Abstract
Background.
Surgery is the only definitive therapy for gastro-entero-pancreatic neuroendocrine tumors (GEP-NETs), and achieving complete tumor resection is an important prognostic factor. Radiopharmaceuticals such as 68Ga-DOTA peptides have been developed that offer superior accuracy for localization of GEPNETs. The study aim was to determine the feasibility of radio-guided surgery (RGS) using 68Ga-DOTATATE in patients with primary and recurrent GEPNETs.
Methods.
Fourteen patients with GEPNETs were enrolled onto a prospective study to determine the feasibility of RGS with 68Ga-DOTATATE. Findings from preoperative imaging, intraoperative exploration, RGS, and pathology were analyzed.
Results.
The median decay corrected target count rate was 172.6 (range 28.15–2341) for tumors, with a tumor-to-background ratio (TBR) of 4.46 (range 1.6–43.56). The median lesion size was 1.55 (range 0.5–15) cm. There was no significant correlation between preoperative imaging maximum standardized uptake value (SUVmax) of the lesions and TBR (Spearman r = − 0.01, p = 0.9), TBR and tumor size (Spearman r = 0.29, p = 0.14), and SUVmax and tumor size (Spearman r = 0.22, p = 0.28). The probe showed correct identification for gastric and small intestine neuroendocrine tumor (NET), including lymph node metastasis in 17 (81.0 %) of 21 cases, with a median TBR of 3.5 (1.6–40.2). For pancreatic NETs and lymph node metastasis, 16 (66.7 %) of 24 were correctly identified by RGS.
Conclusions.
Our study shows that RGS with 68Ga-DOTATATE is feasible and correctly confirms bowel NETs and metastatic mesenteric lymph nodes. Further studies are needed to determine the benefit of RGS with 68Ga-DOTATATE.
The incidence of gastro-entero-pancreatic neuroendocrine tumors (GEPNETs) has increased to about 7.8 cases per 100,000 persons each year, and the prevalence is approximately 35 cases per 100,000 persons.1,2 Surgical resection is the only curative treatment option for patients with early-stage disease, and complete tumor removal is an important prognostic factor in patients with GEPNETs. A recent consensus statement emphasized that resection should be the first-line treatment option for patients with advanced GEPNETs if at least 90 % of the disease burden is resectable.3 Also, the presence of metastatic disease and tumor grade are important prognostic factors.4,5 Thus, an accurate assessment of the extent of disease before surgical therapy and confirming complete resection of disease is important. Further, in patients with recurrent/persistent locoregional GEPNETs, a reoperation can be challenging because of scar tissue, inflammation, altered anatomy, or low volume of disease. In some patients with functioning primary localized GEPNETs (e.g., gastrinoma, insulinoma), small primary or locoregional disease can be difficult to identify at the time of operation. For this reason, several radiopharmaceuticals have been used for radio-guided surgery (RGS) to optimize disease localization during an operation and/or to confirm removal of tumor in patients with GEPNETs.6–9
A unique feature of GEPNETs is the expression of somatostatin receptors (SSTR), which can be targeted with radiolabeled peptides for imaging and treatment.10–12 Positron emission tomography/computed tomography (PET/CT) utilizing somatostatin analogs labeled with positron-emitting 68gallium (68Ga-DOTA peptides) has been shown to be more accurate than other agents for detecting GEPNETs and is an important tool in the clinical management of patients with GEPNETs.13–15 Radiolabeled analogs such as 111In-pentetreotide, 123I-metaiodobenzylguanidine, and 99technetium–sulfur have been used in the past for RGS in patients with GEPNETs.6,16–18 To date, there have been two case reports and one surgical series in nine patients with GEPNETs in which RGS with 68Ga-DOTA peptides were used as preoperative scanning dose but not injected at the time of the operation.19–21 68Ga-DOTA peptides are investigational in the United States, and to our knowledge, there has been no prospective study using 68Ga-DOTATATE for RGS in patients with primary and/or recurrent GEPNETs after selection based on preoperative 68Ga-DOTATATE PET/CT imaging results.
