Abstract
Lead (Pb) in public drinking water supplies has garnered much attention since the outset of the Flint water crisis. Pb is a known hazard in multiple environmental matrices, exposure from which results in long-term deleterious health effects in humans. This discussion paper aims to provide a succinct account of environmental Pb exposures with a focus on water Pb levels (WLLs) in the United States. It is understood that there is a strong correlation between WLLs and blood Pb levels (BLLs) and associated health effects. However, within the Flint water crisis, more than water chemistry and Pb exposure occurred. A cascade of regulatory and bureaucratic failures culminated in the Flint water crisis. This paper will discuss pertinent regulations and responses including their limitations after an overview of the public health effects from Pb exposure as well as discussion on our limitations on monitoring and mitigating Pb in tap water. Increased Legionnares’ disease cases following Flint water change and factors influencing Legionella pneumophila growth are discussed to highlight potential association between water chemistry and L. pneumophila outbreak. As we critically analyze these important aspects of water research, we offer discussions to stimulate future Pb research from a new and systemic perspective to inform and guide public health decision-making.
Keywords: Lead, drinking water, water lead levels, blood lead levels, health effects, regulations
Graphical Abstract
Overview of Lead
Lead (Pb) is a heavy metal ubiquitous in the earth’s crust, the physical properties of which have made it useful to mankind for centuries. It continues to be produced through a variety of industrial processes, although less than in prior decades. Through most of the 20th century, Pb was used extensively in gasoline, paints, pesticides, batteries and plumbing fixtures (ATSDR, 2007). It is estimated that environmental Pb levels have increased more than 1000-fold over the last 300 years due largely to human activities, with the greatest increase occurring between 1950 and 2000 (ATSDR, 2007). A reason for Pb’s persistence in the environment, despite its elimination from consumer products including piping and plumbing fixtures, can be traced to its propensity to bind strongly to soil particles (ATSDR, 2007). A means for improving the removal of Pb in drinking water can be credited to the Safe Drinking Water Act (SDWA), which regulates utilities with regards to Pb and other hazards (SDWA, 1976). As an enforceable standard, the maximum contaminant level (MCL) for Pb has been set by the USEPA at 15 μg/L in drinking water. Since the inception of the MCL, the unenforceable maximum contaminant level goal (MCLG) has been set at 0 mg/L, recognizing that Pb is toxic at any exposure level (USEPA, 1991, 2008; CDC, 2012).
In light of the recurring water crises in the United States, through this discussion paper we aim to offer a succinct account of environmental Pb exposures focusing on water Pb levels (WLLs); its correlation with blood Pb levels (BLLs) and the associated health effects; multiple case studies discussing water Pb crises within the United States with a focus on Flint highlighting failures at various levels leading to water contamination of Pb and Legionella; issues inherent to Pb sampling protocols, potential factors influencing WLLs, and areas of research gaps; and pertinent regulations and responses including their limitations on monitoring and mitigating Pb in drinking water. As we critically appraise various aspects of drinking water research, we offer discussions to stimulate future Pb research from a new and systemic perspective to ultimately guide public health decision-making.
Human Health and Blood Lead Tracking
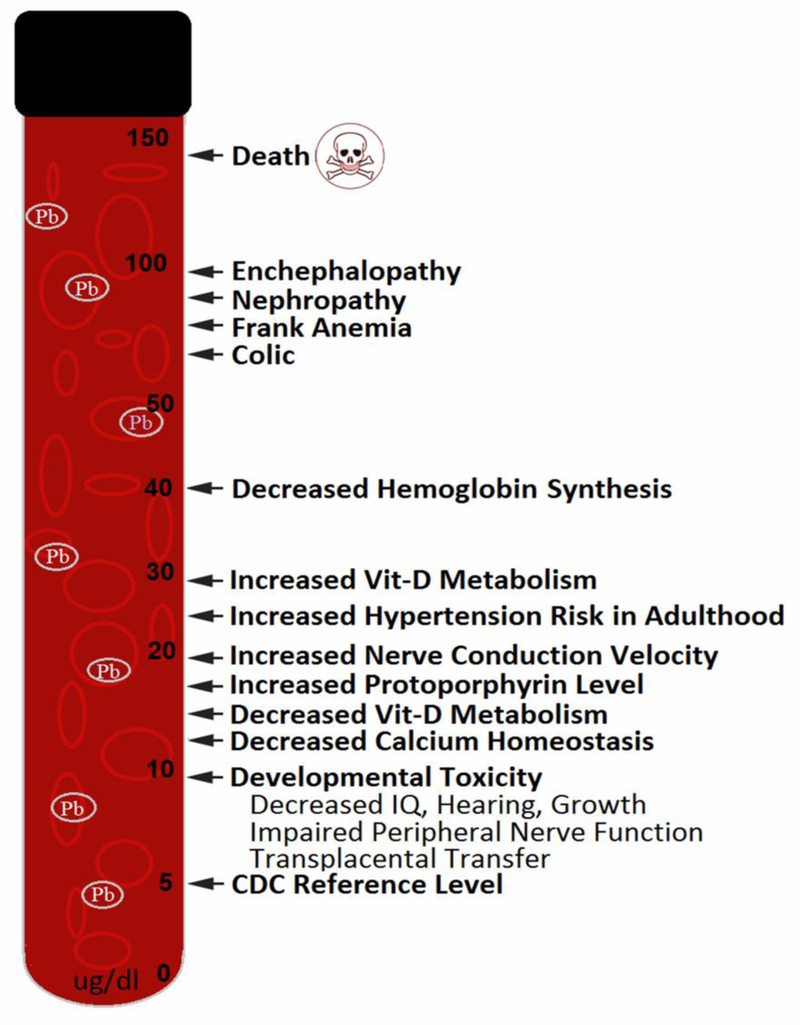
High Pb exposure through air, water, soil or food leads to high blood Pb levels (BLLs) (ATSDR, 2007). The deleterious effects of high BLLs have been well documented as depicted in Fig. 1. Typically, brain, kidneys, (Payton et al., 1994), cardiovascular system (Hu et al., 1996; ATSDR, 2007) and the blood (Roels and Lauwerys, 1987) are the systems mostly affected. With regards to the cardiovascular system both increased incidence of hypertension (Hu et al., 1996) and increased mortality due to heart disease have been observed (Cooper et al., 1985). Another study has shown an increase in overall and cardiovascular mortality in men with BLLs above 2 μg/dL, a much lower level than previously observed (Menke et al., 2006).
Fig. 1.
Known effects of different blood lead levels on human health (Adapted from ATSDR, 2007).
In children, the brain and central nervous system (CNS) are particularly highly sensitive to Pb. This susceptibility persists throughout childhood, however ages 0–3 years tend to show the greatest susceptibility (Chiodo et al., 2004). Children generally absorb a larger fraction of ingested lead than adults. Due to children’s greater susceptibility and larger Pb absorbance their exposure has typically been of greater concern (Lanphear et al., 2005). Pb interferes with the development of connections between neurons and neuronal migration as well as myelin development (ATSDR, 2007). Specific neurodevelopmental problems due to Pb toxicity include: lower IQ scores, behavioral problems, hyperactivity, seizures and impaired coordination (ATSDR, 2007). Studying over 1300 children in different cities, Lanphear et al. (2005) found that an increase in BLL from 2.5 μg/dL to 10 μg/dL was associated with a decrement in IQ of 3.9 points. Children with chronic exposure to higher BLLs are more likely to have lower IQ scores. Further increases in BLLs above 10 μg/dL is associated with additional lowering of average IQ score. No threshold is suggested for the effects of Pb on intellectual function in children (Lanphear et al., 2005). Nevin (2007) reported a strong correlation between BLLs and murder rates in the U.S. over a period of 100 years. This conclusion from Nevin (2007) would seem to corroborate results from Li et al. (2003) that causally linked increased Pb exposure to increased aggression in animal studies.
As could be surmised health impacts from Pb exposure have economic implications. Colborn et al. (1996) demonstrated that a five-point drop in average IQ in a population of 100 million people would increase the diagnosis of intellectual disability from 6 million to 9.4 million, a 57% increase. Grosse et al. (2002) advanced this concept and estimated that a 5–6 point decrease in Pb levels would lead to an annual economic gain between $100 billion and $300 billion.
Due to these wide ranging impacts on human health and the economy, it was recognized in the 1970’s that Pb exposure needed to be tracked. BLL is an important indicator of the level of exposure an individual has had to a source of Pb. It is also a correlated indicator of the health risks that individuals are expected to encounter due to their exposure. Therefore, BLL has been used as a means of describing overall exposure and defending mitigation recommendations and decisions. The reference BLL was developed as a means of standardizing the comparison of BLLs nationally for regulatory and mitigation purposes (ATSDR, 2007; CDC, 2012).
