Abstract
Synthesis of previously inaccessible, potentially liver selective HNO donor V-IPA/NO ([iPrHN3–N1(O1)=N2–O2–R], where R = vinyl) is reported here. A novel fluoride-labile TOM group at O-2 in conjunction with MOM protection at N-3 in IPA/NO is employed. The strategy developed is also extended to synthesis of other NO-releasing prodrugs and has applications in diversity-oriented synthesis of HNO- and NO-prodrugs.
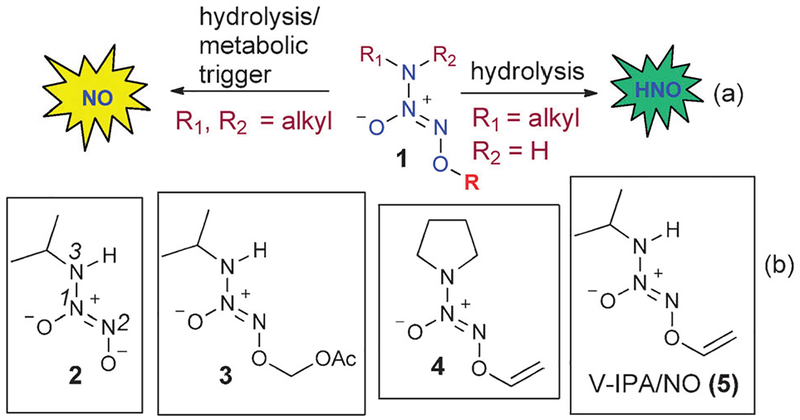
Nitric oxide (NO) and nitroxyl (HNO) chemically differ by one hydrogen atom, but they have different biological properties. NO plays a critical role in a variety of bioregulatory processes like normal physiological control of blood pressure, neurotransmission, and macrophage-induced cytostasis and cytotoxicity.1 On the other hand, HNO has been implicated in the mechanism of the inhibitory effect of cyanamide on aldehyde dehydrogenase in treating alcohol abuse2 and has been demonstrated to enhance contractility in experimental models of heart failure.3 Secondary amine diazeniumdiolate prodrugs are considered reliable sources of NO (Fig. 1a, left arrow).4 More recently, primary amine diazeniumdiolate IPA/NO (2, Fig. 1b) and its prodrug AcOM–IPA/NO (3, Fig. 1b) were found to be sources of HNO.5
Fig. 1.
(a) Activation of diazeniumdiolate prodrugs to release NO and HNO. (b) Structures of IPA/NO (2), AcOM–IPA/NO (3), V-PYRRO/NO (4), and V-IPA/NO (5).
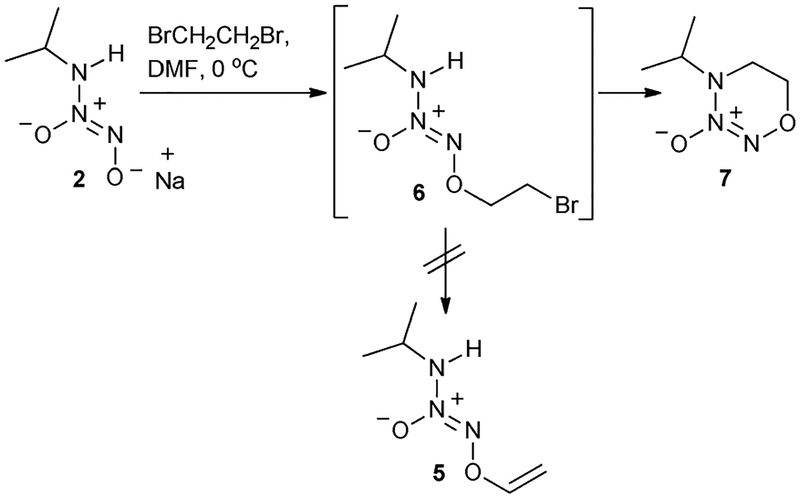
IPA/NO (2) as a potential HNO donor has inclined us to synthesize several additional IPA/NO prodrugs that can be metabolically triggered to release HNO. For example, vinyl protection of a diazeniumdiolate was designed to release NO upon activation by cytochrome P450. Thus, V-PYRRO/NO (4, Fig. 1b) is activated by cytochrome P450 to release NO, and shows hepatoprotective properties in relevant animal models against a variety of life-threatening situations, including the ischemia-reperfusion injury associated with organ transplantation and the toxicity of acetaminophen and other agents.6 We envisioned that such vinyl protection for IPA/NO as in 5 (V-IPA/NO) might lead to a liver selective HNO donor. The conventional synthesis of such vinyl diazeniumdiolate prodrugs involves alkylation of a diazeniumdiolate sodium salt to form a bromoethyl diazeniumdiolate, followed by elimination of HBr.6a However, when IPA/NO sodium salt (2) was reacted with 1,2-dibromoethane, the expected bromide 6 was not isolated (Scheme 1). The free N–H group in 6 interfered to form E-diazeniumdiolate adduct 7 (Scheme 1).7
Scheme 1.
