examined at each timepoint to evaluate group stability over time. Sample size limitations and insufficient data collection timepoints precluded longitudinal modeling. A series of logistic regression models were used to determine whether T1 sociodemographic/medical characteristics predicted profile membership at each timepoint. The relationship between chemotherapy formulation and group membership was only evaluated for groups identified at T2, as there was no theoretical rationale for evaluating this relationship prior to chemotherapy.
Results
Participants
The sample (T1: N = 152; T2: n = 128) is described in Table 1. There were no significant differences between participants who had complete data at both timepoints and those who were only included at T1.
Table 1.
Participant characteristics
| Variable | T1 (N = 152) |
T2 (n = 128) |
|---|---|---|
| Sociodemographic variables | ||
| Whitea | 122 (80.3) | 100 (78.1) |
| Educationa | ||
| < College graduate | 74 (48.7) | 64 (50.0) |
| ≥ College graduate | 78 (51.3) | 64 (50.0) |
| Marrieda | 105 (69.1) | 89 (69.5) |
| Ageb | 50.84 (9.39) | 50.74 (9.19) |
| Medical variables | ||
| Cancer stage at diagnosisa | ||
| I | 42 (27.6) | 31 (24.2) |
| II | 66 (43.4) | 57 (44.5) |
| III | 35 (23.0) | 32 (25.0) |
| Type of chemotherapy receiveda | ||
| AC | 35 (23.0) | 31 (24.2) |
| AC + Taxotere | 30(19.7) | 23 (18.0) |
| AC + Taxol | 45 (29.6) | 41 (32.0) |
| Other | 32(21.1) | 25 (19.5) |
| First treatment receiveda | ||
| Lumpectomy | 62 (40.8) | 53 (41.4) |
| Mastectomy | 63 (41.4) | 52 (40.6) |
| Other | 19(12.5) | 16(12.5) |
| Double mastectomy | 7(4.6) | 7(5.5) |
| Neoadjuvant chemotherapy | 12(7.9) | 9 (7.0) |
| Medications takena | ||
| Analgesic | 102 (67.1) | 45 (35.2) |
| Antidepressant | 29(19.1) | 21 (16.4) |
| Minor tranquilizers | 36(23.7) | 32 (25.0) |
| Medical comorbiditiesa | ||
| Arthritis | 27(17.8) | 20(15.6) |
| Asthma | 16(10.5) | 14(10.9) |
| Other diseases | 21 (13.8) | 24(18.8) |
| BMIb | 28.19(7.11) | 28.11 (7.08) |
Note.
n (%);
M(SD);
BMI: Body Mass Index
Sleep Disturbance and Cancer-Related Fatigue Groups
Groups at T1.
See Table 2 for model fit information for all tested solutions. The two-profile solution fit better than the one-profile solution across all indicators. The AIC, sBIC, and BLRT indicated the three-profile solution fit better than the two-profile solution. Although model fit was similarly good for the four-profile solution, one profile represented only 2% of the sample (n = 3) and was considered spurious. Thus, the three-profile solution was considered the best fit.
Table 2.