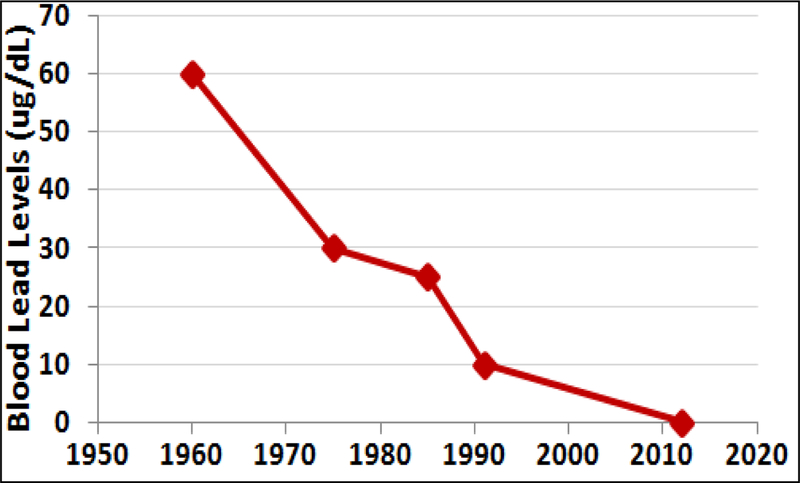
As depicted in Figure 2, prior to 1975 the reference BLL for Pb remained at 60 μg/dL which was then revised to 30 μg/dL in 1975 by the CDC. From 1990 through 2012, the reference BLL was 10 μg/dL, which was lowered to 5 μg/dL in 2012. In 2012, CDC decided to establish that there is no safe BLL (CDC, 2012). These declining reference BLLs were informed by a growing body of literature on the increasing toxicity of infinitesimal amounts of Pb in children.
Fig. 2.
Evolution of reference blood lead levels (BLLs) considered safe by The Centers for Disease Control and Prevention (CDC) over the past six decades (Adapted from Lanphear et al., 2005; ATSDR, 2007; CDC, 2012; Drum, 2016; Raymond and Brown, 2016).
These increasing concerns over the effects of high BLLs were exacerbated by the realization that children’s exposure was increasing nationwide. However, currently, only less than 25% of children under the age of three are screened for BLL (Pennsylvania Department of Health, 2014). Several studies showed 2.5% of American children aged 1–5 years had BLLs of 5 μg/dL or higher with 97.5% having levels below that (CDC, 2012). Currently, the BLL of a child in the United States between the ages 1 and 5 years averages 2 μg/dL (Raymond and Brown, 2016), reduced from 16 μg/dL in 1976 (an 87.5% decrease). In the 1970’s, over 70% of children tested nationwide had BLLs over 10 μg/dL; by 2001, it was less than 1%. These decreases coincide with the phasing out of leaded gasoline and paints (CDC, 2005).
By comparison to the national averages, in 1998, 50% of Flint residents had BLLs over 5 μg/dL and 8.4% had BLLs over 10 μg/dL. By 2013, Flint had BLLs over 5 μg/dL for 3.6% of the population and 0.3% of residents with BLLs over 10 μg/dL. This placed Flint close to the national average for BLL in 2013 (Drum, 2016). Therefore, Flint as with the rest of the United States was progressing forward with Pb exposure reduction and improved public health protections. This frames the juxtaposition of Pb exposure reduction in Flint with the current crises and further highlights the need to examine water Pb exposure routes.
Water Lead Levels (WLLs) and Association with Blood Lead Levels (BLLs)
The elimination of Pb in gasoline and paint led to a marked decrease in average BLLs throughout much of the developed world. Several factors may contribute to Pb concentrations in water including the water chemistry, mineral types, water temperature, aging infrastructure, age of water in the distribution system, and the presence or absence of protective coatings in the pipes (Swistock and Galford, 2016; USEPA, 2016). However, water chemistry with regards to Pb is not a simple matter (Schwake et al., 2016), considering the complications to monitoring and health assessments when the effects of hot water are to be accounted for. Hot water tends to have much higher concentrations of dissolved Pb than in cold water, due to Pb leaching from plumbing fittings being enhanced in hot water (The Cadmus Group, 2007; Masters et al., 2016; CDC, 2016). Therefore, as can be seen when just considering a simple case of water temperature, inferences drawn from the results can be skewed. Alternatively, it can be argued that hot water should be included in monitoring and analysis to develop a realistic picture of Pb exposure in the home (Schwake et al., 2016).
Numerous studies have examined the potential correlation between WLL and BLL. For example, Pocock et al. (1983) examined the link between WLL and BLL in over 900 men in England. The researchers grouped the men into nine bins of first-draw WLLs. The first (lowest) bin contained the men with WLL < 6 μg/dL, while the other eight bins were split out based on increasingly higher WLLs with 50 men in each bin. The data showing a positive correlation between WLL and BLL were sufficient to develop a regression model, where: BLL = 0.699 + 0.0003 * WLL; where WLL is the first draw in units of μg/dL. Pocock et al. (1983) also demonstrated significant inverse correlation between water hardness (defined by amount of dissolved calcium and magnesium in water) and WLL, thus highlighting the complexity of the link between drinking water chemistry and WLL at the tap.
The USEPA’s Water Criteria Document (USEPA, 1986) published formulae for calculating BLLs from WLLs based on prior research assuming linearity. Equation 1 shows a linear relationship between WLL and BLL in children (Ryu et al., 1983; USEPA, 1986), and equation 2 shows the relationship for adults as recommended by the USEPA (1986) and adapted from Pocock et al. (1983).
| (1) |
| (2) |
Since the 1970’s removal of Pb from gasoline and paints, Pb exposure shifted from surface and air exposure to water exposure. This is highlighted by the USEPA’s Criteria Document concluding that approximately 15–40% of the total human burden of Pb is likely due to drinking water (USEPA, 1986). Lanphear et al. (1998) evaluated potential contributions of Pb from water, soil and house dust to the BLLs in urban children. They found that an increase in WLLs from background levels based on the USEPA standard of 15 μg/L was associated with a 13.7% increase in the number of children estimated to have a BLL above 10 μg/dL. Increases in soil Pb levels and dust Pb levels were associated with 11.6% and 22.3% increases in children with BLL above 10 μg/dL, respectively (Lanphear et al., 1998).
Pb in water continues to pose a problem as Renner (2010) highlighted for drinking water. Renner (2010) was able to implicate drinking water resulting in high BLLs in Washington DC, North Carolina, and Maine from 2004 – 2010. These cases as well as Flint demonstrate that a lack of understanding regarding the complexity of a water distribution system (Schock, 1989; Brown et al., 2015) can also lead to increased WLLs and thereby increased BLLs.
Preventable Water Crises in the United States
Flint Water Crisis: Pb Contamination
Flint, Michigan, is a town of 98,000 people located 66 miles northwest of Detroit in Genesee County (US Census Bureau, 2015). It is important to remember during this discussion that Flint residents pay on average the most for their water in the United States (Lynch, 2016). The development of the Flint water crisis first needs to be discussed in the light of why the decisions to change their water supply were made. The cause of the efforts to change the water supply for Flint was economically motivated by State input due to a financial crisis in Flint. However, Flint was not always in poor economic straits. In 1908, General Motors was founded in Flint; after World War II it became a major automobile manufacturing center. However, by the 1980’s almost all of the automobile factories had closed and Flint sank into a deep economic depression that persists today In 2002, after incurring 30 million dollars of debt, an economic emergency was declared in Flint and the state of Michigan appointed an emergency financial manager; he became the city’s chief administrator, superseding the Mayor. The emergency was rescinded in 2004. In 2011, the state again declared a financial emergency. Governor Rick Snyder appointed Michael Brown as the new Emergency Manager. State officials discussed Flint Water Supply alternatives before a Flint City Council meeting in February, 2013. The Council subsequently passed a resolution to purchase water from the Karegrondi Water Authority (KWA) in a cost-saving move. However, the KWA facility was not scheduled to come on-line until 2016. The Detroit Water and Sewer Department (DSWD) objected, but offered to continue providing water to Flint for another year. In April 2014 a notice terminating service was issued (Dixon, 2016). The state managers and Flint City Council decided to reopen Flint’s water treatment plant starting in 2014, using the Flint River for water, until the KWA facility was operating. The DSWD did offer to immediately resume supplying Flint with water in February 2015 once Flint’s water problems started to emerge (Fonger, 2015; Bosman, 2016; U.S. House Committee, 2016).
Shortly after the switch, Flint residents began to complain that the Flint River water looked “bad” and was causing skin problems for some children (Lin et al., 2016). Some of the skin problems could have at that time alerted experts of the increased corrosivity, but this did not occur. In the summer of 2014, three boil-water advisories were issued as coliforms counts in the drinking water dictated this response. In October 2014, General Motors decided to stop using Flint water due to “rust problems” (Lin et al., 2016); this was an obvious sign of high corrosivity that went unanswered again. Scattered complaints of health problems continued into 2015 (Detroit Free Press, 2015). It was obvious at this time that numerous homes had elevated Pb levels in their drinking water (Lin et al., 2016).
Hanna-Attisha et al. (2016) documented that the percentage of children throughout the city of Flint aged 1–5 with BLL above 5 μg/dL rose from a baseline of 2.4% to 4.9% in homes with elevated WLLs. Children aged 1–5 years with BLLs above 5 μg/dL rose from 4% to 10.6% following water source change. Shortly thereafter, the water supply was switched back to the DWSD. However, increased corrosivity of the water resulted in lasting damage to the drinking water distribution system, thus meaning that the crisis is far from over. In Flint drinking water, 17% of the water samples tested had WLLs above the USEPA standard of 15 μg/L (Edwards et al., 2015). The 90th percentile (US EPA’s sampling comparison point) WLL was 25.2 μg/L, which is over the USEPA standard applied to houses with “worse case” Pb plumbing. It is being demonstrated that highly corrosive Flint water might have favored Pb leaching from the pipe connectors used in the Flint water system. It has since been postulated that following standard water treatment protocols (e.g., pH control, use of anticorrosives) could have limited Pb leaching. However, this remains a point of contention as the case of optimal corrosion control is not a well-defined concept (Shock 1989; Brown et al., 2015).