Formation of E-diazeniumdiolate adduct (7) during attempted synthesis of 6 [ref. 7].
This highlighted the need for temporary protections at O-2 and N-3 in IPA/NO, followed by O-2-alkylation (see 2, Fig. 1b; for atom numbering system), to avoid the undesired N-alkylation in the bromide 6. We planned to design a synthetic protecting group at O-2 that can be stable in standard reaction conditions for functional group transformations, and can be converted easily to the required NO-prodrug of biological significance. However, diazeniumdiolate anions are highly sensitive to low pH, and often decompose to release NO and the corresponding amine. Hence, an acid-labile protecting group for O-2 of the diazeniumdiolate is to be avoided. We decided to explore a fluoride-labile synthetic protecting group at the O-2 position of IPA/NO. Diazeniumdiolate salts, when reacted with trialkylsilyl chlorides, give a complex mixture of products. However, diazeniumdiolate salts are known to readily react with primary alkyl chlorides and bromides, hence we decided to explore the reaction with (triisopropylsilyloxy)-methyl chloride (TOMCl). In recent times, TOM has become a popular protecting group in nucleoside chemistry, and has been advantageous over other classical protecting groups.8,9
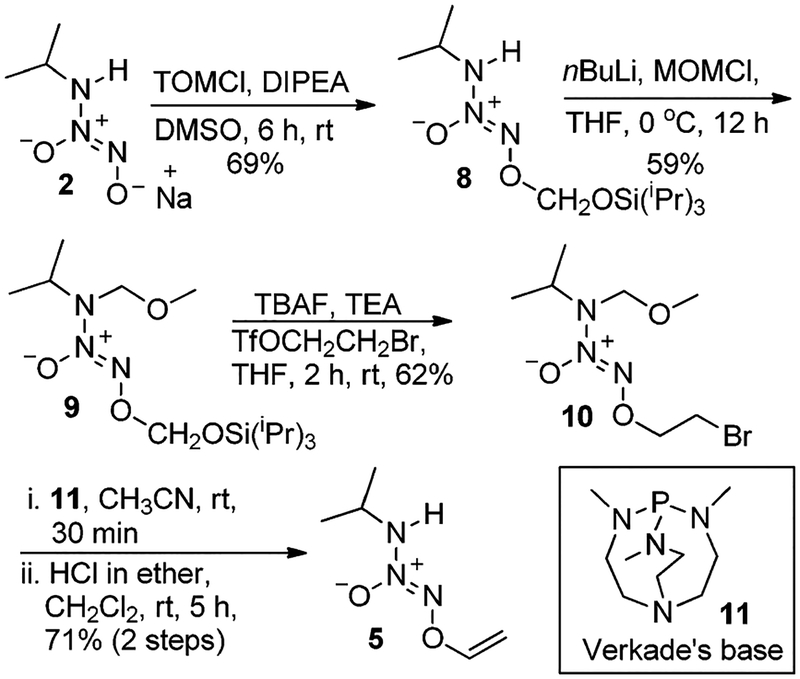
IPA/NO sodium salt 2 was transformed into TOM-ylated IPA/NO 8 by reaction of IPA/NO with TOMCl, DIPEA (N,N-diisopropylethylamine) in DMSO in 69% yield (Scheme 2). Compound 8 on treatment with nBuLi and MOMCl (chloromethyl methyl ether) in THF gave compound 9 (59% yield). Now with the absence of the interfering N–H group in 9 (unlike 2 or 6), the TOM group was removed using tetrabutylammonium fluoride (TBAF) in the presence of a 4-fold excess of 2-bromo-l-[((trifluoromethane)sulfonyl)-oxy]ethane and triethylamine (TEA) to obtain bromide 10 in 62% yield. The elimination of HBr from bromide 10 was carried out using Verkade’s superbase (11) in acetonitrile followed by an acid-induced removal of the MOM group to obtain V-IPA/NO (5) (71% for 2 steps).