The aim of this prospective study was to assess the feasibility of RGS and its utility in detecting GEPNETs using an intraoperative dose of 68Ga-DOTATATE and a handheld detector.
MATERIALS AND METHODS
Patients were enrolled onto a prospective study investigating 68Ga-DOTATATE PET/CT. The decision to perform RGS was made on the basis of positive preoperative 68Ga-DOTATATE PET/CT results. This prospective study was performed under an investigational new drug approval from the United States Food and Drug Administration and approved by the National Cancer Institute review board and the National Institutes of Health Radiation Safety Committee (). Written informed consent was obtained from all study participants.
For preoperative 68Ga-DOTATATE PET/CT imaging, 5 mCi of 68Ga-DOTATATE was administered through a peripheral vein. After approximately 60 min, images from the upper thighs to midskull were obtained (Biograph mCT; Siemens Medical Solutions USA). A low-dose, noncontrast CT was used for attenuation correction and anatomic localization. Maximum standardized uptake values (SUVmax) were measured on the basis of patient total body weight. Imaging was performed within a median of 84 (range 1–214) days before the operation.
At the time of the operation, a median dose of 5.0 (range 4.09–5.33) mCi of 68Ga-DOTATATE was injected intravenously. At an average of 78.2 ± 51.13 (range 10–193) minutes after injection, a Neoprobe handheld gamma detector (model 2300; Neoprobe Corporation, Dublin, OH, USA) was used for the radio-guided explorations. Background count rates (counts per second) were obtained from the liver and mesenteric or omental fat. The gamma probe was used to explore the abdomen in a systematic fashion, and in vivo count rates of each tumor were obtained. The target count was determined on the basis of the highest count rate and normalized to the background count rate [tumor-to-background ratio (TBR)]. When the absolute target count rates are stated, they were decay corrected and normalized to the injected dose of 68Ga-DOTATATE using the following formula: C0 = [Ct × Exp (0.693/half-life × interval time)]/injected dose. Ex vivo counts were performed once the tumor was resected. Before resection, the palpation and visibility of each lesion by the surgeon was recorded. A summary of the steps for the gamma probe protocol is shown in Table 1.
TABLE 1.
68Ga-DOTATATE intraoperative administration protocol
| Step | Description |
|---|---|
| Intraoperative timing of tracer injection to surgical exploration | 68Ga-DOTATATE was injected intravenously at start of surgery in operating room |
| Gamma probe | Gamma probe (high-energy gamma probe with detection capability at 511 keV) |
| Detection setup |
|
| Intraoperative use | Probe verification with survey at counts-per-second mode to select dynamic pitch range and volume
|
The radiation dose to the surgeons was measured by a radiation protection dosimeter (Luxel+, optically stimulated luminescence dosimeter; Landauer, Glenwood, IL) worn on the chest.
For immunohistochemistry, primary antibody for SSTR2 [(UMB1) ab134152; Abcam, Cambridge, MA] and SSTR5 [(UMB4) ab109495; Abcam] were used at 1:60 dilution. GEPNETs were scored using the World Health Organization (WHO) classification system.22 In our study, SSTR2 immunohistochemical staining was assessed on the basis of both the extent of staining and cellular localization using a scoring scale as follows: 0 = no staining, 1 = cytoplasmic only, focal or diffuse, 2 = membranous in <50 % tumor cells (irrespective of cytoplasmic staining), and 3 = membranous in >50 % tumor cells (irrespective of cytoplasmic staining). SSTR5 cases in which staining was present in >10 % tumor cells were scored as positive, as previously described.23
GraphPad Prism 5 software (GraphPad Software, La Jolla, CA) was used for statistical analyses. Data for continuous variables are presented as mean ± standard deviation (SD) or median (range). Spearman correlation was used to measure and test the association between two continuous variables.
