Abstract
Cancer gender disparities have been observed for a variety of human malignancies. Thyroid cancer is one such example where there is a dramatic difference in the incidence, aggressiveness, and death rate by gender. The molecular basis for gender disparity is poorly understood. To address this, we performed genome-wide gene expression profiling in matched papillary thyroid cancer (PTC) samples and identified nine candidate genes differentially expressed by gender. One of these genes was CDC23 that was upregulated in PTC in men compared with women. Because the function and expression of CDC23 is unknown in eukaryotic cells, we further characterized the expression of CDC23 in normal, hyperplastic, and PTC tissue samples. We found CDC23 was overexpressed in PTC and absent in normal and hyperplastic thyroid tissue. In thyroid cancer cells, functional knockdown of CDC23 resulted in an increase in the number of cells in both the S and G2M phases of the cell cycle, and an inhibition of cellular proliferation, tumor spheroid formation, and anchorage-independent growth. Cellular arrest in both S and G2M phases was associated with significant cyclin B1 and securin protein accumulation after CDC23 knockdown. Moreover, the effect of CDC23 on cellular proliferation and cell cycle progression was reversed on triple knockdown studies of CDC23, cyclin B1, and securin. Our data taken together suggests CDC23 has important biologic effects on cell proliferation and cell cycle progression. The effect of CDC23 on cellular proliferation and cell cycle progression is mediated, at least in part, by cyclin B1 and securin protein levels. Therefore, we propose that CDC23 is a critical regulator of cell cycle and cell growth, and may be involved in thyroid cancer initiation and progression, and may explain the different tumor biology observed by gender.
Introduction
Gender-based differences have been observed in the incidence, aggressiveness, and mortality rate for many human malignancies (Naugler et al. 2007, Paggi et al. 2010, Rahbari et al. 2010, Yeh & Chen 2010, Fajkovic et al. 2011). Dietary, environmental, behavioral, and reproductive factors have been implicated in cancer gender disparity, but the molecular basis for this disparity is poorly understood. Thyroid cancer is one such cancer with a dramatically different incidence, aggressiveness, and death rate by gender (Rahbari et al. 2010).
Thyroid cancer arises from follicular (papillary, follicular, hürthle cell cancer, and anaplastic) and parafollicular (medullary) cells. Papillary thyroid cancer (PTC) accounts for ~85% of all thyroid cancer cases. Although the overall incidence of cancer in the United States continues to decrease, the incidence of thyroid cancer has increased dramatically in the last three decades by about 4% annually (Jemal et al. 2004). The gender disparity in thyroid cancer incidence is striking. Incidence rates are particularly high in premenopausal women (four times higher than in men; Sakoda & Horn-Ross 2002, Jemal et al. 2004). On the other hand, thyroid cancer of follicular cell origin in men is more advanced at diagnosis and is associated with a worse outcome, even when confounding factors are accounted for. Furthermore, for the uniformly lethal anaplastic thyroid cancer, the gender distribution is even, unlike that for differentiated thyroid cancer of follicular cell origin (papillary and follicular; Kebebew et al. 2005).
The gender disparity in thyroid cancer incidence has prompted research into the role of sex hormone and reproductive factors in thyroid carcinogenesis. Several epidemiologic studies suggest that early or late menarche increases the risk of thyroid cancer by 50% (Horn-Ross et al. 2001, Iribarren et al. 2001, Sakoda & Horn-Ross 2002). Moreover, recent pregnancy increases the risk of thyroid cancer, whereas the consumption of cruciferous vegetables, antioxidant vitamins, and phytoestrogens protects against thyroid cancer (Horn-Ross et al. 2002). All these studies suggest that hormonal or reproductive, and dietary factors have a role in thyroid carcinogenesis and may account for the gender disparity in thyroid cancer.
Environmental, dietary and reproductive factors have important roles in cancer initiation, promotion and progression in a variety of human malignancies. There is growing evidence that these factors directly regulate gene expression in carcinogenesis (Kaput & Rodriguez 2004). Although epidemiologic and clinical studies suggest the gender disparity in thyroid cancer is influenced by dietary and reproductive factors, the molecular factors that may account for these differences are unknown. Therefore, we studied the expression profile of matched PTC samples by gender. Among several genes, we found CDC23 was overexpressed in PTC in men compared with women, and in PTC compared with normal and hyperplastic thyroid tissue. Sex hormone had no effect on CDC23 expression in thyroid cancer cell lines. Functional studies in thyroid cancer cell lines demonstrated CDC23 had a dramatic effect on cell cycle and cellular proliferation, which were dependent on cyclin B1 and securin protein levels.
Materials and methods
Thyroid tissues
Six normal, ten hyperplastic human thyroid tissues, 96 PTCs, and 86 adrenocortical tumors were snap frozen and stored at −8°C on an Institutional Review Board approved tissue procurement protocol after written consent was obtained. Thirty-five conventional PTCs were used for genome-wide gene expression analysis, 34 separate-independent PTCs were used for validation of the genome-wide gene expression, and 34 PTC samples (27 separate-independent samples and seven repeated samples used in quantitative RT-PCR (qRT-PCR) validation) were used for immunohistochemistry studies. The normal and hyperplastic thyroid tissue were used for immunohistochemistry and qRT-PCR. None of the PTC, normal, or hyperplastic tissue samples had any coexisting lymphocytic thyroiditis.
Cell culture
Human PTC cell line (TPC-1) and follicular thyroid cancer cell line (FTC-133) were maintained in DMEM supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), fungizone (250 ng/ml), TSH (10 IU/l), and insulin (10 μg/ml) in a 5% CO2 atmosphere at 37 °C. The human breast cancer cell line, MCF-7, was maintained in DMEM supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), and fungizone (250 ng/ml).
RNA isolation, microarray, and qRT-PCR
Total RNA was extracted from frozen tissue samples and thyroid cancer cell lines using TRIzol reagent (Invitrogen Life Technologies, Inc.) and the RNeasy Mini Kit (Qiagen).
