Abstract
Nitric oxide (NO) limits formation of neointimal hyperplasia in animal models of arterial injury in large part by inhibiting vascular smooth muscle cell (VSMC) proliferation through cell cycle arrest. The ubiquitin-conjugating enzyme UbcH10 is responsible for ubiquitinating cell cycle proteins for proper exit from mitosis. We hypothesize that NO prevents VSMC proliferation, and hence neointimal hyperplasia, by decreasing levels of UbcH10. Western blotting and immunofluorescent staining showed that NO reduced UbcH10 levels in a concentration-dependent manner in VSMC harvested from the abdominal aortas of Sprague-Dawley rats. Treatment with NO or siRNA to UbcH10 decreased both UbcH10 levels and VSMC proliferation (P < 0.001), while increasing UbcH10 levels by plasmid transfection or angiotensin II stimulation increased VSMC proliferation to 150% (P = 0.008) and 212% (P = 0.002) of control, respectively. Immunofluorescent staining of balloon-injured rat carotid arteries showed a ~4-fold increase in UbcH10 levels, which was profoundly decreased following treatment with NO. Western blotting of carotid artery lysates showed no UbcH10 in uninjured vessels, a substantial increase in the injury alone group, and a significant decrease in the injury + NO group (~3-fold reduction versus injury alone). Importantly, in vitro and in vivo, a marked increase in polyubiquitinated UbcH10 was observed in the NO-treated VSMC and carotid arteries, respectively, indicating that NO may be decreasing unmodified UbcH10 levels by increasing its ubiquitination. Central to our hypothesis, we report that NO decreases UbcH10 levels in VSMC in vitro and following arterial injury in vivo in association with increasing polyubiquitinated-UbcH10 levels. These changes in UbcH10 levels correlate with VSMC proliferation and neointimal hyperplasia, making UbcH10 a promising therapeutic target for inhibiting this proliferative disease.
Keywords: Neointimal hyperplasia, Nitric oxide, Proteasome, UbcH10, Ubiquitin
Introduction
The ubiquitin–proteasome pathway is responsible for the breakdown of the majority of short-lived proteins in the eukaryotic cell, including proteins involved in maintaining normal cell cycle progression [1, 2]. Protein ubiquitination proceeds via the coordinated activities of the ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin ligase (E3) enzymes, linking an ε-amino group of the C-terminal glycine of ubiquitin to a lysine on a protein targeted for degradation [3]. Once a chain of at least four ubiquitin molecules has been added to the target protein, the polyubiquitin chain can be recognized by the 26S proteasome and the protein can be degraded [4]. One of the E2 enzymes, UbcH10, is known to interact with the E3 ligase, the anaphase promoting complex (APC), to sequentially ubiquitinate cell cycle proteins such as cyclin A, securin, and cyclin B, so that cells can exit mitosis [2, 5]. Recently, it has been shown that the UbcH10/APC complex autoubiquitinates UbcH10 in the beginning of G1, targeting UbcH10 for degradation [1]. This autoubiquitination and degradation of UbcH10 is necessary for normal cell cycle progression, as increased levels of UbcH10 have clearly been linked to pro-proliferative cancer disorders [6–8].
Neointimal hyperplasia is a complication of vascular interventions. It is characterized by aggressive cellular proliferation and occurs following procedures to restore flow to occluded arteries, such as angioplasty and stenting, endarterectomy, and bypass grafting. We, and others, have shown that nitric oxide (NO) prevents VSMC proliferation, causes G0/G1 cell cycle arrest, and is effective at inhibiting formation of neointimal hyperplasia in small and large animal models of arterial injury and vein bypass grafting [9–11]. Given the association of UbcH10 with cell cycle progression, and given that neointimal hyperplasia is a pro-proliferative disorder, we hypothesize that NO inhibits neointimal hyperplasia by decreasing UbcH10 levels. We show here, both in vascular smooth muscle cells (VSMC) in vitro and in a rat carotid artery balloon injury model of neointimal hyperplasia in vivo, that levels of UbcH10 directly correlate with proliferation and neointimal hyperplasia. Interestingly, we also show that NO decreases levels of UbcH10 by increasing the ubiquitination and degradation of UbcH10. This makes UbcH10 a promising target for preventing neointimal hyperplasia and restenosis following vascular interventions.
Materials and Methods
Cell Culture
Vascular smooth muscle cells (VSMC) were harvested and cultured from the abdominal aorta of Sprague-Dawley rats (Harlan, Indianapolis, IN), and maintained as previously described [12, 13]. Cultured cells had the characteristic appearance of hills and valleys and were routinely more than 95% pure by smooth muscle cell α-actin staining [14].
