Abstract
A series of pyridine derivatives in the C-region of N-((6-trifluoromethyl-pyridin-3-yl)methyl) 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides were investigated as hTRPV1 antagonists. The SAR analysis indicated that 6-difluorochloromethyl pyridine derivatives were the best surrogates of the C-region for previous leads. Among them, com pound 31 showed excellent antagonism to capsaicin as well as to multiple hTRPV1 activators. It demonstrated strong analgesic activity in the formalin test in mice with full efficacy and it blocked capsaicin-induced hypothermia in vivo.
Keywords: Vanilloid receptor 1, TRPV1 antagonists, Analgesic
1. Introduction
The vanilloid receptor TRPV1 has emerged as a promising therapeutic target for treatment of neuropathic and inflammatory pain as well as for a wide range of other conditions [1–3]. TRPV1 functions as a nociceptor activated by elevated temperature, by low pH, and by both endogenous endovanilloids and exogenous agents such as capsaicin [1,2,4–6]. It is further subject to extensive regulation through signaling pathways, such as protein kinase C or protein kinase D, positioning TRPV1 to integrate the multitude of influences impacting the cell [7,8]. In view of the potential of TRPV1 as a therapeutic target, intense effort by many groups has been directed at the development of potent TRPV1 antagonists [9–17] and at understanding of the structural basis for potent ligand binding [18]. We have used the ultrapotent TRPV1 ligand resiniferatoxin as a structural lead for antagonist development [19]. Using this approach, we have developed antagonists with activities in the low nM to sub-nM range [20–24]. Here, we report our recent efforts at further optimization of our lead structures.
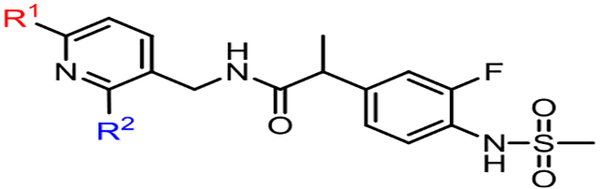
Recently, we reported a series of N-{(6-trifluoromethyl-pyridin-3-yl)methyl} 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides, designed by a pharmacophoric combination approach, which proved to be potent hTRPV1 antagonists (Fig. 1) [20–24]. Structurally the antagonistic template can be divided into three pharmacophoric parts which were designated as the A-region (3-fluoro-4-methylsulfonylaminophenyl), the B-region (propanamide), and the C-region ((6-trifluoromethyl-pyridin-3-yl) methyl). The structure activity relationships of the 2-substituent in the pyridine C-region have been investigated extensively by incorporating various functional groups, including amino [20], oxy [21], thio [22], alkyl [23] and aryl [24] groups. In these series, multiple compounds showed highly potent and stereospecific antagonism to hTRPV1 activators including capsaicin, pH, heat (45 °C) and NADA. In addition, selected compounds were evaluated for antinociceptive activity in neuropathic pain models, where they showed potent activity and were found to block capsaicin-induced hypothermia, consistent with their in vitro mechanism of action. Finally, modeling analysis using our established hTRPV1 homology model [18,20] indicated that, among the receptor binding interactions, the two hydrophobic interactions by the 6-trifluoromethyl group and the 2-substituents in the C-region were critical for their potent antagonism and were made with the hydrophobic pockets composed of Leu547/Thr550 and Met514/Leu515, respectively.
Fig. 1.
Chemical structure of previously reported TRPV1 antagonist.
In continuation of our effort to further optimize the pyridine C-region, we herein have investigated the structure activity relationships for hTRPV1 antagonism of various pyridine derivatives, including isosteres of the 6-trifluoromethyl moiety and isomers of pyridine. In this investigation, 2-substituents in the pyridine were fixed, using selected representative groups which had provided high potency in previous reports. With selected potent antagonists in the series, we have further characterized their analgesic activity and inhibition of capsaicin-induced hypothermia in animal models.
2. Result and discussion
2.1. Chemistry
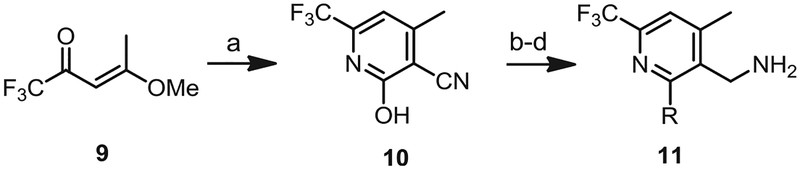
A series of 6-substituted pyridine C-regions (6, 8), containing R1 = CH3, CF2H, CF2Cl, cyclopropyl, and Ph-4-F, were synthesized starting from the corresponding anhydride 1 by a modification of the previously reported procedure (Scheme 1) [20]. For the synthesis of the 2-amino (R2 = NR2) and 2-thio (R2 = SR) analogs, 3-cyanopyridone 3 was prepared and then converted into the corresponding 2-chloropyridine 4, which was condensed with a library of amines and thiols and then reduced to provide the C-region amines 6. For the synthesis of 2-oxy analogs, 3-amidopyridone 7 was synthesized instead and then converted to the C-region amines 8 via an O-alkylation/reduction sequence. A series of 5-methylpyridine C-regions 11 were synthesized from 9, a β-methylated form of 2, employing the same sequences described in Scheme 1 (Scheme 2).
Scheme 1. Syntheses of 6-substituted pyridine C-region analogs.
Reagents and conditions: (a) CH2=CHOEt, pyridine, CHCl3, 0 °C to rt; (b) 2-cyanoacetamide, NaOEt, EtOH, reflux, 3 h; (c) POCl3, sealed tube, 120 °C, 6 h; (d) K2CO3, R–NH2 (or R–SH), CH3CN; (e) BH3–SMe2, THF, 6 h; (f) malonamide, NaOMe, MeOH, reflux, 2 h; (g) RX, K2CO3,18-crown-6 ether, CH3CN/DMF, reflux, 12 h; (h) BH3–SMe2, THF, reflux, 12 h.
Scheme 2. Syntheses of 4-methyl-6-CF3 pyridine C-region analogs.