Total RNA (1 μg) was amplified and labeled using the MessageAmp aRNA Kit (Ambion, Inc., Foster City, CA, USA). Twelve micrograms of labeled and fragmented complimentary RNA was hybridized to the Affymetrix Human Genome U133 plus 2.0 GeneChip (Santa Clara, CA, USA) for 16 h at 45 °C. The probe intensities were measured using argon laser confocal GeneArray Scanner (Hewlett-Packard, Palo Alto, CA, USA).
For qRT-PCR, 0.5–1 μg of total RNA was used for first-strand cDNA synthesis using the Superscript III First-strand Synthesis SuperMix for qRT-PCR (Invitrogen). TaqMan primer probes for CDC23, GUSB, and GAPDH were purchased from Applied Biosystems (Foster City, CA, USA), and relative expression was determined by the ΔCt method described by the manufacturer (Applied Biosystems). All reactions were performed in triplicate.
Sex hormone treatment
TPC-1, FTC-133, and MCF-7 cells were plated in six-well plates (1×105, 4×104, and 5×104 cells/well respectively). After 24 h, cells were washed with PBS and cultured in phenol red-free medium. After 24 h of hormone deprivation (time 0) samples were collected. The cells were treated with four different conditions (1 nM estradiol, 1 nM testosterone, 1% DSMO vehicle, and medium only) in phenol red-free medium and harvested at 24 and 48 h after treatment. Total RNAs were extracted for qRT-PCR. All assays were conducted in biological and technical triplicates.
Small interfering RNA transfection, quantitative PCR, and immunoblotting
The small interfering (si)RNA for human CDC23 (siRNA ID- s16570 and s16572) and scrambled negative controls (Part #: 4390843) were purchased from Applied Biosystems. The siRNA for human cyclin B1 and PTTG (securin) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). FTC-133 and TPC-1 cells were reverse transfected with each individual siRNA at a concentration of 60 or 90 nmol/l using Lipofectamine RNAiMAX (Invitrogen). Total RNA was isolated and the level of CDC23 mRNA was determined by quantitative PCR as described earlier. Whole cell lysate was prepared with 1% SDS plus 10 mM Tris (pH 7.5), and was used for CDC23, cyclin B1, and securin protein detection by western blot (rabbit anti-CDC23, from Calbiochem (Darmstadt, Germany), mouse anti-cyclin B1 from Santa Cruz Biotechnology, Inc., and rabbit anti-securin from Abcam (Cambridge, MA, USA)).
Cell proliferation assay
Cell proliferation experiments were performed in 96-well in triplicates or quadruplicates. Cells were reverse transfected with individual siRNA in 96-well black plates at 2×103 cells/well and maintained in 200 μl serum-free media (DMEM/Ham’s F-12 (1:1) supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), somatostatin (10 ng/ml), and hydrocortisone (0.36 ng/ml)) in a humidified incubator. CyQuant proliferation assays were performed at each day after transfection according to the manufacturer’s instructions (Invitrogen). The cell densities in the 96-well black plates were determined using a 96-well fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 485/538 nm.
Apoptosis assay
Cells (5×104 cells/well) were reverse transfected with different siRNA and maintained in serum-free medium as described earlier. Apoptosis was detected using ApoAlert Annexin-V-FITC Apoptosis kit (Clontech) following the manufacturer’s instructions. Flow cytometric analysis for apoptosis was performed on a FACScan (BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
Cells (5×104 cells/well) were reverse transfected and maintained in serum-free medium as described earlier. Cells were harvested, washed, and resuspended with PBS, and fixed with ice-cold 70% ethanol at 4 °C. After washing with PBS, ribonuclease A was added to the cell suspension and incubated at 37 °C for 20 min. Then propidium iodide (PI; 50 μg/ml in PBS) was added, and samples were stored at 4 °C.
Flow cytometric analysis for cell cycle was performed on a FACScan using CellQuest software (BD Biosciences). Data files were generated for 10 000 events (cells) or more per sample gated on single cells. Doublets, cell clumps, and debris were excluded by PI fluorescence pulse width and pulse area measurements. Cell cycle analysis on the gated PI distribution was performed by Modfit software (Verity Software House, Inc., Topsham, ME, USA).
Soft agar assay for colony formation
Three days after siRNA transfection, FTC-133 cells were trypsinized, counted, and resuspended in culture media. Two-layered soft agar assays were performed in six-well plates. The bottom layer of agar (2 ml/well) contained 0.6% agar (Difco agar noble, Becton, Dickinson and Company, Sparks, MD, USA) in Ham’s F-12 medium supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), and fungizone (250 ng/ml).
Thirty thousand cells were mixed with 1 ml of upper agar solution (0.35% agar in culture media). Thirty minutes later, 1 ml of the culture media was added into each well. The plates were cultured at 37 °C in 5% CO2, and the media was changed twice a week. After 18 days of culture, cell colonies were stained with Crystal Violet and examined by microscopy. Colony counting was performed in five different fields (5×10) per well.
Spheroid culture
Three days after siRNA transfection, FTC-133 cells were trypsinized, counted, and resuspended in culture media and plated in Ultra Low Cluster plate (Costar, Corning, NY, USA) at 3.5×104/well/24-well plates. The plates were cultured at 37 °C in 5% CO2, and the medium was changed every 2–3 days. After 2 weeks of culture, cells were stained with Crystal Violet and photographed under microscope.
Immunohistochemistry
Paraffin-embedded tumor samples were cut into 5 mm thick sections and deparaffinized in xylene. The CDC23 protein expression was examined using a rabbit anti-CDC23 antibody (Purified anti-APC8, BioLegend, San Diego, CA, USA). Staining was detected using vectastain ABC and DAB kits (Vector Laboratories, Inc., Burlingame, CA, USA).
Statistical analysis
ANOVA post hoc tests (StatView) and t-test were used for statistical analysis. Significance for P values between 0.05 and 0.01 is indicated with one asterisk (*), values of P between 0.01 and 0.001 are indicated with two asterisks (**), and values of P<0.001 are indicated with three asterisks (***). All in vitro experiments were repeated two to five times.