Indicators of model fit across all LPA models
| # profiles | Time 1 | Time 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| AIC | sBIC | BLRT p-value |
Entropy | AIC | sBIC | BLRT p-value |
Entropy | |
| 1 | 4727.18 | 4724.92 | --- | --- | 4299.32 | 4294.36 | --- | --- |
| 2 | 4447.28 | 4443.75 | < .001 | 0.95 | 4045.47 | 4037.71 | < .01 | 0.89 |
| 3 | 4379.65 | 4374.85 | < .001 | 0.85 | 3949.54 | 3938.99 | < .01 | 0.92 |
| 4 | 4322.86 | 4316.80 | < .001 | 0.88 | 3920.24 | 3906.89 | < .01 | 0.92 |
| 5 | --- | --- | --- | --- | 3905.39 | 3889.24 | .01 | 0.87 |
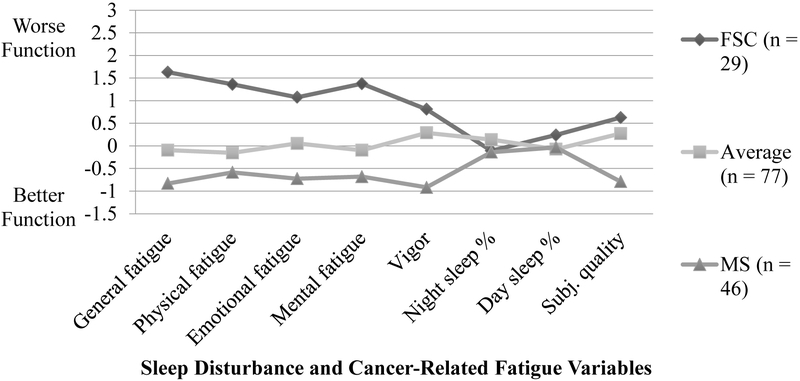
Table 3 and Figure 1 describe conditional response means and relative scores on measures of sleep disturbance and CRF. Profile groups were labeled: (1) Fatigued with sleep complaints, (2) Average, and (3) Minimal symptoms. The Fatigued with sleep complaints (n= 29) group was characterized by higher CRF and average sleep disturbance relative to the rest of the sample. The Average (n = 77) group had relatively average symptoms across all domains. The Minimal symptoms (n = 46) group demonstrated less symptomatology across all domains.
Table 3.
Sample means and group conditional response means
| M (SE) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 Groups | T2 Groups | |||||||||
| Full sample (n =152) |
FSC (n = 29) |
Average (n = 77) |
MS (n = 46) |
Full sample (n = 128) |
SFPS (n =11) |
EFAS (n =15) |
PFAS (n =17) |
Average (n = 31) |
MS (n = 54) |
|
| Objective sleep measures | ||||||||||
| Night sleep % | 78.4 (0.01) |
79.7 (0.02) |
76.8 (0.02) |
80.1 (0.02) |
79.0 (0.01) |
76.6 (0.04) |
80.6 (0.02) |
71.7 (0.03) |
79.7 (0.02) |
80.9 (0.02) |
| Day sleep % | 6.5 (0.01) |
8.3 (0.02) |
6.0 (0.01) |
6.3 (0.01) |
8.6 (0.01) |
15.8 (0.04) |
10.4 (0.02) |
7.6 (0.01) |
6.8 (0.02) |
7.9 (0.02) |
| Subjective sleep quality: PSQI | ||||||||||
| Global score | 7.31 (0.31) |
9.63 (0.69) |
8.32 (0.67) |
4.37 (0.41) |
8.03 (0.35) |
13.00 (0.91) |
9.93 (1.21) |
9.89 (0.88) |
8.02 (0.77) |
5.81 (0.61) |
| Five dimensions of fatigue: MFSI-SF Subscales | ||||||||||
| General | 6.32 (0.46) |
15.64 (0.71) |
5.77 (0.59) |
1.56 (0.40) |
10.35 (0.58) |
19.94 (1.02) |
15.29 (1.65) |
14.72 (1.22) |
10.49 (1.71) |
5.41 (1.03) |
| Physical | 2.66 (0.27) |
7.25 (0.79) |
2.14 (0.32) |
0.69 (0.17) |
4.01 (0.42) |
16.12 (0.87) |
4.18 (0.51) |
8.22 (0.64) |
2.39 (0.43) |
1.01 (0.28) |
| Emotional | 6.06 (0.39) |
11.19 (0.99) |
6.32 (0.55) |
2.60 (0.47) |
5.37 (0.45) |
12.63 (1.57) |
12.94 (1.86) |
4.85 (0.69) |
5.56 (1.27) |
1.77 (0.44) |
| Mental | 4.28 (0.32) |
9.62 (0.74) |
3.90 (0.39) |
1.63 (0.34) |
6.03 (0.47) |
14.92 (1.19) |
11.99 (2.06) |
6.92 (1.00) |
5.42 (1.30) |
2.55 (0.92) |
| Vigor | 11.08 (0.45) |
6.62 (0.74) |
9.49 (0.62) |
16.12 (1.59) |
10.23 (0.51) |
4.00 (1.17) |
4.57 (1.94) |
8.17 (0.96) |
7.94 (2.61) |
15.14 (0.86) |
Note. FSC = Fatigued with sleep complaints; MS = Minimal symptoms;; SFPS = Severe fatigue with poor sleep; EFAS = Emotional fatigue with average sleep; PFAS = Physical fatigue with average sleep; M = Mean; SE = Standard error; PSQI = Pittsburgh Sleep Quality Index; MFSI-SF = Multidimensional Fatigue Symptom Inventory–Short Form
Figure 1.