Scheme 2.
Synthesis of V-IPA/NO (5).
The reaction conditions enabling temporary protection of the N–H group in IPA/NO as in compound 9 would allow one to tag a polymer, peptide or fluorescent group directly to the N-3 of IPA/NO. Efforts are underway to synthesize such IPA/NO derivatives, measure their NO- and HNO-releasing profiles and evaluate their biological properties.
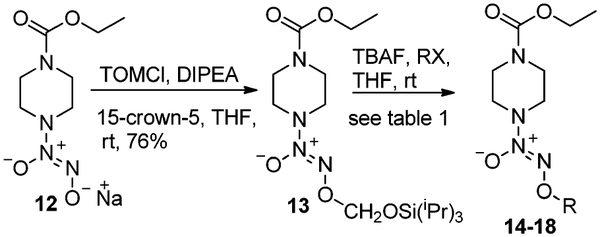
TOM-ylated diazeniumdiolates can have further applications in making various NO-prodrugs. To explore this chemistry, carboethoxy piperazine diazeniumdiolate 1210 was reacted with TOMCl, DIPEA and 15-crown-5 in THF, to afford TOM-ylated diazeniumdiolate 13 in good yield (Table 1). TBAF in THF was added to compound 13 in THF, readily inducing removal of the TOM group, and forming anionic 12 as the tetra-n-butylammonium salt, which was found to be highly soluble in THF, unlike its sodium counterpart.11 When this reaction was carried out in the presence of an electrophile RX, the desired NO-prodrugs were achieved in 59–92% yields (Table 1). Syntheses of prodrugs 14,10 15,10 and JS-K (18)12 are reported in the literature, and the method developed provides an alternate route for their synthesis. The method developed also allowed synthesis of novel compounds 16 and 17, and is useful in structure activity relationship (SAR) studies of diazeniumdiolate prodrugs, where one TOM-ylated diazeniumdiolate derivative can be transformed into several NO-prodrugs, leading to diversity-oriented synthesis.
Table 1.
Transformation of a TOM-diazeniumdiolate into different prodrugs
 | |||
|---|---|---|---|
| Entry | RX | Product | % Yield |
| 1 | (CH3)2SO4 | 14 | 78 |
| 2 | TfOCH2CH2Br | 15a | 65 |
| 3 | AcOCH2Br | 16 | 74 |
| 4 | 1-Chloro-N-acetyl-glucosamine triacetate | 17 | 59 |
| 5 | 2,4-Dinitrophenyl fluorobenzene | 18 (JS-K) | 92 |
Triflate is the leaving group in this reaction to form bromide 15.
In conclusion, we have developed a method for the synthesis of the TOM-protected diazeniumdiolates. These TOM-protected diazeniumdiolates were transformed into different biologically significant NO-releasing prodrugs. The TOM-protection in conjunction with the MOM protecting group was utilized in the synthesis of V-IPA/NO (5), which could not be synthesized by conventional procedures. Efforts are underway to study the cytochrome P450 activation of V-IPA/NO to release HNO. The protection–deprotection strategy developed has potential applications in SAR studies of diazeniumdiolate prodrugs and may enable a TOM-derivative to be transformed into several prodrugs activated by different mechanisms to release NO and/or HNO.