Diethylenetriamine NONOate (DETA/NO) Preparation
Diethylenetriamine NONOate (DETA/NO) was synthesized by L.K.K. and J.E.S. as previously described [15]. Stock solutions (10 mM) were prepared by dissolving solid DETA/NO in the appropriate complete culture medium just before use.
Cell Proliferation
Vascular smooth muscle cells (VSMC) plated in 12-well plates (4 × 104 cells/well) were growth arrested for 24 h in medium without fetal bovine serum (FBS). Cells were then incubated in complete medium containing tritiated [3H]-thymidine (5 μCi/ml, PerkinElmer, Wellesley, MA) and DETA/NO (125–1000 μM) for 24 h. Tritiated [3H]-thymidine incorporated into trichloroacetic-acid-precipitated DNA was measured with a Wallac WinSpectral 1414 liquid scintillation counter (Wallac, Turku, Finland).
Western Blot Analysis
Whole-cell suspensions of DETA/NO-treated, angiotensin II-treated (AngII; Sigma-Aldrich; St. Louis, MO), and untreated VSMC were prepared as previously described [16] and protein concentrations determined by bicinchoninic acid assays performed according to the manufacturer’s instructions (Pierce, Rockford, IL). Whole-cell suspensions were subjected to acrylamide gel electrophoresis on 8–13% gels, after which proteins were transferred to a nitrocellulose membrane. Protein levels were determined using antibodies to UbcH1, UbcH2, UbcH3, UbcH5, UbcH6, UbcH7, UbcH9, UbcH10, and UbcH12 (1:500 to 1:2000; Boston Biochem, Boston, MA). Blotting for β-actin served as a loading control.
Immunoprecipitation
For immunoprecipitations, DETA/NO-treated and untreated VSMC were lysed using lysis buffer A (20 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethyleneglycoltetraacetic acid (EGTA), 1% (v/v) Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, and 1 mM PMSF), and 50 μg of lysate was incubated with 1 μg of goat IgG plus 20 μl of protein A/G Sepharose (both from Santa Cruz) for 1 h at 4°C on a rotating wheel. The beads were then pelleted by centrifugation for 5 min at 1500 rpm and 4°C in an Eppendorf 5417R centrifuge (Westbury, NY), and the supernatant transferred to a fresh tube, to which was added 20 ll of protein A/G Sepharose and 2.5 μg of UbcH10 antibody (Santa Cruz). After overnight incubation at 4°C on a rotating wheel, the beads were pelleted at 2500 rpm for 15 min at 4°C and the supernatants carefully removed. The beads were then subjected to acrylamide gel electrophoresis, transferred to nitrocellulose membranes, and protein levels were detected using the antibody concentrations listed above.
siRNA Transfection
Vascular smooth muscle cells (VSMC) were plated in 12-well plates (4 × 104 cells/well) with 1 ml of complete medium. Transfection reactions were prepared in OptiMEM Medium (Invitrogen) and consisted of 75 pmol of UbcH10 siRNA (sequence GGAGAACCCAACAUCGAGAUU; Dharmacon, Lafeyette, CO) or a scrambled control siRNA (D-001210-01-05, Dharmacon) and a 1:50 dilution of RNAiMax (Invitrogen). One hundred microliters of this transfection reaction mixture was added to each well 16–24 h after initial plating. After 3.5 h, the transfection reaction mixture was aspirated off and replaced with complete medium treated with or without DETA/NO. After 48–72 h, [3H]-thymidine was added to each well for 16 h, and incorporation was measured as described above.
Plasmid Transfection
Vascular smooth muscle cells (VSMC) were plated in 12-well plates (4 × 104 cells/well) with 1 ml of complete medium. The next day, transfection reactions were prepared in serum-free medium consisting of 5 μg of UbcH10 plasmid (Origene; Rockville, MD) or 5 μg of a control plasmid and 1.2 μl of MegaTran 1.0 transfection reagent (Origene). The plating medium was then aspirated off and the transfection reaction mixture was added to cells. After 4 h, the transfection mixture was aspirated off, complete medium containing [3H]-thymidine was added to each well for 16 h, and incorporation was measured as described above.
Immunofluorescent Cell Staining
Vascular smooth muscle cells (VSMC) plated on coverslips placed inside 6-well plates (1 × 105 cells/well) were growth arrested for 16–24 h in serum-free medium. The cells were then treated with 1000 μM DETA/NO for 24 h, followed by fixation in 5% formaldehyde. The coverslips were permeabilized with 0.3% Triton X-100 in PBS for 10 min, blocked with donkey serum (1:20 in BSA) for 30 min, exposed to an anti-UbcH10 antibody (1:50, sc-47545, Santa Cruz) for 1 h, rinsed, exposed to an AlexaFluor 594 secondary antibody (1:2000 in PBS, Invitrogen, Eugene, OR) for 30 min, rinsed, then stained with DAPI (1:500 in PBS) for 30 s. Coverslips were then cured to slides using Pro Long Anti Fade Reagent (Invitrogen). Digital images were acquired using Spot Advanced software (Diagnostic Instruments; Sterling Heights, MI) on a Nikon Eclipse 50i Microscope (Nikon Instruments, Inc.; Melville, NY) with a ×40 objective.