Reagents and conditions: (a) 2-cyanoacetamide, NaOEt, EtOH, reflux, overnight; (b) POCl3, sealed tube, 120 °C, 6 h; (c) K2CO3, R-NH2, CH3CN or K2CO3, R-OH, CH3CN; (d) Pd/C (10%), H2 (1 atm), MeOH, rt, 8 h or BH3–SMe2, THF, 6 h.
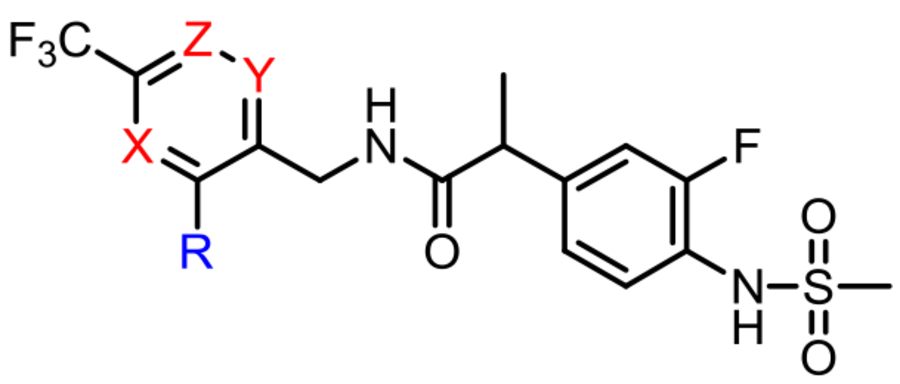
The two regioisomers of the 6-CF3 pyridine C-region, 15 (5-CF3-pyridine) and 19 (2-CF3-pyridine), were synthesized efficiently from appropriate starting material (Scheme 3). For the synthesis of the 5-CF3-pyridine C-region 15, 2,3-dichloropyridine 12 was subjected to 2-cyano substitution followed by a Buchwald–Hartwig amination/reduction sequence to provide 15. For the 2-CF3-pyridine C-region, only one example 19 (R = Ph) was prepared. Pyridone 17 was synthesized from the ketal 16 in 2 conventional steps and then converted to the corresponding 2-chloropyridine 18, which was reduced to the amine 19, concomitant with dehalogenation.
Scheme 3. Syntheses of regioisomeric pyridine C-region analogs.
Reagents and conditions: (1) (a) NaCN, DMF, 120 °C, 8 h; (b) PdCl2, DPPF, t-BuONa, R–NH2, toluene; (c) Pd/C (10%), H2 (1 atm), MeOH, rt, 8 h or BH3–SMe2, THF, 6 h; (2) (a) TFAA, Py, CHCl3, rt; (b) NaOEt, EtOH, 2-cyanoacetamide; (c) POCl3, sealed tube, 120 °C, 6 h; (d) Pd/C (10%), H2 (1 atm), MeOH, rt, 8 h or BH3–SMe2, THF, 6 h.
The synthesized amines (6, 8, 11, 15, 19) were coupled with propionic acid [20] as previously reported to afford the final compounds 20–46 (Scheme 4).
Scheme 4. Syntheses of final compounds.
Reagents and conditions: (a) HOBt, EDC, CH3CN, rt.
2.2. In vitro activity
The synthesized TRPV1 ligands were evaluated in vitro for antagonism as measured by inhibition of activation by capsaicin (CAP). The assays were conducted using a fluorometric imaging plate reader (FLIPR) with human TRPV1 heterologously expressed in Chinese hamster ovary (CHO) cells [20]. The results are summarized in Tables 1–4, together with the potencies of the previously reported antagonists I–V [20–24].
Table 1.
In vitro hTRPV1 antagonistic activities for 2,6-substituted pyridine C-region derivatives.
Values from Ref. [20].
Table 4.
In vitro hTRPV1 antagonistic activities for 2 (or 5)-trifluoromethylpyridine derivatives.
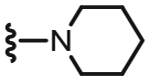
First, we investigated the SAR of the 6-position (R1) in the pyridine C-region by substituting the 6-trifluoromethyl moiety with the corresponding isosteres, including the methyl, difluoromethyl, difluorochloromethyl, cyclopropyl and 4-fluorophenyl groups (Table 1). The 2-position (R2) in the pyridine was held fixed as either the 4-methylpiperidinyl or the piperidinyl group, which had provided high antagonistic potency as reference compounds I and II from the previous report.
The 6-methyl derivative 20 showed a ca. 240-fold reduction in activity compared to the parent I. However, the 6-difluoromethyl 21 and the 6-difluorochloromethyl 22 derivatives retained high potency, with 22 showing the same potency as I. To investigate the halogen effect of the above two substituents, a cyclopropyl group, which has a lipophilicity similar to that of trifluoromethyl group, was incorporated into the 6-position. The 6-cyclopropyl derivative 23 still exhibited potent antagonism comparable to 21. We conclude that the lipophilicity of the 6-substituent was more important for potent antagonism than was its electronic effect. This result is likewise predicted from our molecular modeling, in which the 6-trifluoromethyl group in the pyridine C-region made a hydrophobic interaction with a pocket composed of Leu547 and Thr550. To confirm the relative potency of the 6-difluorochloromethyl and cyclopropyl groups, their 2-piperidinyl derivatives 25 and 26 were also prepared and compared to their parent II. As was the case with 22 and 23, compounds 25 and 26 showed potent and comparable antagonism to their parent compound (II), indicating that the lipophilicity of the substituent at the 6-position was critical for potent antagonism.
Since the 6-difluorochloromethyl group proved to be a promising bioisostere of the 6-trifluoromethyl group above, we explored the SAR of 2-substituents in the 6-difluorochloromethyl pyridine C-region to further evaluate its activity. The various 2-substituents including amino, oxy and thio groups were selected based on their potencies in previous reports [20–24] and activities were compared to those for the corresponding 6-trifluoromethyl surrogates (Table 2). As expected, most 6-difluorochloromethyl pyridine derivatives exhibited very potent antagonism in the low nanomolar range comparable with those of the corresponding 6-trifluoromethyl surrogates. Among them, compounds 31 and 34 with 2-butyloxy and 2-cyclopentyloxy groups were selected as the most promising antagonists for further study.