Flint Water Crisis: Legionella Contamination
While seeming to have been a coincidental incident, during the water crisis Flint also endured a significant spike in Legionnaire’s disease, caused by the bacterium Legionella pneumophila (L. pneumophila). This demonstrates a need to understand water systems from a systems perspective rather than from a single or class of contaminant basis.
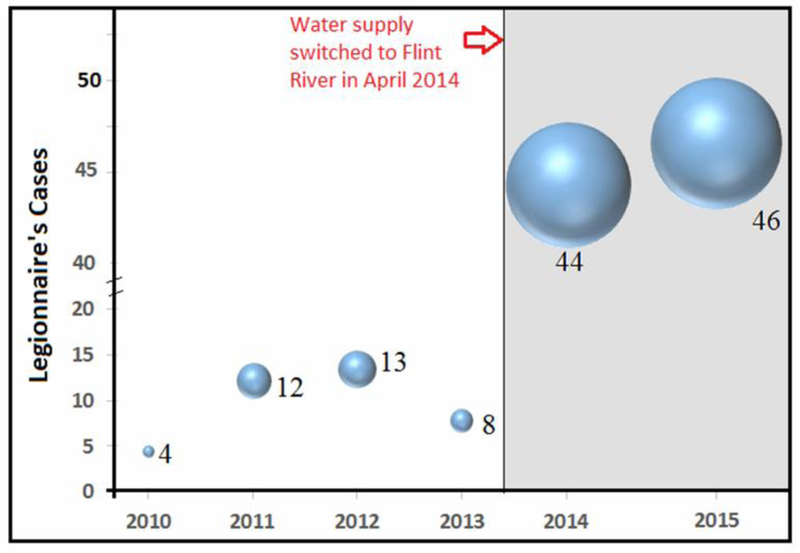
In a one-year period spanning May 2014 to May 2015, there were 87 documented Legionnaires’ disease cases in Flint where in an average year there are 6 to 13 cases (Fig. 3; Schumaker, 2016). ). Not all cases are residents whose domicile falls within the city limits; however, a complete epidemiological study would determine whether these cases resulted from people who commuted in for work or business in Flint, thus providing the real exposure.
Fig. 3.
Legionnaires’ disease (LD) cases (represented by the width of bubble) in Genesee County reported for 2010 through 2015 showing significant rise in LD cases following the switch of water from Detroit-supplied Lake Huron to Flint River in April 2014 (Adapted from: Schumaker, 2016).
It is important to note that the outbreak in Flint has not been causally linked to the water system. However, what we know of L. pneumophila’s growth in water systems allows us to make some inferences. Water temperature, pH, and water-metal content are factors known to influence L. pneumophila growth in water. Studies have shown that water temperatures between 25 and 42 °C and a pH between 6 and 8 are optimal for L. pneumophila growth and persistence (Solimini et al., 2014). A water environment rich in other microorganisms (particularly amoeba and protozoa) and bacterial biofilms can foster the growth and further blooming of L. pneumophila (Taylor et al., 2009; Schwake et al, 2016). Biofilm-embedded L. pneumophila are protected from free chlorine residual in the distribution system (Kuchta, 1983; LeChevallier et al., 1987).
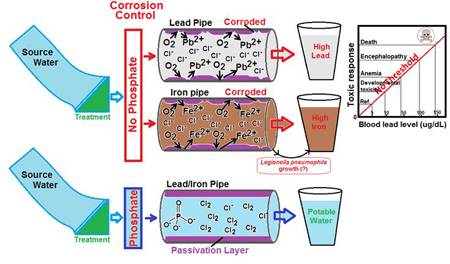
For Flint water, its relatively low pH (which was not adjusted, but is a requirement as part of the corrosion control plan for water utilities) coupled with the presence of oxidants (e.g., dissolved oxygen, chlorine) in the water reacting with iron (Fe) or Pb within the old pipes led to their leaching in the form of dissolved/ionic and particulates into the water, thereby increasing Pb and Fe concentrations in the community water supplied (Torrice, 2016). It has also been postulated that the highly corrosive Flint water depleted free chlorine that was added as a disinfectant (Falkinham, 2015; Torrice, 2016) further complicating the ability to treat biofilm pathogens such as L. pneumophila (LeChevallier et al., 1988). High turbidity (resulting from suspended particulates and organic matter) coupled with Fe leaching from the corroded pipes might have facilitated L. pneumophila growth in the remaining biofilms in the Flint water supplies (Schwake et al., 2016). This has been clearly depicted in our Graphical Abstract provided earlier. Simple measures to reduce water turbidity and corrosivity, as mandated by the USEPA’s Lead and Copper Rule (LCR) (USEPA, 1991), could have controlled the Legionellosis outbreaks and the associated health outcomes.
Other Water Crises in the United States
The Flint water crisis has some similarities to the D.C. water crisis of the early 2000’s. Periodic testing of water required by the LCR showed an abnormal number of D.C. homes with high WLLs (Cohn, 2005a). Pragmatic actions meant to safeguard public health were not implemented; by 2003, almost half of the 6,118 homes tested had WLLs over 50 μg/L. The cause was eventually determined to be the substitution of a disinfectant chloramine for chlorine. The CDC had recommended this switch in disinfectant a few years before due to carcinogenic disinfection byproduct (DBP) concerns related to chlorine. It took approximately a year after resuming usage of chlorine for WLLs to begin to return to baseline (Cohn, 2005b). In 2004, there was an increase from 2.8% to 7.6% (an increase of 4.8%), in the number of children in the affected homes with BLLs above 10 μg/dL (CDC, 2010). Federal and state authorities came under criticism for their slow action against the Pb toxicity crisis both in the Washington DC and Flint (Leonig, 2004, 2009; Hanna-Attisha et al., 2016).
There have been a large number of additional cases of elevated WLLs in the United States since 2000; the exact causes, however, vary. In the North Carolina (NC) towns of Greenville and Durham, numerous homes were noted to have high WLLs starting in 2006 (Renner, 2009). Alum had been used to reduce water turbidity for years, but a switch to ferric chloride interfered with the anti-corrosive treatment used, leading to Pb leaching from the pipes (Renner, 2009). In Wayne County, NC, a switch in disinfectant from chlorine to chloramine led to high tap WLLs. Routine monitoring of water sources failed to detect high WLLs; it was realized only after several children were noted to have very high BLLs (Miranda, 2007). In Jackson, Mississippi, fluctuating pH of the water supply increased the corrosiveness of the water, leading to elevated WLLs in 2015 (Wolfe, 2016). Twenty percent of homes tested had levels exceeding EPA’s action level of 15μg Pb/L. City officials indicated it to be mostly an issue with lead pipes within residents’ homes, but have not confirmed this publicly. Prior to 2015, there were no corrosion control measures in place, but they are now trying to find a regimen that will stabilize water pH (Wolfe, 2016). In South Carolina, 24 different communities were noted to have elevated Pb levels in 2005 (Fretwell, 2016). No corrosion control plan was being implemented at the time, but has since been instituted (Fretwell, 2016). In schools in the Ithaca, NY area, elevated Pb levels were noted in most drinking water fountains starting in 2015 (Hart, 2016). Most of these fountains received their water supply from groundwater as opposed to a municipal water supply. The cause has not been determined as of yet, but all of the schools affected have at least some lead pipes (Hart, 2016).
The similarities of the Flint and Washington, D.C. crises highlight two primary overlaps. First was the choice to change a major portion of the drinking water system. In the case of Flint it was a massive change – the complete switch of their source water. In the case for Washington DC what was theorized as a less impactful change – changing disinfectant chemicals. These changes highlight the second overlap: a lack of a complete understanding of drinking water system complexity and interactions. In both cases due to the lack of corrosion control science, what might have been something that could have been predicted or prevented was not. In the case of Flint there was also a complex mixture of toxic metal and pathogenic exposures (Pb and L. pneumophila), therefore highlighting a continued lack of corrosion control and corrosion science knowledge for drinking water systems but also the lack of knowledge in microbial dynamics and ecology in drinking water systems and their biofilms.
Identifying Issues Related to Pb Research in Drinking Water and Future Prospects
The amount of Pb in our environment is markedly lower now than it was 40 years ago. This is largely due to the elimination of leaded gasoline and paint, which has resulted in a decrease in average BLLs of around 80%. However, Pb adheres to soil particles and persists for long periods of time (ATSDR, 2007). This persistence makes complete elimination of Pb from our environment difficult. This means children are now exposed to low levels of Pb in the air and soil throughout their lives. The persistence of Pb in the environment combined with a lack of complete abatement, especially in urban and economically disadvantaged areas, still present a significant exposure problem. This consistent exposure continues even when not accounting for water Pb exposure.
As of 2010, approximately 81 million homes in the United States has Pb solder, accounting for about 77% of the total housing stock (Triantafyllidou and Edwards, 2012). Six million homes still have Pb service lines. While the vast majority of water taps in homes now test under the USEPA standard of 15 μg/L, only a very small percentage of homes are regularly tested for WLLs. It is, therefore, imperative to limit unnecessary Pb exposures, since there is no safe threshold for cognitive effects (Lanphear et al., 2005).
Issues with Pb Sampling Protocols and Factors Influencing WLLs
The Lead and Copper Rule (LCR) is often used to demonstrate a means of protecting public health with regards to Pb in public drinking water. The LCR was designed to determine the health of a water system, not to identify individual portions of distribution system at high risk (Trianatafyllidou and Edwards, 2012; USEPA, 1991). For example, the rule mandates that only 100 homes in a large city needs to be tested in order to be compliant with the LCR, thus resulting in less than 1 in 1000 homes being tested (USEPA, 2000). The LCR action level of 10 μg/L applies to the 90th percentile of the sample set, but not to individual measurements. Additional details on LCR and tap water monitoring are presented in Tables 1 and 2 (USEPA, 2008).