Standardized conditional response means (z-scores) for groups defined by sleep disturbance and cancer-related fatigue variables at T1. FSC = Fatigued with sleep complaints; MS = Minimal symptoms. Z-scores for percentage of the night spent asleep and the Vigor subscale of the MFSI-SF were reverse-coded, so higher scores indicate worse functioning across all indicators.
Groups at T2.
See Table 2 for model fit information for all tested solutions. Across all indicators the two-profile solution fit better than the one-profile solution, and the three-profile solution fit better than the two-profile solution. The AIC, sBIC, and BLRT indicated that the four-profile solution fit better than the three-profile solution, and the five-profile solution fit better than the four-profile solution. A six-profile solution was attempted; however, the model failed to converge. Therefore, the five-profile solution was considered the best fit to the data.
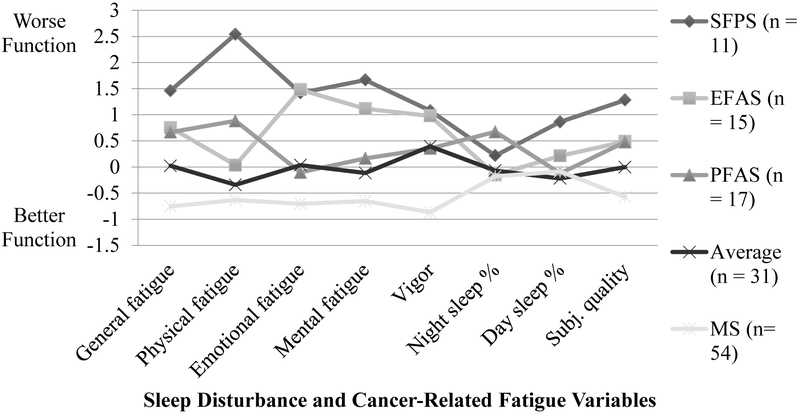
Table 3 and Figure 2 describe conditional response means and relative scores on measures of sleep disturbance and CRF. Profile groups were labeled: (1) Severely fatigued with poor sleep (SFPS), (2) Emotionally fatigued with average sleep (EFAS), (3) Physically fatigued with average sleep (PFAS), (4) Average, and (5) Minimal symptoms. The SFPS (n = 11) group was characterized by relatively poorer symptomatology overall. The EFAS (n = 15) group demonstrated somewhat worse sleep quality, more severe ratings on General, Emotional, and Mental fatigue and Vigor but not Physical fatigue, less nighttime sleep disturbance, and more daytime sleepiness. The PFAS (n = 17) group demonstrated relatively poorer sleep quality, more severe ratings on General and Physical fatigue but not on Emotional and Mental fatigue or Vigor, the most severe nighttime sleep disturbance, and slightly elevated daytime sleepiness. The Average (n = 31) group demonstrated relatively average symptoms across all domains. The Minimal symptoms (n = 54) group demonstrated less symptomatology across all domains.
Figure 2.
Standardized conditional response means (z-scores) for groups defined by sleep disturbance and cancer-related fatigue variables at T2. SFPS = Severe fatigue with poor sleep; EFAS = Emotional fatigue with average sleep; PFAS = Physical fatigue with average sleep; MS = Minimal symptoms. Z-scores for percentage of the night spent asleep and the Vigor subscale of the MFSI-SF were reverse-coded, so higher scores indicate worse functioning across all indicators.
Stability of group membership from T1 to T2.