Table 2.
In vitro hTRPV1 antagonistic activities for 6-difluorochloropyridine derivatives.
Next, we sought to evaluate 4-methyl derivatives of selected 6-trifluoromethyl pyridine antagonists to investigate their steric tolerance by the receptor. The four representative derivatives 40–43 were synthesized and compared to their corresponding parents (Table 3). The incorporation of a 4-methyl group to the pyridine led to ca. 50–250 fold reductions in potency compared to those of the corresponding parent antagonists, indicating that the 4-methyl group was poorly tolerated probably by inducing steric repulsion with the receptor or unfavorable conformation of B/C-regions.
Table 3.
In vitro hTRPV1 antagonistic activities for 4-methylpyridine derivatives.
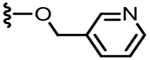
 | |||
|---|---|---|---|
| R2 | R1 = CH3 | R1 = H | |
| Ki [cap] (nM) | Ki [cap] (nM)a | ||
| 40 | 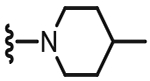 |
17.5 | 0.3 |
| 41 | 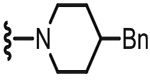 |
9.2 | 0.2 |
| 42 | 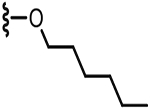 |
100 | 0.4 |
| 43 |  |
44 | 0.9 |
Finally, the SAR of pyridine isomers in the C-region was investigated. Due to limitations of synthetic accessibility, only a few representative compounds were prepared and compared to the parents (Table 4). Relative to the parent compounds III and IV (X = N, Y = Z = H), the shift of a nitrogen to the 4-position led to a dramatic reduction in antagonism, with 44 and 45 (Y = N, X = Z = H) proving to be weak and moderate antagonists, respectively. Likewise, relative to the parent compound V, the shift of a nitrogen to the 5-position to provide 46 (Z = N, X = Y = H) caused a 5-fold reduction in potency. These results indicate that among the pyridine isomers the 6-trifluoromethyl pyridine was optimal for antagonism.
Detailed in vitro activities of 31 and 34, the most potent antagonists in this study, were investigated for multiple TRPV1 activators, namely capsaicin, pH, heat (45 °C) and N-arachidonoyl dopamine (NADA), and were compared to the activity of lead compound I (Table 5). Compound 31 showed excellent antagonism toward all activators comparable to I, but compound 34 exhibited 2–3 fold less potency than 31.
Table 5.
In vitro hTRPV1 antagonistic activities of 31 and 34 for multiple activators.
| Activators, parameter | I | 31 | 34 |
|---|---|---|---|
| CAP (f)Ki (nM) | 0.3 | 0.3 | 0.5 |
| pH, IC50 (nM) | 15.8 | 8.3 | 18.5 |
| Heat 45 °C, IC50 (nM) | 2.56 | 7.0 | 19.3 |
| NADA (f)Ki (nM) | 0.02 | 0.02 | 0.04 |
2.3. In vivo activity
Consistent with its in vitro mechanism of action as an hTRPV1 antagonist, in vivo 31 and 34 likewise blocked response to capsaicin (Table 6). Compounds 31 and 34 were administered orally at a dose 0.3 mg/kg 15 min before intraperitoneal injection of 3 mg/kg capsaicin, following the procedure described previously [20]. This dose of 31 and 34 inhibited the hypothermic response to capsaicin, assayed 30 min after capsaicin injection, by 43% and 41%, respectively. Compound 34 showed a dose-dependent inhibition in capsaicin-induced hypothermia and almost completely blocked the response at a dose of 10 mg/kg.
Table 6.
Effect of compounds 31 and 34 on capsaicin-induced hypothermia in mice.
| Dose (mpk) | 0.1 | 0.3 | 1 | 10 |
|---|---|---|---|---|
| % Inhibition | ||||
| 31 | 43 | |||
| 34 | 23 | 41 | 60 | 92 |
We evaluated the in vivo analgesic activities of the two selected antagonists, 31 and 34, in the formalin test [25] in mice upon oral administration (Table 7). Compound 31 showed a significant antinociceptive effect, with 56% and 73% inhibition of response at the doses of 0.1 and 0.3 mg/kg, respectively. Since we had observed that TRPV1 knock-out mice showed approximately 50% of the magnitude of response in the formalin test as was seen in wild-type mice (unpublished observations), the inhibition of the formalin response that we found for this compound would correspond to the expected result for full TRPV1 blockade. Compound 34 showed weaker activity than that of 31 in the formalin assay, inhibiting the nociceptive response by 29% and 15% at the doses of 0.3 and 1.0 mg/kg, respectively.
Table 7.
Analgesic activity of compound 31 and 34 on formalin model after oral administration in mice.
| Dose (mpk) | 0.1 | 0.3 | 1 |
|---|---|---|---|
| MPE | |||
| 31 | 56 | 73 | |
| 34 | 29 | 15 |
3. Conclusion
The structure activity relationship of pyridine derivatives in the C-region of N-(6-trifluoromethyl-pyridin-3-ylmethyl) 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides was investigated for hTRPV1 antagonism. The analysis indicated that among the current series the 6-difluorochloromethyl pyridine C-region was the most adequate surrogate of the C-region for the leads described previously. The two selected antagonists 31 and 34 showed excellent antagonism to multiple hTRPV1 activators and blocked capsaicin-induced hypothermia, consistent with their actions in vitro being through TRPV1. Compound 31 demonstrated strong analgesic activity in the formalin test in mice with full TRPV1 efficacy.