Table 1:
Lead and Copper Tap and Water Quality Parameter (WQP) Tap Monitoring (USEPA, 2008).
| Size Category |
System Size | Number of Pb/Cu Tap Sample Sites1 | Number of WQP Tap Sample Sites2 | ||
|---|---|---|---|---|---|
| Standard | Reduced | Standard | Reduced | ||
| Large | > 100K | 100 | 50 | 25 | 10 |
| 50,001 – 100K | 60 | 30 | 10 | 7 | |
| Medium | 10,001 – 50K | 60 | 30 | 10 | 7 |
| 3,301 – 10K | 40 | 20 | 3 | 3 | |
| Small | 501 – 3,300 | 20 | 10 | 2 | 2 |
| 101 – 500 | 10 | 5 | 1 | 1 | |
| ≤100 | 5 | 5 | 1 | 1 | |
With written State approval, Public Water Supplies (PWSs) can collect < 5 samples if all taps used for human consumption are sampled.
Two WQP tap samples are collected at each sampling site.
WQPs that must be measured include: pH, alkalinity, calcium (initial only, unless calcium carbonate stabilization is used), conductivity (initial monitoring only), orthophosphate (if inhibitor is phosphate-based); silica (if inhibitor is silicate-based); and temperature (initial monitoring only).
Addition details on LCR can be found at: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt.
Table 2.
Criteria for Reduced Pb/Cu Tap Monitoring (USEPA, 2008).
| Monitoring Time | Criteria for Reduced Pb/Cu Tap Monitoring |
|---|---|
| Annual | 1. PWS serves ≤ 50,000 people and is ≤ both action levels (ALs) for 2 consecutive years of monitoring; or 2. Any PWS that meets optimal water quality parameters (OWQPs) and is ≤ Pb AL for 2 consecutive 6-month monitoring periods. |
| Triennial | 1. PWS serves ≤ 50,000 people and is ≤ both ALs for 3 consecutive years of monitoring; or 2. Any PWS that meets OWQPs and is ≤ Pb AL for 3 consecutive years of monitoring; or 3. Any PWS with 90th percentile Pb and Cu levels ≤ 0.005 mg/L and ≤ 0.65 mg/L, respectively, for 2 consecutive 6-month monitoring periods. (i.e., accelerated reduced Pb/Cu tap monitoring). |
| Every 9 years | PWS serves ≤ 3,300 people and meets monitoring waiver criteria found at 40 CFR 141.86(g). |
Addition details on LCR can be found at: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt.
The ability to appropriately characterize the Pb concentration that consumers are exposed to is a continuing debate. Research has shown that the typical sampling and analysis strategies outlined by USEPA may not be appropriate to develop an accurate picture of the overall water Pb exposure (EET Inc., 2015). These sampling uncertainties also need to be considered within our lack of knowledge with regards to corrosion control and corrosion sciences for drinking water distribution systems. This lack of knowledge in corrosion control is especially glaring since the LCR regulates corrosion control as a means of protection from Pb and a justification for reduced sampling in the distribution system (USEPA, 1991). To protect consumers, a more systematic method of tap water sampling under consistent conditions may be required (Renner, 2010; EET Inc., 2015; Goovaerts, 2016).
This lack of specific guidance on when or how to test for WLLs leaves the public drinking water supply at risk (Renner, 2010; Goovaerts, 2016). Concerns have been raised about WLLs in school and daycare centers, as well as BLLs in young infants, both representing populations most sensitive to Pb toxicity (Triantafyllidou and Edwards, 2012). When WLLs are tested, whether at homes or schools, the measurements may not reflect the actual amount of ingestible Pb in water (Trianatafyllidou et al., 2007). For instance, it may not take into account the amount of Pb particulates in water―particulates that can become lodged in the gastrointestinal system, thus causing acute Pb toxicity as the particulates dissolve in the gastric (low pH) environment. Pb particulates can over time slowly dissolve in water leading to chronic health effects owing to low-dose repeated exposures (Schock et al., 2008). Research has also shown that the assay method used for Pb may not result in the most accurate depiction of ingested Pb. Trianatafyllidou et al. (2007) postulated that a simulated gastric fluid (SGF) acid digestion may allow for a more realistic picture of Pb concentration that the exposed individual experiences. The SGF is an acid digestion protocol to mimic the pH and conditions of the human stomach, thus giving credence to the method use.
There is also inherent sample variability in WLLs, thus requiring multiple measurements at a given site. Schock (1990) notes that serial measurements of WLLs from the same site in a potable drinking water system will show frequent, unpredictable spikes in WLLs. This result has been verified by EET Inc. (2015) that demonstrated how Pb profiles could change during the sampling event. This temporal dependency on WLLs is also highlighted by CDC’s recommendation of running the tap for one to two minutes prior to use, if the water has been stagnant for six or more hours (CDC, 2016). This consideration of the temporal dependency also highlights the number and frequency of samples collected during a sampling event. First liter sampling has traditionally been used with or without some period of temperature stabilization for the water. First liter sampling has been shown in many cases to not represent the entire peak Pb (high levels) in the water (Triantafyllidou et al., 2015; EET Inc., 2015). However, these results are still affected by large amounts of uncertainty and variability that require more research to address.
Adding to the sample variability is the flow rate of water at time of sampling. Most testing protocols call for lower flow rates than are generally used by consumers when using their tap. Faster flow rates may dislodge more Pb deposits and result in both higher WLLs and more Pb particulates. There may be ten times more Pb particulates found in water at high flow rates than at low flow rates (Triantafyllidou et al., 2007).
These issues have continued the discussions on the effectiveness of sampling methods, corrosion control and premise plumbing effects (Edwards and Dudi, 2004; Triantafyllidou et al., 2007; Triantafyllidou et al., 2015; EET Inc., 2015). A consistent sampling protocol is needed, but research needs to verify which protocol is best and that could serve as a national standard. This consistent sampling protocol needs to account for the current knowledge of: (a) Pb particulates accumulating in the system; (b) SGF or other acidification in the assay; (c) water age in premise plumbing; (d) premise plumbing flushing time prior to sampling; and (e) number of samples to take in one sampling event.
Another factor impacting Pb concentration in potable water is temperature. WLLs tend to increase with increasing temperature (The Cadmus Group, 2007; Masters et al., 2016). In the presence of natural organic matter (NOM), increased reductive dissolution led to 36 times more PbO dissolved at 20o C than at 4o C in a given exposure period (Master, 2016). This is further substantiated by the pipe-loop studies performed in Washington (District of Columbia) in 2005 and in Providence (Rhode Island) in 2013, showing that about 2–3 times more dissolved Pb and 2–6 times more particulate Pb were observed during the summer than in winter months (The Cadmus Group, 2007; Masters et al., 2016). NOM is virtually always present in community drinking water, albeit at low levels as dictated by Disinfectants and Disinfection Byproducts Rules (DBPRs) (USEPA 1998), and therefore the potential for elevated levels of Pb presence due to NOM-facilitated high dissolution in warm water is of public health concern.
Potential Path Forward for Pb Sampling
An alternative approach to Pb sampling from consumer taps is to understand the sources of uncertainty and variability that occur from the consumer’s premise plumbing. By evaluating where the Pb service line (LSL) is and its length until reaching the main, samples from inside the LSL can be taken, by timing when the sample is collected. While the concept is consistent across all homes with a LSL, the actual timing will vary based on the specific home where the sample is being collected. This allows for a more personalized and realistic assessment of the Pb exposure from drinking water in that home.
An additional source of uncertainty in Pb sampling and quantification arises from the use of the faucet aerator (Edwards and Dudi, 2004). To date there has not been any assessment of the effects of the aerator being removed or not, other than an assessment of the particulates that can be lodged in the aerator (Triantafyllidou et al., 2007). This has thus sparked a debate as to whether the aerator should remain in place or be removed during Pb sampling from faucets. The faucet aerator is used primarily to: (1) reduce actual flow rate to minimize water use; (2) avoid splashing; (3) streamline flow from the faucet; and (4) minimize faucet noise during use. However, the aerator may risk obscuring a realistic picture of Pb concentration in potable water systems when engaging in standard USEPA Pb sampling protocol (Edwards and Dudi, 2004). Recent guideline documentation from the USEPA has clarified this issue to an extent, recommending the retention of the aerator during sample collection (USEPA, 2016). This point is also becoming increasingly moot given that modern faucets most typically have an aerator that cannot be removed. These new faucets, however, may pose another risk in that the aerators cannot be removed for cleaning. If the aerators are cleaned any Pb particulates that were trapped in them would thus be removed, and thereby reducing Pb ingestion risks.