Animal Surgery
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85–23, 1996) and approved by the Northwestern University Animal Care and Use Committee. Male Sprague-Dawley rats weighing between 350 and 400 g were anesthetized with inhaled isoflurane (0.5–3%). Atropine was administered subcutaneously (0.1 mg/kg) to decrease airway secretions. After a midline neck incision, the left common carotid artery (CCA), external carotid artery (ECA), and the internal carotid artery (ICA) were dissected and proximal and distal control obtained with microclips. A transverse arteriotomy was created on the ECA. A 2 French Fogarty catheter (generously provided by Edwards Lifesciences) was inserted into the CCA through the ECA, and the CCA injured by inflating the balloon to 5 atmospheres of pressure for 5 min. Following injury, the catheter was removed, the ECA ligated, and flow restored to the CCA and ICA, as previously described [10, 11, 17]. Immediately after injury and restoration of flow, 20 mg of proline NONOate (PROLI/NO) powder was applied evenly to the periadventitial surface of the injured CCA of rats in the treatment group as previously described [10, 11, 17]. The neck incision was closed and carotid arteries were harvested at 3 or 14 days post-injury. Control groups included no injury and injury alone (n = 3/group).
Tissue Processing for Histology
Carotid arteries were harvested 14 days after balloon injury as follows. Rats were anesthetized with isoflurane and euthanized with 0.5 ml euthasol, and a bilateral thoracotomy was performed. A long midline incision was made to expose both carotid arteries. Vessels were perfused and fixed in situ using cold 1× PBS (250 ml) and 2% paraformaldehyde (500 ml), and then explanted en bloc from the aortic arch to the carotid bifurcation. The injured segment of the carotid arteries were frozen in TissueTek O.C.T. compound (Sakura Finetek USA, Torrance, CA) and cut into 5-μm sections throughout the entire injured area, as previously described [9].
Tissue Processing for Lysates
For carotid lysates, vessels were harvested 3 days after balloon injury. Rats were anesthetized, euthanized, and the vessels exposed as described above; however, the rats did not undergo in situ perfusion and fixation. Each carotid artery was ligated at the aortic arch and explanted en bloc as quickly as possible. Vessels were identified, washed in cold 1× PBS, opened en face, and the injured section of the CCA was excised, snap-frozen, and stored in liquid nitrogen until lysed via mechanical means (ceramic mortar and pestle, CoorsTek; Golden, CO) and resuspended in lysis buffer (50 mM Hepes [pH 7.5], 150 mM NaCl, 10% glycerol (v/v), 10 mM sodium pyrophosphate [Na4P2O7], 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid [EGTA], 1% Triton X-100, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 mM sodium fluoride [NaF], 1 mM sodium orthovanadate [Na3VO4], and 1 mM PMSF). Protein concentration was determined via BCA assay, and samples were stored at ‒80°C until western blot analysis.
Immunofluorescent Tissue Staining
Carotid artery sections of uninjured, injured, and NO-treated rats were fixed in 2% paraformaldehyde. Slides were permeabilized with 0.3% Triton X-100 in PBS for 10 min. Slides were then blocked with donkey serum (1:20 in BSA) for 30 min, exposed to an antibody toUbcH10 (1:50 in BSA, Santa Cruz) for 1 h, rinsed, exposed to an AlexaFluor 594 secondary antibody (1:2000 in PBS, Invitrogen) for 30 min, and stained with DAPI (1:500 in PBS) for 30 s. The slides were then coverslipped with Pro Long Anti Fade Reagent (Invitrogen). Digital images were acquired using Spot Advanced software (Diagnostic Instruments; Sterling Heights, MI) on a Nikon Eclipse 50i Microscope (Nikon Instruments, Inc.; Melville, NY) with a ×20 objective.
Statistical Analysis
Results are expressed as the mean ± standard error (SE) of the mean. Differences between groups were analyzed by one-way analysis of variance (ANOVA) with the Student–Newmann–Keuls post hoc test for all pairwise comparisons (SigmaStat; SPSS. Chicago, IL). Statistical significance was assumed for P < 0.05.