4. Experimental
4.1. Chemistry
4.1.1. General
All chemical reagents were commercially available. Melting points were determined on a Büchi Melting Point B-540 apparatus and are uncorrected. Silica gel column chromatography was performed on silica gel 60, 230–400 mesh, Merck. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded on JEOL JNM-LA 300 [300 MHz (1H), 75 MHz (13C)] and Bruker Avance 400 MHz FT-NMR [400 MHz (1H), 100 MHz (13C)] spectrometers. Chemical shifts are reported in ppm units with Me4Si as a reference standard. Infrared (IR) spectra were recorded on a JASCO FT/IR-4200 spectrometer. Mass spectra were recorded on a VG Trio-2 GC-MS and 6460 Triple Quad LC/MS. All final compounds were purified to >95% purity, as determined by high-performance liquid chromatography (HPLC). HPLC was performed on an Agilent 1120 Compact LC (G4288A) instrument using an Agilent Eclipse Plus C18 column (4.6 × 250 mm, 5 μm) and a Daicel Chiralcel OD-H column (4.6 × 250 mm, 5 μm).
4.1.2. General procedure for chemistry
The general synthetic procedure for the syntheses of the final compounds was described in previous reports [20–22].
4.1.2.1. N-((6-Methyl-2-(4-methylpiperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(20).
75% yield, white solid, mp = 78–80 °C, 1H NMR (300 MHz, CDCl3) δ 7.51 (t, 1H, J = 8.2 Hz), 7.13 (d, 1H, J = 8.6 Hz), 7.07 (d, 2H, J = 8.0 Hz), 6.76 (s, 1H), 6.74 (s, 1H), 6.46 (brs, 1H), 4.41 (t, 2H, J = 4.4 Hz), 3.51 (q, 1H), 3.18 (m, 2H), 3.02 (s, 3H), 2.78 (m, 2H), 2.42 (s, 3H), 1.73 (m, 2H), 1.50 (d, 3H, J = 7.1 Hz), 1.26 (m, 2H), 0.97 (d, 3H, J = 6.4 Hz); MS (FAB) m/z 463 (M+H).
4.1.2.2. N-((6-(Difluoromethyl)-2-(4-methylpiperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(21).
48% yield, white solid, mp = 105–107 °C, 1H NMR (300 MHz, CDCl3) δ 7.50 (t, 2H, J = 4.7 Hz), 7.17 (d, 1H, J = 8.1 Hz), 7.09 (d, 1H, J = 6.0 Hz), 6.48 (s, 1H), 6.42 (brs, 1H), 4.46 (brs, 1H), 3.55 (q, 1H), 3.26 (t, 2H, J = 13.2 Hz), 3.02 (s, 3H), 2.79 (t, 2H, J = 11.9 Hz), 1.71 (brs, 2H), 1.52 (d, 3H, J = 6.6 Hz), 1.19 (m, 2H), 0.97 (d, 3H, J = 6.0 Hz); MS (FAB) m/z 499 (M+H).
4.1.2.3. N-((6-(Chlorodifluoromethyl)-2-(4-methylpiperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (22).
77% yield, white solid, mp = 175–176 °C, 1H NMR (300 MHz, CDCl3) δ 7.45–7.53 (m, 2H), 7.07–7.18 (m, 3H), 6.72 (bs, 1H), 6.37 (bt, 1H), 4.46 (d, 2H, J = 5.7 Hz), 3.56 (q,lH, J = 6.9 Hz), 3.32 (m, 2H), 3.02 (s, 3H), 2.82 (m, 2H), 1.71 (m, 2H), 1.53 (d, 3H, J = 7.5 Hz), 1.23 (m, 3H), 0.97 (d, 3H, J = 6.9 Hz); MS (FAB) m/z 534 (M+H).
4.1.2.4. N-((6-Cyclopropyl-2-(4-methylpiperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(23).
58% yield, white solid, mp = 84 °C, 1H NMR (300 MHz, CDCl3) δ 7.50 (t, 1H, J = 8.3 Hz), 7.23–7.06 (m, 3H), 6.74 (d, 2H, J = 7.5 Hz), 6.47 (bs, 1H), 4.40 (t, 2H, J = 5.9 Hz), 3.50 (q, 1H,J = 7.1 Hz), 3.18 (m, 2H), 3.01 (s, 3H), 2.72 (m, 2H), 1.90 (m,1H), 1.69 (m, 2H), 1.50 (d, 3H, J = 7.1 Hz), 1.26–1.13 (m, 3H), 0.95 (d, 3H, J = 6.4 Hz); MS (FAB) m/z 489 (M+H).
4.1.2.5. N-((6-(4-Fluorophenyl)-2-(4-methylpiperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (24).
52% yield, white solid, mp = 154–156 °C, 1H NMR (300 MHz, CDCl3) δ 7.49–7.54 (m, 2H), 7.51 (dd, 1H, J = 8.1 and 8.1 Hz), 7.43 (d, 1H, 7.8 Hz), 7.29 (d, 1H, J = 7.8 Hz), 7.09–7.17 (m, 4H), 6.64 (bt, 1H), 4.48 (d, 2H, J = 5.7 Hz), 3.52 (q, 1H, J = 6.9 Hz), 3.30 (m, 2H), 3.03 (s, 3H), 2.88 (m. 2H), 1.76 (m, 2H), 1.51 (d, 3H, J = 6.9 Hz), 1.24 (m, 3H), 0.99 (d, 3H, J = 6.6 Hz); MS (FAB) m/z 543 (M+H).
4.1.2.6. N-((6-(Chlorodifluoromethyl)-2-(piperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (25).
70% yield, pale yellow solid, mp = 63–65 °C, 1H NMR (300 MHz, CDCl3) δ 7.45–7.52 (m, 2H), 7.06–7.18 (m, 3H), 6.46 (bt, 1H), 4.47 (d, 2H, J = 5.1 Hz), 3.58 (q, 1H, J = 6.9 Hz), 3.05 (m, 4H), 3.00 (s, 3H), 1.61 (m, 6H), 1.53 (d, 3H, J = 7.1 Hz); MS (FAB) m/z 520 (M+H).
4.1.2.7. N-((6-Cyclopropyl-2-(piperidin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (26).
57% yield, white solid, mp = 65–75 °C, 1H NMR(300 MHz, CDCl3) δ 7.51 (t, 1H,J = 8.4 Hz), 7.24–7.06 (m, 3H), 6.74 (d, 2H,J = 7.5 Hz), 6.47 (bs, 1H), 4.39 (t, 2H, J = 5.3 Hz), 3.50 (q,1H, J = 7.0 Hz), 3.01 (s, 3H), 2.92 (m, 4H), 1.90 (m, 1H), 1.55 (m, 2H), 1.50 (d, 3H, J = 7.1 Hz), 1.01–0.86 (m, 3H); MS (FAB) m/z 475 (M+H).