Among 23 participants in the Fatigued with sleep complaints group at T1, 14 (60.9%) remained in one of the two groups characterized by the most severe symptoms (SFPS or EFAS) at T2, while the remainder (39.1%) were in a group indicating less severe symptoms at T2. Among 65 participants in the Average group at T1, 30 (46.1%) remained in one of the two groups characterized by more moderate symptoms (PFAS or Average), 10 (15.4%) were in a group indicating more severe symptoms (i.e., SFPS or EFAS), and 25 (38.5%) were in a group indicating less severe symptoms (i.e., Minimal symptoms) at T2. Finally, of the 40 participants in the Minimal symptoms group at T1, 29 (72.5%) remained in the Minimal symptoms group, while the rest (27.5%) were in a group indicating more severe symptoms at T2.
Associations of Sleep Disturbance and Cancer-Related Fatigue Groups with T1 Levels of Sociodemographic Variables
Results of logistic regression analyses comparing symptom burden groups on sociodemographic and medical variables are presented in Table 4. At T1, participants in the Fatigued with sleep complaints (M = 49.69, SD = 9.49) and Average groups (M = 49.06, SD = 8.33) were younger than those in the Minimal symptoms group (M = 54.52, SD = 10.13). Additionally, there were more married participants in the Average group (76.6%) as opposed to the Fatigued with sleep complaints group (51.7%). Race and education were not associated with group membership at T1 (see Table 4)
Table 4.
Significant odds ratios (95% confidence intervals) from logistic regression analyses comparing symptom burden groups on sociodemographic and medical variables
| T1 Groups | T2 Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSC vs. Average |
FSC vs. MS | Average vs. MS |
A vs. EFAS | A vs. SFPS | SFPS vs. PFAS |
EFAS vs. MS | PFAS vs. MS | SFPS vs. MS | PFAS vs. A | |
| Sociodemographic | ||||||||||
| Age | --- | 0.95 (0.90, 1.00) |
0.94 (0.90, 0.98) |
--- | --- | --- | --- | --- | --- | --- |
| Married vs. not married | 0.33 (0.13, 0.80) |
--- | --- | 4.55 (1.13, 18.35) |
6.24 (1.36, 28.66) |
--- | --- | --- | --- | --- |
| Medical | ||||||||||
| Stage at dx: II (vs. III) | --- | --- | 2.92 (1.13, 7.58) |
--- | --- | --- | --- | --- | --- | --- |
| Surgical intervenion | ||||||||||
| Lumpect vs. “Other” | --- | --- | 3.24 (1.01, 10.42) |
--- | --- | --- | --- | --- | --- | --- |
| Mastect vs. “Other” | --- | --- | 3.43 (1.07, 11.01) |
--- | --- | --- | --- | --- | --- | --- |
| BMI | --- | --- | --- | --- | --- | 1.17 (1.02, 1.33) |
--- | --- | --- | --- |
| Current medications | ||||||||||
| Analgesics | --- | 4.22 (1.26, 14.16) |
--- | --- | --- | --- | --- | --- | --- | --- |
| Antidep | --- | --- | --- | --- | --- | --- | 4.38 (1.19, 16.13) |
--- | --- | --- |
| Minor tranq | --- | --- | --- | 0.17 (0.04, 0.68) |
--- | --- | 8.95 (2.38, 33.62) |
--- | --- | --- |
| Comorbidities | ||||||||||
| Asthma | --- | 6.14 (1.14, 33.07) |
--- | --- | --- | --- | 18.91 (1.92, 185.95) |
11.14 (1.08, 115.54) |
19.50 (1.80, 211.21) |
|
| Arthritis | --- | --- | --- | --- | --- | --- | --- | 3.58 (1.00, 12.81) |
--- | 5.09 (1.08, 24.02) |
| “Other” disease | --- | 4.90 (1.15, 20.96) |
--- | --- | 0.09 (0.01, 0.98) |
--- | --- | --- | --- | 12.50 (1.32, 118.48) |
Note. Only pairwise comparisons that were statistically significantly different are presented in this table (all ps ≤ .05). FSC = Fatigued with sleep complaints; MS – Minimal symptoms; SFPS = Severely fatigued with poor sleep; EFAS = Emotionally fatigued with average sleep; PFAS = Physically fatigued with average sleep; A = Average; Lumpect = Lumpectomy; Mastect = Mastectomy; Antidep = Antidepressants; Minor tranq = minor tranquilizers. For surgical intervention “other’ indicates double mastectomy or neoadjuvant chemotherapy. For comorbidities “other’ indicates any disease not specifically queried.