RESULTS
A total of 14 patients underwent RGS for GEPNETs using 68Ga-DOTATATE. The clinical characteristics and operations performed are summarized in Table 2. Nine patients had pancreatic primary tumors, 3 had primary ileal tumors, and 2 had primary gastric/duodenal tumors, and 9 of 14 patients had undergone previous abdominal surgeries.
TABLE 2.
Clinical characteristics, histology, and procedures in study cohort
| Patient no. | Age/sex | Tumor type, histology, and stage | Type of operation |
|---|---|---|---|
| 1 | 50 male | MEN1, metastatic gastrinoma, WHO 1, pT2 N1 (13/24) | Duodenotomy and resection of primary tumor, LN resection |
| 2 | 51 female | MEN1, PNET WHO 2, pT2 N0 (0/3) | Pancreatic EN, LN dissection |
| 3 | 49 male | VHL, PNET with LN: Giant cell reaction/traumatic neuroma | Retroperitoneal and pericaval LN dissection |
| 4 | 40 male | MEN1, PNET WHO 1, pT2 N0 (0/10) | Pancreatic EN, LN dissection |
| 5 | 41 male | MEN1, PNET metastatic to LN, WHO 1 | Periportal and retropancreatic LN dissection |
| 6 | 38 female | MEN1, PNET WHO 1, gastrinomas, N1 (10/11); uterus: NEC, pT3 N1 (4/7) | Pancreatic EN, duodenotomy, peripancreatic and periaortic LN dissection, and salphingo-oophorectomy |
| 7 | 31 female | VHL, PNET WHO 2 pT2 N0 (0/4) | Extended distal pancreatectomy |
| 8 | 65 male | MEN1, gastric and duodenal NET, WHO 2, N1 (9/29), liver: NET | Aortocaval, retropancreatic and porta hepatis LN dissection, resection of duodenal gastrinoma, gastric wedge resection, and biopsy liver segment III lesion |
| 9 | 47 female | Sporadic insulinoma, PNET, WHO 1 | Pancreatic EN |
| 10 | 43 male | Metastatic ileal NET, multiple, WHO 1, N1 (3/5) | Small bowel resection and mesenteric LN resection |
| 11 | 47 female | VHL, PNET | Exploration and resection of periduodenal lesion |
| 12 | 39 male | VHL, PNET WHO 2, N0 (0/1) | Total pancreatectomy |
| 13 | 58 female | Unknown primary lesion, ileal NET, WHO 1, pT2 N0 (0/13) M1 (liver) | Small bowel and mesenteric LN dissection, nonanatomic resection of lesions in segment II and IV of liver |
| 14 | 70 female | Positive margins at anastomosis from prior ileal NET (WHO 1, N1 [4/18]). No tumor on histology | Resection of ileocolic anastomosis |
Tumor, node, metastasis classification stage according to American Joint Committee on Cancer28,29 and World Health Organization (WHO) histologic classification for PNET (1 low-grade NET, 2 intermediate NET, 3 high-grade NEC)22
MEN1 multiple endocrine neoplasia type 1, EN enucleation, LN lymph node, PNET pancreatic neuroendocrine tumor, VHL von Hippel–Lindau, T tumor size, N lymph node
There were 44 lesions identified on pathology. Thirty-five (79.6 %) of 44 lesions were concordant among pathology, probe, and imaging results, and 9 (20.4 %) of 44 lesions were discordant, with preoperative imaging and RGS being positive but pathology being negative (eight lymph nodes and one pancreas tail lesion). The median SUVmax of the target lesions on 68Ga-DOTATATE PET/CT was 46.8 (range 5.4–307). The median corrected target count rate was 172.6 (range 28.15–2341) counts per second, with a TBR of 4.46 (range 1.6–43.56) for resected lesions. The overall median lesion (tumors and lymph nodes) size was 1.55 (range 0.5–15) cm. There was no significant correlation between SUVmax and TBR (Spearman r = −0.01, p = 0.9), TBR and tumor size (Spearman r = 0.29, p = 0.14), and SUVmax and tumor size (Spearman r = 0.22, p = 0.28).