Funding Issues Related to Pb Mitigation and Research
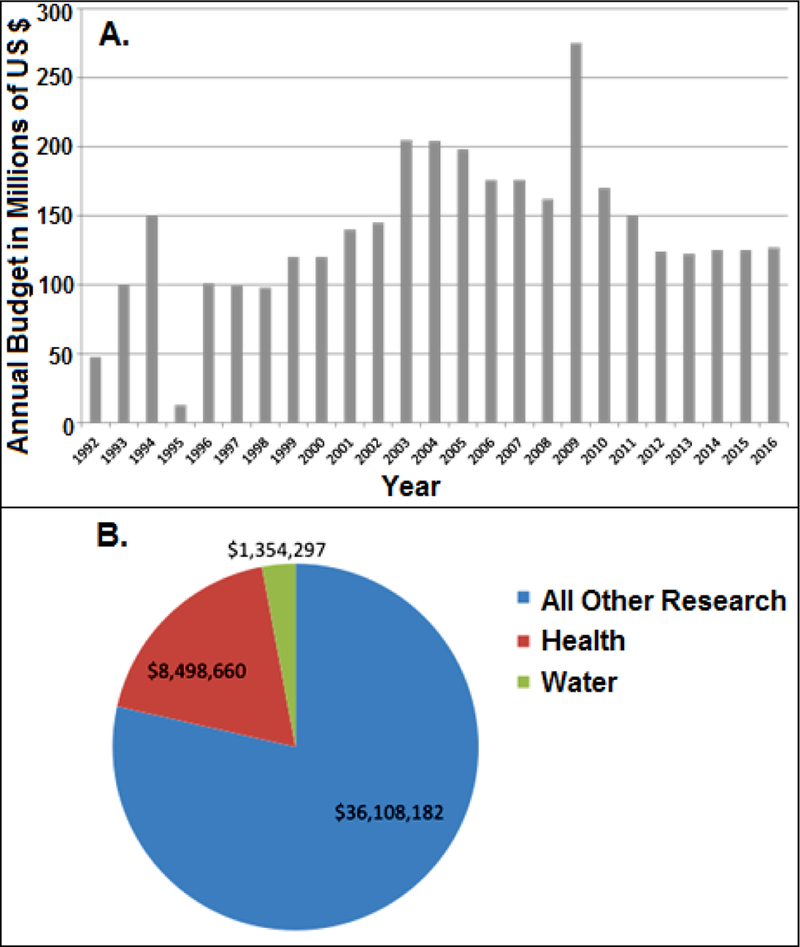
With this more complete understanding of the mixture of exposures that children and the general population face with regards to Pb, federal funding must be highlighted. Specifically the funding for prevention of Pb poisoning will be considered. The CDC and the US Department of Housing and Urban Development (HUD) are appropriated funds annually for Pb poisoning prevention. These funds are seeing a decline since an anomalous peak in 2009, a result of the American Recovery and Reinvestment Act of 2009 (Fig. 4A). What can also be seen in Figure 4A is the high degree of variability of these appropriations, making long-term planning and abatement difficult at best, if not impossible.
Fig. 4.
US Federal funding for lead poisoning prevention from CDC Healthy Homes/Lead Poisoning Prevention Program (A) (Adapted from: https://www.cdc.gov/nceh/information/healthy_homes_lead.htm); and US Federal research expenditures related to Pb for the period 2008–2016 (B) (data sources: AAAS, 2016; www.usaspending.gov).
This lack of continual or sufficient funding for lead poisoning prevention is compounded via a lack of research funding as well. Insufficient public resources devoted to Pb research may have resulted in unintended competition between sources of Pb exposure (e.g., water versus surfaces). A possible realization of this can be seen in a recent USEPA report suggested that Pb in drinking water as an exposure source is becoming a more significant percentage of total human exposure (Davis, 2010).
For the period between 2008 and 2016, Federal non-defense spending in the US accounted for $648.87 billion of which $343.34 billion was dedicated to health research (AAAS, 2016). However, in this same time frame of Federal research or research and development (R&D), a total of $45.96 million was spent on grants where the driving focus was Pb related (USA Spending, 2016). Once this value is parsed further, we can Research has research projects such as advanced batteries and other technology development. What is quite startling is the lack of water Pb research. In total from 2008 to 2016 (years for which data are readily available to the public), only $1,354,297 was spent on projects researching Pb in water, whether being related to health or not. This lack of Federal R&D support tied with the inability for long-term abatement and prevention planning (Fig. 4A) has likely resulted in the untenable set of health impacts.
Methods for Pb Quantitation
Despite the identified uncertainties in Pb sampling protocols, Pb can be fairly easily quantified in drinking water or other environmental samples using instrumentations such as atomic absorption spectroscopy (AAS), inductively coupled plasma-atomic emission spectroscopy (ICP-AES), inductively coupled plasma-mass spectroscopy (ICP-MS), laser microprobe mass analysis (LAMMA), electron probe x-ray microanalysis (EPXMA), among others (ATSDR, 2007). ICP-MS is preferred over other techniques due to its high resolution and sensitivity (ng/L; using quadruple) and its potential for separating different ionic species of Pb (Al-Rashdan et al., 1991). If inorganic lead (Pb2+) and organometallic lead are of particular interest to be detected and measured separately, 30% methanol mobile phase is considered optimal for chromatographic (HPLC) separation of differing Pb species, which can be subsequently quantified using one of the above techniques, albeit ICP-MS shows over multiple orders of magnitude improved detection compared to other techniques that are currently available (Al-Rashdan et al., 1991). It is imperative that the samples are subjected to acid digestion following standard methods such as Method 3050B before being analyzed for Pb by ICP-MS or ICP-AES (USEPA, 1996).
Placing Flint in Broader Context
Environmental Justice
Flint’s water crisis raised important questions regarding socioeconomic fairness of America’s infrastructure. There is ample evidence that areas of low socioeconomic status are at much higher risk of experiencing problems following environmental catastrophe. This was very evident following Hurricanes Katrina and Andrew (Donner and Rodríguez, 2008). New Orleans residents in the lower socioeconomic strata lived in areas more susceptible to extreme weather than more affluent residents. More importantly, the ability of those in lower socioeconomic levels to recover from disaster is much less than the more affluent. It can be argued that the physical damage incurred by Katrina was widespread, affecting all economic strata. However, the more affluent had the resources to recover quickly and independent of government response, while the less affluent are still rebuilding. Transportation and financing issues are identified as the major impediments to recovery (Masozera et al., 2007). Additionally, facilities with hazardous characteristics are more likely to be located in regions with higher income inequality and more minority residents (Elliott et al., 2004). The State of Michigan’s poor response as well as the willingness to change water suppliers without performing an appropriate evaluation first, fits a preexisting pattern of less affluent communities bearing the brunt of environmental problems (Fothergill and Peek, 2004).
Failure of environmental justice in Flint fits a pre-existing pattern of less affluent communities bearing the brunt of environmental problems. One of the most egregious aspects was the failure of the Michigan Department of Environmental Quality (MDEQ) and Flint officials to take seriously the concerns about water quality and the health complaints voiced by the Flint residents after the change in water supplier in 2014 (Bosman, 2016). This led to a delay of nearly one year in beginning to remedy the situation. By April, 2015, the U.S. EPA was aware of high WLLs in Flint drinking water (Bosman, 2016; U.S. House Committee, 2016). According to the U.S. House Committee (2016), an EPA staff member tried multiple occasions to get the EPA to intervene, but was unsuccessful until January, 2016. This apparently is against the spirit of the SDWA, which states that the EPA must step in to enforce water laws once it’s clear that the state in question is failing to enforce the laws (US EPA, 2000). The MDEQ incorrectly instructed community water suppliers to preflush residents’ taps before testing for lead; this is a violation of the LCR (Renner, 2009; Butler, 2016; Felton, 2016). In addition, the MDEQ did not abide by the LCR’s provision to preferentially sample homes most at risk of Pb contamination (Renner, 2009; Felton, 2016; Goovaerts, 2016). This noncompliance, combined with slow bureaucratic reaction to a potential Pb crisis and a marked tendency for elevated WLLs to occur mostly in poor communities with high minority populations, support the idea that environmental justice ideals are not being met (Bosman, 2016; Butler et al., 2016; Campbell et al., 2016). This nonadherence to the SDWA and the LCR led to what was an avoidable delay in the resolution of the crisis and the potential for long-term health effects for the populations affected (Butler et al., 2016; Katner et al., 2016).
Decision Making and Policy Problem
Several decisions enabled the development of the Flint crisis. It began with the decision to change drinking water supplier due to economic considerations. This was followed by the decision not to add inexpensive anticorrosive agents to the water after the switch. Once reports of water problems were encountered, inadequate water sampling procedures delayed detection of the magnitude of the Pb problem. These combined to make many Flint residents felt municipal and state authorities were not seriously addressing their concerns (Bosman, 2016; Butler et al., 2016; Campbell et al., 2016; Felton, 2016; Goovaerts, 2016; Hanna-Attisha et al., 2016).
Environmental and water policy are not simple concepts that are addressed to the best of the regulatory agencies’ capabilities. However, what can and does often get overlooked is the human element in policy and regulatory development. As an example that will be discussed here is sometimes referred to as the public notification regulation in the LCR is actually the Public Education and Supplemental Monitoring Requirements Section (40 CFR §141.85). In Subpart I where §141.85 resides, the regulation is fairly proscriptive as to what needs to be reported but there are essential gaps that can lead to confusing communication.
In an economically depressed community such as Flint, access to a family physician can be challenging. This combined into a risk communications problem where vital information could not be passed along since those channels (e.g., contact with family physician) might be non-existent in some cases. This may be highlighting the need for other stakeholders and experts not always engaged in policy and regulation development to be engaged such as community organizers and activists and environmental psychologists (Tietenberg and Lewis, 2009). This may also prevent us from forgetting that not only do complex sciences need to be addressed but that complex communities need to be accounted for as well (Smith, 2012).
Concluding Remarks
What happened in Flint, Michigan, is clearly disturbing and a reminder that even though tremendous progress has been achieved in the fight against environmental Pb toxicity, we cannot declare it solved. It is imperative that those in charge of private and public water supplies need to be vigilant and systematic in their assessments of drinking water quality.