At T2, there were more married participants in the Average group (83.9%) than the SFPS (45.5%) or the EFAS (53.3%) groups. Age, education, and race were not associated with group membership at T2 (see Table 4).
Associations of Sleep Disturbance and Cancer-Related Fatigue Groups with T1 Levels of Medical Variables
At T1, participants in the Average group were more likely to have Stage II (vs. III) cancer at diagnosis (49.4%) than those the Minimal symptoms group (32.6%). Those in the Average group were also more likely to have received a lumpectomy (42.9%) or single mastectomy (45.5%) followed by chemotherapy (vs. a double mastectomy followed by chemotherapy or neoadjuvant chemotherapy) compared to those in the Minimal symptoms group (lumpectomy: 37.0%; single mastectomy: 37.0%). Finally, participants in the Minimal symptoms group were less likely to be diagnosed with asthma (4.3%) or a disease not specifically queried at T1 (6.5%), or using analgesics according to standard medication classes (58.7%) than those in the Fatigued with sleep complaints group (asthma: 20.7%; other diseases: 24.1%; analgesics: 82.8%; see Table 4).
For groups identified at T2, participants in the SFPS group had higher BMI (M = 31.49, SD = 7.04) than those in the PFAS group (M = 25.09, SD = 4.40). Those in the EFAS group were more likely to be using antidepressants (40.0%) and minor tranquilizers according to standard medication classes (53.3%) than those in the Minimal symptoms group (antidepressants: 13.0%; minor tranquilizers: 11.1%), and more likely to be using minor tranquilizers than those in the Average group (16.1%). Participants in the SFPS (27.3%) and PFAS groups (29.4%) were more likely to be diagnosed with a disease not specifically queried than participants in the Average group (3.2%). Those in the SFPS (27.3%), EFAS (26.7%), and PFAS groups (17.6%) were all more likely to be diagnosed with asthma than those in the Minimal symptoms group (1.9%). Finally, participants in the PFAS group (35.3%) were more likely to be diagnosed with arthritis than participants in the Average (9.7%) or the Minimal symptoms groups (13.0%; see Table 4).
Discussion
Sleep Disturbance and Cancer-Related Fatigue Symptom Cluster Groups
This study’s first aim was to identify sleep disturbance and CRF groups of breast cancer patients prior to chemotherapy and at the last week of the fourth cycle of chemotherapy. This is the first known study to identify groups of breast cancer patients based on multidimensional, objective and subjective indicators of sleep disturbance and CRF. Groups were named based on symptom dimensions that were heightened relative to the rest of the sample. Three groups were identified at T1 (i.e., prior to chemotherapy). At T1 the full sample reported greater emotional fatigue, but lesser fatigue in the other domains, as compared to Stein and colleagues’ MFSI-SF development sample [28]. Evaluating each group separately, the Fatigued with sleep complaints group reported greater CRF across all dimensions, while the Average group reported more emotional fatigue, similar mental fatigue, and less general fatigue, mental fatigue, and vigor than Stein and colleagues’ [28] development sample. Conversely, the Minimal symptoms group reported less CRF across all five MFSI-SF subscales as compared to not only the 275 breast cancer patients, but also the 70 non-cancer comparison participants described by Stein and colleagues [28]. Additionally, only the Minimal symptoms group demonstrated PSQI global scores below the clinical cutoff of five, while the Fatigued with sleep complaints and Average groups, as well as the full sample, demonstrated PSQI global scores above this cutoff. Despite these variations, each individual group and the full sample demonstrated less than 85% of the night spent asleep, which is the commonly accepted cutoff for “normal” sleep in the greater insomnia literature [39].