Raw microarray data were analyzed using the affy package (R/Bioconductor). The robust multiarray average method was used to quantitate intensity values in log2 scale for each probe set (Bolstad et al. 2003, Irizarry et al. 2003). Hierarchical clustering was done using the Euclidean distance and complete linkage on the most variably expressed genes, as defined by the median absolute deviation (MAD, a robust measure of variance) across all arrays. For class comparison (female vs male), we used the limma package in R/Bioconductor to calculate the moderated t-statistics and the associated P values, as well as the log posterior odds ratio (B statistics) that a gene is differentially expressed vs not differentially expressed. P values were adjusted for multiple testing by controlling for false discovery rate using the Benjamini–Hochberg method. For the cross-validation of microarray data in PTC to that of the adrenocortical tumor samples, we examined the gender-specific expression of the probe sets for the genes (42 genes by B statistics >0) that showed significant differential expression in PTC by gender in the adrenocortical tumors as a function of gender. We classified the probe sets into three classes: those that show significant gender-specific adrenocortical tumor expression (Student’s t-test P value <0.0002); those that show no evidence of gender-specific expression (P value >0.30); and those that could not be confidently assigned to either class (0.0002<P<0.30).
Results
Gene expression profile of PTC by gender
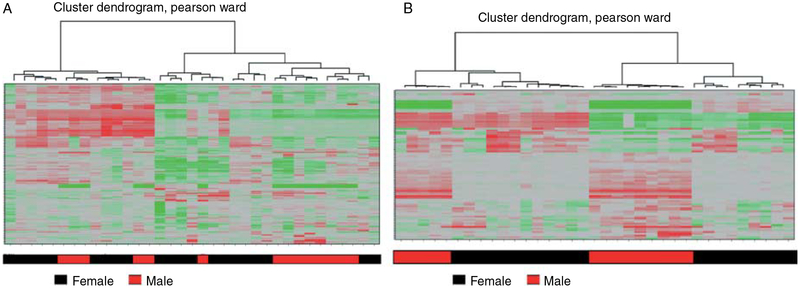
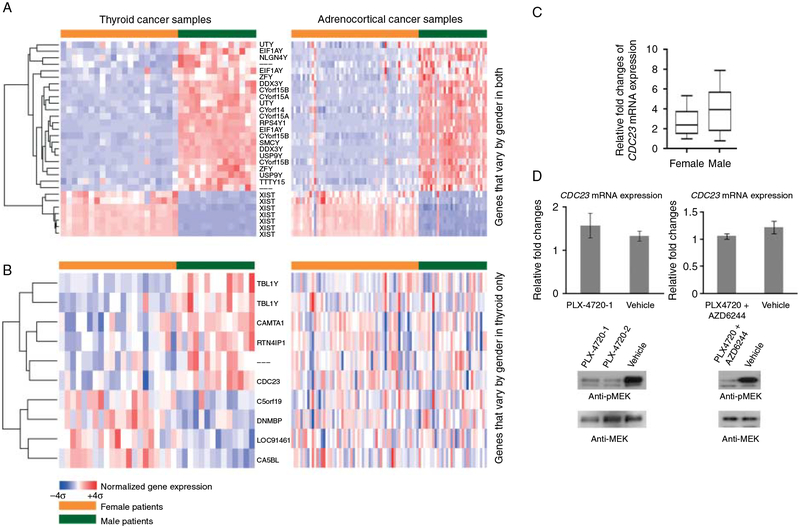
In 35 samples of PTC, we found a number of significantly differentially expressed genes by gender. By hierarchical cluster analysis, the tumor samples were segregated into two broad groups with tumor samples from women and men clustering together (Fig. 1). To eliminate gender-specific gene expression differences that occur in all cell types, such as Y- and X-linked gene expressions, we compared the differentially expressed genes in PTC to a genome-wide gene expression dataset of 86 adrenocortical tumors (56 women and 30 men). Adrenocortical tumors are endocrine tumors without significant gender disparity and thus are well suited for this type of analysis. We first examined 42 genes (B statistics >0) that showed significant PTC gender-specific expression in adrenocortical tumors as a function of gender. The majority of genes that showed increased expression in samples from male patients with thyroid or adrenocortical tumors were genes that were present on the Y chromosome. Conversely, many of the genes that showed increased expression in samples from female patients with either thyroid or adrenocortical tumors were genes that were involved in X-chromosome inactivation (Fig. 2A). There were, however, nine differentially expressed genes that showed gender-specific expression only in PTCs suggesting that these genes may contribute to gender-specific differences in thyroid biology and/or papillary thyroid tumorigenesis (Fig. 2B). Furthermore, we validated the differential expression of CDC23 by gender in 34 independent PTC samples using qRT-PCR (Fig. 2C). We found CDC23 was upregulated in PTC in men (1.4-fold) compared with PTC in women. We found no significant difference in CDC23 immunoreactivity in PTC samples by gender but most of the samples had strong staining. We also found no significant association between CDC23 mRNA expression and other clinicopathologic variables or by common somatic mutation status (14 wild type, 15 BRAF V600E, and 5 RET/PTC3). Even though, we did not see a difference in the tissue samples, we also tested the idea of whether activating mutation in the MAPK pathways that are common in PTC may modulate CDC23 mRNA expression. However, we found no significant change in CDC23 mRNA expression when using a selective BRAF V600E and or MEK inhibitor in the 8505c BRAF V600E mutant cell line (Fig. 2D).
Figure 1.
Dendrograms of clustering from 35 papillary thyroid cancer samples. (A) Unsupervised hierarchical cluster analysis of the top 200 most variably expressed genes. (B) Supervised hierarchical cluster analysis showing differentially expressed genes by gender using the log posterior odds ratio (B statistics >0) statistical criterion. Each row represents the mean adjusted expression level of an individual gene and each column represents an individual tumor sample. Overexpressed genes are indicated in red and underexpressed genes are indicated in green.
Figure 2.