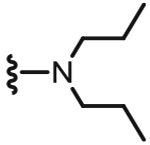
4.1.2.8. N-((6-(Chlorodifluoromethyl)-2-(dipropylamino)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (27).
58% yield, white solid, mp = 101–103 °C, 1H NMR (300 MHz, CDCl3) δ 7.53 (dd, 1H,J = 8.1 and 8.1 Hz), 7.43 (d,1H, J = 7.8 Hz), 7.16 (dd, 1H, J = 2.1 and 10.8 Hz), 7.08–7.12 (m, 2H), 6.46 (bs, 1H), 6.15 (bt, 1H), 4.44 (d, 2H, J = 5.7 Hz), 3.53 (q,1H, J = 6.9 Hz), 3.10 (m, 4H),3.02 (s, 3H), 1.44–1.54 (m, 4H), 0.83 (t, 6H, J = 7.2 Hz), MS (FAB) m/z 535 (M+H).
4.1.2.9. N-((6-(Chlorodifluoromethyl)-2-(3,5-(cis/trans)-dimethylpi-peridin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methyl-sulfonamido)phenyl)propanamide (28).
58% yield, colorless oil, 1H NMR (300 MHz, CDCl3) δ 7.45–7.54 (m, 2H), 7.06–7.15 (m, 3H), 6.62 (bs, 1H), 6.31 (bt, 1H), 4.46 (d, 2H, J = 5.7 Hz), 3.54 (q, 1H, J = 7.2 Hz), 3.25 (m, 2H), 3.02 (s, 3H), 2.36 (m, 2H), 2.03 (m, 1H), 1.53–1.65 (m, 3H), 1.52 (d, 3H, J = 7.2 Hz) 0.92 (d, 3H, J = 6.6 Hz), 0.88 (d, 3H, J = 6.6 Hz); MS (FAB) m/z 548 (M+H).
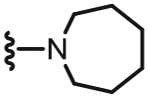
4.1.2.10. N-((2-(Azepan-1-yl)-6-(chlorodifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (29).
58% yield, white solid, mp = 59–61 °C, 1H NMR (300 MHz, CDCl3) δ 7.52 (dd, 1H, J = 8.1 and 8.1 Hz), 7.38 (d,1H, J = 7.5 Hz), 7.17 (dd, 1H, J = 1.8 and 11.1 Hz), 7.08 (d, 1H, J = 8.1 Hz), 6.99 (d, 1H, J = 7.5 Hz), 6.57 (bs, 1H), 5.87 (bt, 1H), 4.42 (d, 2H, J = 5.7 Hz), 3.56 (q, 1H, J = 6.9 Hz), 3.39 (t, 4H, J = 6.0 Hz), 3.02 (s, 3H), 1.75 (m, 4H),1.56 (m, 4H), 1.52 (d, 3H, J = 6.9 Hz); MS (FAB) m/z 534 (M+H).
4.1.2.11. N-((6-(Chlorodifluoromethyl)-2-(4-phenylpiperazin-1-yl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (30).
65% yield, white solid, mp = 108 °C, 1H NMR (300 MHz, CD3OD) δ 8.60 (m, 1H), 7.58 (d, 1H, J = 7.5 Hz), 7.47–7.25 (m, 4H), 7.25–7.00 (m, 4H), 4.57–4.35 (m, 2H), 3.73 (d, 1H, J = 7.1 Hz), 3.45–3.32 (m, 8H), 2.95 (bs, 3H), 1.47 (d, 3H, J = 7.1 Hz); MS (FAB) m/z 597 (M+H).
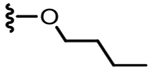
4.1.2.12. N-((2-Butoxy-6-(chlorodifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(31).
70% yield, white solid, mp = 75–77 °C, 1H NMR (300 MHz, CDCl3) δ 7.49–7.54 (m, 2H), 7.14 (d, 1H, J = 7.5 Hz), 7.05–7.09 (m, 2H), 6.46 (bs, 1H), 6.02 (bt, 1H), 4.36 (m, 4H), 3.52 (q, 1H,J = 6.9 Hz),3.03 (s, 2H), 1.71 (m, 2H), 1.48 (m, 5H), 0.97 (t, 3H, J = 7.2 Hz); MS (FAB) m/z 508 (M+H).
4.1.2.13. N-((6-(Chlorodifluoromethyl)-2-(pentyloxy)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(32).
48% yield, white solid, mp = 86 °C, 1H NMR (400 MHz, CDCl3) δ 7.58–7.47 (m, 2H), 7.17–7.03 (m, 3H), 6.50 (bs, 1H), 5.98 (bt, 1H), 4.43–4.25 (m, 4H), 3.51 (q, 1H, J = 8.6 Hz), 3.03 (s, 3H), 1.78–1.67 (m, 2H), 1.49 (d, 3H, J = 6.8 Hz), 1.46–1.27 (m, 6H), 0.94–0.87 (m, 3H); MS (FAB) m/z 522 (M+H).
4.1.2.14. N-((6-(Chlorodifluoromethyl)-2-isobutoxypyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(33).
57% yield, white solid, mp = 105 °C,1H NMR(300 MHz, CDCl3) δ 7.58–7.48 (m, 2H), 7.16 (d, 1H, J = 7.5 Hz), 7.11 (m, 1H), 7.06 (m, 1H), 6.46 (m, 1H), 5.95 (bt, 1H), 4.44–4.32 (m, 2H), 4.19–4.06 (m, 2H), 3.51 (q, 1H, J = 7.1 Hz), 3.04 (s, 3H), 2.05 (m, 1H), 1.49 (d, 3H, J = 7.1 Hz), 0.99 (d, 6H, J = 6.8 Hz); MS (FAB) m/z 509 (M+H).