Results
NO Decreased Levels of the Ubiquitin-Conjugating Enzymes Required for Proper Cell Cycle Advancement
To begin our investigation into the effects of NO on protein ubiquitination, we performed western blotting analysis on extracts of VSMC treated in the presence or absence of the NO donor DETA/NO (500–1000 μM) for 24 h. As seen in Fig. 1, while NO had little to no effect on UbcH1, UbcH2, UbcH5, UbcH6, UbcH7, or UbcH9, it decreased levels of UbcH3, UbcH10, and UbcH12 (Fig. 1 arrows), all of which are crucial to proper cell cycle advancement. UbcH3 breaks down the cyclin-dependent kinase inhibitor p27 [18], but is also autoubiquitinated to insure the cell moves out of G1 and into S phase [19]. UbcH12 ubiquitinates the cullin protein that is part of the Skp-cullin-F-box ubiquitin ligase [20], which is the E3 partner of UbcH3. Finally, UbcH10 is required for ubiquitination and degradation of cyclin A, securin, and cyclin B, which allows exit from mitosis [5]. Since NO induced the greatest decrease from baseline in UbcH10 levels, we decided to pursue this relationship further.
Fig. 1.
Nitric oxide (NO) reduced levels of ubiquitin-conjugating (E2) enzymes important for cell cycle progression. Vascular smooth muscle cells (VSMC) were treated with the NO donor diethylenetriamine NONOate (DETA/NO, 500–1000 μM), then subjected to western blot analysis using antibodies to the E2 enzymes shown on the left. Equal loading was verified by staining for β-actin. NO caused decreased levels of UbcH3, UbcH10, and UbcH12 (arrows), with the greatest decrease from baseline seen in UbcH10. t = 24 h. Images are representative of three separate experiments
Decreased UbcH10 Levels Correlated with Decreased VSMC Proliferation
Since NO is known to inhibit VSMC proliferation, and we observed that NO decreases UbcH10 levels, we performed [3H]-thymidine incorporation assays and VSMC protein collections in tandem to examine this relationship further. As seen in Fig. 2a, the NO-mediated decrease in UbcH10 levels was concentration dependent. Interestingly, NO caused an increase in polyubiquitinated UbcH10. As shown in Fig. 2b, this decrease in UbcH10 directly correlated with inhibition of VSMC proliferation (59% inhibition with DETA/NO 1000 μM, P < 0.001). Immunofluorescent staining for UbcH10 in VSMC revealed a mostly cytoplasmic and perinuclear pattern of distribution and confirmed that NO decreased levels of UbcH10 in VSMC (Fig. 2c, red staining).
Fig. 2.
NO decreased VSMC proliferation and levels of UbcH10 in a concentration-dependent manner. a VSMC treated with DETA/NO (62.5–1000 μM) were subjected to western blot analysis for UbcH10. As seen in Fig. 1, 500 and 1000 μM DETA/NO caused the greatest reduction in UbcH10 levels. NO was also noted to increase polyubiquitinated UbcH10 (Ub-UbcH10). b Proliferation of VSMC was assessed via [3H]-thymidine incorporation. NO treatment decreased VSMC proliferation 59% compared to control, * P = 0.001. c Immunofluorescent staining of VSMC for UbcH10 (red) showed a distinct localization to the cytoplasm in treated and untreated cells. While NO did not alter the subcellular localization of UbcH10, it markedly decreased UbcH10 levels. Nuclei were stained with DAPI (blue). Data shown are representative of three separate experiments. t = 24 h, n = 3/treatment group
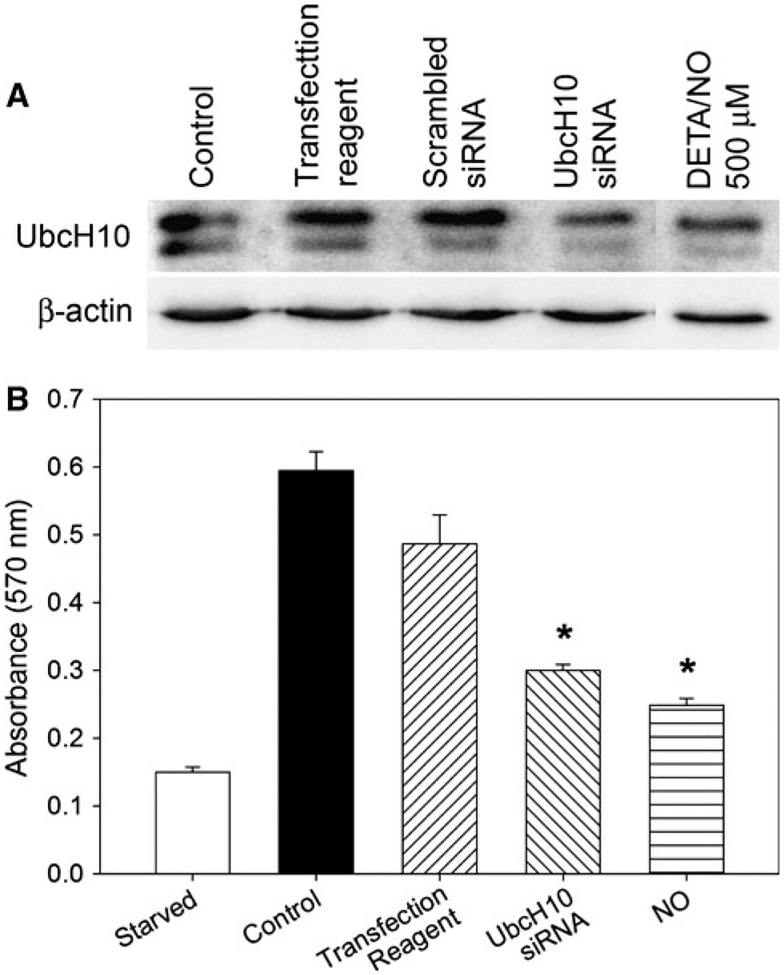
Since NO lowered UbcH10 levels and inhibited proliferation in VSMC, we sought to determine whether lowering UbcH10 levels independent of NO would have a similar effect. To that end, we transfected VSMC with siRNA against UbcH10 and assessed protein levels and proliferation. As seen in Fig. 3a, we achieved knockdown of UbcH10 on par with that caused by 0.5 mM DETA/NO. As shown in Fig. 3b, decreased UbcH10 levels caused by either siRNA or NO correlated with inhibition of VSMC proliferation (50% and 58% inhibition, respectively; P < 0.001).