CDC23 was differentially expressed by gender in thyroid cancer. (A) The gender-specific genes identified by microarray analysis in both thyroid and adrenocortical tumors. (B) Genes with gender-specific expression in papillary thyroid cancer (PTC) only. (C) Quantitative RT-PCR (qRT-PCR) validation of CDC23 mRNA expression in independent PTC samples. Seventeen paired PTC samples (matched for age at diagnosis (21–45 years, same age or 1 year difference in each pair) and tumor size) were analyzed. CDC23 mRNA expression was normalized to β-glucuronidase. Box plot is for mean±95% interval. P<0.01. (D) Inhibiting the MAPK pathway does not significantly alter CDC23 mRNA expression. 8505c cells were treated with 3000 nM of PLX 4720 (Braf inhibitor) (left panels), 3000 nM of PLX 4720 plus 80 nM of AZD6244 (MEK inhibitor) (right panels), or vehicle for 3.5 h. CDC23 expression was determined by qRT-PCR using β-glucuronidase as a reference gene (mean±s.e.m.). By t-test P value was >0.05. Effective inhibition of the MAPK pathway was detected by immunoblotting with anti-phospho-MEK or anti-MEK antibodies.
Sex hormones do not regulate CDC23 expression in thyroid and breast cancer cell lines
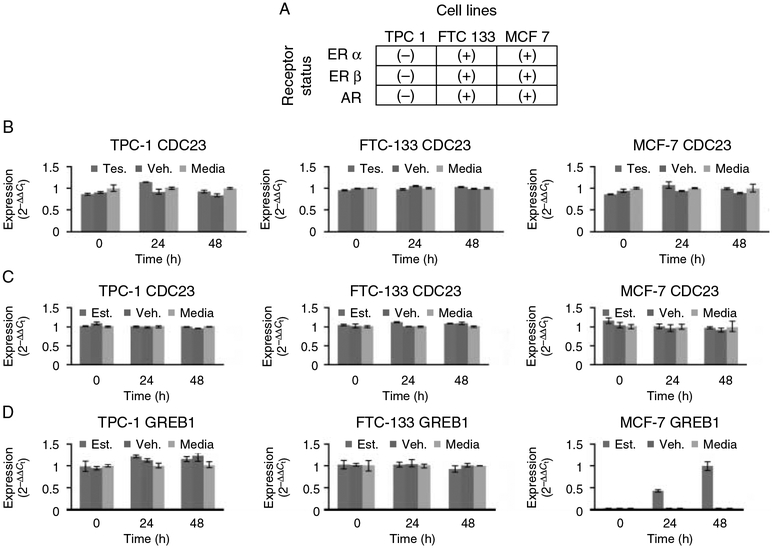
One of the genes, CDC23, was of particularly interest to us since the function of the gene in higher eukaryotic cells is unknown (Irniger & Nasmyth 1997, Zhao et al. 1998, Matyskiela & Morgan 2009). We first tested the hypothesis of whether the gender-specific difference in CDC23 gene expression in PTC (which was upregulated in male tumor samples) was due to sex hormone gene expression regulation. To determine the effect of estrogen and testosterone on CDC23 gene expression, we used thyroid cancer cell lines representing the common histologies of thyroid cancer (TPC-1 for PTC and FTC-133 for follicular thyroid cancer) and the breast cancer cell line MCF-7. Treatment of cells with either estradiol or testosterone had no significant effect on CDC23 gene expression levels compared with their effect on a known sex hormone target gene (GREB1; Fig. 3A–D). This result suggests that the differential expression of CDC23 in thyroid cancer we observed cannot be explained simply by differences in sex hormones in males vs females.
Figure 3.
Sex hormone effect on CDC23 mRNA expression. Estradiol-17β and testosterone have no significant effect on CDC23 mRNA expression in TPC-1, FTC-133, and MCF-7 cell lines. (A) Estrogen receptor and androgen receptor expression status in cell lines. Estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and androgen receptor (AR). (B) Relative expression levels of CDC23 compared with control in three cell lines (FTC-133, TPC-1, and MCF-7) after exposure to 1 nM testosterone (blue), vehicle (1% DSMO) (red), and medium only (green). (C) Relative expression levels of CDC23 compared with control in three cell lines after exposure to 1 nM 17β-estradiol, vehicle, and medium only. (D) Relative expression levels of GREB1 compared with control in three cell lines after exposure to 1 nM 17β-estradiol, vehicle, and medium only. GREB1 was used as a positive control target gene for 17β-estradiol.
CDC23 protein expression in thyroid tissue
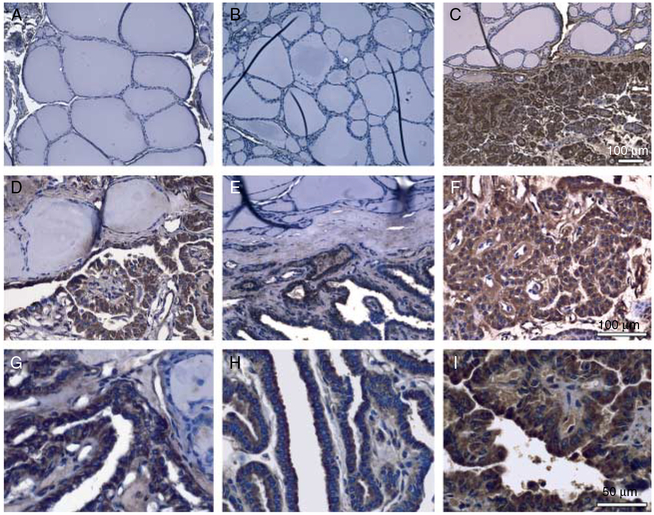
Immunohistochemistry staining was performed to examine the expression of CDC23 protein in normal thyroid tissue and PTC samples from 34 individual patient samples. In normal thyroid tissue, the anti-CDC23 antibody showed no staining in normal follicular cells (Fig. 4A and B). In contrast, PTC cells showed strong CDC23 immunoreactivity. Moreover, in cancer samples with adjacent normal thyroid follicles, strong staining was observed only in cancer cells, while the adjacent normal cells were negative for CDC23. Areas of hyperplastic thyroid tissue, follicular cells with increased proliferation and cell cycling, showed no CDC23 immunoreactivity. These findings suggest that CDC23 protein overexpression is specific to PTC cells.