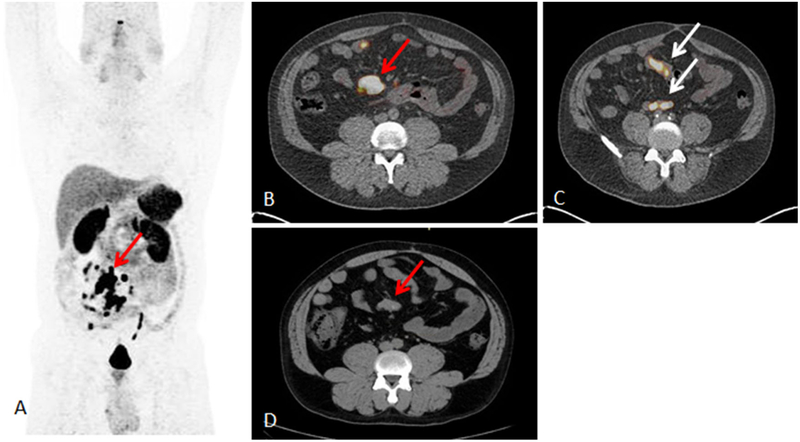
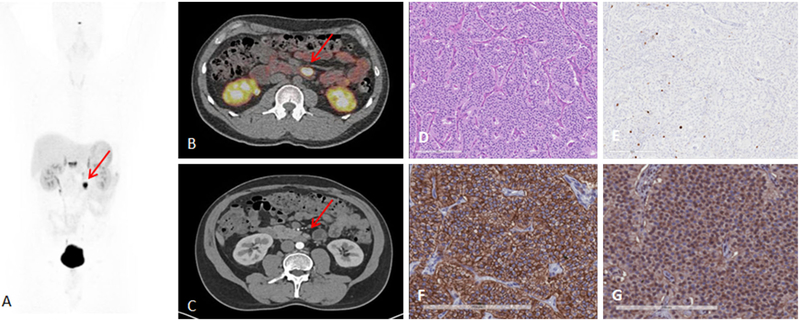
When we performed analysis by the organs involved, RGS had the highest correct identification by pathology for gastric and small bowel neuroendocrine tumors (NETs), including mesenteric lymph nodes (17 of 21, 81.0 %). For these 21 lesions (7 primary lesions and 14 lymph nodes), the median TBR was 3.5 (range 1.6–40.2). An example of a patient in whom 68Ga-DOTATATE PET/CT showed a known mesenteric mass and found multiple unknown bowel lesions is presented in Fig. 1 (Table 2, patient 10). RGS revealed a primary small bowel NET with multiple sites with a TBR ranging from 1.6 to 3.5 and a mesenteric mass with a TBR of 6.3. A mesenteric lymph node from a primary pancreatic NET that had a high TBR [3.4 for a 0.8 cm lesion (WHO grade 1 on pathology)] and that was found with the probe is shown in Fig. 2 (Table 2, patient 5). This patient had undergone previous surgery for a pancreatic NET and was found to have metastatic lymph nodes seen on preoperative 68Ga-DOTATATE PET/CT.
FIG. 1.
A 43-year-old man in whom 68Ga-DOTATATE PET/CT showed known mesenteric mass but also multiple bowel lesions (Table 2, patient 10). Surgery revealed primary small bowel NET with multiple sites of intestinal lesions, and mesenteric mass showed TBR of 6.3. A 68Ga-DOTATATE PET maximum intensity projection image showing mesenteric mass (red arrow), additional small bowel lesions, and multiple mesenteric lymph nodes. B 68Ga-DOTATATE PET/CT image showing mesenteric mass with SUVmax of 117.4 (red arrow). C 68Ga-DOTATATE PET/CT image showing small bowel uptake (white arrows). D Noncontrast CT showing corresponding mesenteric mass (red arrow), but small bowel lesions were not seen on CT or magnetic resonance imaging
FIG. 2.