4.1.2.15. N-((6-(Chlorodifluoromethyl)-2-(cyclopentyloxy)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(34).
71%yield, white solid, mp = 106 °C,1H NMR(300 MHz, CDCl3) δ 7.57–7.48 (m, 2H), 7.15–7.03 (m, 3H), 6.56 (bs, 1H), 6.01–5.90 (bt, 1H), 5.46 (m, 1H), 4.42–4.27 (m, 2H), 3.52 (q,1H, J = 7.1 Hz), 3.03 (s, 3H), 2.08–1.91 (m, 2H), 1.78–1.56 (m, 6H), 1.49 (d, 3H, J = 7.1 Hz); MS (FAB) m/z 520 (M+H).
4.1.2.16. N-((6-(Chlorodifluoromethyl)-2-(cyclohexyloxy)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(35).
58% yield, white solid, mp = 97 °C,1H NMR (300 MHz, CDCl3) δ 7.59–7.48 (m, 2H), 7.14–7.02 (m, 3H), 6.49 (bs, 1H), 6.01 (bt, 1H),5.13 (m, 1H), 4.47–4.29 (m, 2H), 3.52 (q, 1H, J = 7.3 Hz), 3.03 (s, 3H), 1.99–1.85 (m, 2H), 1.77–1.62 (m, 2H), 1.52–1.38 (m, 9H); MS (FAB) m/z 534 (M+H).
4.1.2.17. N-((2-(Benzyloxy)-6-(chlorodifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide(36).
52% yield, white solid, mp = 62 °C, 1H NMR (300 MHz, CDCl3) δ 7.59 (d, 1H, J = 7.3 Hz), 7.50–7.30 (m, 6H), 7.20 (d, 1H, J = 7.8 Hz),7.05 (dd, 1H, J = 11.2 and 2.0 Hz), 6.97 (d, 1H, J = 7.9 Hz), 6.52 (bs, 1H), 6.00 (bt, 1H), 5.49–5.36 (m, 2H), 4.46–4.30 (m, 2H), 3.42 (q, 1H, J = 7.1 Hz), 3.00 (bs, 3H), 1.43 (d, 3H, J = 7.1 Hz); MS (FAB) m/z 542 (M+H).
4.1.2.18. N-((6-(Chlorodifluoromethyl)-2-(pyridin-3-ylmethoxy)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)prop-anamide (37).
61% yield, white solid, mp = 78 °C, 1H NMR (400 MHz, CDCl3) δ 8.61–8.56 (m, 2H), 7.77 (m, 1H), 7.62 (d, 1H, J = 7.6 Hz), 7.48 (dd, 1H, J = 8.0 and 8.0 Hz), 7.31 (m,1H), 7.21 (d,1H, J = 7.6 Hz), 7.09–6.92 (m, 2H), 5.88 (bt, 1H), 5.47–5.37 (m, 2H),4.43–4.30 (m, 2H), 3.49 (q, 1H, J = 6.8 Hz), 3.03 (s, 3H), 1.28 (d, 3H, J = 6.8 Hz); MS (FAB) m/z 543 (M+H).
4.1.2.19. N-((6-(Chlorodifluoromethyl)-2-(pyridin-2-ylmethoxy)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (38).
52% yield, white solid, mp = 67 °C, 1H NMR (400 MHz, CDCl3) δ 8.62 (d, 1H, J = 4.4 Hz), 7.75 (dd, 1H, J = 7.6 and 7.6 Hz), 7.67 (d, 1H, J = 7.2 Hz), 7.51–7.41 (m, 2H), 7.27–7.19 (m, 2H),7.13 (dd, 1H, J = 11.2 and 1.6 Hz), 7.04 (d, 1H, J = 8.4 Hz), 6.50 (bs, 1H), 5.63–5.48 (m, 2H), 4.61–4.40 (m, 2H), 3.60 (q, 1H, J = 7.2 Hz),3.05 (s, 3H), 1.49 (d, 3H, J = 7.2 Hz); MS (FAB) m/z 543 (M+H).
4.1.2.20. N-((6-(Chlorodifluoromethyl)-2-(cyclohexylthio)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (39).
66% yield, white solid, mp = 70–73 °C, 1H NMR (300 MHz, CD3OD) δ 7.35–7.48 (m, 3H), 7.26 (d, 1H, J = 7.8 Hz), 7.16 (dd, 1H, J = 1.8 and 11.1 Hz), 7.10 (d, 1H, J = 8.4 Hz), 6.13 (bs, 1H), 4.35 (d, 2H, J = 5.7 Hz), 3.82 (m, 1H), 3.56 (q, 1H,J = 7.2 Hz), 3.02 (s, 3H), 2.06 (m, 2H), 1.75 (m, 2H), 1.49 (d, 3H, J = 7.2 Hz), 1.26–1.33 (m, 6H); MS (FAB) m/z 550 (M+H).
4.1.2.21. N-((4-Methyl-2-(4-methylpiperidin-1-yl)-6-(tri-fluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methyl-sulfonamido)phenyl)propanamide (40).
66% yield, white solid, 1H NMR (300 MHz, CDCl3) δ 7.51–7.46 (t, 1H, J = 8.0 Hz), 7.14 (s, 1H), 7.07–7.02 (m, 2H), 6.80 (brs, 1H), 4.52 (d, 2H, J = 5.3 Hz), 3.48 (q, 1H), 3.17 (d, 1H, J = 11.9 Hz), 3.02 (s, 3H), 2.78 (tt, 2H, J = 11.0 and 2.6 Hz), 2.38 (s, 3H), 1.71 (m, 2H), 1.47 (d, 3H, J = 7.0 Hz), 1.26–1.13 (m, 2H), 0.97 (d, 3H, J = 6.6 Hz), 0.88 (m, 1H); MS (FAB) m/z 531 (M+H).
4.1.2.22. N-((2-(4-Benzylpiperidin-1-yl)-4-methyl-6-(tri-fluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methyl-sulfonamido)phenyl)propanamide (41).