Figure 4.
CDC23 was overexpressed in papillary thyroid cancer (PTC). Representative immunohistochemistry of (A and B) normal thyroid tissues from two different patients with PTC (magnification 10×) and (C–I) PTC samples from six individual patients (magnification: 10× for C; 20× for D and E, F; 40× for G–I).
Function of CDC23 in thyroid cancer cells
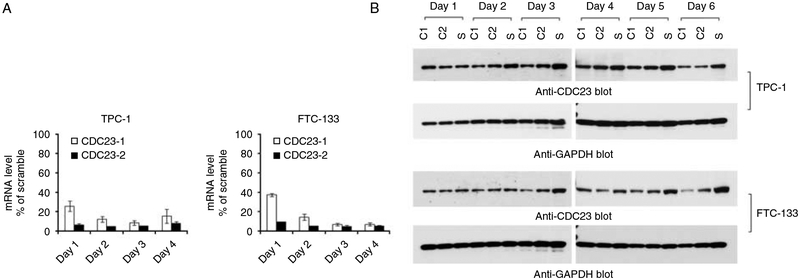
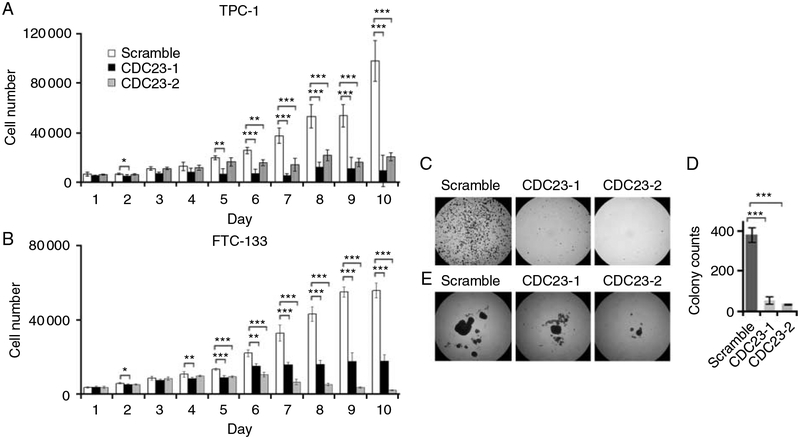
Human CDC23 has a 30% homology with Saccharomyces cerevisiae Cdc23, a tetratricopeptide repeat protein component of anaphase-promoting complex (APC; Zhao et al. 1998). Since the function of CDC23 in higher eukaryotic cells is unknown and CDC23 showed dramatic overexpression in PTC, we were interested in evaluating the role of CDC23 in thyroid cancer cells. SiRNA directed CDC23 knockdown was performed in thyroid cancer cell lines (TPC-1 and FTC-133) and qRT-PCR and immunoblot were used to confirm good CDC23 knockdown (Fig. 5). CDC23 knockdown dramatically inhibited thyroid cancer cell growth (Fig. 6A and B). A significant difference in cellular proliferation between control and CDC23 knockdown cells was observed after 4 or 5 days of transfection, which were later than the knockdown of CDC23 protein expression (Figs 5 and 6A and B). This delayed effect on cellular proliferation suggested that the effect of CDC23 may depend on cell cycling and/or due to its regulation of downstream substrates that mediate its effect.
Figure 5.
SiRNA-induced CDC23 knockdown in thyroid cancer cells. The transfection of CDC23 specific siRNA reduced CDC23 mRNA (A) and protein (B) expression in both TPC-1 and FTC-133 cells. After 24 h of transfection, the CDC23 mRNA was reduced more than 60% with CDC23-siRNA1 and more than 90% with CDC23-siRNA2 in both cell lines, while the level of CDC23 protein was reduced after 48 h (TPC-1 cells) or 72 h (FTC-133 cells) suggesting that this protein had a relatively long half-life.
Figure 6.
CDC23 knockdown decreases thyroid cancer cell proliferation and anchorage-independent growth. Cellular proliferation of TPC-1 cells (A) and FTC-133 cells (B) transfected by either scrambled or two different CDC23 specific siRNAs (CDC23–1 and CDC23–2). Data are presented as the mean±s.d. (C) Soft agar colony formations of FTC-133 cells following transfection with the indicated siRNA. (D) Quantitative measures of soft agar assay in four different transfections. Data are the mean±s.d. (E) Spheroid formation of FTC-133 cells after transfection with the indicated siRNA. FTC-133 cells, but not TPC-1 cells, formed colonies in soft agar and grew as spheroids in suspension culture. *, 0.01<P<0.05; **, 0.001<P<0.01; ***, P<0.001.
Given that CDC23 was overexpressed in PTC and regulated thyroid cancer cell proliferation; we next examined the role of this gene in regulating other cancer cell phenotypes, including anchorage-independent growth. Compared with the negative control cells, CDC23 knockdown significantly reduced the colony formation and size of FTC-133 cells (Fig. 6C and D). Specifically, in suspension cultures, cell aggregates were formed 16 h after plating and after 2 weeks a large solid spheroid with a diameter of 1 mm and a few separate small cell cluster balls were observed in the negative control cultures (Fig. 6E). The CDC23 deficient cells also formed spheroids, however, their sizes were only about 1/3 (CDC23 siRNA#1 transfected cells) or 1/10 (CDC23 siRNA#2 transfected cells) of the control cells.