Fig. 3.
Knockdown of UbcH10 levels decreased VSMC proliferation. a VSMC were transfected with siRNA against UbcH10, a scrambled control, transfection reagent only (Dharmafect), or treated with DETA/NO (500 μM), then subjected to western blot analysis using antibodies to UbcH10 or β-actin. UbcH10 knockdown using siRNA was similar to that observed with DETA/NO. b Proliferation of VSMC transfected as in a was assessed via [3H]-thymidine incorporation. NO treatment and siRNA transfection and NO treatment caused similar decreases in VSMC proliferation (50% and 58% versus control, respectively, * P < 0.001). t = 48 h. Data are representative of three separate experiments
Increased UbcH10 Levels Correlated with Increased VSMC Proliferation
Since decreased UbcH10 levels led to decreased VSMC proliferation, we wanted to determine whether increased UbcH10 levels would lead to increased VSMC proliferation. As shown in Fig. 4a, VSMC transfected with a plasmid (5 μg) bearing the gene for UbcH10 showed a marked increase in UbcH10 levels. Increased UbcH10 levels correlated with increased VSMC proliferation (150% of control, P = 0.008), as seen in Fig. 4b.
Fig. 4.
Increased UbcH10 levels increased VSMC proliferation. a VSMC transfected with a plasmid bearing the gene for UbcH10 (pUbcH10) were subjected to western blot analysis using antibodies to UbcH10 or β-actin. Transfection caused a significant increase in UbcH10 expression over baseline. b Proliferation of VSMC transfected as in a was assessed via [3H]-thymidine incorporation, and showed that pUbcH10 increased proliferation to 150% of control. * P = 0.008, t = 24 h. Data are representative of three separate experiments
Angiotensin II (AngII) is commonly used to stimulate VSMC proliferation in culture. Since we observed increased proliferation when we increased UbcH10 levels via plasmid transfection, we wanted to ascertain whether UbcH10 levels increased in AngII-treated VSMC. As seen in Fig. 5a, treatment with AngII (5000 nM) caused a significant increase in UbcH10 levels, which was accompanied by a sharp increase in VSMC proliferation (212% of control, P = 0.002), as shown in Fig. 5b. Interestingly, NO still decreased UbcH10 levels and proliferation in VSMC treated with AngII.
Fig. 5.
NO inhibited angiotensin II-stimulated VSMC proliferation and increased UbcH10 levels. a VSMC treated with angiotensin II (AngII, 1000 or 5000 nM) to stimulate proliferation were treated with DETA/NO (500 or 1000 μM) and subjected to western blot analysis using antibodies to UbcH10 or β-actin. AngII treatment caused a significant increase in UbcH10 levels, which was abrogated by treatment with NO. b Proliferation of VSMC treated as in a was assessed via [3H]-thymidine incorporation. AngII increased VSMC proliferation to 212% of control, which was substantially decreased upon exposure to DETA/NO. * P = 0.002 vs. control, t = 24 h. Data are representative of three separate experiments
NO Decreased Injury-Induced Increases in UbcH10 Levels
To study the effects of NO on UbcH10 levels in vivo, we employed the rat carotid artery balloon injury model. Immunofluorescent staining of artery sections (Fig. 6) showed that injury significantly increased UbcH10 levels (red staining) 14 days post-injury. This increase in UbcH10 was observed throughout all three layers of the arterial wall (i.e., neointima, media, and adventitia). Periadventitial treatment with the NO donor PROLI/NO (20 mg) caused a substantial decrease in injury-induced UbcH10 levels throughout all three layers of the arterial wall.