There is the hope that if Flint’s WLLs are maintained below the LCR standard that the community’s health may not continue to be adversely affected. However, due to the very long duration of Pb’s persistence in the body this one event’s effects will be felt for the rest of the citizens’ lives. It is important to investigate how risk communication and regulatory standards as well as response strategies are developed and outlined for effective health protection based on the circumstances of the crisis. It is also important to understand that infrastructure and public health decision-making based on economic considerations over that of public health protection is detrimental to public health.
Flint water crisis also teaches us that our lack of funding to public health sciences and programs is detrimental to public health. A lack of funding in corrosion science and water based Pb exposure limited experts to a select few who could volunteer their time to react and help. A lack of funding of the county health departments further limits the capabilities of public health first responders and experts. Without the response and efforts of the Genesee County Health Department and their volunteer academic experts this crisis could have been even worse for the citizens of Flint. These conclusions seem obvious in funding public health research and first responders improving public health. However, since the Flint water crisis does not exist in a vacuum and is not the first of its kind, this conclusion needs to be stated.
Human health is affected by a spectrum of risks from a spectrum of hazards. Focusing on the chemical hazard of current greatest concern, or microbial agent in current vogue leads to a boutique approach to research. Rather since humans are exposed to a spectrum of hazards as is highlighted in both Pb and L. pneumophila affecting Flint in the same water crisis. Therefore, this spectrum of realistic exposures, hazards and risks needs to be understood to a greater degree for public health and safety.
Highlights:
-
1)
A succinct discussion on environmental lead (Pb) exposures with a focus on Pb in drinking water is presented.
-
2)
Water Pb level (WLL) is a strong correlate for blood Pb level and associated health effects.
-
3)
Multiple cases of water Pb crises for the United States are presented including the Flint crisis.
-
4)
Pertinent regulations/responses and their limitations for monitoring/mitigating WLLs are critiqued.
-
5)
Potential role of water chemistry influencing Legionnaire’s disease outbreak is highlighted.
-
6)
Issues in Pb sampling protocols, factors influencing WLLs and future prospects are discussed.
Acknowledgements
LRP gratefully acknowledges the support from Temple University’s College of Public Health. Funding for this research was provided by the Quantitative Microbial Risk Assessment Interdisciplinary Instructional Institute supported by National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award Number R25GM108593 to MHW.
References
- AAAS - The World’s Largest General Scientific Society. (2016). Historical trends in Federal R&D. 11–June–2016. Retrieved from: https://www.aaas.org/page/historical-trends-federal-rd.
- Al-Rashdan A, Heitkemper D, Caruso AA (1991). Lead speciation by HPLC—ICP—AES and HPLC—ICP—MS. Chromatogr. Sci, 29(3): 98–102. doi: 10.1093/chromsci/29.3.98. [DOI] [PubMed] [Google Scholar]
- Bernard SM, & McGeehin MA (2003, 12; 2016/5). Prevalence of blood lead levels greater than or equal to] 5 micro]g/dL among US children 1 to 5 years of age and socioeconomic and demographic factors associated with blood of lead levels 5 to 10 ug/dL, third national health and nutrition examination survey, 1988–1994, 112, 1308+. [DOI] [PubMed] [Google Scholar]
- Bosman J, Davey M, & Smith M. (2016, January 20). As water problems grew, officials belittled complaints in flint. The New York Times; Retrieved from: http://www.nytimes.com/2016/01/21/us/flint-michigan-lead-water-crisis.html [Google Scholar]
- Bosman J. (2016, October 20). EPA waited too long to warn of Flint water danger, report says. The New York Times; Retrieved from: http://www.nytimes.com/2016/10/21/us/epa-waited-too-long-to-warn-of-flint-water-danger-report-says.html?action=click&contentCollection=us&module=NextInCollection®ion=Footer&pgtype=article&version=newsevent&rref=collection%2Fnews-event%2Fflint-water-crisis [Google Scholar]
- Brown MJ, & Margolis S. (2012). Lead in drinking water and human blood lead levels in the United States Morbidity and Mortality Weekly Report. Surveillance Summaries; (Washington, D.C: 2002), 61, 1–9. [Google Scholar]
- Brown R, McTigue N, & Cornwell D. (2015). Controlling lead in drinking water Water Research Foundation, Denver, CO, USA, Water Research Foundation Grant Report 4409. [Google Scholar]
- Butler LJ; Scammell MK; Benson EB (2016). The Flint, Michigan, water crisis: A case study in regulatory failure and environmental injustice. Environ. Justice. 9(4), 93–97. [Google Scholar]
- Campbell C; Greenberg R; Mankikar D; Ross RD (2016). A case study of environmental injustice: The failure in Flint. Int. J. Environ. Res. Public Health 13, 951, doi: 10.3390/ijerph13100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Low level lead exposure harms children. A renewed call for primary prevention. Report of the Advisory Committee on Childhood Lead Poisoning Prevention. Centers for Disease Control and Prevention; 2012. Available at: http://www.cdc.gov/nceh/lead/acclpp/final_document_030712.pdf. [Google Scholar]
- Centers for Disease Control and Prevention. (February 18, 2016). Water. Retrieved from: http://www.cdc.gov/nceh/lead/tips/water.htm
- Chiodo LM, Jacobson JL, & Jacobson SW (2004). Neurodevelopmental effects of postnatal lead exposure at very low levels. Neurotoxicology and Teratology, 26(3), 359–371. doi: 10.1016/j.ntt.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Cooper WC, Wong O, & Kheifets L. (1985). Mortality among employees of lead battery plants and lead-producing plants, 1947—1980. Scandinavian Journal of Work, Environment & Health, 11(5), 331–345. [DOI] [PubMed] [Google Scholar]
- Cutter S, Boruff BJ, & Shirley WL (2006). Social vulnerability to environmental hazards. Hazards, Vulnerability, and Environmental Justice, 115–132. [Google Scholar]
- Cohn D. (2005a). Investigating Washington D.C.’s Water Quality. (2005). Niemann Report, Harvard University; Retrieved from: http://niemanreports.org/articles/investigating-washington-d-c-s-water-quality/ [Google Scholar]
- Cohn D. (2005b). D.C. tests show drops in level of lead. Washington Post, 2005: March 12; B01. Retrieved from: http://www.washingtonpost.com/wp-dyn/articles/A28213-2005Mar11.html [Google Scholar]
- Davis M. (2010). Strict lead air, dust rules spur EPA to focus on drinking water risks. Inside EPA’s Risk Policy Report. Retrieved from: http://search.proquest.com.libproxy.temple.edu/docview/1011479139?accountid=14270 [Google Scholar]
- Detroit Free Press. Flint City Councilman “We got bad water”. (January 14, 2015). Retrieved from: http://www.freep.com/story/news/local/michigan/2015/01/1.4/flint-water-resident-complaints/21743465/
- Dixon J. (2016). Flint water crisis timeline. Detroit Free Press; Retrieved from: http://www.freep.com/pages/interactives/flint-water-crisis-timeline/ [Google Scholar]
- Donner W, & Rodríguez H. (2008). Population composition, migration and inequality: The influence of demographic changes on disaster risk and vulnerability. Social Forces, 87(2), 1089–1114. [Google Scholar]
- Drum K. (2016). Raw data: Lead poisoning in Flint. Mother Jones. 2016: Jan 25. Retrieved from: http://www.motherjones.com/kevin-drum/2016/01/raw-data-lead-poisoning-kids-flint.