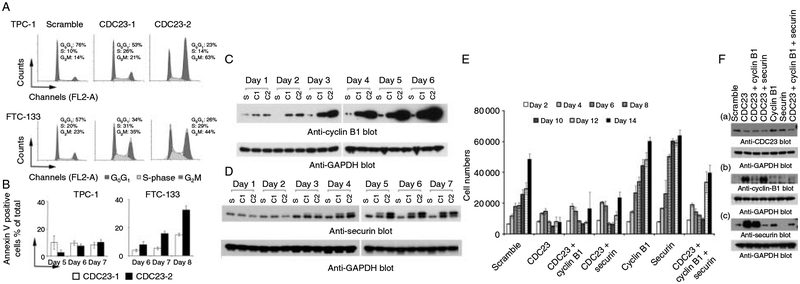
To investigate the possible mechanism of cell growth inhibition that occurs with CDC23 knockdown, cell cycle and apoptosis analyses were performed. Compared with the negative control, CDC23 deficiency resulted in an increase in the number of cells in both the S (4–19%) and G2M (7–49%) phases of cell cycle (Fig. 7A). Annexin V staining was used to examine the effects of CDC23 deficiency on thyroid cancer cell apoptosis. In TPC-1 cells, CDC23 knockdown increased the percentage of Annexin V positive cells moderately (3–10%) after 5 days of transfection. Similarly, in FTC-133 cells, the deficiency in CDC23 resulted in increased apoptosis (5–32%) 6–8 days after transfection (Fig. 7B). This time frame for increased apoptosis matched that of the reduction in cellular proliferation. In addition, these phenotypic changes occurred later than CDC23 protein knockdown, further suggesting that a downstream target of CDC23 may mediate this effect.
Figure 7.
CDC23 deficiency induced cell cycle arrest, cell apoptosis, and the accumulation of cyclin B1 and securin proteins. (A) Cell cycle analysis after 5 days (TPC-1 cells) or 7 days (FTC-133 cells) of transfection with the indicated siRNAs. (B) The percentages of Annexin V positive cells in the CDC23 siRNA transfected cells compared with control cells. Data are presented as the mean±s.e.m. (C) Cyclin B1 expression assessed by immunoblotting of whole cell lysates prepared from FTC-133 cells transfected with the indicated siRNAs. S, Scramble; C1, CDC23-1; C2, CDC23-2. (D) Securin protein expression assessed by immunoblotting of whole cell lysates prepared from FTC-133 cells transfected with the indicated siRNAs. (E) Effect of CDC23, cyclin B1, and/or securin knockdown on cell proliferation. Simultaneous cyclin B1 and/or securin knockdown reversed the cell growth inhibition effect of CDC23 deficiency as expected. (F) Western blot analysis of CDC23, cyclin B1, and/or securin knockdown.
Given the profound effect of CDC23 on thyroid cancer cell proliferation and cell cycle progression and the previous observation in yeast that mutations in APC genes lead to abnormal levels of cell cycle regulatory proteins (Peters 2006, Sullivan & Morgan 2007), especially the B-type cyclins, we explored the idea that cyclin B1 may be a substrate of CDC23 protein (Wasch & Cross 2002, Herbert et al. 2003, Thornton & Toczyski 2003, Soni et al. 2008). In both FTC-133 and TPC-1 cells, CDC23 knockdown resulted in a dramatic buildup of cyclin B1 protein, starting 3 days after transfection (Fig. 7C). Of the two different CDC23 siRNAs used to induce knockdown, siRNA#2 induced a more dramatic accumulation of cyclin B1 protein, which is consistent with this siRNA’s more dramatic functional effects on thyroid cancer cell proliferation and cell cycle arrest.
Another important substrate of APC in yeast is securin, a protein involved in sister chromatids separation (Nasmyth 2005). The phosphorylation of securin by Cdk1 is critical for its binding with separase and also reduces the ubiquitination of itself by APC (Holt et al. 2008). In Homo sapiens, pituitary tumor-transforming 1 (PTTG1) is a homolog of yeast securin protein for which the function is unknown. Therefore, we examined the protein expression of securin following CDC23 knockdown. Compared with control cells, CDC23 deficiency resulted in the accumulation of securin. There was also a change in the migration pattern of the securin protein; a second slower migrating band was present (Fig. 7D). We speculate this may be due to increased phosphorylation of the securin protein. The time course of securin accumulation and the change in migration matched that of cyclin B1 protein accumulation and also the functional changes in these cells after CDC23 knockdown. We next performed triple knockdown studies of CDC23, cyclin B1 and securin proteins to determine if the effect of CDC23 on cellular proliferation were dependent on these proteins. We found that the effect of CDC23 knockdown on cellular proliferation could be reversed by the simultaneous knockdown of cyclin B1 and securin (Fig. 7E and F). These findings, taken together with the overexpression of CDC23 in PTC provide a molecular basis by which the differential expression of CDC23 in PTC by gender may result in differences in PTC progression.
Discussion
We studied the gene expression profile of PTC tumor samples by gender. Among several genes, we found CDC23 was overexpressed in PTC in men compared with women. Furthermore, CDC23 was overexpressed in PTC and was absent in normal and hyperplastic thyroid tissue. We found sex hormone treatment of thyroid and breast cancer cell lines had no effect on CDC23 expression. Functional studies in thyroid cancer cell lines demonstrated CDC23 had a dramatic effect on cell cycle, cellular proliferation, anchorage-independent growth, and tumor spheroid formation. The effect of CDC23 on cellular proliferation and cell cycle was, at least in part, dependent on cyclin B1 and securin protein levels.
Although gender-based differences in the incidence, aggressiveness, and mortality rate for many human malignancies have been observed, the molecular basis for this is under studied and poorly understood. Thyroid cancer is an excellent tumor type to study the molecular basis of cancer gender disparity as it has a dramatically different incidence, aggressiveness, and death rate by gender. Because environmental, dietary, and reproductive factors have important roles in cancer initiation, promotion, and progression and these factors may directly regulate gene expression, we used genome-wide gene expression profiling to identify candidate genes differentially expressed in PTC by gender (Mutter et al. 2001, Arimoto et al. 2003, Steinberg et al. 2008, Masotti et al. 2010). To minimize possible confounding factors and false-positive results, the samples were matched for extent of disease (TNM stage) and age (tumor samples from premenopausal women). We found nine candidate differentially expressed genes once we cross-validated the array data to exclude X- and Y-linked genes. This suggests that these candidate genes may account for the different epidemiologic and tumor behavior observed in thyroid cancer by gender (higher incidence in women but more aggressive tumors in men).