Fig. 6.
NO decreased the injury-mediated increase of UbcH10 levels in vivo. Following balloon injury and treatment with or without the nitric oxide donor proline NONOate (PROLI/NO, 20 mg, t = 14 days), rat carotid artery cross sections underwent immunofluorescent staining for UbcH10 levels (red). UbcH10 levels increased following arterial injury, and was significantly decreased by the addition of NO. Nuclei were stained with DAPI (blue). Auto-fluorescence of the internal elastic lamina is also shown (green). Data are representative of three separate stains
To further study the effects of NO on UbcH10 levels and determine if NO decreased UbcH10 by increasing polyubiquitination of UbcH10, we homogenized arteries 3 days after injury and subjected them to western blot analysis. As seen in Fig. 7, balloon injury increased UbcH10 levels, correlating with the histology data. NO treatment reduced UbcH10 levels but increased polyubiquitinated UbcH10, supporting our in vitro data.
Fig. 7.
NO reversed the injury-mediated increase in UbcH10 levels and increased polyubiquitinated UbcH10 levels in the carotid artery in vivo. Following balloon injury and treatment with or without PROLI/NO (20 mg, t = 3 days), rat carotid arteries were homogenized and subjected to western blot analysis using antibodies to UbcH10 or β-actin. As seen in VSMC culture, baseline levels of UbcH10 were low (uninjured group), but increased dramatically following balloon injury. Also similar to VSMC culture, treatment with NO almost completely reversed the increase in UbcH10 levels. Interestingly, balloon injury alone increased levels of polyubiquitinated UbcH10, but the addition of NO treatment significantly increased these higher molecular weight bands, indicating that NO may be causing the breakdown of UbcH10 by increasing the polyubiquitination of UbcH10. n = 3 rats/group. Data are representative of three replicates
Discussion
Here we show for the first time that levels of UbcH10 in the vasculature directly correlate with cellular proliferation in vitro and in vivo. Depletion of UbcH10 levels by NO or siRNA treatment caused decreased VSMC proliferation. Elevation of UbcH10 levels using plasmid transfection or angiotensin II stimulation led to increased VSMC proliferation. Balloon-injured arteries showed increased UbcH10 levels, especially in the neointima, an area of aggressive proliferation. When balloon-injured arteries were treated with NO, neointima formation was abrogated, and UbcH10 levels dropped markedly. Interestingly, we showed that while treatment with NO decreased levels of unmodified UbcH10, it increased levels of polyubiquitinated UbcH10, indicating that NO may be preventing proliferation by causing UbcH10 degradation by the 26S proteasome.
Much is known about UbcH10 and its role in regulating cell cycle progression by aiding the APC to degrade cyclin A, securin, and cyclin B, thus allowing cells to exit mitosis [5]. Recent work has shown that autoubiquitination of UbcH10 allows it to be degraded in a timely manner by the APC at the beginning of G1, leading to inactivation of the APC [1, 2]. The inactivation of the APC allows UbcH10 to accumulate through S phase and peak in mitosis, restarting the cycle [5]. Disruption of UbcH10 levels leads to derangements in the cell cycle, with many of the effects of increasing or decreasing levels of UbcH10 documented in cancer. Cancers derived from many different tissues, including lung, stomach, ovary, breast, uterus, bladder, and brain, have increased levels of UbcH10 [8]. Increasing levels of UbcH10 in NIH-3T3 fibroblasts causes them to become more invasive and proliferative [7], while knockdown of UbcH10 levels using siRNA decreases cellular proliferation and invasiveness [6, 8]. Taken together, these results show that UbcH10 is clearly important in regulating proliferation.
Regarding the vasculature, many studies have shown the beneficial effects of NO, including maintenance of vascular tone, upregulation of endothelial cell proliferation, prevention of endothelial cell apoptosis, prevention of leukocyte chemotaxis, prevention of VSMC proliferation and migration, and enhancement of VSMC apoptosis [21]. The effects of temporary inhibition of the proteasome in the vasculature are also well-known, and are similar to those of NO administration following balloon injury. They include prevention of neointimal hyperplasia, increased cellular apoptosis, reduction in macrophage infiltration, and accumulation of ubiquitinated proteins [22, 23]. The buildup of ubiquitinated proteins due to chronic inhibition of the proteasome has been linked to a number of cardiovascular dysfunctions, including cardiomyopathy, unstable atherosclerotic plaques, and increased intimal hyperplasia of vein grafts [24–29]. Thus, while temporary inhibition of the proteasome by NO can be beneficial, chronic inhibition causes derangements in protein clearance and can lead to disease in the vasculature.