- Dye BA, Hirsch R, & Brody DJ (2002). The relationship between blood lead levels and periodontal bone loss in the United States, 1988–1994. Environmental Health Perspectives, 110(10), 997–1002. doi: 10.1289/ehp.02110997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Dudi A. Role of chlorine and chloramine in corrosion of lead-bearing plumbing materials. J. Am. Water Works Assoc 2004, 96(10), 69–81. [Google Scholar]
- Edwards M. (2009). Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001−2004. Environmental Science & Technology, 43(5), 1618; 1618–1623; 1623. [DOI] [PubMed] [Google Scholar]
- Edwards M, Parks J, Matha A. (2015, December 1). Flint water study update. Retrieved from: http://flintwaterstudy.org/2015/12/complete-dataset-lead-results-in-tap-water-for-271-flint-samples/. [Google Scholar]
- EET Inc., Evaluation of Lead Sampling Strategies. WRF, Final Grant Report 4569, 2015. http://www.waterrf.org/PublicReportLibrary/4569.pdf. [Google Scholar]
- Elliott MR, Wang Y, Lowe RA, & Kleindorfer PR (2004). Environmental justice: Frequency and severity of US chemical industry accidents and the socioeconomic status of surrounding communities. Journal of Epidemiology and Community Health, 58(1), 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert N, & Höring H. (1994). Lead concentration in tap-water and in blood of selected schoolchildren in southern saxonia. Toxicology Letters, 72(1), 325–331. doi: 10.1016/0378-4274(94)90044-2 [DOI] [PubMed] [Google Scholar]
- Etchevers A, Le Tertre A, Lucas JP, Bretin P, Oulhote Y, Le Bot B, & Glorennec P. (2015). Environmental determinants of different blood lead levels in children: A quantile analysis from a nationwide survey. Environment International, 74, 152–159. doi: 10.1016/j.envint.2014.10.007 [doi] [DOI] [PubMed] [Google Scholar]
- Falkinham J, Hilborn E, Arduno M, Pruden A, Edwards M. (2015), Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophilia, Mycobacterum avium and Pseudomonas aeruginosa. Environ. Health Perspect. 123(8), 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter SP, Daston GP, Euling SY, Piersma AH, & Tassinari MS (2015). Assessment of health risks resulting from early-life exposures: Are current chemical toxicity testing protocols and risk assessment methods adequate? Critical Reviews in Toxicology, 45(3), 219–244. [DOI] [PubMed] [Google Scholar]
- Felton R. (2016, April 27). Michigan official suggested gaming water tests to ‘bump out’ lead results. The Guardian; Retrieved from: https://www.theguardian.com/us-news/2016/apr/27/michigan-employees-manipulate-water-samples-lead-testing [Google Scholar]
- Fertmann R, Hentschel S, Dengler D, Janßen U, & Lommel A. (2004). Lead exposure by drinking water: An epidemiologial study in Hamburg, Germany. International Journal of Hygiene and Environmental Health, 207(3), 235–244. doi: 10.1078/1438-4639-00285 [DOI] [PubMed] [Google Scholar]
- Fretwell S. (2016, Feb 19). Lead tainted water in South Carolina communities. Retrieved from: http://www.thestate.com/news/local/article61283287.html
- Flint Water Advisory Task Force (FWATF). (2016, March 21). Final Report. Retrieved from: https://www.michigan.gov/documents/snyder/FWATF_FINAL_REPORT_21March2016_517805_7.pdf
- Fonger R. (2015, February 25). Detroit offer Flint alternative of using river as long-term water backup. M-Live Michigan; Retrieved from: http://www.mlive.com/news/flint/index.ssf/2015/02/detroit_offers_flint_water_bac.html [Google Scholar]
- Fothergill A, & Peek LA (2004). Poverty and disasters in the United States: A review of recent sociological findings. Natural Hazards, 32(1), 89–110. doi: 10.1023/B:NHAZ.0000026792.76181.d9 [DOI] [Google Scholar]
- Glymph C, Knuckles ME, Calhoun T, Moses MS, Stokes L, Lum G, … Goldsmith DF (2007). Elevated lead in drinking water in washington, DC, 2003–2004: The public health response. Environmental Health Perspectives, 115(5), 695–701. doi: 10.1289/ehp.8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goovaerts P. 2016. The drinking water contamination crisis in Flint: Modeling temporal trends of lead level since returning to Detroit Water System. Science of the Total Environment, 10.1016/j.scitotenv.2016.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, Matte TD, Schwartz J, Jackson PJ. (2002). Economic gains resulting from the reduction in childhood exposure to lead in the U.S. Environ. Health Perspect. June; 110(6), 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. (2016). An abundance of caution regarding lead in the water. Retrieved from: http://www.ithaca.com/news/tompkins_county/an-abundance-of-caution-regarding-lead-in-water/article_a3a2186e-3e26-11e6-b91c-0bb2d3a3a13d.html [Google Scholar]
- Hanna-Attisha M, LaChance J, Sadler RC, & Champney Schnepp A. (2016). Elevated blood lead levels in children associated with the flint drinking water crisis: A spatial analysis of risk and public health response. American Journal of Public Health, 106(2), 283–290. doi: 10.2105/AJPH.2015.303003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, & Rotnitzky A. (1996). The relationship of bone and blood lead to hypertension: The normative aging study. Jama, 275(15), 1171–1176. doi: 10.1001/jama.1996.03530390037031 [DOI] [PubMed] [Google Scholar]
- Jean Brown M, Raymond J, Homa D, Kennedy C, & Sinks T. (2011). Association between children’s blood lead levels, lead service lines, and water disinfection, washington, DC, 1998–2006. Environmental Research, 111(1), 67–74. doi: 10.1016/j.envres.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Katner A, Pieper KJ, Lambrinidou Y, Brown K, Hu C, Mielke HW, & Edwards MA (2016). Weaknesses in federal drinking water regulations and public health policies that impede lead poisoning prevention and environmental justice. Environmental Justice, 9(4), 19–117. doi: 10.1089/env.2016.0012 [DOI] [Google Scholar]
- Kaufman AS (2001). Do low levels of lead produce IQ loss in children? A careful examination of the literature. Archives of Clinical Neuropsychology, 16(4), 303–341. doi: 10.1016/S0887-6177(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Kuchta JM, States SJ, McNamara AM, Wadowsky RM, & Yee RB (1983). Susceptibility of Legionella pneumophila to chlorine in tap water. Appl Environ Microbiol, 46(5), 1134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RF, Moore MR, & Richards WN (1985). Lead in water, infant diet and blood: The glasgow duplicate diet study. Science of the Total Environment, 41(3), 235–257. doi: 10.1016/0048-9697(85)90144-5. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Burgoon DA, Rust SW, Eberly S, & Galke W. (1998). Environmental exposures to lead and urban children’s blood lead levels. Environmental Research, 76(2), 120–130. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Burgoon DA, Rust SW, Eberly S, & Galke W. (1998). Environmental exposures to lead and urban children’s blood lead levels. Environmental Research, 76(2), 120–130. [DOI] [PubMed] [Google Scholar]
- Lanphear BP (2005). Childhood lead poisoning prevention: Too little, too late. JAMA, 293(18), 2274–2276. doi:293/18/2274 [pii] [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, .. . Roberts R. (2005). Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environmental Health Perspectives, 113(7), 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Weitzman M, Winter NL, Eberly S, Yakir B, Tanner M, … Matte TD (1996). Lead-contaminated house dust and urban children’s blood lead levels. American Journal of Public Health, 86(10), 1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonnig C. (2004). Senators Urge Probe of EPA on Lead in Water. Washington Post; 2004: October 6; B03. Retrieved from: http://www.washingtonpost.com/wp-dyn/articles/A9550-2004Oct5.html. [Google Scholar]
- Leonnig C. (2009). D.C., U.S. Underreported Number of Kids with High Lead Levels by more than half. Washington Post; 2009: August 4. Retrieved from: http://www.washingtonpost.com/wp-dyn/content/article/2009/08/03/AR2009080303003.html. [Google Scholar]
- Levin R. (1986). Reducing lead in drinking water: A benefit analysis. EPA Report 230–09-86–019. [Google Scholar]
- Li W, Han S, Gregg TR, Kemp FW, Davidow AL, Louria DB, Siegel A, Bogden JD. (2003). Lead exposure potentiates predatory attack behavior in the cat. Environ Res 92(3), 197–206. [DOI] [PubMed] [Google Scholar]
- LeChevallier MW, Babcock TM, & Lee RG (1987). Examination and characterization of distribution system biofilms. Appl. Environ. Microbiol, 53(12), 2714–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier MW, Cawthon CD, & Lee RG (1987). Factors promoting survival of bacteria in chlorinated water supplies. Appl. Environ. Microbiol, 54(3), 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Rutter J, & Park H. (2016, February 4). Events that led to the flint water crisis. New York Times. [Google Scholar]
- Lynch J. (2016, July 22). Officials: Flint water rates could double in five years. The Detroit News; Retrieved from: http://www.detroitnews.com/story/news/michigan/flint-water-crisis/2016/07/22/officials-flint-water-rates-double-five-years/87461748/ [Google Scholar]
- Mahaffey KR, Annest JL, Roberts J, & Murphy RS (1982). National estimates of blood lead levels: United states, 1976–1980: Association with selected demographic and socioeconomic factors. The New England Journal of Medicine, 307(10), 573–579. doi: 10.1056/NEJM198209023071001 [DOI] [PubMed] [Google Scholar]
- Mandour RA, Ghanem A, & El-Azab SM (2013). Correlation between lead levels in drinking water and mothers’ breast milk: Dakahlia, Egypt. Environmental Geochemistry and Health, 35(2), 251–256. [DOI] [PubMed] [Google Scholar]
- Masozera M, Bailey M, & Kerchner C. (2007). Distribution of impacts of natural disasters across income groups: A case study of New Orleans. Ecological Economics, 63(2), 299–306. doi: 10.1016/j.ecolecon.2006.06.013 [DOI] [Google Scholar]
- Masters S, Welter GJ, & Edwards M. (2016). Seasonal variations in lead release to potable water. Environmental Science & Technology, 50(10), 5269–5277. [DOI] [PubMed] [Google Scholar]
- Menke A, Muntner P, Batuman V, Silbergeld EK, & Guallar E. (2006). Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation, 114(13), 1388–1394. doi:CIRCULATIONAHA.106.628321 [pii] [DOI] [PubMed] [Google Scholar]