CDC23 was one of the nine candidate genes differentially expressed by gender in PTC; 1.3-fold higher in tumor samples from men. We were interested in studying CDC23 because its expression and function in cancer cells is unknown (Irniger & Nasmyth 1997, Zhao et al. 1998, Matyskiela & Morgan 2009). We first determined whether CDC23 expression could be regulated by sex hormones as we found it to be differentially expressed by gender in PTC. In thyroid and breast cancer cell lines, treatment of cells with either estradiol or testosterone had no significant effect on CDC23 gene expression levels. This suggests that the difference in CDC23 expression level by gender is not likely to be due to sex hormone difference between women and men. Other possible reasons for the difference in CDC23 expression in PTC by gender may be due to activating oncogenic changes which may occur at different frequencies by gender, differential effect of environmental and dietary factors by gender, and secondary changes that occur differently during cancer progression by gender (Nakagawa et al. 2009, Duma et al. 2010, Rahbari et al. 2010, Lista et al. 2011, Sighoko et al. 2011). The complex possible mechanisms that may regulate CDC23 expression would need to be studied in the future to explain why this gene is differentially expressed in PTC by gender.
To determine if CDC23 was specifically mis-expressed in cancer, we next characterized the expression of CDC23 in normal and hyperplastic thyroid tissue samples, and PTC. We found CDC23 to be overexpressed in PTC. CDC23 protein was absent in normal thyroid follicular cells and hyperplastic follicular thyroid tissue. This finding suggested that CDC23 may be a positive regulator of PTC initiation and or progression. Thus, we tested the hypothesis if CDC23 had any effect on cancer cell phenotype (cell proliferation, tumor spheroid formation, cell cycle, apoptosis, and anchorage-independent growth). We found dramatic inhibition of cell growth, anchorage-independent growth, and tumor spheroid formation with cell cycle arrest as a result of CDC23 knockdown in thyroid cancer cells. Taken together, these findings all suggest that CDC23 may promote thyroid cancer initiation and or progression.
Given the profound effect of CDC23 on thyroid cancer cell proliferation and cell cycle progression, and the observation that alterations in APC genes lead to abnormal levels of cell cycle regulatory proteins, we explored the idea that cyclin B1 and securin may be a substrate of CDC23 protein and mediate its function (Wasch & Cross 2002, Herbert et al. 2003, Thornton & Toczyski 2003, Peters 2006, Sullivan & Morgan 2007, Soni et al. 2008). We show for the first time that the effect of CDC23 knockdown on cellular proliferation could be reversed by the simultaneous knockdown of cyclin B1 and securin. This finding suggests that the effect of CDC23 is mediated, at least in part, by cyclin B1 and securin which is consistent with the role of these two proteins in cancer progression (Bowers & Boylan 2004, Borlak et al. 2005, Winnepenninckx et al. 2006, Salehi et al. 2008).
In summary, our results demonstrate CDC23 is differentially expressed by gender and is over-expressed in PTC. Although sex hormones do not regulate CDC23 gene expression, our functional studies indicate that CDC23 has important biologic effects on cell proliferation and cell cycle progression. The effect of CDC23 on cancer cell phenotype is mediated, at least in part, by cyclin B1 and securin. Therefore, we propose that CDC23 is a critical regulator of cell cycle and cancer cell growth, and may be involved in thyroid cancer initiation and/or progression.
Funding
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Arimoto T, Katagiri T, Oda K, Tsunoda T, Yasugi T, Osuga Y, Yoshikawa H, Nishii O, Yano T, Taketani Y et al. 2003. Genome-wide cDNA microarray analysis of gene-expression profiles involved in ovarian endometriosis. International Journal of Oncology 22 551–560. [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M & Speed TP 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 185–193. (doi: 10.1093/bioinformatics/19.2.185) [DOI] [PubMed] [Google Scholar]
- Borlak J, Meier T, Halter R, Spanel R & Spanel-Borowski K 2005. Epidermal growth factor-induced hepatocellular carcinoma: gene expression profiles in precursor lesions, early stage and solitary tumours. Oncogene 24 1809–1819. (doi: 10.1038/sj.onc.1208196) [DOI] [PubMed] [Google Scholar]
- Bowers AJ & Boylan JF 2004. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene 328 135–142. (doi: 10.1016/j.gene.2003.12.002) [DOI] [PubMed] [Google Scholar]
- Duma D, Collins JB, Chou JW & Cidlowski JA 2010. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Science Signaling 3 ra74. (doi: 10.1126/scisignal.2001077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajkovic H, Halpern JA, Cha EK, Bahadori A, Chromecki TF, Karakiewicz PI, Breinl E, Merseburger AS & Shariat SF 2011. Impact of gender on bladder cancer incidence, staging, and prognosis. World Journal of Urology 29 457–463. (doi: 10.1007/s00345-011-0709-9) [DOI] [PubMed] [Google Scholar]
- Herbert M, Levasseur M, Homer H, Yallop K, Murdoch A & McDougall A 2003. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nature Cell Biology 5 1023–1025. (doi: 10.1038/ncb1062) [DOI] [PubMed] [Google Scholar]
- Holt LJ, Krutchinsky AN & Morgan DO 2008. Positive feedback sharpens the anaphase switch. Nature 454 353–357. (doi: 10.1038/nature07050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn-Ross PL, Morris JS, Lee M, West DW, Whittemore AS, McDougall IR, Nowels K, Stewart SL, Spate VL, Shiau AC et al. 2001. Iodine and thyroid cancer risk among women in a multiethnic population: the Bay Area Thyroid Cancer Study. Cancer Epidemiology, Biomarkers & Prevention 10 979–985. [PubMed] [Google Scholar]