59% yield, white solid, mp = 167 °C, 1H NMR (300 MHz, CDCl3) δ 7.49 (dd, 1H, J = 8.3 and 8.3 Hz), 7.35–7.27 (m, 2H), 7.25–7.11 (m, 4H), 7.10–6.98 (m, 6.70 (bt, 1H), 4.58–4.42 (m, 2H), 3.45 (q, 1H, J = 7.1 Hz), 3.21–3.02 (m, 2H), 2.99 (s, 3H), 2.83–2.68 (m, 2H), 2.58 (d, 2H, J = 6.6 Hz), 2.37 (s, 3H), 1.80–1.64 (m, 3H), 1.47 (d, 3H, J = 7.1 Hz), 1.32–1.78 (m, 2H); MS (FAB) m/z 607 (M+H).
4.1.2.23. N-((2-(Hexyloxy)-4-methyl-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (42).
44% yield, white solid, 1H NMR (300 MHz, ClCl3) δ 7.50 (dd, 1H, J = 8.3 Hz), 7.03 (m, 3H), 6.03 (bt, 1H), 4.42 (m, 2H), 4.29 (m, 2H), 3.45 (q, 1H, J = 7.6 Hz), 3.02 (s, 3H), 2.47 (s, 3H), 1.69 (m, 2H),1.45 (d, 3H, J = 7.1 Hz), 1.35 (m, 2H), 1.25 (m, 2H), 0.92 (m, 5H); (FAB) m/z 534 (M+H).
4.1.2.24. N-((2-(Cyclopentyloxy)-4-methyl-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (43).
75% yield, white solid, mp = 92–98 °C, 1H NMR (300 MHz, CDCl3) δ 7.51 (t, 1H, J = 8.3 Hz), 7.03 (m, 3H), 5.96 (bs, 1H), 5.43 (m,1H), 4.45–4.33 (m, 2H), 3.46 (q, 1H, J = 7.1 Hz), 3.02 (s, 3H), 2.48 (s, 3H), 1.99–1.96 (m, 2H), 1.65–1.55 (m, 6H), 1.46 (d, 3H, J = 6.9 Hz); MS (FAB) m/z 518 (M+H).
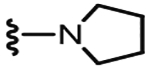
4.1.2.25. N-((3-(Pyrrolidin-1-yl)-5-(trifluoromethyl)pyridin-2-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (44).
85% yield, white solid, 1H NMR (300 MHz, CDCl3) δ 8.21 (s, 1H), 7.52 (t,1H, J = 8.4 Hz), 7.41 (bs,1H), 7.19 (t, 2H, J = 8.4 Hz), 6.41 (bs, 1H), 4.59–4.54 (dq, 2H, J = 12.1 and 4.2 Hz), 3.68 (q, 1H, J = 7.3 Hz), 3.30 (m, 4H), 3.01 (s, 3H), 1.99 (m, 4H), 1.55 (d, 3H, J = 7.1 Hz); MS (FAB) m/z 489 (M+H).
4.1.2.26. N-((2-(4-Methylbenzylthio)-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (45).
81% yield, white solid, 1H NMR (300 MHz, CDCl3) δ 8.43 (s, 1H), 7.54–7.49 (m, 2H), 7.23–7.15 (m, 2H), 6.63 (bs, 1H), 4.67–4.52 (dq, 2H, J = 13.5 and 4.4 Hz), 3.68 (q, 1H, J = 7.5 Hz), 3.01 (s, 3H), 2.96–2.83 (m, 4H), 1.74–1.60 (m, 4H), 1.59 (m, 2H), 1.55 (d, 2H, J = 7.1 Hz); MS (FAB) m/z 503 (M+H).
4.1.2.27. N-((4-Phenyl-6-(trifluoromethyl)pyridin-3-yl)methyl)-2-(3-fluoro-4-(methylsulfonamido)phenyl)propanamide (46).
54% yield, colorless oil, 1H NMR (300 MHz, CD3OD) δ 8.55 (s, 1H), 7.46–7.53 (m, 5H), 7.24–7.27 (m, 2H), 7.05 (dd,1H, J = 11.1 and 1.8 Hz), 6.99 (d, 1H, J = 8.1 Hz), 6.62 (bs 1H), 5.67 (bt, 1H), 4.49 (d, 2H, J = 5.7 Hz),3.45 (q, 1H, J = 6.9 Hz), 3.04 (s, 3H), 1.45 (d, 3H, J = 6.9 Hz); MS (FAB) m/z 496 (M+H).
4.2. Biological assay
The methods for in vitro and in vivo assays were reported in the previous literature [16,17]. All animal protocols were approved by the institutional review committee at Grunenthal Innovations.
Acknowledgments
This research was supported by Research Grants from Grunenthal in Germany, Grants from the National Research Foundation of Korea (NRF) (R11-2007-107-02001-0) and the Korea Science and Engineering Foundation (KOSEF) (2014M3A9B5073755) in South Korea, and in part by the Intramural Research Program of NIH, Center for Cancer Research, NCI (Project Z1A BC 005270) in USA.