Five groups were identified at T2 (i.e., during the last week of the fourth cycle of chemotherapy). The two groups characterized by the most severe symptoms (i.e., SFPS and EFAS), and the full sample considered in aggregate, demonstrated notably more severe sleep disturbance and CRF across all dimensions as compared to prior samples of cancer patients [28, 40, 41]. For the two groups with more moderate symptoms (i.e., PFAS and Average), CRF was slightly more severe than Stein and colleagues’ [28] validation sample. Conversely, the Minimal symptoms group demonstrated similar or lower CRF across all dimensions compared to both the cancer patient validation sample and the non-cancer comparison participants. Additionally, though all five groups and the full sample demonstrated PSQI global scores greater than five, the Minimal symptoms group mean was lower than what has been observed among other cancer samples [40]. Finally, average percentage of the night spent asleep remained below the 85% cutoff for all groups and when the sample was considered in aggregate. Results suggest that the Minimal symptoms groups identified at both time points reflected cancer patients who were less fatigued than individuals with no history of cancer, and at T2 these participants also reported better subjective sleep quality than prior samples of cancer patients, despite experiencing objectively-measured sleep disturbance.
Most participants in the two groups characterized by more (Fatigued with sleep complaints) and less (Minimal symptoms) severe symptoms at T1 were also in a group characterized by more or less severe symptoms at T2. Dodd and colleagues [11] identified a similar result in their analysis of a symptom cluster informed by unidimensional assessments of subjective sleep disturbance, fatigue, depression, and pain in cancer. Three-quarters (73%) of participants who were in the group with the least severe symptoms at the beginning of biotherapy remained in such a group one month later. The present study’s results further support the relative stability of group membership among patients reporting low symptom severity, and extend this by suggesting relative stability among individuals reporting more severe symptoms as well. In the present analysis, the Average group was less stable than the other two T1 groups. Future evaluation of this sleep disturbance and CRF symptom cluster, informed by more assessment timepoints, is needed with larger samples to enable statistical comparison of change trajectories over time. Such work will enable better identification of those who are likely to improve without intervention, as well as those at greater risk for increases in symptom burden. The relative stability of the Fatigued with sleep complaints group also raises the question of whether patients in this group would benefit from personalized interventions initiated prior to or at the start of their chemotherapy.
Sociodemographic Characteristics Associated with Group Membership
This study’s second aim was to evaluate if T1 sociodemographic and medical characteristics were significantly associated with profile membership at T1 and T2. Group membership was associated with different sociodemographic variables at both timepoints. Consistent with the literature [11,21], younger participants were more likely to be in the Fatigued with sleep complaints or Average group as opposed to the Minimal symptoms group prior to chemotherapy.
Consistent with the findings of Miaskowski et al. [42], the present study found married participants were more likely to be in a group characterized by less severe symptoms at both T1 and T2. Married patients often have better cancer outcomes, including lower mortality rates and less psychosocial symptoms [43], possibly indicating increased social support. Future research would benefit from directly examining the ability of social support to distinguish sleep disturbance and CRF symptom cluster groups among breast cancer patients.
Medical Characteristics Associated with Group Membership
As with sociodemographic variables, group membership was associated with distinct medical variables at both timepoints. Patients reporting more comorbidities, more use of medications, and other indicators of worse health (e.g., higher BMI) were more likely to be in a group characterized by higher symptom severity, consistent with the broader literature [2, 44-47]. In addition, the present findings provide valuable information regarding associations of cancer-specific details, such as stage at diagnosis and first treatment received, with symptom cluster group membership. Interestingly, participants with more advanced disease at diagnosis and who had undergone double mastectomy prior to chemotherapy or were receiving neoadjuvant chemotherapy, as opposed to lumpectomy or single mastectomy prior to chemotherapy, were more likely to be classified into the group reporting the least severe symptoms at T1. Unfortunately information about time from diagnosis and/or surgery (for those women who received adjuvant treatment) to the start of chemotherapy was not available for all women, and, therefore, could not be considered in this analysis. It is possible that women who reported more severe symptoms were still recovering from psychological adjustment to diagnosis and/or surgical intervention, and therefore reported more severe sleep disturbance and CRF symptoms. Future research would benefit from considering these variables when evaluating medical correlates of symptom cluster group membership.