A 41-year-old man with metastatic lymph nodes on 68Ga-DOTATATE PET/CT. He had undergone previous surgery for pancreatic NET (Table 2, patient 5). Mesenteric lymph node from primary pancreatic NET was found with probe with TBR of 3.4 for this 0.8 cm lesion (WHO grade 1). A 68Ga-DOTATATE PET maximum intensity projection image showing mesenteric/duodenal lesion in segment 3 (red arrow) and multiple retroperitoneal lymph nodes. B 68Ga-DOTATATE PET/CT image showing mesenteric mass with SUVmax of 72.8 (red arrow). C Arterial phase CT showing corresponding mesenteric mass (red arrow). D Representative hematoxylin and eosin staining of tumor showing NET in lymph node, WHO grade 1. E Representative immunohistochemistry for MIB-1 staining showing <2 % of cells positive. F Immunohistochemistry for SSTR2 showing representative image for score 3 (membranous pattern of SSTR2 staining in >50 % of tumor cells) (original magnification, ×20). G Immunohistochemistry for SSTR5 showing representative image of tumor with >10 % of tumor cells positive (original magnification, ×20)
We found that lesions in the pancreas and peripancreatic lymph nodes were difficult to detect with the probe as a result of high relative background counts. In such cases, palpation and intraoperative ultrasound were most useful. The gamma probe correctly identified 16 (66.7 %) of 24 lesions. However, there was one case of a sporadic insulinoma (Table 2, patient 9) in which the probe was helpful in confirming the location of a single lesion with a TBR of 43.6 (WHO grade 1 insulinoma). We did not find that the probe was helpful for detecting liver lesions: in 3 patients with liver metastases found on 68Ga-DOTATATE PET/CT, the background count for the liver was high, with an average of 499.7 ± 89.0, and target lesions therefore could not be distinguished. Resection was guided by ultrasound.
When we analyzed the surgeon’s ability to identify lesions intraoperatively by palpation or visualization of the lesions and compared it to findings of the probe and pathology results, we found that 3 (8.6 %) of 35 concordant lesions had not been seen or palpated by the surgeon and could have been missed (present on imaging, but not seen or palpated by the surgeons before probe scanning); they represented three small bowel lesions (smallest size, 0.7 cm). For the nine discordant lesions with negative pathology, four of them had not been seen or palpated, and five were either palpated or both palpated and visualized.
We did not find a significant difference in the target count rate by WHO tumor grade (mean target count rate 504.9 ± 534 for WHO grade 1 versus 205 ± 147 for WHO grade 2 lesions; Mann–Whitney p = 0.5). SSTR2 staining was performed in 12 of the resected samples, with a higher TBR in the group with more membranous SSTR2 staining (SSTR2 score 3: median TBR 6.5 [3.4–14.9] vs. SSTR2 score 2: 3.7 [3.5–6.3]), but it was not statistically significant (Mann–Whitney p = 0.23) (Fig. 2d–g).23
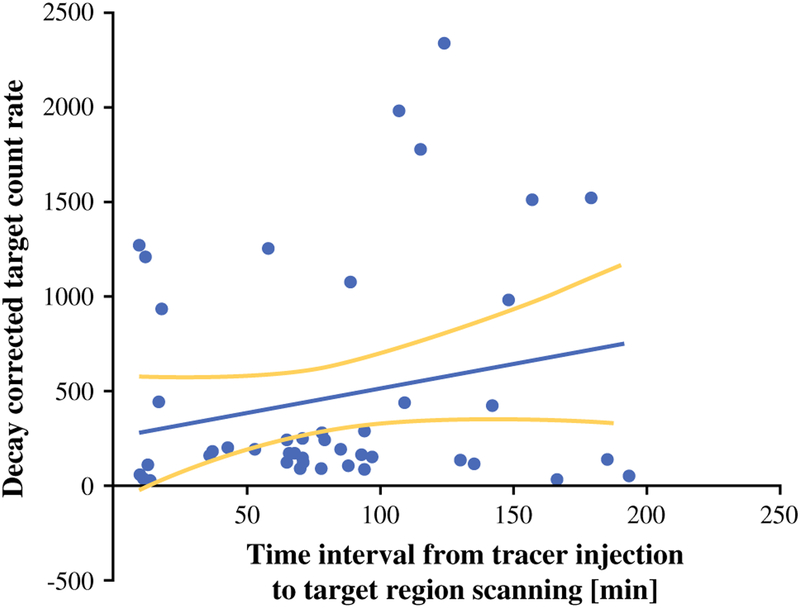
The average interval between the times of injection to measurements with the probe was 78.2 ± 51.13 (range 10–193) min. Even though 68Ga tracers have a short half-life of 68 min, we found no significant correlation between the length of time between tracer injection to the time point of gamma probe scanning and the level of target count rate (Spearman r = 0.19, p = 0.18, Fig. 3) or between the time elapsed from tracer dose injection and background count and TBR value.