References
- [1].Szallasi A, Blumberg PM, Vanilloid (Capsaicin) receptors and mechanisms, Pharmacol. Rev 51 (1999) 159–211. [PubMed] [Google Scholar]
- [2].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D, The cloned capsaicin receptor integrates multiple pain-producing stimuli, Neuron 21 (1998) 531–543. [DOI] [PubMed] [Google Scholar]
- [3].Szallasi A, Vanilloid (Capsaicin) receptors in health and disease, Am. J. Clin. Pathol 118 (2002) 110–121. [DOI] [PubMed] [Google Scholar]
- [4].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D, The capsaicin receptor: a heat-activated ion channel in the pain pathway, Nature 389 (1997) 816–824. [DOI] [PubMed] [Google Scholar]
- [5].Morales-Lazaro SL, Simon SA, Rosenbaum T, The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1), J. Physiol 591 (2013) 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Szolcsányi J, Sándor Z, Multisteric TRPV1 nocisensor: a target for analgesics, Trend Pharmacol. Sci 33 (2012) 646–655. [DOI] [PubMed] [Google Scholar]
- [7].Blumberg PM, Pearce LV, Lee J, TRPV1 activation is not an all-or-none event: TRPV1 partial agonism/antagonism and its regulatory modulation, Curr. Top. Med. Chem 11 (2011) 2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xia R, Samad TA, Btesh J, Jiang LH, Kays I, Stjernborg L, Dekker N, TRPV1 signaling: mechanistic understanding and therapeutic potential, Curr. Top. Med. Chem 11 (2011) 2180–2191. [DOI] [PubMed] [Google Scholar]
- [9].Kym PR, Kort ME, Hutchins CW, Analgesic potential of TRPV1 antagonists, Biochem. Pharmacol 78 (2009) 211–216. [DOI] [PubMed] [Google Scholar]
- [10].Wong GY, Gavva NR, Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks, Brain Res. Rev 60 (2009) 267–277. [DOI] [PubMed] [Google Scholar]
- [11].Gunthorpe MJ, Chizh BA, Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway, Drug Disc. Today 14 (2009) 56–67. [DOI] [PubMed] [Google Scholar]
- [12].Lazar J, Gharat L, Khairathkar-Joshi N, Blumberg PM, Szallasi A, Screening TRPV1 antagonists for the treatment of pain: lessons learned over a decade, Exp. Opin. Drug Discov 4 (2009) 159–180. [DOI] [PubMed] [Google Scholar]
- [13].Voight EA, Kort ME, Transient receptor potential vanilloid-1 antagonists: a survey of recent patent literature, Exp. Opin. Ther. Pat 20 (2010) 1–16. [DOI] [PubMed] [Google Scholar]
- [14].Szolcsányi J, Sándor Z, Multisteric TRPV1 nocisensor: a target for analgesics, Trend Pharmacol. Sci 33 (2012) 646–655. [DOI] [PubMed] [Google Scholar]
- [15].Szallasi A, Sheta M, TargetingTRPV1 for pain relief: limits, losers and laurels, Exp. Opin. Invest. Drug 21 (2012) 1351–1369. [DOI] [PubMed] [Google Scholar]
- [16].De Petrocellis L, Moriello AS, Modulation of the TRPV1 channel: current clinical trials and recent patents with focus on neurological conditions, Recent Pat. CNS Drug Discov 8 (2013) 180–204. [DOI] [PubMed] [Google Scholar]
- [17].Szallasi A, Sheta M, Targeting TRPV1 for pain relief: limits, losers and laurels, Exp. Opin. Invest. Drug 21 (2012) 1351–1369. [DOI] [PubMed] [Google Scholar]
- [18].Lee JH, Lee Y, Ryu H, Kang DW,Lee J,Lazar J, Pearce LV, Pavlyukovets VA, Blumberg PM, Choi S, Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies, J. Comput. Aided Mol. Des 25 (2011) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim MS, Ki Y, Ahn S, Yoon S, Kim S-E, Park H-G, Sun W, Son K, Cui M, Choi S, Pearce LV, Esch TE, DeAndrea-Lazarus IA, Blumberg PM, Lee J, Asymmetric synthesis and receptor activity of chiral simplified resiniferatoxin (sRTX) analogues as transient receptor potential vanilloid 1 (TRPV1) ligands, Bioorg. Med. Chem. Lett 24 (2014) 382–385 (and references therein). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim MS, Ryu H, Kang DW, Cho S-H, Seo S, Park YS, Kim M-Y, Kwak EJ, Kim YS, Bhondwe RS, Kim HS, Park S-g., Son K, Choi S, DeAndrea-Lazarus I, Pearce LV, Blumberg PM, Frank R, Bahrenberg G, Stockhausen H, Kögel BY, Schiene K, Christoph T, Lee J, 2-(3-Fluoro-4-methylsulfonylaminophenyl)propanamides as potent transient receptor potential vanilloid 1 (TRPV1) antagonists: structure–activity relationships of 2-amino derivatives in the N-(6-Trifluoromethylpyridin-3-ylmethyl) C-region, J. Med. Chem 55 (2012) 8392–8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thorat SA, Kang DW, Ryu H, Kim MS, Kim HS, Ann J, Ha T-H, Kim SE, Son K, Choi KS, Blumberg PM, Frank R, Bahrenberg G, Schiene K, Christoph T, Lee J, 2-(3-Fluoro-4-methylsulfonylaminophenyl)propanamides as potent TRPV1 antagonists: structure activity relationships of the 2-oxy pyridine C-region, Eur. J. Med. Chem 64 (2013) 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ha T-H, Ryu H, Kim S-E, Kim HS, Ann J, Tran P-T, Hoang V-H, Son K, Cui M, Choi S, Blumberg PM, Frank R, Bahrenberg G, Schiene K, Christoph T, Frormann S, Lee J, TRPV1 antagonist with high analgesic efficacy: 2-thio pyridine C-region analogues of 2-(3-fluoro-4-methylsulfonylaminophenyl) propanamides, Bioorg. Med. Chem 21 (2013) 6657–6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ryu H, Seo S, Cho S-H, Kim HS, Jung A, Kang DW, Son K, Cui M, Hong S-h., Sharma PK, Choi S, Blumberg PM, Frank-Foltyn R, Bahrenberg G, Stockhausen H, Schiene K, Christoph T, Frormann S, Lee J, 2-Alkyl/alkenyl substituted pyridine C-region analogues of 2-(3-fluoro-4-methylsulfonylaminophenyl)propanamides as highly potent TRPV1 antagonists, Bioorg. Med. Chem. Lett 24 (2014) 4039–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ryu H, Seo S, Cho S-H, Kim MS, Kim M-Y, Kim HS, Ann J, Tran P-T, Hoang V-H, Byun J, Cui M, Son K, Sharma PK, Choi S, Blumberg PM, Frank-Foltyn R, Bahrenberg G, Koegel B-Y, Christoph T, Frormann S, Lee J, 2-Aryl substituted pyridine C-region analogues of 2-(3-fluoro-4-methyl-sulfonylaminophenyl)propanamides as highly potent TRPV1 antagonists, Bioorg. Med. Chem. Lett 24 (2014) 4044–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dubuisson D, Dennis SG, The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats, Pain 4 (1977) 161–174. [DOI] [PubMed] [Google Scholar]