Study Limitations
This study has limitations. Longitudinal modeling techniques could not be used to assess trajectories of group membership over time due to sample size constraints and insufficient data collection timepoints. Also due to sample size constraints, the relationship between symptom cluster group membership and each medical and sociodemographic variable was examined in a separate logistic regression model, and precision was diminished leading to wide confidence intervals. Therefore, results of logistic regression analyses must be interpreted cautiously. Additionally, there were no measures of pain or sleep disordered breathing, which may play an important role in sleep disturbance and CRF.
Conclusions
This study extends the literature on oncology symptom clusters. These findings can help clinicians better understand the complex and interrelated symptoms of sleep disturbance and CRF among women receiving chemotherapy for early-stage breast cancer, identify subgroups of patients at risk for sleep disturbance and elevated CRF, and identify those who may be more likely to need intervention. Understanding how these groups vary across the chemotherapy treatment trajectory can clarify ways in which symptom experiences change over time for some patients, but not others. These findings can help identify which patients may benefit from additional assessment and early intervention to prevent or reduce sleep disturbances and CRF during chemotherapy, and can inform targeted interventions to improve patients’ overall cancer experiences.
Acknowledgments:
Funding for this research was provided by the National Cancer Institute [grant numbers R01 CA112035 and UL1RR031980], the National Institutes of Health (grant number M01 RR00827], the Stein Institute for Research on Aging, and the Department of Veterans Affairs Center of Excellence for Stress and Mental Health. The authors would like to thank Dr. Lianqi Liu for his contributions to this study, and all patients for their time and participation.
Footnotes
Conflict of Interest
Sonia Ancoli-Israel consults for Merck, Eisai, Purdue, and Pfizer. All other authors have no conflicts of interest to disclose. The authors have full control of all primary data and agree to allow the journal to review these data if requested.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Rina S. Fox is currently affiliated with Northwestern University Feinberg School of Medicine, Chicago, IL, USA
References
- 1.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol 2010;28(2):292–298. doi: 10.1200/JCO.2009.22.5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw 2015;13(8):1012–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs 2002;25(4):283–297. [DOI] [PubMed] [Google Scholar]
- 5.Bennett B, Goldstein D, Friedlander M, et al. The experience of cancer-related fatigue and chronic fatigue syndrome: A qualitative and comparative study. J Pain Symptom Manage 2007;34(2):126–135. doi: 10.1016/j.jpainsymman.2006.10.014 [DOI] [PubMed] [Google Scholar]
- 6.Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams HJG, Gielissen MFM, Verhagen CAHHVM, Knoop H. The relationship of fatigue in breast cancer survivors with quality of life and factors to address in psychological interventions: A systematic review. Clin Psychol Rev 2018;63:1–11. doi: 10.1016/j.cpr.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Dickerson SS, Connors LM, Fayad A, Dean GE. Sleep-wake disturbances in cancer patients: narrative review of literature focusing on improving quality of life outcomes. Nat Sci Sleep 2014;6:85–100. doi: 10.2147/NSS.S34846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res 2009;18(2):148–158. doi: 10.1111/j.13652869.2008.00732.x [DOI] [PubMed] [Google Scholar]
- 10.Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J Natl Cancer Inst 2017;109(4):djw253. doi: 10.1093/jnci/djw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum 2011;38(1):33–42. Doi: 10.1188/11.ONF.33-42 [DOI] [PubMed] [Google Scholar]