FIG. 3.
Time interval between injection of 68Ga-DOTATATE and decay-corrected, normalized target lesion count rate detected with gamma probe (n = 47, Spearman r = 0.19, p = 0.18)
The radiation exposure to the surgeons was measured quarterly with dosimeters applied to the chest, and included two attending and three clinical fellow surgeons. For the 14 cases, the average exposure for the deep dose (internal organs) was 13.2 ± 9.1 (range 4–27) mrem per surgeon (annual limit for occupational workers is 5000 mrem per year).
After the lesions with positive TBR were resected, we performed ex vivo counts. The overall median ex vivo count rate was 133 (14–1300). However, there were 11 positive lesions on pathology that had a negative ex vivo count.
DISCUSSION
We show that RGS is feasible using 68Ga-DOTATATE. Our results showed that TBR is independent of size and preoperative SUVmax on 68Ga-DOTATATE PET/CT. Further, we showed that small bowel NET and metastatic mesenteric lymph nodes are detected with the probe in more than 81 % of lesions, and that the probe was helpful in confirming multiple locations of NET and found additional lesions. In cases of pancreatic NETs and liver metastases, RGS with 68Ga-DOTATATE was not as helpful as a result of relatively high background counts.
Surgical resection is the only curative treatment option for patients with well-differentiated GEPNETs. Complete or near-complete tumor resection in patients with advanced and metastatic GEPNET provides survival benefit, improves quality of life, and can reduce recurrence.4,24,25 Therefore, it is important to accurately determine the extent of disease before operations and to confirm that all sites of disease have been removed. A significant number of patients may have undetected residual low-volume disease, especially in patients who have undergone previous operations and who develop recurrent/persistent GEPNET disease. In such cases, RGS can facilitate the identification and removal of all or most sites of disease and allow confirmation of tumor removal by obtaining ex vivo counts.
Several studies using analogs such as 111In-pentetreotide, 123I-metaiodobenzylguanidine, or 99technetium–sulfur have been performed.6,16–18,26 They showed improved detection using RGS.16 Because of the higher sensitivity and superiority of 68Ga-DOTA peptides than PET/CT to detect GEPNET, the application of this radiopharmaceutical in RGS could be helpful to surgeons in detecting small, non-visible lesions or when trying to identify and remove tumors in patients who have undergone previous operations.13,14 To date, there have been 2 case reports with pancreatic NETs and one series of nine patients using 68Ga-DOTA peptides for RGS.19–21 Kaemmerer et al. reported a pilot study with nine patients (seven ileal NET, one pancreatic lesion, and one patient with an unknown primary lesion) using either 68Ga-DOTATOC or DOTATATE. They reported the detection of small lesions (0.5 cm) and additional lesions with the probe.21 However, in that study, only one patient had a pancreatic NET, and furthermore, the time interval between injection and probe use were not analyzed.