In contrast to the wealth of information regarding the effects of NO in the vasculature and UbcH10 in cancer, very little is known about the actions of UbcH10 in the vasculature and even less is known about the effect of NO on UbcH10 in the vasculature. To our knowledge, this is the first study to evaluate the effects of NO on UbcH10 levels in the vasculature. Though our results show an essential action for UbcH10 in cellular proliferation, there is one limitation of the current study; namely, the mechanism by which NO acts on UbcH10 is not known. We showed that NO caused increased polyubiquitination of UbcH10, but do not know how NO is causing this reaction. NO could either be increasing ubiquitination of UbcH10 or preventing deubiquitination of UbcH10, thus keeping UbcH10 in an inactive state and preventing cell cycle progression. With respect to increasing ubiquitination of UbcH10, NO may act to increase the activity of the APC through altering APC activators (i.e., Cdc20 or Cdh1) or APC inhibitors (i.e., Emi1, Mad2, or Bubr1). Furthermore, given that the majority of deubiquitinating enzymes (DUBs) are cysteine proteases, it is possible that NO is inhibiting the DUB that deubiquitinates UbcH10 through S-nitrosylation. More work in our lab is currently underway to determine the deubiquitinating enzyme(s) responsible for acting on UbcH10.
In conclusion, we showed that UbcH10 is an important mediator of the effects of NO in the vasculature. Specifically, increased UbcH10 caused increased proliferation and neointimal hyperplasia, while decreased UbcH10 led to decreased proliferation and neointimal hyperplasia. NO also caused increased levels of polyubiquitinated UbcH10 to form, providing a glimpse at the mechanism by which NO acts to inhibit neointimal hyperplasia. This important work could lead to a small molecule therapy to target UbcH10 and prevent neointimal hyperplasia and restenosis without the side effects of systemic NO or proteasome inhibitor administration.
Acknowledgments
The authors would like to express their thanks to the Northwestern University Institute for BioNanotechnology in Medicine, the Northwestern University Feinberg Cardiovascular Research Institute, to Lynnette Dangerfield for her administrative support, and to Edwards Lifesciences for providing the Fogarty balloon catheters. This work was supported in part by funding from the National Institutes of Health (1K08HL0842-03 to MRK), the American Vascular Association (Mentored Clinical Scientist Development Award to MRK), the American Heart Association (0725766Z and 09POST2230028 to NDT), and by the generosity of Mrs. Hilda Rosenbloom and Mrs. Eleanor Baldwin. In addition, part of this research was supported with federal funds from the National Cancer Institute, NIH, under Contract N01-CO-2008-00001 with SAIC-Frederick, Inc. and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Contributor Information
Larry K. Keefer, Laboratory for Comparative Carcinogenesis/Center for Cancer Research, National Cancer Institute-Frederick, Frederick, MD, USA
Melina R. Kibbe, Division of Vascular Surgery, Northwestern University, 676 N. St Clair, #650, Chicago, IL 60611, USA
References
- 1.Rape M, & Kirschner MW (2004). Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature, 432(7017), 588–595. [DOI] [PubMed] [Google Scholar]
- 2.Rape M, Reddy SK, & Kirschner MW (2006). The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell, 124(1), 89–103. [DOI] [PubMed] [Google Scholar]
- 3.Hershko A, & Ciechanover A (1998). The ubiquitin system. Annual Review of Biochemistry, 67, 425–479. [DOI] [PubMed] [Google Scholar]
- 4.Crews CM (2003). Feeding the machine: Mechanisms of proteasome-catalyzed degradation of ubiquitinated proteins. Current Opinion in Chemical Biology, 7(5), 534–539. [DOI] [PubMed] [Google Scholar]
- 5.De GA, Ganier O, & Cohen-Fix O (2006). Before and after the spindle assembly checkpoint—an APC/C point of view. Cell Cycle, 5(18), 2168–2171. [DOI] [PubMed] [Google Scholar]
- 6.Berlingieri MT, Pallante P, Sboner A, Barbareschi M, Bianco M, Ferraro A, et al. (2007). UbcH10 is overexpressed in malignant breast carcinomas. European Journal of Cancer, 43(18), 2729–2735. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, & Nakagawara A (2003). UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Research, 63(14), 4167–4173. [PubMed] [Google Scholar]
- 8.Wagner KW, Sapinoso LM, El-Rifai W, Frierson HF, Butz N, Mestan J, et al. (2004). Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene, 23(39), 6621–6629. [DOI] [PubMed] [Google Scholar]