- 12.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs 2015;17(3):237–247. doi: 10.1177/1099800414550394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine 2012;58(3):437–447. doi: 10.1016/j.cyto.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer 2014;120(15):2371–2378. doi: 10.1002/cncr.28699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage 2015;50(1):28–37. doi: 10.1016/j.jpainsymman.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trudel-Fitzgerald C, Savard J, Ivers H. Longitudinal changes in clusters of cancer patients over an 18-month period. Health Psychol 2014;33(9):1012–1022. doi: 10.1037/a0033497 [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell D, Silva EJ, Munch M, et al. Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. J Sleep Res 2009;18(2):254–263. doi: 10.1111/j.1365-2869.2008.00719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thong MSY, Mols F, van de Poll-Franse LV, et al. Identifying the subtypes of cancer-related fatigue: results from the population-based PROFILES registry. J Cancer Surviv 2018;12(1):38–46. doi: 10.1007/s11764-017-0641-0 [DOI] [PubMed] [Google Scholar]
- 19.Dirksen SR, Belyea MJ, Epstein DR. Fatigue-based subgroups of breast cancer survivors with insomnia. Cancer Nurs 2009;32(5):404–411. doi: 10.1097/NCC.0b013e3181a5d05e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower JE, Wiley J, Petersen L, et al. Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories. Health Psychol 2018;37(11):1025–1034. doi: 10.1037/hea0000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Onselen C, Cooper BA, Lee K, et al. Identification of distinct subgroups of breast cancer patients based on self-reported changes in sleep disturbance. Support Care Cancer 2012;20(10):2611–2619. doi: 10.1007/s00520-012-1381-3 [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer 2014;22(9):2535–2545. doi: 10.1007/s00520-014-2204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26(3):342–392. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med 2015;13(sup1):S4–S38. doi: 10.1080/15402002.2015.1046356 [DOI] [PubMed] [Google Scholar]
- 26.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep 2006;29(2):232–239. [PubMed] [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 28.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract 1998;6(3):143–152. [DOI] [PubMed] [Google Scholar]
- 29.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage 2004;27(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: the roles of emotion regulation and inattention. Dev Psychol 2006;42(5):913–928. doi: 10.1037/0012-1649.42.5.913 [DOI] [PubMed] [Google Scholar]
- 31.Muthén LK, Muthén BO. Mplus User's Guide. Sixth Edition. Los Angeles, CA: Muthén & Muthén, 1998–2011. [Google Scholar]
- 32.Enders CK. Applied missing data analysis. New York: Guilford Press, 2010. [Google Scholar]
- 33.Akaike H A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 34.Schwarz G Estimating the dimension of a model. Ann Stat 1978;6(2):461–464. [Google Scholar]
- 35.Arminger G, Stein P, Wittenberg J. Mixtures of conditional mean-and covariance-structure models. Psychometrika 1999;64(4):475–494. doi: 10.1007/BF02294568 [DOI] [Google Scholar]
- 36.McLachlan G, Peel D. Finite mixture models. John Wiley & Sons, 2004. [Google Scholar]
- 37.Ramaswamy V, DeSarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Science 1993;12(1):103–124. [Google Scholar]
- 38.Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: a commentary on Nooner et al., Pears et al., and looking beyond. Child Abuse Negl 2010;34(3):155–160. doi: 10.1016/j.chiabu.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 39.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep 2004;27(8):1567–1596. [DOI] [PubMed] [Google Scholar]
- 40.Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 41.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage 2007;33(4):398–409. doi: 10.1016/j.jpainsymman.2006.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: A cluster analysis. Oncol Nurs Forum 2006;33(5):E79–89. doi: 10.1188/06.ONF.E79-E89 [DOI] [PubMed] [Google Scholar]
- 43.Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31(31):3869–3876. doi: 10.1200/JCO.2013.49.6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meeske K, Smith AW, Alfano CM, et al. Fatigue in breast cancer survivors two to five years post diagnosis: a HEAL Study report. Qual Life Res 2007;16(6):947–960. doi: 10.1007/s11136-007-9215-3 [DOI] [PubMed] [Google Scholar]
- 45.Hwang SS, Chang VT, Rue M, Kasimis B. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 2003;26(1):604–614. [DOI] [PubMed] [Google Scholar]
- 46.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain 2005;21(5):422–431. [DOI] [PubMed] [Google Scholar]
- 47.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010 [DOI] [PubMed] [Google Scholar]