To our knowledge, our study is the largest prospective study using RGS using 68Ga-DOTATATE in patients with GEPNETs. We found that the probe successfully detected lesions as small as 0.5 cm. It is known that the uptake of 68Ga-DOTA analogs in normal tissue is highly variable and the high physiologic retention of radiopharmaceutical in the liver, kidney, spleen, and pancreas can compromise the detection capability of RGS, as has been reported by Wang et al. using the somatostatin analog 111In-pentetreotide.6,27 Similar to that observation, we also found a lower detection rate for primary pancreatic lesions and peripancreatic lymph nodes, as well as liver metastases.
An important variable with RGS for any tumor is the timing of tracer injection relative to surgical access of the target lesion or lesions. This is especially important in the setting of reoperations (as in 65 % of our cohort), as lysis of adhesions can take a long time before arriving to the target tumor region. 68Ga-DOTA peptides have a half-life of about 68 min; thus, there is a risk of scanning tissues intraoperatively either too early or too late. The average time interval in our study was 78.2 min, ranging from 10 min to 3 h maximum from the time of tracer injection to obtaining TBR values. We did not find an optimal time point for peak counts, but most importantly, the TBR was not significantly different by the time interval from injection to target count determination. Several factors also may influence the TBR or target count rate values during RGS using 68Ga-DOTATATE and include primary and metastatic tumor site, tumor grade, geometry of scanning (angle of probe and distance to target), and time-dependent washout of 68Ga-DOTATATE, which can be different for each organ and patient. The latter might also explain the negative ex vivo count rate encountered in histologically confirmed positive tumors with positive in vivo TBR counts. Furthermore, long-term follow-up is needed to definitively determine the true value of the probe and the completeness of the resection, as lesions could have been missed and account for the discordant findings.
In conclusion, our study revealed that RGS with 68Ga-DOTATATE in patients with GEPNETs is safe and feasible. The identification of small bowel NETs and metastatic mesenteric lymph nodes were best. However, performing further studies in a larger cohort and randomizing patients to operations with and without RGS could better determine whether it benefits patients with GEPNETs.
ACKNOWLEDGMENT
We would like to thank Candice Cottle-Delisle, RN, and Roxanne Merkel, RN, for their help coordinating the clinical protocol and study subjects; Lily A. Yang for data management; Maureen George for technical surgical support for the gamma probe; and the staff at the U.S. National Institutes of Health PET department. This research was supported by the intramural research programs of the Center for Cancer Research, National Cancer Institute.
Footnotes
DISCLOSURE The authors declare no conflict of interest.