This project has been funded with Federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR spectra for all new compounds. See DOI: 10.1039/c1cc12130h
Notes and references
- 1.Moncada S, Palmer RMJ and Higgs EA, Pharmacol. Rev, 1991, 43, 109–142. [PubMed] [Google Scholar]
- 2.Nagasawa HT, Kawle SP, Elberling JA, DeMaster EG and Fukuto JM, J. Med. Chem, 1995, 38, 1865–1871. [DOI] [PubMed] [Google Scholar]
- 3.Paolocci N, Katori T, Champion HC, St. John ME, Miranda KM, Fukuto JM, Wink DA and Kass DA, Proc. Natl. Acad. Sci. U. S. A, 2003, 100, 5537–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hrabie JA and Keefer LK, Chem. Rev, 2002, 102, 1135–1154. [DOI] [PubMed] [Google Scholar]
- 5.(a) Salmon DJ, de Holding CLT, Thomas L, Peterson KV, Goodman GP, Saavedra JE, Srinivasan A, Davies KM, Keefer LK and Miranda KM, Inorg. Chem, 2011, 50, 3262–3270; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Andrei D, Salmon DJ, Donzelli S, Wahab A, Klose JR, Citro ML, Saavedra JE, Wink DA, Miranda KM and Keefer LK, J. Am. Chem. Soc, 2010, 132, 16526–16532; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Miranda KM, Katori T, de Holding CLT, Thomas L, Ridnour LA, McLendon WJ, Cologna SM, Dutton AS, Champion HC, Mancardi D, Tocchetti CG, Saavedra JE, Keefer LK, Houk KN, Fukuto JM, Kass DA, Paolocci N and Wink DA, J. Med. Chem, 2005, 48, 8220–8228. [DOI] [PubMed] [Google Scholar]
- 6.(a) Hong SY, Saavedra JE, Keefer LK and Chakrapani H, Tetrahedron Lett., 2009, 50, 2069–2071; [Google Scholar]; (b) Inami K, Nims RW, Srinivasan A, Citro ML, Saavedra JE, Cederbaum AI and Keefer LK, Nitric Oxide, 2006, 14, 309–315; [DOI] [PubMed] [Google Scholar]; (c) Qu W, Liu J, Fuquay R, Shimoda R, Sakurai T, Saavedra JE, Keefer LK and Waalkes MP, Nitric Oxide, 2005, 12, 114–120; [DOI] [PubMed] [Google Scholar]; (d) Liu J, Li C, Waalkes MP, Clark J, Myers P, Saavedra JE and Keefer LK, Hepatology, 2003, 37, 324–333; [DOI] [PubMed] [Google Scholar]; (e) Liu J, Saavedra JE, Lu T, Song J-G, Clark J, Waalkes MP and Keefer LK, J. Pharmacol. Exp. Ther, 2002, 300, 18–25; [DOI] [PubMed] [Google Scholar]; (f) Ricciardi R, Foley DP, Quarfordt SH, Saavedra JE, Keefer LK, Wheeler SM, Donohue SE, Callery MP and Meyers WC, Transplantation, 2001, 71, 193–198; [DOI] [PubMed] [Google Scholar]; (g) Saavedra JE, Billiar TR, Williams DL, Kim Y-M, Watkins SC and Keefer LK, J. Med. Chem, 1997, 40, 1947–1954. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y-N, Bohle DS, Bonifant CL, Chmurny GN, Collins JR, Davies KM, Deschamps J, Flippen-Anderson JL, Keefer LK, Klose JR, Saavedra JE, Waterhouse DJ and Ivanic J, J. Am. Chem. Soc, 2005, 127, 5388–5395. [DOI] [PubMed] [Google Scholar]
- 8.(a) Pitsch S, Weiss PA, Jenny L, Stutz A and Wu X, Helv. Chim. Acta, 2001, 84, 3773–3795; [Google Scholar]; (b) Pitsch S, Weiss PA, Wu X, Ackermann D and Honegger T, Helv. Chim. Acta, 1999, 82, 1753–1761; [Google Scholar]; (c) Wu X and Pitsch S, Nucleic Acids Res, 1998, 26, 4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Cramer H, Rhea R, Okicki J, Yirava K and Silverman R, Nucleosides, Nucleotides Nucleic Acids, 2003, 22, 1733–1736; [DOI] [PubMed] [Google Scholar]; (b) Micura R, Angew. Chem., Int. Ed, 2002, 41, 2265–2269. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra JE, Booth MN, Hrabie JA, Davies KM and Keefer LK, J. Org. Chem, 1999, 64, 5124–5131. [DOI] [PubMed] [Google Scholar]
- 11.Reaction of an amine and NO to form a diazeniumdiolate tetraalkylammonium salt has not been found to be practical or successful in our hands thus far.
- 12.Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flippen-Anderson JL, Rice WG, Turpin JA, Davies KM and Keefer LK, J. Org. Chem, 2001, 66, 3090–3098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.