- 9.Shears LL, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, et al. (1998). Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. Journal of the American College of Surgeons, 187(3), 295–306. [DOI] [PubMed] [Google Scholar]
- 10.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, et al. (2008). Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radical Biology and Medicine, 44(1), 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, et al. (2008). Nitric oxide and nanotechnology: A novel approach to inhibit neointimal hyperplasia. Journal of Vascular Surgery, 47(1), 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu SM, Hung LM, & Lin CC (1997). cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation, 95(5), 1269–1277. [DOI] [PubMed] [Google Scholar]
- 13.Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, et al. (2000). Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. Journal of Vascular Surgery, 31(6), 1214–1228. [DOI] [PubMed] [Google Scholar]
- 14.Chamley JH, Campbell GR, McConnell JD, & Groschel-Stewart U (1977). Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell and Tissue Research, 177(4), 503–522. [DOI] [PubMed] [Google Scholar]
- 15.Hrabie JA, Klose JR, Wink DA, & Keefer LK (1993). New nitric oxide-releasing zwitterions derived from polyamines. Journal of Organic Chemistry, 58(6), 1472–1476. [Google Scholar]
- 16.Kibbe MR, Nie S, Seol DW, Kovesdi I, Lizonova A, Makaroun M, et al. (2000). Nitric oxide prevents p21 degradation with the ubiquitin-proteasome pathway in vascular smooth muscle cells. Journal of Vascular Surgery, 31(2), 364–374. [DOI] [PubMed] [Google Scholar]
- 17.Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, et al. (2008). Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. American Journal of Physiology - Heart and Circulatory Physiology, 295(6), H2388–H2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butz N, Ruetz S, Natt F, Hall J, Weiler J, Mestan J, et al. (2005). The human ubiquitin-conjugating enzyme Cdc34 controls cellular proliferation through regulation of p27Kip1 protein levels. Experimental Cell Research, 303(2), 482–493. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A, Gregori L, Xu Y, & Chau V (1993). The bacterially expressed yeast CDC34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. Journal of Biological Chemistry, 268(8), 5668–5675. [PubMed] [Google Scholar]
- 20.Osaka F, Kawasaki H, Aida N, Saeki M, Chiba T, Kawashima S, et al. (1998). A new NEDD8-ligating system for cullin-4A. Genes and Development, 12(15), 2263–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahanchi SS, Tsihlis ND, & Kibbe MR (2007). The role of nitric oxide in the pathophysiology of intimal hyperplasia. Journal of Vascular Surgery, 45(Suppl A), A64–A73. [DOI] [PubMed] [Google Scholar]
- 22.Meiners S, Laule M, Rother W, Guenther C, Prauka I, Muschick P, et al. (2002). Ubiquitin-proteasome pathway as a new target for the prevention of restenosis. Circulation, 105(4), 483–489. [DOI] [PubMed] [Google Scholar]
- 23.Kapadia MR, Eng JW, Jiang Q, Stoyanovsky DA, & Kibbe MR (2009). Nitric oxide regulates the 26S proteasome in vascular smooth muscle cells. Nitric Oxide, 20(4), 279–288. [DOI] [PubMed] [Google Scholar]
- 24.Faries PL, Rohan DI, Wyers MC, Marin ML, Hollier LH, Quist WC, et al. (2001). Relationship of the 20S proteasome and the proteasome activator PA28 to atherosclerosis and intimal hyperplasia in the human vascular system. Annals of Vascular Surgery, 15(6), 628–633. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann J, Saguner AM, Versari D, Peterson TE, Chade A, Olson M, et al. (2007). Chronic proteasome inhibition contributes to coronary atherosclerosis. Circulation Research, 101(9), 865–874. [DOI] [PubMed] [Google Scholar]
- 26.Weekes J, Morrison K, Mullen A, Wait R, Barton P, & Dunn MJ (2003). Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics, 3(2), 208–216. [DOI] [PubMed] [Google Scholar]
- 27.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, et al. (2010). Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation, 121(8), 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, et al. (2006). Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(9), 2132–2139. [DOI] [PubMed] [Google Scholar]
- 29.Stone DH, Sivamurthy N, Contreras MA, Fitzgerald L, LoGerfo FW, & Quist WC (2001). Altered ubiquitin/proteasome expression in anastomotic intimal hyperplasia. Journal of Vascular Surgery, 34(6), 1016–1022. [DOI] [PubMed] [Google Scholar]