REFERENCES
- 1.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a SEER analysis. J Cancer. 2012;3:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18. [DOI] [PubMed] [Google Scholar]
- 3.Plockinger U, Rindi G, Arnold R, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology. 2004;80:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- 5.Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut. 2005;54(suppl 4):iv1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YZ, Diebold A, Woltering E, et al. Radioguided exploration facilitates surgical cytoreduction of neuroendocrine tumors. J Gastrointest Surg. 2012;16:635–40. [DOI] [PubMed] [Google Scholar]
- 7.Hodolic M, Fettich J, Repse S, Peitl P, Lezaic L, Hojker S. Factors influencing radioguided surgery of neuroendocrine tumours using 99mTc-ethylenediamine N,N0-diacetic acid/6-hydrazinopyridine-3-carboxylic acid-D-Phe1-Tyr3-octreotide. Nucl Med Commun 2008;29:311–7. [DOI] [PubMed] [Google Scholar]
- 8.Grossrubatscher E, Vignati F, Dalino P, et al. Use of radioguided surgery with [111In]-pentetreotide in the management of an ACTH-secreting bronchial carcinoid causing ectopic Cushing’s syndrome. J Endocrinol Invest. 2005;28:72–8. [DOI] [PubMed] [Google Scholar]
- 9.Albertario S, Forti P, Bianchi C, et al. Radioguided surgery for gastrinoma: a case report. Tumori. 2002;88:S41–3. [DOI] [PubMed] [Google Scholar]
- 10.Reubi JC, Kvols L, Krenning E, Lamberts SW. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990;39(9 suppl 2):78–81. [DOI] [PubMed] [Google Scholar]
- 11.de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10:451–8. [DOI] [PubMed] [Google Scholar]
- 12.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24:389–427. [DOI] [PubMed] [Google Scholar]
- 13.Falconi M, Bartsch DK, Eriksson B, et al. ENETS Consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–34. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of gallium-68 DOTATOC and gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol 2014;55:389–98. [DOI] [PubMed] [Google Scholar]
- 15.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imag Radiat Oncol. 2012;56:40–7. [DOI] [PubMed] [Google Scholar]
- 16.Ohrvall U, Westlin JE, Nilsson S, et al. Intraoperative gamma detection reveals abdominal endocrine tumors more efficiently than somatostatin receptor scintigraphy. Cancer. 1997;80(12 suppl):2490–4. [DOI] [PubMed] [Google Scholar]
- 17.Adams S, Baum RP. Intraoperative use of gamma-detecting probes to localize neuroendocrine tumors. Q J Nucl Med. 2000;44:59–67. [PubMed] [Google Scholar]
- 18.Gulec SA, Daghighian F, Essner R. PET-probe: evaluation of technical performance and clinical utility of a handheld high-energy gamma probe in oncologic surgery. Ann Surg Oncol. 2006. doi: 10.1245/ASO.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 19.Freesmeyer M, Wurst C, Uberrueck T, et al. Intraoperative identification of a neuroendocrine tumour diagnosed by 68Ga-DOTATOC PET but undetectable by surgical palpation or conventional imaging. Nuklearmedizin. 2009;48:N50–1. [PubMed] [Google Scholar]
- 20.Kunikowska J, Slodkowski M, Koperski L, et al. Radioguided surgery in patient with pancreatic neuroendocrine tumour followed by PET/CT scan as a new approach of complete resection evaluation—case report. Nucl Med Rev Cent East Eur. 2014;17:110–4. [DOI] [PubMed] [Google Scholar]
- 21.Kaemmerer D, Prasad V, Daffner W, et al. Radioguided surgery in neuroendocrine tumors using Ga-68-labeled somatostatin analogs: a pilot study. Clin Nucl Med 2012;37:142–7. [DOI] [PubMed] [Google Scholar]
- 22.Bosman F, Carneiro F, Hruban R, Theise N. WHO classification of tumors of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 23.Volante M, Brizzi MP, Faggiano A, et al. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 2007;20:1172–82. [DOI] [PubMed] [Google Scholar]
- 24.Hodul PJ, Strosberg JR, Kvols LK. Aggressive surgical resection in the management of pancreatic neuroendocrine tumors: when is it indicated? Cancer Control. 2008;15:314–21. [DOI] [PubMed] [Google Scholar]
- 25.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–36. [DOI] [PubMed] [Google Scholar]
- 26.van Hulsteijn LT, Corssmit EP, van der Hiel B, Smit JW, Stokkel MP. Is there a role for radioguided surgery with iodine-labeled metaiodobenzylguanidine in resection of neuroendocrine tumors? Clin Nucl Med 2012;37:1083–8. [DOI] [PubMed] [Google Scholar]
- 27.Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging. 2010;54:61–7. [PubMed] [Google Scholar]
- 28.Bilimoria KY, Bentrem DJ, Merkow RP, et al. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg. 2007;205:558–63. [DOI] [PubMed] [Google Scholar]
- 29.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edition New York: Springer, 2010. [Google Scholar]