- Horn-Ross PL, Hoggatt KJ & Lee MM 2002. Phytoestrogens and thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiology, Biomarkers & Prevention 11 43–49. [PubMed] [Google Scholar]
- Iribarren C, Haselkorn T, Tekawa IS & Friedman GD 2001. Cohort study of thyroid cancer in a San Francisco Bay area population. International Journal of Cancer 93 745–750. (doi: 10.1002/ijc.1377) [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B & Speed TP 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Research 31 e15. (doi: 10.1093/nar/gng015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger S & Nasmyth K 1997. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. Journal of Cell Science 110 1523–1531. [DOI] [PubMed] [Google Scholar]
- Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN & Edwards BK 2004. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 101 3–27. (doi: 10.1002/cncr.20288) [DOI] [PubMed] [Google Scholar]
- Kaput J & Rodriguez RL 2004. Nutritional genomics: the next frontier in the postgenomic era. Physiological Genomics 16 166–177. (doi: 10.1152/physiolgenomics.00107.2003) [DOI] [PubMed] [Google Scholar]
- Kebebew E, Greenspan FS, Clark OH, Woeber KA & McMillan A 2005. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer 103 1330–1335. (doi: 10.1002/cncr.20936) [DOI] [PubMed] [Google Scholar]
- Lista P, Straface E, Brunelleschi S, Franconi F & Malorni W 2011. On the role of autophagy in human diseases: a gender perspective. Journal of Cellular and Molecular Medicine 15 1443–1457. (doi: 10.1111/j.1582-4934.2011.01293.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masotti A, Da Sacco L, Bottazzo GF & Alisi A 2010. Microarray technology: a promising tool in nutrigenomics. Critical Reviews in Food Science and Nutrition 50 693–698. (doi: 10.1080/10408390903044156) [DOI] [PubMed] [Google Scholar]
- Matyskiela ME & Morgan DO 2009. Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Molecular Cell 34 68–80. (doi: 10.1016/j.molcel.2009.02.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter GL, Baak JP, Fitzgerald JT, Gray R, Neuberg D, Kust GA, Gentleman R, Gullans SR, Wei LJ & Wilcox M 2001. Global expression changes of constitutive and hormonally regulated genes during endometrial neoplastic transformation. Gynecological Oncology 83 177–185. (doi: 10.1006/gyno.2001.6352) [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N et al. 2009. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. International Journal of Cancer 125 2264–2269. (doi: 10.1002/ijc.24720) [DOI] [PubMed] [Google Scholar]
- Nasmyth K 2005. How do so few control so many? Cell 120 739–746. (doi: 10.1016/j.cell.2005.03.006) [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM & Karin M 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317 121–124. (doi: 10.1126/science.1140485) [DOI] [PubMed] [Google Scholar]
- Paggi MG, Vona R, Abbruzzese C & Malorni W 2010. Gender-related disparities in non-small cell lung cancer. Cancer Letters 298 1–8. (doi: 10.1016/j.canlet.2010.08.009) [DOI] [PubMed] [Google Scholar]
- Peters JM 2006. The anaphase promoting complex/cyclo-some: a machine designed to destroy. Nature Reviews. Molecular Cell Biology 7 644–656. (doi: 10.1038/nrm1988) [DOI] [PubMed] [Google Scholar]
- Rahbari R, Zhang L & Kebebew E 2010. Thyroid cancer gender disparity. Future Oncology 6 1771–1779. (doi: 10.2217/fon.10.127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda LC & Horn-Ross PL 2002. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiology, Biomarkers & Prevention 11 51–57. [PubMed] [Google Scholar]
- Salehi F, Kovacs K, Scheithauer BW, Lloyd RV & Cusimano M 2008. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocrine-Related Cancer 15 721–743. (doi: 10.1677/ERC-08-0012) [DOI] [PubMed] [Google Scholar]
- Sighoko D, Curado MP, Bourgeois D, Mendy M, Hainaut P & Bah E 2011. Increase in female liver cancer in the gambia, west Africa: evidence from 19 years of population-based cancer registration (1988–2006). PLoS ONE 6 e18415. (doi: 10.1371/journal.pone.0018415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni DV, Sramkoski RM, Lam M, Stefan T & Jacobberger JW 2008. Cyclin B1 is rate limiting but not essential for mitotic entry and progression in mammalian somatic cells. Cell Cycle 7 1285–1300. (doi: 10.4161/cc.7.9.5711) [DOI] [PubMed] [Google Scholar]
- Steinberg CE, Sturzenbaum SR & Menzel R 2008. Genes and environment – striking the fine balance between sophisticated biomonitoring and true functional environmental genomics. Science of the Total Environment 400 142–161. (doi: 10.1016/j.scitotenv.2008.07.023) [DOI] [PubMed] [Google Scholar]
- Sullivan M & Morgan DO 2007. Finishing mitosis, one step at a time. Nature Reviews. Molecular Cell Biology 8 894–903. (doi: 10.1038/nrm2276) [DOI] [PubMed] [Google Scholar]
- Thornton BR & Toczyski DP 2003. Securin and B-cyclin/CDK are the only essential targets of the APC. Nature Cell Biology 5 1090–1094. (doi: 10.1038/ncb1066) [DOI] [PubMed] [Google Scholar]
- Wasch R & Cross FR 2002. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418 556–562. (doi: 10.1038/nature00856) [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V, Debiec-Rychter M, Belien JA, Fiten P, Michiels S, Lazar V, Opdenakker G, Meijer GA, Spatz A & van den Oord JJ 2006. Expression and possible role of hPTTG1/securin in cutaneous malignant melanoma. Modern Pathology 19 1170–1180. (doi: 10.1038/modpathol.3800627) [DOI] [PubMed] [Google Scholar]
- Yeh SH & Chen PJ 2010. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology 78 (Suppl 1) 172–179. (doi: 10.1159/000315247) [DOI] [PubMed] [Google Scholar]
- Zhao N, Lai F, Fernald AA, Eisenbart JD, Espinosa R, Wang PW & Le Beau MM 1998. Human CDC23: cDNA cloning, mapping to 5q31, genomic structure, and evaluation as a candidate tumor suppressor gene in myeloid leukemias. Genomics 53 184–190. (doi: 10.1006/geno.1998.5473) [DOI] [PubMed] [Google Scholar]