- Mielke HW, & Reagan PL (1998). Soil is an important pathway of human lead exposure. Environmental Health Perspectives, 106 Suppl 1, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Kim D, Hull AP, Paul CJ, & Galeano MAO (2007). Changes in blood lead levels associated with use of chloramines in water treatment systems. Environmental Health Perspectives, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngueta G, Prevost M, Deshommes E, Abdous B, Gauvin D, & Levallois P. (2014). Exposure of young children to household water lead in the montreal area (canada): The potential influence of winter-to-summer changes in water lead levels on children’s blood lead concentration. Environment International, 73, 57–65. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Le Tertre A, Etchevers A, Le Bot B, Lucas J, Mandin C, … Glorennec P. (2013). Implications of different residential lead standards on children’s blood lead levels in france: Predictions based on a national cross-sectional survey. International Journal of Hygiene and Environmental Health, 216(6), 743–750. [DOI] [PubMed] [Google Scholar]
- Payton M, Payton M, Hu H, Hu H, Sparrow D, Sparrow D, … Weiss ST (1994). Low-level lead exposure and renal function in the normative aging study. American Journal of Epidemiology, 140(9), 821–829. [DOI] [PubMed] [Google Scholar]
- Pennsylvania Department of Health. (2014). Childhood lead surveillance annual report 2014. Retrieved from: http://www.health.pa.gov/My%20Health/Infant%20and%20Childrens%20Health/Lead%20Poisoning%20Prevention%20and%20Control/Documents/2014%20Lead%20Surveillance%20Annual%20Report%20r2.pdf [Google Scholar]
- Pocock SJ, Shaper AG, Walker M, Wale CJ, Clayton B, Delves T, … Powell P. (1983). Effects of tap water lead, water hardness, alcohol, and cigarettes on blood lead concentrations. Journal of Epidemiology and Community Health, 37(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. (2005). The 1991 Lead/Copper drinking water rule and the 1995 decision not to revise the arsenic drinking water rule: Two case studies in EPA’s use of science. Discussion Paper 97–05. Retrieved from: http://ageconsearch.umn.edu/bitstream/10454/1/dp970005.pdf
- Raymond J, Brown MJ. (2016). Blood Lead Levels in Children Aged <5 Years — United States, 2007–2013. MMWR Morb Mortal Wkly Rep, 63, 66–72. DOI: 10.15585/mmwr.mm6355a6 [DOI] [PubMed] [Google Scholar]
- Renner R. (2009). Out of plumb: When water treatment causes lead contamination. Environmental Health Perspectives, 117(12), A542–A547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner R. (2010). Exposure on tap drinking water as an overlooked source of lead. Environmental Health Perspectives, 118(2), A68–A74. doi: 10.1289/ehp.118-a68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, & Lauwerys R. (1987). Evaluation of dose-effect and dose-response relationships for lead exposure in different belgian population groups (fetus, child, adult men and women). Trace Elements in Medicine, 4(2), 80–87. [Google Scholar]
- Rothenberg SJ, Kondrasho V, Manalo M, Jiang J, Cuellar R, et al. (2002). Increases in hypertension and blood pressure during pregnancy with increased bone lead levels. American Journal of Epidemiology, 156(12), 1079–1087. doi: 10.1093/aje/kwf163 [DOI] [PubMed] [Google Scholar]
- Ryu JE, Ziegler EE, Nelson SE, & Fomon SJ (1983). Dietary intake of lead and blood lead concentration in early infancy. American Journal of Diseases of Children, 137(9), 886–891. doi: 10.1001/archpedi.1983.02140350060015 [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Beaudet N, Omri K, & Karr C. (2006). Predicting children’s blood lead levels from exposure to school drinking water in Seattle, Washington, USA. Ambulatory Pediatrics, 6(5), 288–292. doi: 10.1016/j.ambp.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Schock MR, & Schock MR (1990). Causes of temporal variability of lead in domestic plumbing systems. Environmental Monitoring and Assessment, 15(1), 59–82. doi: 10.1007/BF00454749 [DOI] [PubMed] [Google Scholar]
- Schock MR, Hyland RN, & Welch MM (2008). Occurrence of contaminant accumulation in lead pipe scales from domestic drinking-water distribution systems. Environmental Science and Technology, 42(12), 4285–4291. doi: 10.1021/es702488v [DOI] [PubMed] [Google Scholar]
- Schock MR (1989). Understanding corrosion control strategies for lead. Journal-American Water Works Association 81(7), 88–100. [Google Scholar]
- Schumaker E. Flint’s Legionnaires’ outbreak may be tied to its contaminated water. When will Flint catch a break? Huff Post Healthy Living, January 19, 2016. Available at: http://www.huffingtonpost.com/entry/flint-water-legionnaires-lead-crisis_us_569d09d6e4b0ce4964252c33 [Google Scholar]
- Schwake DO, Garner E, Storm OW, Pruden A, & Edwards MA (2016) Legionella DNA Markers in Tap Water Coincident with a Spike in Legionnaires’ Disease in Flint, MI. Environmental Science and Technology Letters, 3, 311–315. [Google Scholar]
- Schwartz J. (1994). Societal benefits of reducing lead exposure. Environmental Research, 66(1), 105–124. doi: 10.1006/enrs.1994.1048 [DOI] [PubMed] [Google Scholar]
- Smith ZA (2012). Environmental Policy Paradox, 6th ed Boston: Routledge. [Google Scholar]
- Solimini AG, Cottarelli A, Marinelli L, De Giusti M. (2014). Factors influencing persistence of Legionella pneumophila serogroup 1 in laboratory cocultures. BMC Microbiology, 14, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretesky PB, & Lynch MJ (2001). The relationship between lead exposure and homicide. Archives of Pediatrics & Adolescent Medicine, 155(5), 579–582. [DOI] [PubMed] [Google Scholar]
- Swistock B, Galford A. Lead in Drinking Water. (2016, January). Retrieved from: http://extension.psu.edu/natural-resources/water/drinking-water/water-testing/pollutants/lead-in-drinking-water [Google Scholar]
- Taylor M, Ross K, Bentham R. (2009). Legionella, protozoa, and biofilms: interactions within complex microbial systems. Microb Ecol, 58, 538–547. [DOI] [PubMed] [Google Scholar]
- The Cadmus Group. Review of the Interim Optimal Corrosion Control Treatment for Washington, D.C.; Report for the U.S. Environmental Protection Agency; The Cadmus Group: Arlington, VA, 2007. [Google Scholar]
- Tietenberg T, & Lewis L. (2009). Environmental Economics & Policy, 6th ed Pearson. [Google Scholar]
- Torrice M. (2016) How lead ended up in Flint’s tap water. Chemical & Engineering News 94(7), 26–29. [Google Scholar]
- Triantafyllidou S, Lambrinidou Y, & Edwards M. (2009). Lead (Pb) exposure through drinking water: Lessons to be learned from recent US experience. Glob NEST J, 11(3), 341–348. [Google Scholar]
- Triantafyllidou S, & Edwards M. (2012). Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Critical Reviews in Environmental Science and Technology, 42(13), 1297–1352. [Google Scholar]
- Triantafyllidou S, Parks J, & Edwards M. (2007). Lead particles in potable water. Journal (American Water Works Association), 99(6), 107–117. [Google Scholar]
- Triantafyllidou S, Schock MR, DeSantis MK, & White C. (2015). Low contribution of PbO2-coated lead service lines to water lead contamination at the tap. Environ. Sci. Technol, 49(6), 3746–3754. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2015). 2015 Flint Michigan demographic information. Retrieved from: http://www.census.gov/quickfacts/table/PST045215/2629000
- United States House Committee on Oversight and Government Reform. (2016). Examining the administration of the safe drinking water act in Flint, Michigan. Retrieved from: https://oversight.house.gov/hearing/examining-federal-administration-of-the-safe-drinking-water-act-in-flint-michigan-part-2/
- United States Environmental Protection Agency. Office of Policy Planning and Evaluation. (1986). Reducing lead in drinking water: A benefit analysis. Retrieved from: https://nepis.epa.gov/reducingleadindrinkingwater
- United States Agency for Toxic Substances and Disease Registry. (2007). Toxicological profile for lead. Atlanta, Ga: U.S. Dept. of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; Retrieved from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22 [Google Scholar]
- United States Environmental Protection Agency. (1996). Acid digestion of sediments, sludges, and soils. Method 3050B. Retrieved from: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf
- United States Environmental Protection Agenc y; (1998). National primary drinking water regulations: disinfectants and disinfection byproducts. 40 CFR Parts 9, 141, and 142. December 16. Retrieved from: https://www.gpo.gov/fdsys/pkg/FR-1998-12-16/pdf/98-32887.pdf#page=1 [Google Scholar]
- United States Environmental Protection Agency. Office of Water. (2005). Is there lead in my drinking water?: You can reduce the risk of lead exposure from drinking water in your home: Tips for protecting your family’s health. Washington, D.C: U.S. Environmental Protection Agency, Office of Water; Retrieved from: https://nepis.epa.gov/Adobe/PDF/500025PW.PDF [Google Scholar]
- United States Environmental Protection Agency (2000). National primary drinking water regulations for lead and copper; 40 CFR parts 9, 141, and 142. Retrieved from: https://www.gpo.gov/fdsys/pkg/FR-2000-01-12/pdf/00-3.pdf
- United States Environmental Protection Agency. Lead and Copper Rule: A quick reference guide. June 2008. EPA 816-F-08–018. Retrieved from: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=60001N8P.txt
- United States Environmental Protection Agency. (March 17, 2016). Basic information about lead in drinking water. Retrieved from: https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-lead-drinking-water#getinto
- United States Environmental Protection Agency. (February 29, 2016). Clarification of Recommended Tap Sampling Procedures for Purposes of the Lead and Copper Rule. Retrieved from: https://www.epa.gov/sites/production/files/2016-02/documents/epa_lcr_sampling_memorandum_dated_february_29_2016_508.pdf
- USA Spending. (2016). Retrieved from: https://www.usaspending.gov/Pages/Default.aspx
- Wolfe A. (2016, September 13). Jackson still experiencing elevated lead levels in water. The Clarion-Ledger. Retrieved from: http://www.clarionledger.com/story/news/local/2016/09/13/jackson-still-experiencing-elevated-lead-levels-water/90302838/ [Google Scholar]
- World Health Organization (United Nations). (2011). Global health risks: Mortality and burden of disease attributable to selected major risks; 2011 IIS 4640-M116; ISBN 978–92-4–156387-1 (internet). Retrieved from: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf
- Yankel AJ, von Lindern IH, & Walter SD (1977). The silver valley lead study: The relationship between childhood blood lead levels and environmental exposure. Journal of the Air Pollution Control Association, 27(8), 763–767. [DOI] [PubMed] [Google Scholar]